Six Decades of Research on Human Fetal Gonadal Steroids
Abstract
1. Introduction
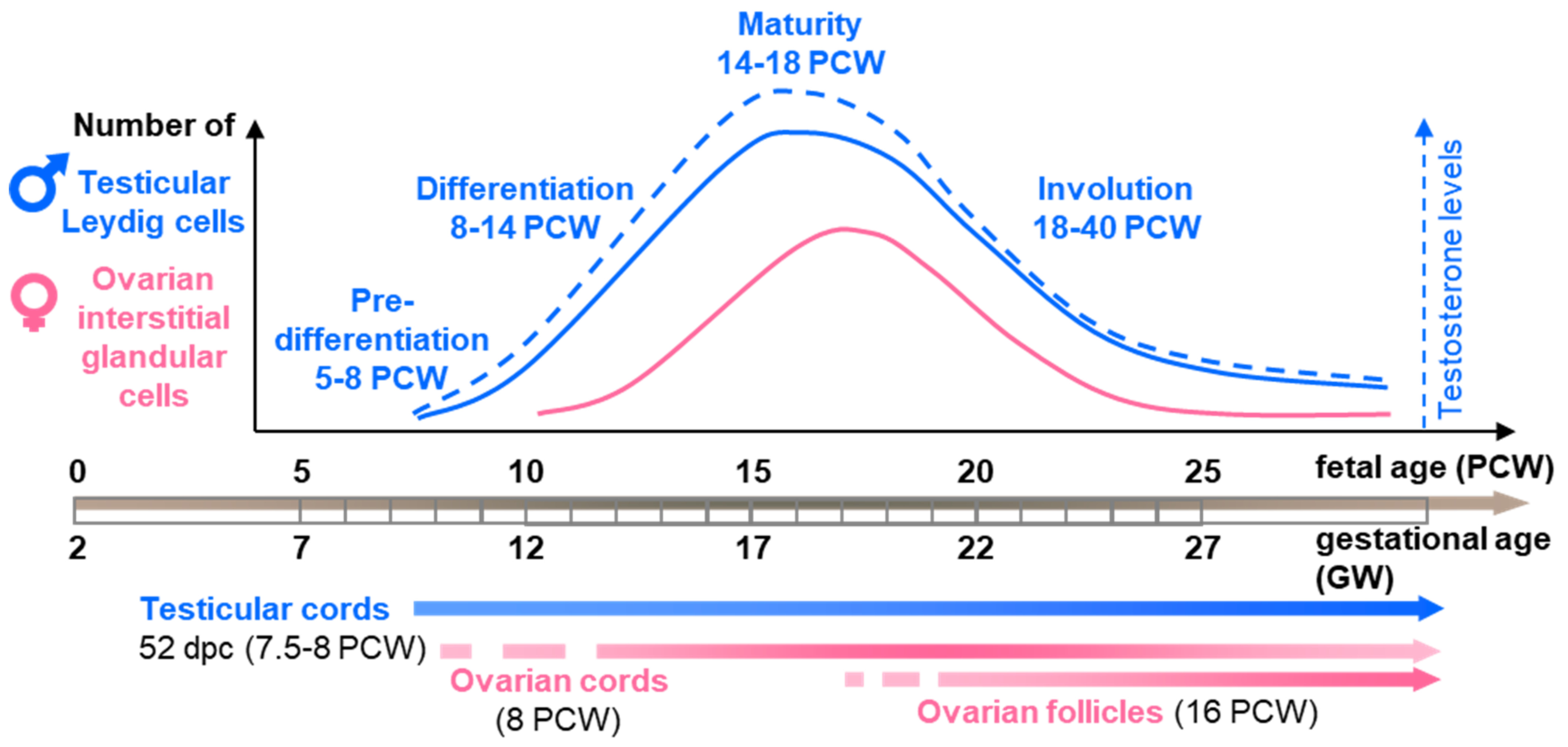
2. Histological and Ultrastructural Features of Steroidogenic Cells during Human Gonad Development in the Spotlight
3. 1960s–1970s: Conversion of Radioactive Precursors
3.1. Principle
3.2. Methodology
3.3. Conversion of Steroids in Human Fetal Gonads
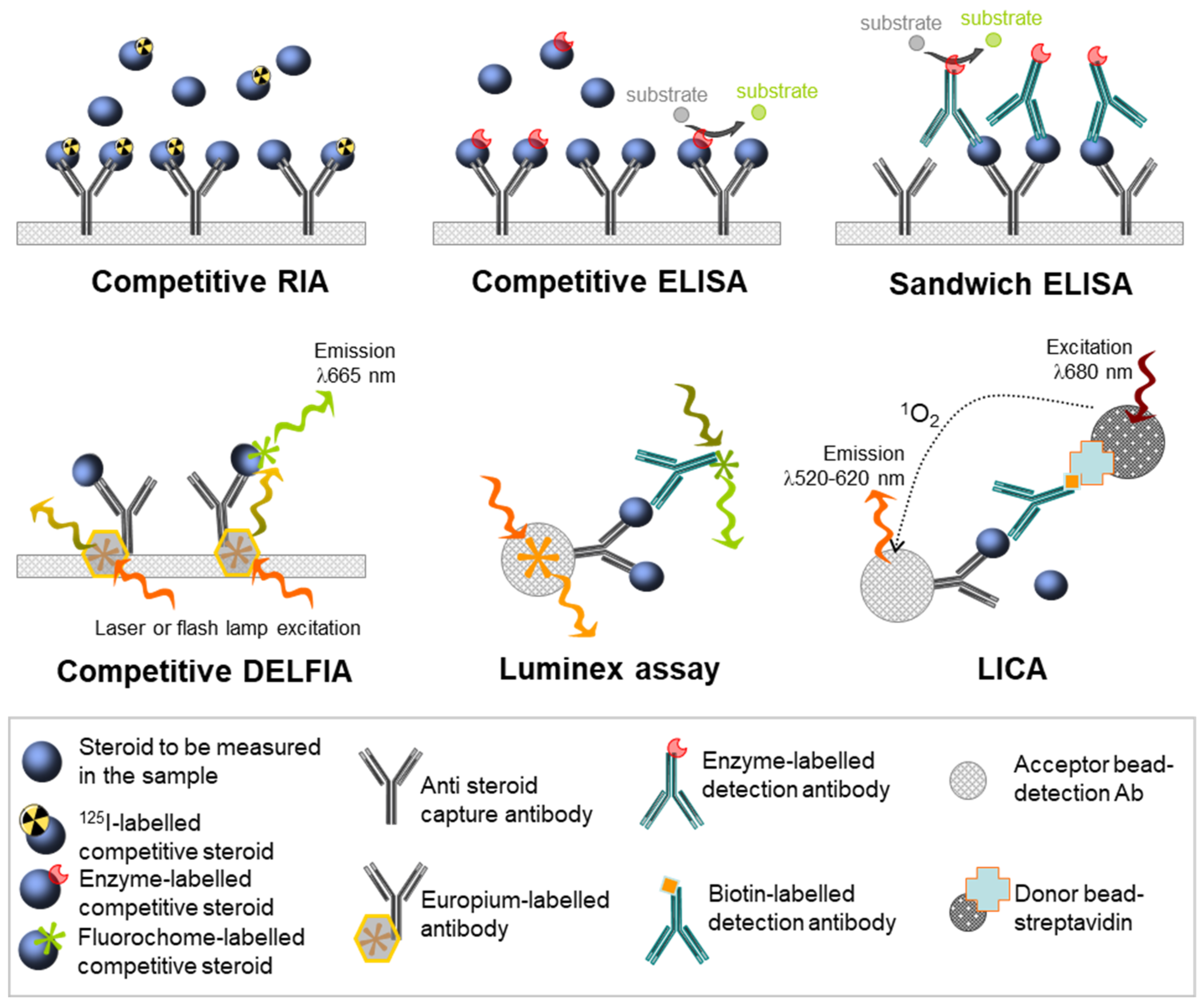
4. Advent of Antibody-Based Assays
4.1. Principle
4.2. Methodologies
4.3. Antibody-Based Assays for Human Fetal Gonadal Steroids
| Year | Technique | Chemical/Injury | Measurement Technique | Measured Steroids | Reference |
|---|---|---|---|---|---|
| 1991 | injection of hormones before abortion | Injection of norethindrone acetate and ethinyl estradiol before abortion | Conversion of radiolabeled DHEA | T, androstenedione | [67] |
| 2006 | culture | (−) Culture validation (+/− hLH) Retinoic acid | RIA RT-QtPCR (CYP11A1, CYP17A1, STAR) | T | [68] |
| 2007 | culture | Dieldrin +/− hLH | EIA DELFIA®/fluo IHC (STAR) | T | [69] |
| 2007 | culture | di(n-butyl) phthalate (DBP) monobutyl phthalate (MBP) +/− hCG, +/− 22R-OH | RIA | T | [70] |
| 2007 | culture | irradiation | RIA RT-QtPCR (CYP11A1, CYP17A1) | T | [71] |
| 2009 | culture | mono-2-ethylhexyl phthalate (MEHP) Ketoconazole | RIA RT-QtPCR (CYP11A1, CYP17A1, STAR) | T | [72] |
| 2010 | culture | Cadmium (+/− hCG) | RIA | T | [73] |
| 2012 | culture | Metformin | RIA | T | [74] |
| 2012 | xenografts | di-n-butyl phthalate (DBP) monobutyl phthalate (MBP) | RIA Seminal Vesicle weight (SVW) RT-QtPCR (STAR, CYP11A1) | T Androgen action | [75] |
| 2012 | xenografts | phthalates | RIA RT-QtPCR (SCARB1, STAR, CYP11A1, CYP17A1) | [76] | |
| 2012 | culture | Bisphenol A Diethylstilbestrol | RIA | T | [62] |
| 2013 | culture | Paracetamol Aspirin Indomethacin | RIA | T | [77] |
| 2013 | xenografts | Diethylstilbestrol | RIA SVW | T Androgen action | [78] |
| 2014 | xenograft | abiraterone acetate di-n-butyl phthalate (DBP) | RIA Androgen sensitive organ weights | T, P4 Androgen action | [79] |
| 2015 | xenografts | Acetaminophen (paracetamol) | RIA SVW | T Androgen action | [80] |
| 2015 | culture | Bisphenol A (BPA), BPS, PBF | RIA | T | [64] |
| 2017 | culture xenografts | Ibuprofen | RIA GC-MS/MS RT-QtPCR (STAR, BZRP, CYP11A1, CYP17A1, HSD17B3, SRD5A3) RIA | T Endogenous: T, DHEA, Preg Produced: 17OH-Preg; DHEA; 17OH-P4; T; DHT androstenedione; T | [66] |
| 2017 | culture | 27 chemicals (caffeine; ethanol; paraxanthine; theobromine; theophylline; 1,3,7 trimethyluric acid; atrazine; bitertanol; chlordecone; glyphosate; imazalil; orto-phenylphenol; prochloraz; propiconazole; aniline; BPA; BPB; BPE; BPF; BPS; aspirin; clomiphene; ibuprofen; indomethacine; ketoconazole; paracetamol; valproic acid) | RIA | T | [81] |
| 2018 | culture xenografts | Specific ALK4/5/7 inhibitor SB431542 | LC-MS/MS RIA SVW | 17OH-P4, T; DHEA; androstenedione; P4, E1 sulphate, T | [82] |
| 2018 | culture xenografts | BPA | RIA RT-QtPCR (STAR, (CYP11A1, CYP17A1, CYP19A1) SVW | T | [65] |
| 2019 | culture | recombinant FGF9 tyrosine kinase inhibitor SU5402 | LC-MS/MS | P4; 17OH-P4; T; DHEA; androstenedione | [83] |
5. Steroidogenic Fingerprints
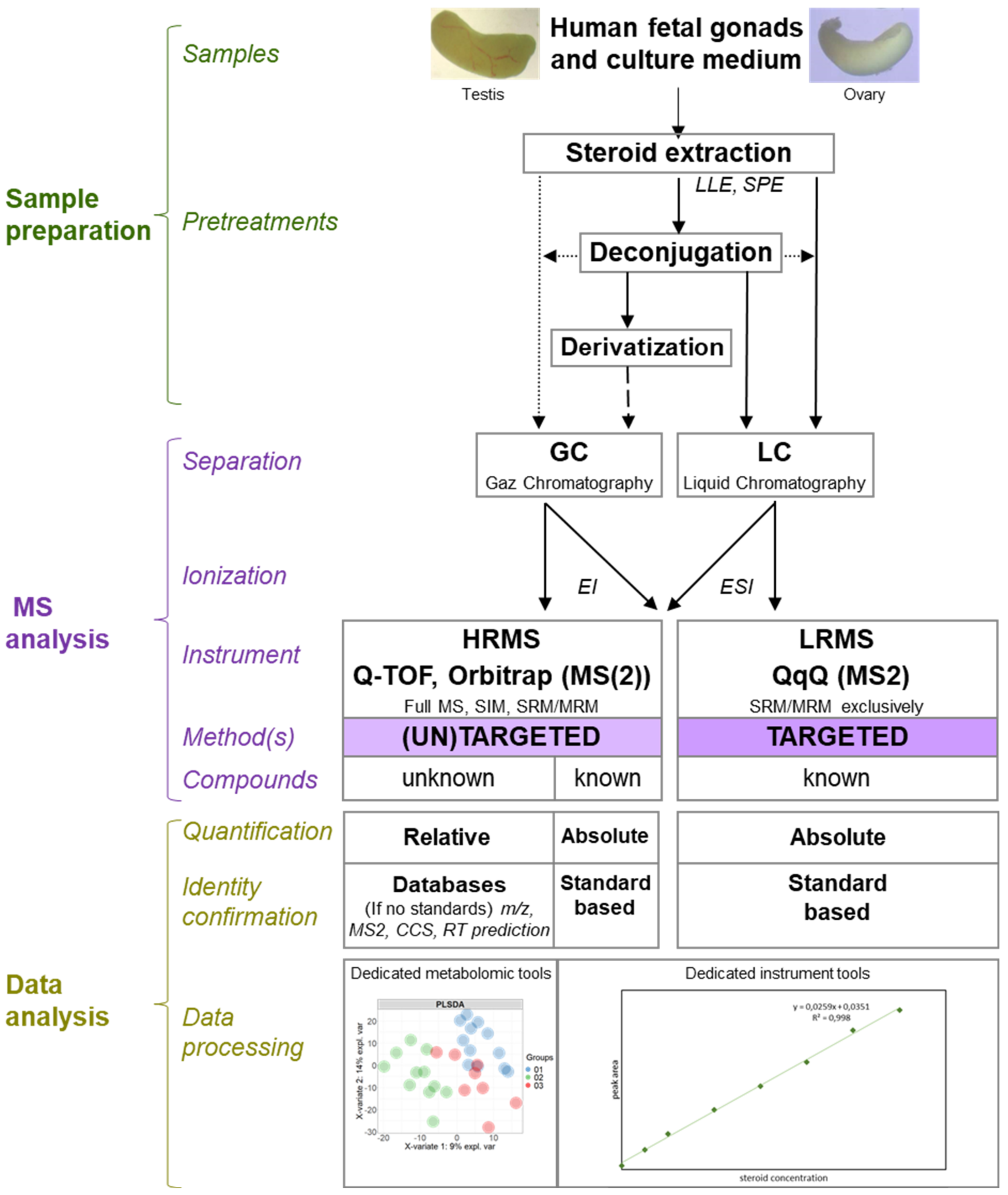
6. XXIth Century and Mass Spectrometry
6.1. Principle
6.2. Methodologies
6.3. Mass Spectrometry in the Service of Human Fetal Gonad Research
7. Beyond the Gonads: A Universe of Possibilities
8. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Skakkebæk, N.E.; Rajpert-De Meyts, E.; Main, K.M. Testicular Dysgenesis Syndrome: An Increasingly Common Developmental Disorder with Environmental Aspects. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef]
- Biason-Lauber, A.; Miller, W.L.; Pandey, A.v.; Flück, C.E. Of Marsupials and Men: “Backdoor” Dihydrotestosterone Synthesis in Male Sexual Differentiation. Mol. Cell. Endocrinol. 2013, 371, 124–132. [Google Scholar] [CrossRef]
- Fevold, H.R.; Lorence, M.C.; McCarthy, J.L.; Trant, J.M.; Kagimoto, M.; Waterman, M.R.; Mason, J.I. Rat P450 17α from Testis: Characterization of a Full-Length CDNA Encoding a Unique Steroid Hydroxylase Capable of Catalyzing Both Δ 4-and Δ 5-Steroid-17,20-Lyase Reactions. Mol. Endocrinol. 1989, 3, 968–975. [Google Scholar] [CrossRef]
- Shaw, G.; Renfree, M.B.; Leihy, M.W.; Shackleton, C.H.L.; Roitman, E.; Wilson, J.D. Prostate Formation in a Marsupial Is Mediated by the Testicular Androgen 5α-Androstane-3α,17β-Diol. Proc. Natl. Acad. Sci. USA 2000, 97, 12256–12259. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Auchus, R.J.; Leihy, M.W.; Guryev, O.L.; Estabrook, R.W.; Osborn, S.M.; Shaw, G.; Renfree, M.B. 5α-Androstane-3α,17β-Diol Is Formed in Tammar Wallaby Pouch Young Testes by a Pathway Involving 5α-Pregnane-3α,17α-Diol-20-One as a Key Intermediate. Endocrinology 2003, 144, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Motta, P.M.; Makabe, S. Development of the Ovarian Surface and Associated Germ Cells in the Human Fetus—A Correlated Study by Scanning and Transmission Electron Microscopy. Cell Tissue Res. 1982, 226, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Konishi, I.; Fujii, S.; Okamura, H. Development of Interstitial Cells and Ovigerous Cords in the Human Fetal Ovary: An Ultrastructural Study. J. Anat. 1986, 148, 121–135. [Google Scholar] [PubMed]
- Stegner, H.E.; Pape, C.; Günther, P. The Ultrastructure of the Interstitial Cells in Human Fetal Ovaries. Archiv für Gynäkologie 1976, 221, 289–298. [Google Scholar] [CrossRef]
- Codesal, J.; Regadera, J.; Nistal, M.; Regadera-Sejas, J.; Paniagua, R. Involution of Human Fetal Leydig Cells. An Immunohistochemical, Ultrastructural and Quantitative Study. J. Anat. 1990, 172, 103–114. [Google Scholar] [PubMed]
- Pelliniemi, L.J.; Niemi, M. Fine Structure of the Human Foetal Testis–I. The Interstitial Tissue. Zeitschrift für Zellforschung und Mikroskopische Anatomie 1969, 99, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Leydig, F. Zur Anatomie der männlichen Geschlechtsorgane und Analdrüsen der Säugethiere. In Zeitschrift für Wissenschaftliche Zoologie; Akademische Verlagsgesellschaft: Leipzig, Germany, 1850; Volume 2, pp. 1–57. [Google Scholar]
- Christensen, A.K. A History of Leydig Cell Research. In The Leydig Cell in Health and Disease; Humana Press: Totowa, NJ, USA, 2007; pp. 3–30. [Google Scholar]
- Guraya, S.S. Histochemical Observations on the Human Foetal Testis with Reference to Steroid Hormone Synthesis. Cell Tissue Res. 1974, 150, 497–503. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J.; Baker, P.J.; Monteiro, A.; Cassie, S.; Bhattacharya, S.; Fowler, P.A. Developmental Changes in Human Fetal Testicular Cell Numbers and Messenger Ribonucleic Acid Levels during the Second Trimester. J. Clin. Endocrinol. Metab. 2007, 92, 4792–4801. [Google Scholar] [CrossRef]
- Makabe, S.; Naguro, T.; Heyn, R.; Motta, P.M. Ultrastructure of Human Leydig Cells at Early Gonadal Embryogenesis. Ital. J. Anat. Embryol 1995, 100, 525–533. [Google Scholar]
- Heyn, R.; Makabe, S.; Motta, P.M. Ultrastructural Dynamics of Human Testicular Cords from 6 to 16 Weeks of Embryonic Development. Study by Transmission and High Resolution Scanning Electron Microscopy. Ital. J. Anat. Embryol. 1998, 103, 17–29. [Google Scholar] [PubMed]
- Kuopio, T.; Paranko, J.; Pelliniemi, L.J. Basement Membrane and Epithelial Features of Fetal-Type Leydig Cells in Rat and Human Testis. Differentiation 1989, 40, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.A.; Mehmet, H.; Thomas, N.S.B.; Cox, P.M.; Ralph, D.J.; Bajoria, R.; Chatterjee, R. Ontogeny of Human Fetal Testicular Apoptosis during First, Second, and Third Trimesters of Pregnancy. J. Clin. Endocrinol. Metab. 2002, 87, 1189–1193. [Google Scholar] [CrossRef]
- Gondos, B.; Hobel, C.J. Interstitial Cells in the Human Fetal Ovary1. Endocrinology 1973, 93, 736–739. [Google Scholar] [CrossRef]
- Nottola, S.A.; Makabe, S.; Stallone, T.; Macchiarelli, G.; Correr, S.; Motta, P.M. Ultrastructure and Distribution of Interstitial Glandular Cells and Associated Elements in Human Fetal Ovaries. Arch. Histol. Cytol. 2000, 63, 345–355. [Google Scholar] [CrossRef][Green Version]
- Krauser, J.A. A Perspective on Tritium versus Carbon-14: Ensuring Optimal Label Selection in Pharmaceutical Research and Development. J. Label. Compd. Radiopharm. 2013, 56, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Elmore, C.S.; Bragg, R.A. Isotope Chemistry; A Useful Tool in the Drug Discovery Arsenal. Bioorganic Med. Chem. Lett. 2015, 25, 167–171. [Google Scholar] [CrossRef]
- Acevedo, H.F.; Axelrod, L.R.; Ishikawa, E.; Takaki, F. Steroidogenesis in the Human Fetal Testis: The Conversion of Pregnenolone-7alpha-H3 to Dehydroepiandrosterone, Testosterone and 4-Androstene-3,17-Dione. J. Clin. Endocrinol. Metab. 1961, 21, 1611–1613. [Google Scholar] [CrossRef]
- Siiteri, P.K.; Wilson, J.D. Testosterone Formation and Metabolism during Male Sexual Differentiation in the Human Embryo. J. Clin. Endocrinol. Metab. 1974, 38, 113–125. [Google Scholar] [CrossRef]
- George, F.W.; Wilson, J.D. Conversion of Androgen to Estrogen by the Human Fetal Ovary. J. Clin. Endocrinol. Metab. 1978, 47, 550–555. [Google Scholar] [CrossRef]
- Bloch, E. Metabolism of 4-14C-Progesterone by Human Fetal Testis and Ovaries. Endocrinology 1964, 74, 833–845. [Google Scholar] [CrossRef]
- Bloch, E.; Romney, S.L.; Klein, M.; Lippiello, L.; Cooper, P.; Goldring, I.P. Steroid Synthesis by Human Fetal Adrenals and Ovaries Maintained in Organ Culture. Proc. Soc. Exp. Biol. Med. 1965, 119, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Rice, B.F.; Johanson, C.A.; Sternberg, W.H. Formation of Steroid Hormones from Acetate-1-14C by a Human Fetal Testis Preparation Grown in Organ Culture. Steroids 1966, 7, 79–90. [Google Scholar] [CrossRef]
- Mathur, R.S.; Wigvist, N.; Diczfalusy, E. De Novo Synthesis of Steroids and Steroid Sulphates by the Testicles of the Human Foetus at Midgestation. Acta Endocrinol. 1972, 71, 792–800. [Google Scholar] [CrossRef][Green Version]
- Taylor, T.; Coutts, J.R.T.; Macnaughton, M.C. Human Foetal Synthesis of Testosterone from Perfused Progesterone. J. Endocrinol. 1974, 60, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Jaffe, R.B. Androgen Formation from Pregnenolone Sulfate by Fetal, Neonatal, Prepubertal and Adult Human Testes. J. Clin. Endocrinol. Metab. 1975, 40, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.E.; Friedrich, E. Steroid Metabolism of Foetal Tissues. I. Metabolism of Pregnenolone 4 14C by Human Foetal Ovaries. Endokrinologie 1975, 65, 72–79. [Google Scholar] [PubMed]
- Sano, Y.; Okinaga, S.; Arai, K. Metabolism of [14C]-C21 Steroids in the Human Fetal Ovaries. J. Steroid Biochem. 1982, 16, 721–724. [Google Scholar] [CrossRef]
- Azoury, R.; Eyal, F.; Springer, C. 5α-Reductase Activity in Human Fetal Testis. Int. J. Biochem. 1982, 14, 577–580. [Google Scholar] [CrossRef]
- Hammar, M.; Berg, A.; Läckgren, G. On the In Vitro Metabolism of Androstenedione and Progesterone in Human Testicular Tissue from Fetal Age to Senescence. Andrologia 1984, 16, 283–288. [Google Scholar] [CrossRef]
- Flück, C.E.; Miller, W.L.; Auchus, R.J. The 17, 20-Lyase Activity of Cytochrome P450c17 from Human Fetal Testis Favors the Δ5 Steroidogenic Pathway. J. Clin. Endocrinol. Metab. 2003, 88, 3762–3766. [Google Scholar] [CrossRef] [PubMed]
- Reisch, N.; Taylor, A.E.; Nogueira, E.F.; Asby, D.J.; Dhir, V.; Berry, A.; Krone, N.; Auchus, R.J.; Shackleton, C.H.L.; Hanley, N.A.; et al. Alternative Pathway Androgen Biosynthesis and Human Fetal Female Virilization. Proc. Natl. Acad. Sci. USA 2019, 116, 22294–22299. [Google Scholar] [CrossRef] [PubMed]
- Hammar, M.; Ahlstrand, C.; Berg, A.Å.; Läckgren, G. In Vitro Conversion of 3h-Pregnenolone by Human Testicular Tissue from Fetal Age to Senescence. Syst. Biol. Reprod. Med. 1984, 13, 203–212. [Google Scholar] [CrossRef]
- Newman, A.E.M.; Chin, E.H.; Schmidt, K.L.; Bond, L.; Wynne-Edwards, K.E.; Soma, K.K. Analysis of Steroids in Songbird Plasma and Brain by Coupling Solid Phase Extraction to Radioimmunoassay. Gen. Comp. Endocrinol. 2008, 155, 503–510. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Lee, J.S.; Santen, R.J. Standardization of Steroid Hormone Assays: Why, How, and When? Cancer Epidemiol. Biomark. Prev. 2007, 16, 1713–1719. [Google Scholar] [CrossRef]
- Rinaldi, S.; Déchaud, H.; Biessy, C.; Morin-Raverot, V.; Toniolo, P.; Zeleniuch-Jacquotte, A.; Akhmedkhanov, A.; Shore, R.E.; Secreto, G.; Ciampi, A.; et al. Reliability and Validity of Commercially Available, Direct Radioimmunoassays for Measurement of Blood Androgens and Estrogens in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 757–765. [Google Scholar]
- Habert, R.; Devif, I.; Gangnerau, M.N.; Lecerf, L. Ontogenesis of the in Vitro Response of Rat Testis to Gonadotropin-Releasing Hormone. Mol. Cell. Endocrinol. 1991, 82, 199–206. [Google Scholar] [CrossRef]
- Picon, R.; Ktorza, A. Effect of LH on Testosterone Production by Foetal Rat Testes in vitro. FEBS Lett. 1976, 68, 19–22. [Google Scholar] [CrossRef]
- Wang, C.; Catlin, D.H.; Demers, L.M.; Starcevic, B.; Swerdloff, R.S. Measurement of Total Serum Testosterone in Adult Men: Comparison of Current Laboratory Methods Versus Liquid Chromatography-Tandem Mass Spectrometry. J. Clin. Endocrinol. Metab. 2004, 89, 534–543. [Google Scholar] [CrossRef]
- Scalas, D.; Squadrone, S.; Gili, M.; Marchis, D.; Prearo, M.; Abete, M.C. Validation of a Dissociation Enhanced Lanthanide Fluorescence Immunoassay for the Screening of 17 β-Estradiol in Bovine Serum According to European Union Decision 2002/657/EC. J. AOAC Int. 2007, 90, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.T.; Francis, K.S.; Shortt, H.D.; McCaughey, W.J. Determination of the Concentrations of the Steroids Estradiol, Progesterone and Testosterone in Bovine Sera: Comparison of Commercial Dissociation Enhanced Lanthanide Fluorescence Immunoassay Kits with Conventional Radio and Enzyme Immunoassays. Analyst 1995, 120, 1827–1830. [Google Scholar] [CrossRef]
- Fulton, R.J.; McDade, R.L.; Smith, P.L.; Kienker, L.J.; Kettman, J.R. Advanced Multiplexed Analysis with the FlowMetrix (TM) System. Clin. Chem. 1997, 43, 1749–1756. [Google Scholar] [CrossRef]
- Martins, T.B. Development of Internal Controls for the Luminex Instrument as Part of a Multiplex Seven-Analyte Viral Respiratory Antibody Profile. Clin. Diagn. Lab. Immunol. 2002, 9, 41–45. [Google Scholar] [CrossRef]
- Dong Huy, G.; Jin, N.; Yin, B.C.; Ye, B.C. A Novel Separation and Enrichment Method of 17β-Estradiol Using Aptamer-Anchored Microbeads. Bioprocess Biosyst. Eng. 2011, 34, 189–195. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Bian, Y.; Yu, Y.; Qiang, Z.; Zhang, Y.; Li, H. Quantitation of Estradiol by Competitive Light-Initiated Chemiluminescent Assay Using Estriol as Competitive Antigen. J. Clin. Lab. Anal. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chon, H.; Lee, S.; Cheng, Z.; Hong, S.H.; Yoon, Y.H.; Choo, J. Highly Sensitive Detection of Hormone Estradiol E2 Using Surface-Enhanced Raman Scattering Based Immunoassays for the Clinical Diagnosis of Precocious Puberty. ACS Appl. Mater. Interfaces 2016, 8, 10665–10672. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Taxvig, C.; Pedersen, M.; Nellemann, C.; Hass, U.; Vinggaard, A.M. Concentration Addition, Independent Action and Generalized Concentration Addition Models for Mixture Effect Prediction of Sex Hormone Synthesis In Vitro. PLoS ONE 2013, 8, e70490. [Google Scholar] [CrossRef]
- Abramovich, D.R.; Rowe, P. Foetal Plasma Testosterone Levels at Mid-Pregnancy and at Term: Relationship to Foetal Sex. J. Endocrinol. 1973, 56, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Abramovich, D.R.; Baker, T.G.; Neal, P. Effect of Human Chorionic Gonadotrophin on Testosterone Secretion by the Foetal Human Testis in Organ Culture. J. Endocrinol. 1974, 60, 179–185. [Google Scholar] [CrossRef]
- Reyes, F.I.; Winter, S.D.; Faiman, C. Studies on Human Sexual Development. I. Fetal Gonadal and Adrenal Sex Steroids. J. Clin. Endocrinol. Metab. 1973, 37. [Google Scholar] [CrossRef]
- Huhtaniemi, I.T.; Korenbrot, C.C.; Jaffe, R.B. HCG Binding and Stimulation of Testosterone Biosynthesis in the Human Fetal Testis. J. Clin. Endocrinol. Metab. 1977, 44, 963–967. [Google Scholar] [CrossRef]
- Word, R.A.; George, F.W.; Wilson, J.D.; Carr, B.R. Testosterone Synthesis and Adenylate Cyclase Activity in the Early Human Fetal Testis Appear to Be Independent of Human Chorionic Gonadotropin Control. J. Clin. Endocrinol. Metab. 1989, 69, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, P.J.; Jaffe, R.B. Leydig Cell Desensitization by Human Chorionic Gonadotropin Does Not Occur in the Human Fetal Testis. J. Clin. Endocrinol. Metab. 1985, 61, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.A.; Jawad, M.J. The Effect of Trophic Agents on Fetal Ovarian Steroidogenesis in Organ Culture. Fertil. Steril. 1979, 32, 73–79. [Google Scholar] [CrossRef]
- Tapanainen, J.; Kellokumpu-Lehtinen, P.; Pelliniemi, L.; Huhtaniemi, I. Age-Related Changes in Endogenous Steroids of Human Fetal Testis during Early and Midpregnancy. J. Clin. Endocrinol. Metab. 1981, 52, 98–102. [Google Scholar] [CrossRef]
- Lambrot, R.; Livera, G.; Coffigny, H.; Pairault, C.; Frydman, R.; Habert, R.; Rouiller-Fabre, V. A New Method for Toxicity Assays on Human and Mouse Fetal Testis. Biochimie 2006, 88, 1831–1835. [Google Scholar] [CrossRef]
- N’Tumba-Byn, T.; Moison, D.; Lacroix, M.; Lecureuil, C.; Lesage, L.; Prud’homme, S.M.; Pozzi-Gaudin, S.; Frydman, R.; Benachi, A.; Livera, G.; et al. Differential Effects of Bisphenol A and Diethylstilbestrol on Human, Rat and Mouse Fetal Leydig Cell Function. PLoS ONE 2012, 7, e51579. [Google Scholar] [CrossRef]
- Maamar, M.B.; Lesne, L.; Desdoits-Lethimonier, C.; Coiffec, I.; Lassurguère, J.; Lavoué, V.; Deceuninck, Y.; Antignac, J.P.; le Bizec, B.; Perdu, E.; et al. An Investigation of the Endocrine-Disruptive Effects of Bisphenol A in Human and Rat Fetal Testes. PLoS ONE 2015, 10, e0117226. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A New Chapter in the Bisphenol a Story: Bisphenol S and Bisphenol F Are Not Safe Alternatives to This Compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef]
- Eladak, S.; Moison, D.; Guerquin, M.J.; Matilionyte, G.; Kilcoyne, K.; N’Tumba-Byn, T.; Messiaen, S.; Deceuninck, Y.; Pozzi-Gaudin, S.; Benachi, A.; et al. Effects of Environmental Bisphenol a Exposures on Germ Cell Development and Leydig Cell Function in the Human Fetal Testis. PLoS ONE 2018, 13, e0191934. [Google Scholar] [CrossRef] [PubMed]
- Ben Maamar, M.; Lesné, L.; Hennig, K.; Desdoits-Lethimonier, C.; Kilcoyne, K.R.; Coiffec, I.; Rolland, A.D.; Chevrier, C.; Kristensen, D.M.; Lavoué, V.; et al. Ibuprofen Results in Alterations of Human Fetal Testis Development. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kellokumpu-Lehtinen, P.; Pelliniemi, L.J.; Pulkkinen, M.O.; Schweikert, H.U. Androgen Synthesis in Human Fetal Testis Exposed in Utero to a Combination of Norethindrone Acetate and Ethinyl Estradiol. Horm. Res. Paediatr. 1991, 35, 242–245. [Google Scholar] [CrossRef]
- Lambrot, R.; Coffigny, H.; Pairault, C.; Donnadieu, A.C.; Frydman, R.; Habert, R.; Rouiller-Fabre, V. Use of Organ Culture to Study the Human Fetal Testis Development: Effect of Retinoic Acid. J. Clin. Endocrinol. Metab. 2006, 91, 2696–2703. [Google Scholar] [CrossRef][Green Version]
- Fowler, P.A.; Abramovich, D.R.; Haites, N.E.; Cash, P.; Groome, N.P.; Al-Qahtani, A.; Murray, T.J.; Lea, R.G. Human Fetal Testis Leydig Cell Disruption by Exposure to the Pesticide Dieldrin at Low Concentrations. Hum. Reprod. 2007, 22, 2919–2927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hallmark, N.; Walker, M.; McKinnell, C.; Mahood, I.K.; Scott, H.; Bayne, R.; Coutts, S.; Anderson, R.A.; Greig, I.; Morris, K.; et al. Effects of Monobutyl and Di(n-Dutyl) Phthalate in Vitro on Steroidogenesis and Leydig Cell Aggregation in Fetal Testis Explants from the Rat: Comparison with Effects in Vivo in the Fetal Rat and Neonatal Marmoset and in Vitro in the Human. Environ. Health Perspect. 2007, 115, 390–396. [Google Scholar] [CrossRef]
- Lambrot, R.; Coffigny, H.; Pairault, C.; Lécureuil, C.; Frydman, R.; Habert, R.; Rouiller-Fabre, V. High Radiosensitivity of Germ Cells in Human Male Fetus. J. Clin. Endocrinol. Metab. 2007, 92, 2632–2639. [Google Scholar] [CrossRef]
- Lambrot, R.; Muczynski, V.; Lécureuil, C.; Angenard, G.; Coffigny, H.; Pairault, C.; Moison, D.; Frydman, R.; Habert, R.; Rouiller-Fabre, V. Phthalates Impair Germ Cell Development in the Human Fetal Testis in Vitro without Change in Testosterone Production. Environ. Health Perspect. 2009, 117, 32–37. [Google Scholar] [CrossRef]
- Angenard, G.; Muczynski, V.; Coffigny, H.; Pairault, C.; Duquenne, C.; Frydman, R.; Habert, R.; Rouiller-Fabre, V.; Livera, G. Cadmium Increases Human Fetal Germ Cell Apoptosis. Environ. Health Perspect. 2010, 118, 331–337. [Google Scholar] [CrossRef]
- Tartarin, P.; Moison, D.; Guibert, E.; Dupont, J.; Habert, R.; Rouiller-Fabre, V.; Frydman, N.; Pozzi, S.; Frydman, R.; Lecureuil, C.; et al. Metformin Exposure Affects Human and Mouse Fetal Testicular Cells. Hum. Reprod. 2012, 27, 3304–3314. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.T.; Childs, A.J.; Anderson, R.A.; van den Driesche, S.; Saunders, P.T.K.; McKinnell, C.; Wallace, W.H.B.; Kelnar, C.J.H.; Sharpe, R.M. Do Phthalates Affect Steroidogenesis by the Human Fetal Testis? Exposure of Human Fetal Testis Xenografts to Di-n-Butyl Phthalate. J. Clin. Endocrinol. Metab. 2012, 97. [Google Scholar] [CrossRef]
- Heger, N.E.; Hall, S.J.; Sandrof, M.A.; Mcdonnell, E.V.; Hensley, J.B.; McDowell, E.N.; Martin, K.A.; Gaido, K.W.; Johnson, K.J.; Boekelheide, K. Human Fetal Testis Xenografts Are Resistant to Phthalate-Induced Endocrine Disruption. Environ. Health Perspect. 2012, 120, 1137–1143. [Google Scholar] [CrossRef]
- Mazaud-Guittot, S.; Nicolaz, C.N.; Desdoits-Lethimonier, C.; Coiffec, I.; Maamar, M.B.; Balaguer, P.; Kristensen, D.M.; Chevrier, C.; Lavoué, V.; Poulain, P.; et al. Paracetamol, Aspirin, and Indomethacin Induce Endocrine Disturbances in the Human Fetal Testis Capable of Interfering with Testicular Descent. J. Clin. Endocrinol. Metab. 2013, 98. [Google Scholar] [CrossRef]
- Mitchell, R.T.; Sharpe, R.M.; Anderson, R.A.; McKinnell, C.; Macpherson, S.; Smith, L.B.; Wallace, W.H.B.; Kelnar, C.J.H.; van den Driesche, S. Diethylstilboestrol Exposure Does Not Reduce Testosterone Production in Human Fetal Testis Xenografts. PLoS ONE 2013, 8, e61726. [Google Scholar] [CrossRef]
- Spade, D.J.; Hall, S.J.; Saffarini, C.M.; Huse, S.M.; McDonnell, E.V.; Boekelheide, K. Differential Response to Abiraterone Acetate and Di-n-Butyl Phthalate in an Androgen-Sensitive Human Fetal Testis Xenograft Bioassay. Toxicol. Sci. 2014, 138, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Van den Driesche, S.; Macdonald, J.; Anderson, R.A.; Johnston, Z.C.; Chetty, T.; Smith, L.B.; McKinnell, C.; Dean, A.; Homer, N.Z.; Jorgensen, A.; et al. Prolonged Exposure to Acetaminophen Reduces Testosterone Production by the Human Fetal Testis in a Xenograft Model. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Gaudriault, P.; Mazaud-Guittot, S.; Lavoué, V.; Coiffec, I.; Lesné, L.; Dejucq-Rainsford, N.; Scholze, M.; Kortenkamp, A.; Jégou, B. Endocrine Disruption in Human Fetal Testis Explants by Individual and Combined Exposures to Selected Pharmaceuticals, Pesticides, and Environmental Pollutants. Environ. Health Perspect. 2017, 125. [Google Scholar] [CrossRef]
- Jørgensen, A.; Macdonald, J.; Nielsen, J.E.; Kilcoyne, K.R.; Perlman, S.; Lundvall, L.; Langhoff Thuesen, L.; Juul Hare, K.; Frederiksen, H.; Andersson, A.M.; et al. Nodal Signaling Regulates Germ Cell Development and Establishment of Seminiferous Cords in the Human Fetal Testis. Cell Rep. 2018, 25, 1924–1937.e4. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.H.; Nielsen, J.E.; Frederiksen, H.; Melau, C.; Hare, K.J.; Thuesen, L.L.; Perlman, S.; Lundvall, L.; Mitchell, R.T.; Juul, A.; et al. Dysregulation of FGFR Signalling by a Selective Inhibitor Reduces Germ Cell Survival in Human Fetal Gonads of Both Sexes and Alters the Somatic Niche in Fetal Testes. Hum. Reprod. 2019, 34, 2228–2243. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, R.; Miller, W.L. Developmental Expression of Genes for the Stereoidogenic Enzymes P450scc (20,22-Desmolase), P450cl7 (17αhydroxylase/17,20-Lyase), and P450c21 (21-Hydroxylase) in the Human Fetus xsxs. J. Clin. Endocrinol. Metab. 1986, 63, 1145–1150. [Google Scholar] [CrossRef]
- Savchuk, I.; Morvan, M.L.; Antignac, J.P.; Kurek, M.; le Bizec, B.; Söder, O.; Svechnikov, K. Ontogenesis of Human Fetal Testicular Steroidogenesis at Early Gestational Age. Steroids 2019, 141, 96–103. [Google Scholar] [CrossRef]
- Pezzi, V.; Mathis, J.M.; Rainey, W.E.; Carr, B.R. Profiling Transcript Levels for Steroidogenic Enzymes in Fetal Tissues. J. Steroid Biochem. Mol. Biol. 2003, 87, 181–189. [Google Scholar] [CrossRef]
- Pollack, S.E.; Furth, E.E.; Kallen, C.B.; Arakane, F.; Kiriakidou, M.; Kozarsky, K.F.; Strauss, J.F. Localization of the Steroidogenic Acute Regulatory Protein in Human Tissues. J. Clin. Endocrinol. Metab. 1997, 82, 4243–4251. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.J.; Fowler, P.A.; Abramovich, D.R.; Haites, N.; Lea, R.G. Human Fetal Testis: Second Trimester Proliferative and Steroidogenic Capacities. J. Clin. Endocrinol. Metab. 2000, 85, 4812–4817. [Google Scholar] [CrossRef]
- Dharia, S.; Slane, A.; Jian, M.; Conner, M.; Conley, A.J.; Parker, C.R. Colocalization of P450c17 and Cytochrome B5 in Androgen-Synthesizing Tissues of the Human. Biol. Reprod. 2004, 71, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Boukari, K.; Ciampi, M.L.; Guiochon-Mantel, A.; Young, J.; Lombès, M.; Meduri, G. Human Fetal Testis: Source of Estrogen and Target of Estrogen Action. Hum. Reprod. 2007, 22, 1885–1892. [Google Scholar] [CrossRef]
- Shapiro, E.; Huang, H.; Masch, R.J.; McFadden, D.E.; Wu, X.R.; Ostrer, H.; Reiner, W. Immunolocalization of Androgen Receptor and Estrogen Receptors α and β in Human Fetal Testis and Epididymis. J. Urol. 2005, 174, 1695–1698. [Google Scholar] [CrossRef]
- Andersson, S.; Sundberg, M.; Pristovsek, N.; Ibrahim, A.; Jonsson, P.; Katona, B.; Clausson, C.M.; Zieba, A.; Ramström, M.; Söderberg, O.; et al. Insufficient Antibody Validation Challenges Oestrogen Receptor Beta Research. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Cole, B.; Hensinger, K.; Maciel, G.A.R.; Chang, R.J.; Erickson, G.F. Human Fetal Ovary Development Involves the Spatiotemporal Expression of P450c17 Protein. J. Clin. Endocrinol. Metab. 2006, 91, 3654–3661. [Google Scholar] [CrossRef]
- Fowler, P.A.; Anderson, R.A.; Saunders, P.T.; Kinnell, H.; Mason, J.I.; Evans, D.B.; Bhattacharya, S.; Flannigan, S.; Franks, S.; Monteiro, A.; et al. Development of Steroid Signaling Pathways during Primordial Follicle Formation in the Human Fetal Ovary. J. Clin. Endocrinol. Metab. 2011, 96, 1754–1762. [Google Scholar] [CrossRef]
- Houmard, B.; Small, C.; Yang, L.; Naluai-Cecchini, T.; Cheng, E.; Hassold, T.; Griswold, M. Global Gene Expression in the Human Fetal Testis and Ovary. Biol. Reprod. 2009, 81, 438–443. [Google Scholar] [CrossRef]
- Mamsen, L.S.; Ernst, E.H.; Borup, R.; Larsen, A.; Olesen, R.H.; Ernst, E.; Anderson, R.A.; Kristensen, S.G.; Andersen, C.Y. Temporal Expression Pattern of Genes during the Period of Sex Differentiation in Human Embryonic Gonads. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Lecluze, E.; Rolland, A.D.; Filis, P.; Evrard, B.; Leverrier-Penna, S.; Maamar, M.B.; Coiffec, I.; Lavoué, V.; Fowler, P.A.; Mazaud-Guittot, S.; et al. Dynamics of the Transcriptional Landscape during Human Fetal Testis and Ovary Development. Hum. Reprod. 2020, 35, 1099–1119. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell 2017, 20, 858–873.e4. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sosa, E.; Chitiashvili, T.; Nie, X.; Rojas, E.J.; Oliver, E.; Plath, K.; Hotaling, J.M.; Stukenborg, J.B.; Clark, A.T.; et al. Single-Cell Analysis of the Developing Human Testis Reveals Somatic Niche Cell Specification and Fetal Germline Stem Cell Establishment. Cell Stem Cell 2021, 28. [Google Scholar] [CrossRef]
- Munkboel, C.H.; Christensen, L.R.; Islin, J.; Bonomo, S.; Olsen, L.; Jørgensen, F.S.; Styrishave, B. The Anti-Epileptic Drug Lamotrigine Inhibits the CYP17A1 Lyase Reaction in Vitro. Biol. Reprod. 2018, 99, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Czerwinska, J.; Wojciechowicz, B.; Nynca, A.; Ciereszko, R. Genistein and Daidzein Affect in Vitro Steroidogenesis but Not Gene Expression of Steroidogenic Enzymes in Adrenals of Pigs. J. Physiol. Pharmacol. 2014, 65, 127–133. [Google Scholar] [PubMed]
- Ohno, S.; Shinoda, S.; Toyoshima, S.; Nakazawa, H.; Makino, T.; Nakajin, S. Effects of Flavonoid Phytochemicals on Cortisol Production and on Activities of Steroidogenic Enzymes in Human Adrenocortical H295R Cells. J. Steroid Biochem. Mol. Biol. 2002, 80, 355–363. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; D’Atri, V.; Barknowitz, G.; Fanuel, M.; Pezzatti, J.; Dreolin, N.; Ropartz, D.; Monteau, F.; Vigneau, E.; Rudaz, S.; et al. Interlaboratory and Interplatform Study of Steroids Collision Cross Section by Traveling Wave Ion Mobility Spectrometry. Anal. Chem. 2020, 92, 5013–5022. [Google Scholar] [CrossRef]
- Pozo, O.J.; van Eenoo, P.; Deventer, K.; Elbardissy, H.; Grimalt, S.; Sancho, J.V.; Hernandez, F.; Ventura, R.; Delbeke, F.T. Comparison between Triple Quadrupole, Time of Flight and Hybrid Quadrupole Time of Flight Analysers Coupled to Liquid Chromatography for the Detection of Anabolic Steroids in Doping Control Analysis. Anal. Chim. Acta 2011, 684, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, A.; Pozo, O.J. Determination of Steroid Profile in Hair by Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1624. [Google Scholar] [CrossRef]
- Kushnir, M.M.; Rockwood, A.L.; Roberts, W.L.; Yue, B.; Bergquist, J.; Meikle, A.W. Liquid Chromatography Tandem Mass Spectrometry for Analysis of Steroids in Clinical Laboratories. Clin. Biochem. 2011, 44, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Olisov, D.; Lee, K.; Jun, S.H.; Song, S.H.; Kim, J.H.; Lee, Y.A.; Shin, C.H.; Song, J. Measurement of Serum Steroid Profiles by HPLC-Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1117, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rousu, T.; Tolonen, A. Comparison of Unit Resolution SRM and TOF-MS at 12,000 Mass Resolution for Quantitative Bioanalysis of 11 Steroids from Human Plasma. Bioanalysis 2012, 4, 555–563. [Google Scholar] [CrossRef]
- Athanasiadou, I.; Angelis, Y.S.; Lyris, E.; Georgakopoulos, C.; Athanasiadou, I.; Georgakopoulos, C. Chemical Derivatization to Enhance Ionization of Anabolic Steroids in LC-MS for Doping-Control Analysis. TrAC Trends Anal. Chem. 2013, 42, 137–156. [Google Scholar] [CrossRef]
- Kito, K.; Ito, T. Mass Spectrometry-Based Approaches Toward Absolute Quantitative Proteomics. Curr. Genom. 2008, 9, 263–274. [Google Scholar] [CrossRef]
- Hopfgartner, G.; Varesio, E.; Tschäppät, V.; Grivet, C.; Bourgogne, E.; Leuthold, L.A. Triple Quadrupole Linear Ion Trap Mass Spectrometer for the Analysis of Small Molecules and Macromolecules. J. Mass Spectrom. 2004, 39, 845–855. [Google Scholar] [CrossRef]
- Loos, G.; van Schepdael, A.; Cabooter, D. Quantitative Mass Spectrometry Methods for Pharmaceutical Analysis. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef] [PubMed]
- Boesl, U. Time-of-Flight Mass Spectrometry: Introduction to the Basics. Mass Spectrom. Rev. 2017, 36, 86–109. [Google Scholar] [CrossRef]
- Perry, R.H.; Cooks, R.G.; Noll, R.J. Orbitrap Mass Spectrometry: Instrumentation, Ion Motion and Applications. Mass Spectrom. Rev. 2008, 27, 661–699. [Google Scholar] [CrossRef] [PubMed]
- Matysik, S.; Liebisch, G. Quantification of Steroid Hormones in Human Serum by Liquid Chromatography-High Resolution Tandem Mass Spectrometry. J. Chromatogr. A 2017, 1526, 112–118. [Google Scholar] [CrossRef]
- Delvaux, A.; Rathahao-Paris, E.; Alves, S. Different Ion Mobility-Mass Spectrometry Coupling Techniques to Promote Metabolomics. Mass Spectrom. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Giles, K.; Ujma, J.; Wildgoose, J.; Pringle, S.; Richardson, K.; Langridge, D.; Green, M. A Cyclic Ion Mobility-Mass Spectrometry System. Anal. Chem. 2019, 91, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, A.R.; DeLaney, K.; Johnson, J.; Li, L. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem. 2018, 90, 240–265. [Google Scholar] [CrossRef]
- Sun, N.; Wu, Y.; Nanba, K.; Sbiera, S.; Kircher, S.; Kunzke, T.; Aichler, M.; Berezowska, S.; Reibetanz, J.; Rainey, W.E.; et al. High-Resolution Tissue Mass Spectrometry Imaging Reveals a Refined Functional Anatomy of the Human Adult Adrenal Gland. Endocrinology 2018, 159, 1511–1524. [Google Scholar] [CrossRef]
- Cobice, D.F.; Livingstone, D.E.W.; MacKay, C.L.; Goodwin, R.J.A.; Smith, L.B.; Walker, B.R.; Andrew, R. Spatial Localization and Quantitation of Androgens in Mouse Testis by Mass Spectrometry Imaging. Anal. Chem. 2016, 88, 10362–10367. [Google Scholar] [CrossRef]
- Shimma, S.; Kumada, H.O.; Taniguchi, H.; Konno, A.; Yao, I.; Furuta, K.; Matsuda, T.; Ito, S. Microscopic Visualization of Testosterone in Mouse Testis by Use of Imaging Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 7607–7615. [Google Scholar] [CrossRef]
- Huhtaniemi, I.; Ikonen, M.; Vihko, R. Presence of Testosterone and Other Neutral Steroids in Human Fetal Testes. Biochem. Biophys. Res. Commun. 1970, 38, 715–720. [Google Scholar] [CrossRef]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass Spectrometry and Immunoassay: How to Measure Steroid Hormones Today and Tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef]
- Acevedo, H.F.; Axelrod, L.R.; Ishikawa, E.; Takaki, F. Studies in Fetal Metabolism. II. Metabolism of Progesterone-4-C 14 and Pregnenolone-7α-H 3 in Human Fetal Testes. J. Clin. Endocrinol. Metab. 1963, 23, 885–890. [Google Scholar] [CrossRef]
- Huhtaniemi, I. Studies on Steroidogenesis and Its Regulation in Human Fetal Adrenal and Testis. J. Steroid Biochem. 1977, 8, 491–497. [Google Scholar] [CrossRef]
- Albalushi, H.; Sahlin, L.; Åkesson, E.; Kurek, M.; Kjartansdóttir, K.R.; Lindh, R.; Söder, O.; Rotstein, E.; Hovatta, O.; Stukenborg, J.B. Hormone Production by Human First-Trimester Gonads in a Functional in Vitro System. Endocrinology 2019, 160, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.L.; Meredith, W.; Snape, C.E.; Sephton, M.A. Analysis of Conjugated Steroid Androgens: Deconjugation, Derivatisation and Associated Issues. J. Pharm. Biomed. Anal. 2009, 49, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Shima, Y.; Miyabayashi, K.; Haraguchi, S.; Arakawa, T.; Otake, H.; Baba, T.; Matsuzaki, S.; Shishido, Y.; Akiyama, H.; Tachibana, T.; et al. Contribution of Leydig and Sertoli Cells to Testosterone Production in Mouse Fetal Testes. Mol. Endocrinol. 2013, 27, 63–73. [Google Scholar] [CrossRef]
- Fowler, P.A.; Childs, A.J.; Courant, F.; Mackenzie, A.; Rhind, S.M.; Antignac, J.P.; le Bizec, B.; Filis, P.; Evans, F.; Flannigan, S.; et al. In Utero Exposure to Cigarette Smoke Dysregulates Human Fetal Ovarian Developmental Signalling. Hum. Reprod. 2014, 29, 1471–1489. [Google Scholar] [CrossRef] [PubMed]
- Johnston, Z.C.; Bellingham, M.; Filis, P.; Soffientini, U.; Hough, D.; Bhattacharya, S.; Simard, M.; Hammond, G.L.; King, P.; O’Shaughnessy, P.J.; et al. The Human Fetal Adrenal Produces Cortisol but No Detectable Aldosterone throughout the Second Trimester. BMC Med. 2018, 16, 23. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J.; Antignac, J.P.; le Bizec, B.; Morvan, M.L.; Svechnikov, K.; Söder, O.; Savchuk, I.; Monteiro, A.; Soffientini, U.; Johnstonid, Z.C.; et al. Alternative (Backdoor) Androgen Production and Masculinization in the Human Fetus. PLoS Biol. 2019, 17, e3000002. [Google Scholar] [CrossRef]
- Reyes, F.I.; Boroditsky, R.S.; Winter, J.S.D.; Faiman, C. Studies on Human Sexual Development. II. Fetal and Maternal Serum Gonadotropin and Sex Steroid Concentrations. J. Clin. Endocrinol. Metab. 1974, 38, 612–617. [Google Scholar] [CrossRef]
- Dawood, M.Y.; Saxena, B.B. Testosterone and Dihydrotestosterone in Maternal and Cord Blood and in Amniotic Fluid. Am. J. Obstet. Gynecol. 1977, 129, 37–42. [Google Scholar] [CrossRef]
- Abramovich, D.R.; Herriot, R.; Stott, J. Dihydrotestosterone Levels at Midpregnancy and Term: A Comparison with Testosterone Concentrations. BJOG An. Int. J. Obstet. Gynaecol. 1983, 90, 232–234. [Google Scholar] [CrossRef]
- Imperato-McGinley, J.; Guerrero, L.; Gautier, T.; Peterson, R.E. Steroid 5α-Reductase Deficiency in Man: An Inherited Form of Male Pseudohermaphroditism. Science 1974, 186, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Jost, A.; Vigier, B.; Prepin, J.; Perchellet, J.P. Studies on Sex Differentiation in Mammals. Recent Prog. Horm. Res. 1973, 29, 1–41. [Google Scholar] [CrossRef]
- Avendaño, A.; Paradisi, I.; Cammarata-Scalisi, F.; Callea, M. 5-α-Reductase Type 2 Deficiency: Is There a Genotype-Phenotype Correlation? A Review. Hormones 2018, 17, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhou, M.; Liu, T. Association between SRD5A2 Polymorphism and Hypospadias: A Meta-Analysis. Pharmazie 2019, 74, 125–128. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J.; Monteiro, A.; Bhattacharya, S.; Fraser, M.J.; Fowler, P.A. Steroidogenic Enzyme Expression in the Human Fetal Liver and Potential Role in the Endocrinology of Pregnancy. Mol. Hum. Reprod. 2013, 19, 177–187. [Google Scholar] [CrossRef]
- Auchus, R.J. Steroid 17-Hydroxylase and 17,20-Lyase Deficiencies, Genetic and Pharmacologic. J. Steroid Biochem. Mol. Biol. 2017, 165, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, B.B.; Gomes, N.L.; Costa, E.M.F.; Inacio, M.; Martin, R.M.; Nishi, M.Y.; Carvalho, F.M.; Tibor, F.D.; Domenice, S. 46,XY Disorder of Sex Development (DSD) Due to 17β-Hydroxysteroid Dehydrogenase Type 3 Deficiency. J. Steroid Biochem. Mol. Biol. 2017, 165, 79–85. [Google Scholar] [CrossRef]
- Flück, C.E.; Meyer-Böni, M.; Pandey, A.V.; Kempná, P.; Miller, W.L.; Schoenle, E.J.; Biason-Lauber, A. Why Boys Will Be Boys: Two Pathways of Fetal Testicular Androgen Biosynthesis Are Needed for Male Sexual Differentiation. Am. J. Hum. Genet. 2011, 89, 201–218. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Wartofsky, L. Requirement for Mass Spectrometry Sex Steroid Assays in the Journal of Clinical Endocrinology and Metabolism. J. Clin. Endocrinol. Metab. 2013, 98, 3971–3973. [Google Scholar] [CrossRef] [PubMed]
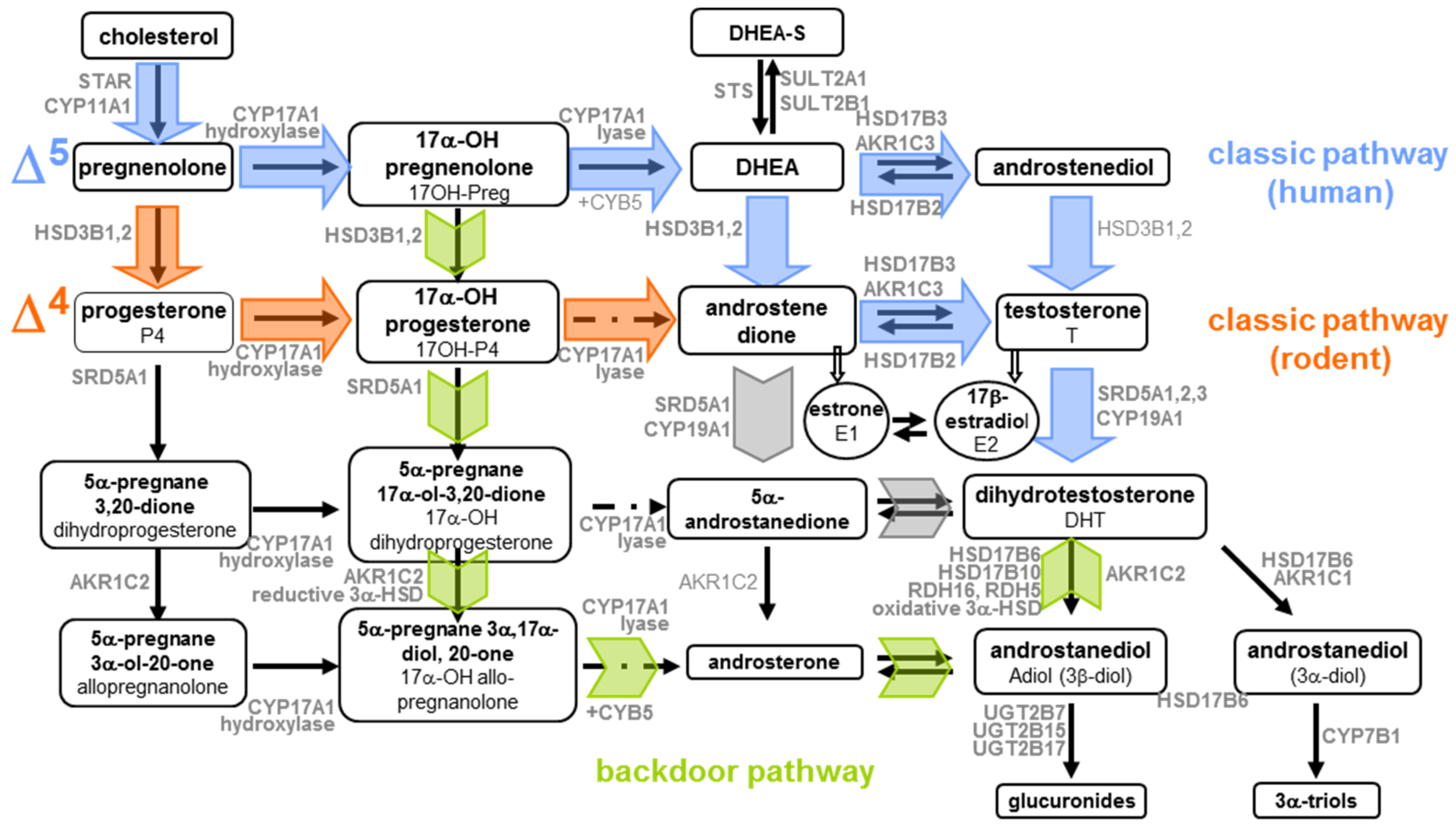
| Year | Testis/Ovary | Endogenous/Produced | Precursor | Steroid Found | Active Enzymes | Reference |
|---|---|---|---|---|---|---|
| 1961 | Testis 21 Weeks, 24 Weeks | endo | Preg-7α-3H | DHEA T | CYP17A1/HSD17B/HSD3B | [23] |
| 1964 | Testis (9–11, 12–15 and 19 Weeks) | endo | 4-14C-P4 | T 17α-hydroxyprogesterone 16α-hydroxyprogesterone | CYP17A1 HSD17B | [26] |
| Ovaries (9–11, 12–15 and 19 Weeks) | endo | 4-14C-P4 | 20α-hydroxy-4-pregnene- 3-one (only 19 Weeks ovary) | |||
| 1965 | Ovaries (11 Weeks) | Culture 4–8 days | 7-3H-P4 | 7-3H-20α-hydroxy-pregnene-3-one | [27] | |
| 1966 | Testis (21 cm) | Culture 8 days | 1-14C-sodium acetate | C21: 3β-hydroxy-pregnene-20-one 17-hydroxy-pregnene-3, 20-dione P4 C19: androstene-3, 17-dione T | [28] | |
| 1972 | Testis mid-gestation | endo | 14C-sodium acetate | Preg, Preg-sulphate DHEA, DHEA-sulphate D5-androstenediol, T | [29] | |
| 1974 | Testis (16–20 Weeks) | Endo (foetus perfusion) | 4-14C-P4 | T, androstenedione | [30] | |
| Ovaries (16–20 Weeks) | Endo (foetus perfusion) | 4-14C-P4 | Neither testosterone nor androstenedione | |||
| 1974 | Testes | 7α-3H-Preg | T | [24] | ||
| (1–21 cm) | 1,2-3H-P4 | T | ||||
| Ovaries (1–21 cm) | 7α-3H-Preg 1,2-3H-P4 | No testosterone | ||||
| 1975 | Testes | endo | 3H-Preg-sulphate | DHEA, T, androstenedione | [31] | |
| 1975 | Ovaries (14–42 Weeks) | 4-14C-Preg | P4, 17OH-Preg DHEA | [32] | ||
| 1978 | Testes (1–20 cm) | endo | radiolabeled androgen | No estrogen | ||
| Ovaries (1–20 cm) | endo | l,2,6,7-3H-T | E1 and E2 (by the 3.1–5-cm stage) | CYP19A1 | [25] | |
| l,2,6,7-3H-androstenedione | E1 and E2 (by the 3.1–5-cm stage) | CYP19A1 | ||||
| 1982 | Ovaries | endo | 14C-Preg | P4, 17OH-Preg, 5-pregnene-3β,20α-diol 5-pregnene-3β,17α,20α-triol 5α-pregnan-3,20-dione | 5-ene-3β-HSD 17-hydroxylase 20α-HSD 5α-reductase | [33] |
| 1982 | Testis (32-weeks) | endo | 3H-Preg | androst-5-ene-3α,17β-diol 3β-hydroxyandrost-5-ene-17-one | no activity of 5 α-reductase | [34] |
| 3H-P4 | Androstenedione 17b-hydroxy-5a-androstan-3-one Unidentified steroid | no activity of 5 α-reductase | ||||
| 1984 | Testis | endo | 3H-androstenedione | T | HSD17B | |
| 3H-P4 | 17OH-P4 | HSD17B | [35] | |||
| 2003 | Testis microsomes | endo | 17OH-Preg | DHEA | CYP17A1 Δ5 preferred pathway (11-fold) | [36] |
| 17OH-P4 | androstenedione | CYP17A Δ4 pathway | ||||
| 2019 | Testis explants (6–10 WPC) | Deuterated 17OH-P4 | Androstenedione T 5α-17OH-pregnanolone | CYP17A1 AKR1C1/3 SRD5A1 | [37] | |
| Deuterated 5α-17OH-pregnanolone | 5α-androsterone 5α-andro-stanediol 5α-andro-standione | CYP17A1 AKR1C/3 HSD17B6 | ||||
| Deuterated 5α-androsterone | 5α-andro-standione 5α-andro-stanediol | HSD17B6 AKR1C1/3 | ||||
| Deuterated 5α-androstanediol | 5α-androsterone 5α-andro-standione DHT | HSD17B6 | ||||
| Ovaries explants (6–10 WPC) | Deuterated 17OH-P4 | Androstenedione 5α-17OH-pregnanolone | CYP17A1 AKR1C1/3 SRD5A1 | |||
| Deuterated 5α-17OH-pregnanolone | 5α-androsterone 5α-andro-stanediol | CYP17A1 AKR1C/3 | ||||
| Deuterated 5α-androsterone | 5α-andro-standione DHT 5α-andro-stanediol | HSD17B6 AKR1C1/3 | ||||
| Deuterated 5α-androstanediol | 5α-androsterone DHT | HSD17B6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connan-Perrot, S.; Léger, T.; Lelandais, P.; Desdoits-Lethimonier, C.; David, A.; Fowler, P.A.; Mazaud-Guittot, S. Six Decades of Research on Human Fetal Gonadal Steroids. Int. J. Mol. Sci. 2021, 22, 6681. https://doi.org/10.3390/ijms22136681
Connan-Perrot S, Léger T, Lelandais P, Desdoits-Lethimonier C, David A, Fowler PA, Mazaud-Guittot S. Six Decades of Research on Human Fetal Gonadal Steroids. International Journal of Molecular Sciences. 2021; 22(13):6681. https://doi.org/10.3390/ijms22136681
Chicago/Turabian StyleConnan-Perrot, Stéphane, Thibaut Léger, Pauline Lelandais, Christèle Desdoits-Lethimonier, Arthur David, Paul A. Fowler, and Séverine Mazaud-Guittot. 2021. "Six Decades of Research on Human Fetal Gonadal Steroids" International Journal of Molecular Sciences 22, no. 13: 6681. https://doi.org/10.3390/ijms22136681
APA StyleConnan-Perrot, S., Léger, T., Lelandais, P., Desdoits-Lethimonier, C., David, A., Fowler, P. A., & Mazaud-Guittot, S. (2021). Six Decades of Research on Human Fetal Gonadal Steroids. International Journal of Molecular Sciences, 22(13), 6681. https://doi.org/10.3390/ijms22136681






