Flavonoid and Phenolic Acids Content and In Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis
2.2. Skin-Related Activities
2.2.1. Antioxidant Activity
2.2.2. Anti-Collagenase Activity
2.2.3. Anti-Elastase Activity
2.2.4. Anti-Hyaluronidase Activity
2.2.5. Antibacterial Activity
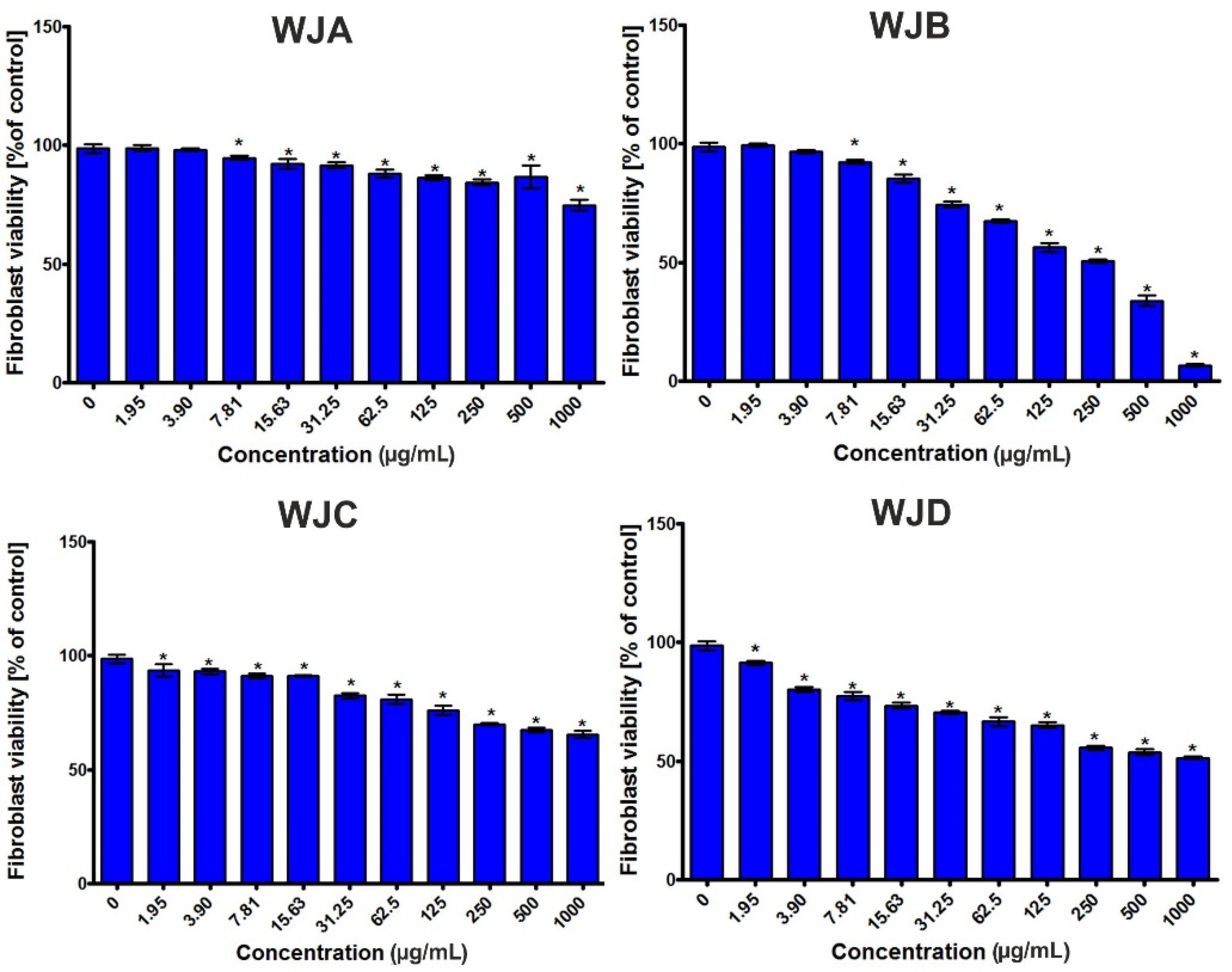
2.2.6. Cytotoxic Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation of the Extracts
3.4. Total Flavonoid and Phenolic and Phenolic Acid Contents
3.5. LC-ESI-MS/MS Analysis
3.6. Antioxidant Activity
3.6.1. DPPH• Assay
3.6.2. ABTS●+ Assay
3.6.3. Metal Chelating Activity (CHEL)
3.7. Enzyme Inhibitory Activity
3.7.1. Anti-Elastase Activity
3.7.2. Anti-Collagenase Activity
3.7.3. Anti-Hyaluronidase Activity
3.8. Antimicrobial Activity
3.8.1. Bacterial Strains
3.8.2. Agar Disc Diffusion Assay
3.8.3. MIC (Minimum Inhibitory Concentration) and MBC (Minimal Bactericidal Concentration) Analysis
3.9. Cytotoxicity Evaluation
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nizioł-Łukaszewska, Z.; Bujak, T. Ocena właściwości kosmetyków myjących zawierających ekstrakt wodny z czarnuszki siewnej (Nigella sativa L.). In Rośliny w Nowoczesnej Kosmetologii; Kiełtyka-Dadasiewicz, A., Ed.; Wydawnictwo Akade-mickie Wyższej Szkoły Społeczno-Przyrodniczej im; Wincentego Pola w Lublinie: Lublin, Poland, 2016; pp. 93–105. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- The Plant List, Version 1.1. Available online: http://www.theplantlist.org/ (accessed on 3 May 2021).
- Miles, C.; Chadwick, C. Growing Wasabi in the Pacific Northwest. Farming the Northwest; PNW0605; Washington State University: Washington, DC, USA, 2008; pp. 1–12. [Google Scholar]
- Kojima, M.; Uchida, M.; Akahori, Y. Studies on the Volatile Components of Wasabia japonica, Brassica juncea and Cocholearia armoracia by Gas Chromatography-Mass Spectrometry. I. Determination of Low Mass Volatile Components. Yakugaku Zasshi 1973, 93, 453–459. [Google Scholar] [CrossRef]
- Etoh, H.; Nishimura, A.; Takasawa, R.; Yagi, A.; Saito, K.; Sakata, K.; Kishima, I.; Ina, K. ω-methylsulfinylalkyl isothiocyanates in wasabi, Wasabia japonica Matsum. Agric. Biol. Chem. 1990, 54, 1587–1589. [Google Scholar] [CrossRef]
- Kumagai, H.; Kashima, N.; Seki, T.; Sakurai, H.; Ishii, K.; Ariga, T. Analysis of volatile components in essential oil of upland Wasabi and their inhibitory effects on platelet aggregation. Biosci. Biotech. Biochem. 1994, 58, 2131–2135. [Google Scholar] [CrossRef]
- Hosoya, T.; Yun, Y.S.; Kunugi, A. Five novel flavonoids from Wasabia japonica. Tetrahedron 2005, 61, 7037–7044. [Google Scholar] [CrossRef]
- Kurata, T.; Misawa, N.; Hosoya, T.; Yamada-Kato, T.; Okunishi, I.; Kumazawa, S. Isolation and Identification of Components from Wasabi (Wasabia japonica Matsumura) Flowers and Investigation of Their Antioxidant and Anti-inflammatory Activities. Food Sci. Technol. Res. 2019, 25, 449–457. [Google Scholar] [CrossRef]
- Hosoya, T.; Yun, Y.S.; Kunugi, A. Antioxidant phenylpropanoid glycosides from the leaves of Wasabia japonica. Phytochemistry 2008, 69, 827–832. [Google Scholar] [CrossRef]
- Yoshida, S.; Hosoya, T.; Inui, S.; Masuda, H.; Kumazawa, S. Component Analysis of Wasabi Leaves and an Evaluation of their Anti-inflammatory Activity. Food Sci. Technol. Res. 2015, 21, 247–253. [Google Scholar] [CrossRef]
- Shimamura, Y.; Iio, M.; Urahira, T.; Masuda, S. Inhibitory effects of Japanese horseradish (Wasabia japonica) on the formation and genotoxicity of a potent carcinogen, acrylamide. J. Sci. Food Agric. 2016, 97, 2419–2425. [Google Scholar] [CrossRef]
- Nagai, M.; Okunishi, I. The effect of wasabi rhizome extract on atopic dermatitis-like symptoms in HR-1 hairless mice. J. Nutr. Sci. Vitaminol. 2009, 55, 195–200. [Google Scholar] [CrossRef][Green Version]
- Morimitsu, Y.; Hayashi, K.; Nakagawa, Y.; Horio, F.; Uchida, K.; Osawa, T. Antiplatelet and anticancer isothiocyanates in Japanese domestic horseradish, wasabi. BioFactors 2000, 13, 271–276. [Google Scholar] [CrossRef]
- Fuke, Y.; Haga, Y.; Ono, H.; Nomura, T.; Ryoyama, K. Anti-carcinogenic activity of 6-methylsulfinylhexyl isothiocyanate-, an active anti-proliferative principal of wasabi (Eutrema wasabi Maxim.). Cytotechnology 1997, 25, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Ogawa, T.; Wang, L.; Katsube, T.; Yamasaki, Y.; Sun, X.; Shiwaku, K. Anti-obesity effects of hot water extract from Wasabi (Wasabia japonica Matsum.) leaves in mice fed high-fat diets. Nutr. Res. Pract. 2013, 7, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, H.; Liu, X. Preliminary study on the functional elements in the mixture of wasabi leaf and leaf stalk and its catharsis effect. J. Chongqing Med. Univ. 2011, 36, 1347–1349. [Google Scholar]
- Kinae, N.; Masuda, H.; Shin, I.S.; Furugori, M.; Shimoi, K. Functional properties of wasabi and horseradish. BioFactors 2000, 13, 265–269. [Google Scholar] [CrossRef]
- Nagai, M.; Akita, K.; Yamada, K.; Okunishi, I. The effect of isosaponarin isolated from wasabi leaf on collagen synthesis in human fibroblasts and its underlying mechanism. J. Nat. Med. 2010, 64, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Gottschalck, T.E.; Bailey, J.E. International Cosmetic Ingredient Dictionary and Handbook, 10th ed.; Introduction INCI Name Monographs A–K; The Cosmetics, Toiletries and Fragrances Association; CTFA: Washington, DC, USA, 2004; Volume 1, p. 1983. [Google Scholar]
- Kang, D.Y.; Hitayezu, E.; Han, I.H.; Kim, J.S.; Jo, Y.M.; Kang, Y.-H. Physicochemical characteristics and antioxidant activities of solvent fractions from ethanol extract of Wasabia koreana Nakai leaf. Korean J. Food Preserv. 2019, 26, 586–593. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, H.S.; Lee, J.; Pan, J.H.; Kim, Y.J.; Kim, J.K.; Woo, S.; Shin, E.-C. Wasabia koreana Nakai: A Preliminary Study on Nutrients and Chemical Compounds That May Impact Sensory Properties. Molecules 2018, 23, 2512. [Google Scholar] [CrossRef]
- Shin, S.W.; Ghimeray, A.K.; Park, C.H. Investigation of total phenolic, total flavonoid, antioxidant and allyl isothiocyanate content in the different organs of Wasabia japonica grown in an organic system. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 38–45. [Google Scholar] [CrossRef]
- Szewczyk, K.; Bogucka-Kocka, A.; Vorobets, N.; Grzywa-Celińska, A.; Granica, S. Phenolic Composition of the Leaves of Pyrola rotundifolia L. and Their Antioxidant and Cytotoxic Activity. Molecules 2020, 25, 1749. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Vorobets, N.; Chrząszcz, M.; Pietrzak, W.; Szewczyk, K. Polyphenol Composition of Extracts of the Fruits of Laserpitium Krapffii Crantz and Their Antioxidant and Cytotoxic Activity. Antioxidants 2019, 8, 363. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Kwon, O.W.; Park, H.B.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Wasabisides A–E, Lignan Glycosides from the Roots of Wasabia japonica. J. Nat. Prod. 2016, 79, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Boran, R. Investigations of anti-aging potential of Hypericum origanifolium Willd. for skincare formulations. Ind. Crop. Prod. 2018, 118, 290–295. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Ismail, I.S. Cosmetic potential of Southeast Asian herbs: An overview. Phytochem. Rev. 2015, 14, 419–428. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Uitto, J. Connective tissue biochemistry of the aging dermis: Age-associated alternations in collagen and elastin. Clin. Geriatr. Med. 1989, 5, 127–148. [Google Scholar] [CrossRef]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-aging potential and anti-tyrosinase activity of three Hypericum species with focus on phytochemical composition by LC–MS/MS. Ind. Crop. Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sussmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Bauman, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef]
- Barla, F.; Higashijima, H.; Funai, S.; Sugimoto, K.; Harada, N.; Yamaji, R.; Fujita, T.; Nakano, Y.; Inui, H. Inhibitive Effects of Alkyl Gallates on Hyaluronidase and Collagenase. Biosci. Biotechnol. Biochem. 2009, 73, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.J.; Chopra, I. Preclinical evaluation of novel antibacterial agents by microbiological and molecular techniques. Expert Opin. Investig. Drugs 2004, 13, 1045–1063. [Google Scholar] [CrossRef] [PubMed]
- Kiba, A.; Saitoh, H.; Nishihara, M.; Omiya, K.; Yamamura, S. C-Terminal Domain of a Hevein-Like Protein from Wasabia japonica has Potent Antimicrobial Activity. Plant Cell Physiol. 2003, 44, 296–303. [Google Scholar] [CrossRef]
- Shin, I.S.; Masuda, H.; Naohide, K. Bactericidal activity of wasabi (Wasabia japonica) against Helicobacter pylori. Int. J. Food Microbiol. 2004, 94, 255–261. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia IX. In PTFarm; Polish Pharmaceutical Society: Warsaw, Poland, 2011; p. 150.
- Guo, J.-T.; Lee, H.-L.; Chiang, S.-H.; Lin, F.-I.; Chang, C.-Y. Antioxidant Properties of the Extracts from Different Parts of Broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar] [CrossRef]
- Nowak, R.; Szewczyk, K.; Gawlik-Dziki, U.; Rzymowska, J.; Komsta, Ł. Antioxidative and cytotoxic potential of some Che-nopodium L. species growing in Poland. Saudi J. Biol. Sci. 2016, 23, 15–23. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crop. Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crop. Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, elastase, hyaluronidase, in-hibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crop. Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Murray, P.R.; Baron, E.J.; Pfaller, M.A.; Tenover, F.C.; Yolke, R.H. Manual of Clinical Microbiology, 6th ed.; Mosby Year Book: London, UK, 1995; pp. 143–260. ISBN 13-978-1555810863. [Google Scholar]
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Eighteenth International Supplement; CLSI document M7-MIC; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Miazga-Karska, M.; Szewczyk, K.; Klimek, K.; Ginalska, G. In vitro activity of peptide fractions from Impatiens glandulifera against caries causing bacteria. Acta Pol. Pharm. 2017, 74, 710–714. [Google Scholar] [PubMed]
- Chrząszcz, M.; Miazga-Karska, M.; Klimek, K.; Granica, S.; Tchórzewska, D.; Ginalska, G.; Szewczyk, K. Extracts from Cephalaria uralensis (Murray) Roem. & Schult. and Cephalaria gigantea (Ledeb.) Bobrov as Potential Agents for Treatment of Acne Vulgaris: Chemical Characterization and In Vitro Biological Evaluation. Antioxidants 2020, 9, 796. [Google Scholar] [CrossRef]
- Pitucha, M.; Woś, M.; Miazga-Karska, M.; Klimek, K.; Mirosław, B.; Pachuta-Stec, A.; Gładysz, A.; Ginalska, G. Synthesis, antibacterial and antiproliferative potential of some new 1-pyridinecarbonyl-4-substituted thiosemicarbazide derivatives. Med. Chem. Res. 2016, 25, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
| Sample | Total Phenolic Content (mg GAE/g DE) | Total Phenolic Acids (mg CAE/g DE) | Total Flavonoid Content (mg QE/g DE) |
|---|---|---|---|
| WJA | 187.79 ± 6.86 | 17.20 ± 0.24 | 45.08 ± 0.18 |
| WJB | 31.55 ± 2.17 | 4.34 ± 0.08 | 9.15 ± 0.10 |
| WJC | 77.92 ± 4.82 | 13.84 ± 0.09 | 28.81 ± 0.11 |
| WJD | 54.93 ± 0.99 | 9.70 ± 0.20 | 29.77 ± 0.04 |
| No | Compound | Amounts (mg/g DE) | |||
|---|---|---|---|---|---|
| WJA | WJB | WJC | WJD | ||
| 1 | salicylic acid | <LOQ | <LOQ | <LOQ | nd |
| 2 | p-coumaric acid | 0.10 ± 0.02 | nd | 0.01 ± 0.01 | <LOQ |
| 3 | caffeic acid | <LOQ | nd | <LOQ | <LOQ |
| 4 | ferulic acid | <LOQ | nd | 1.21 ± 0.07 | <LOQ |
| 5 | chlorogenic acid | 0.01 ± 0.01 | 0.62 ± 0.02 | nd | 0.42 ± 0.02 |
| 6 | cis-sinapic acid | 0.67 ± 0.07 | 0.09 ± 0.00 | 0.77 ± 0.06 | 0.56 ± 0.01 |
| 7 | apigenin | <LOQ | nd | <LOQ | <LOQ |
| 8 | naringenin | <LOQ | nd | nd | nd |
| 9 | kaempferol | <LOQ | nd | nd | nd |
| 10 | eriodictiol | <LOQ | nd | nd | nd |
| 11 | quercetin | <LOQ | nd | nd | nd |
| 12 | taxifolin | <LOQ | nd | nd | nd |
| 13 | isorhamnetin | <LOQ | nd | nd | nd |
| 14 | luteolin | <LOQ | nd | 0.01 ± 0.00 | <LOQ |
| 15 | apigenin 7-O-glucoside | 0.02 ± 85.0 | nd | <LOQ | nd |
| 16 | naringenin 7-O-glucoside | <LOQ | nd | nd | nd |
| 17 | apigenin 6-C-glucoside/apigenin 8-C-glucoside | 0.52 ± 0.07 | nd | 2.57 ± 0.01 | 0.70 ± 0.09 |
| 18 | luteolin 7-O-glucoside | 0.44 ± 0.12 | nd | 0.18 ± 0.00 | 0.13 ± 0.02 |
| 19 | kaempferol 3-O-glucoside | 0.38 ± 0.01 | nd | <LOQ | nd |
| 20 | quercetin 3-O-glucoside | 0.53 ± 0.07 | nd | <LOQ | <LOQ |
| 21 | isorhamnetin 3-O-glucoside | 0.48 ± 0.02 | nd | nd | nd |
| 22 | kaempferol 3-O-rutinoside | 2.50 ± 0.06 | nd | <LOQ | nd |
| 23 | quercetin 3-O-rutinoside | 0.74 ± 0.03 | <LOQ | <LOQ | <LOQ |
| 24 | luteolin 3′,7′-diglucoside | 4.98 ± 0.23 | nd | 3.51 ± 0.13 | 2.74 ± 0.10 |
| 25 | isorhamnetin 3-O-rutinoside | 0.54 ± 0.04 | nd | nd | nd |
| 26 | isovitexin 4’-O-glucoside | 6.02 ± 0.10 | <LOQ | 6.22 ± 0.04 | 5.99 ± 0.22 |
| Sample | IC50 | ||
|---|---|---|---|
| DPPH (μg/mL) | ABTS (μg/mL) | CHEL (μg/mL) | |
| WJA | 28.72 ± 0.24 * | 11.68 ± 0.47 | 12.50 ± 0.36 # |
| WJB | 134.30 ± 1.75 * | 65.18 ± 0.73 | 32.08 ± 0.13 # |
| WJC | 62.84 ± 0.08 * | 33.25 ± 0.31 | 14.27 ± 0.21 # |
| WJD | 69.10 ± 0.16 * | 28.53 ± 0.72 | 13.65 ± 0.29 # |
| AA | 4.92 ± 0.32 | nt | nt |
| Trolox | nt | 3.14 ± 0.17 | nt |
| Na2EDTA*2H2O | nt | nt | 8.75 ± 0.15 |
| Sample | Inhibition (%) | ||
|---|---|---|---|
| Collagenase Inhibition | Elastase Inhibition | Hyaluronidase Inhibition | |
| WJA | 93.34 ± 0.77 * | 88.93 ± 0.16 * | 47.32 ± 0.53 * |
| WJB | 78.42 ± 0.25 * | 90.18 ± 0.54 * | 13.46 ± 0.22 * |
| WJC | 87.51 ± 0.83 * | 84.90 ± 0.60 | 28.95 ± 0.69 * |
| WJD | 90.16 ± 0.51 * | 88.89 ± 0.36 * | 25.78 ± 0.18 * |
| EGCG | 88.49 ± 0.45 | 91.03 ± 0.18 | 62.90 ± 0.12 |
| Bacterial Strains | Zones of Bacterial Growth Inhibition (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WJA | WJB | WJC | WJD | K1 | K2 | K3 | K4 | K5 | K6 | |
| S. aureus ATCC 25923 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 0 |
| S. epidermidis ATCC 12228 | 0 | 0 | 0 | 0 | 0 | 0 | 36 | 0 | 28 | 0 |
| E. coli ATCC 25992 | 7 | 6 | 0 | 0 | 0 | 0 | 12 | 8 | 4 | 4 |
| P. aeruginosa ATCC 27853 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 6 | 4 | 4 |
| S. mutans PCM 2502 | 16.5 | 16 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 |
| P. acnes PCM 2400 | 16 | 19.5 | 15 | 8 | 0 | 0 | 20 | 0 | 0 | 0 |
| P. acnes PCM 2334 | 17 | 21 | 16 | 10 | 4 | 0 | 22 | 0 | 0 | 0 |
| S. sanguinis PCM 2335 | 17 | 18 | 0 | 0 | 0 | 0 | 18 | 0 | 0 | 0 |
| Bacterial Strains | Sample | ||
|---|---|---|---|
| WJA | WJB | K3 | |
| S. aureus ATCC 25923 | - | - | 125 |
| MBC/MIC | 16 | ||
| S. epidermidis ATCC 12228 | - | - | 31.25 |
| MBC/MIC | - | - | 8 |
| E. coli ATCC 25992 | 1000 | 1000 | 500 |
| MBC/MIC | - | - | 8 |
| P. aeruginosa ATCC 27853 | - | - | 1000 |
| MBC/MIC | - | - | - |
| S. mutans PCM 2502 | 500 | 500 | 125 |
| MBC/MIC | - | 8 | 8 |
| P. acnes PCM 2400 | 500 | 250 | 62.5 |
| MBC/MIC | 8 | 8 | 8 |
| P. acnes PCM 2334 | 250 | 125 | 62.5 |
| MBC/MIC | 8 | 16 | 8 |
| S. sanguinis PCM 2335 | 500 | 250 | 62.5 |
| MBC/MIC | - | 8 | 8 |
| Extract | CC50 (μg/mL) |
|---|---|
| WJA | >1000 |
| WJB | 245.45 |
| WJC | >1000 |
| WJD | >1000 |
| Compound | Retention Time (min) | Q1/Q3 (m/z) | DP (V) | EP (V) | CEP (V) | CE (eV) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||
| 5-caffeoylquinic acid (chlorogenic acid) | 9.30 | 352.9/190.8 | −35 | −4.5 | −16 | −24 | −2 |
| 10.42 | 352.9/84.9 | −35 | −4.5 | −16 | −60 | 0 | |
| Caffeic acid | 11.38 | 178.7/88.9 | −30 | −6.5 | −12 | −46 | 0 |
| 178.7/134.9 | −30 | −6.5 | −12 | −16 | 0 | ||
| 4-Hydroxycinnamic acid (p-coumaric acid) | 14.10 | 162.7/119 | −30 | −8 | −12 | −14 | 0 |
| 162.7/93 | −30 | −8 | −12 | −44 | 0 | ||
| Sinapic acid | 14.47 | 222.8/121 | −35 | −8.5 | −10 | −36 | 0 |
| 14.94 | 222.8/148.9 | −35 | −8.5 | −10 | −20 | 0 | |
| Ferulic acid | 14.80 | 192.8/133.9 | −25 | −11.5 | −14 | −16 | 0 |
| 15.22 | 192.8/177.9 | −25 | −11.5 | −14 | −12 | −2 | |
| Salicylic acid | 17.91 | 136.8/93 | −35 | −4 | −10 | −16 | −2 |
| 136.8/75 | −35 | −4 | −10 | −48 | 0 | ||
| Flavonoid aglycones | |||||||
| Naringenin | 14.52 | 270.8/119 | −50 | −11.5 | −12 | −34 | 0 |
| 270.8/150.9 | −50 | −11.5 | −12 | −22 | 0 | ||
| Taxifolin | 15.15 | 302.7/124.9 | −45 | −3.5 | −18 | −26 | 0 |
| 302.7/284.8 | −45 | −3.5 | −18 | −14 | −4 | ||
| Luteolin | 17.82 | 284.7/132.9 | −75 | −9 | −18 | −38 | 0 |
| 284.7/150.9 | −75 | −9 | −18 | −26 | 0 | ||
| Eriodyctiol | 17.89 | 286.7/134.9 | −45 | −6 | −12 | −32 | 0 |
| 286.7/150.9 | −45 | −6 | −12 | −18 | −2 | ||
| Quercetin | 17.94 | 300.7/150.9 | −60 | −2.5 | −12 | −26 | 0 |
| 300.7/178.8 | −60 | −2.5 | −12 | −20 | −2 | ||
| Apigenin | 18.64 | 268.8/117 | −70 | −9.5 | −12 | −44 | 0 |
| 268.8/106.8 | −70 | −9.5 | −12 | −34 | 0 | ||
| Kaempferol | 18.85 | 284.7/116.8 | −70 | −5 | −12 | −46 | 0 |
| 284.7/93 | −70 | −5 | −12 | −52 | 0 | ||
| Isorhamnetin | 18.99 | 314.7/299.7 | −65 | −2.5 | −26 | −20 | −4 |
| 314.7/150.9 | −65 | −2.5 | −26 | −30 | 0 | ||
| Flavonoid glycosides | |||||||
| Isovitexin 4’-O-glucoside (Isosaponarin) | 8.82 | 593.2/282.1 | −25 | −10 | −34 | −30 | −3 |
| 593.2/473.1 | −25 | −10 | −34 | −30 | −3 | ||
| Luteolin 3′,7′-diglucoside | 11.28 | 609.1/285 | −70 | −7.5 | −28 | −50 | −4 |
| 609.1/447 | −70 | −7.5 | −28 | −32 | −18 | ||
| Quercetin 3-O-rutinoside (Rutin) | 11.99 | 608.7/299.6 | −90 | −8 | −30 | −46 | −4 |
| 608.7/270.9 | −90 | −8 | −30 | −60 | −4 | ||
| Apigenin 6-C-glucoside (Isovitexin) | 12.38 | 430.8/310.9 | −65 | −4.5 | −18 | −28 | −4 |
| 430.8/340.9 | −65 | −4.5 | −18 | −26 | −14 | ||
| Apigenin 8-C-glucoside (Vitexin) | 12.40 | 430.8/310.9 | −75 | −4.5 | −20 | −26 | −4 |
| 430.8/340.9 | −75 | −4.5 | −20 | −34 | −14 | ||
| Luteolin 7-O-glucoside (Luteoloside) | 12.87 | 446.8/284.8 | −70 | −10.5 | −20 | −30 | −4 |
| 446.8/132.9 | −70 | −10.5 | −20 | −78 | 0 | ||
| Quercetin 3-O-glucoside (Isoquercetin) | 13.00 | 462.7/299.7 | −85 | −1.5 | −20 | −30 | −4 |
| 462.7/270.7 | −85 | −1.5 | −20 | −44 | −4 | ||
| Kaempferol 3-O-rutinoside (Nicotiflorin) | 13.31 | 592.7/284.8 | −65 | −12 | −30 | −38 | −2 |
| 592.7/226.7 | −65 | −12 | −30 | −68 | −2 | ||
| Isorhamnetin 3-O-rutinoside (Narcissoside) | 13.52 | 622.8/314.9 | −90 | −4.5 | −30 | −40 | −4 |
| 622.8/298.8 | −90 | −4.5 | −30 | −52 | −4 | ||
| Kaempferol 3-O-glucoside (Astragalin) | 14.66 | 446.7/226.8 | −75 | −9 | −20 | −54 | −2 |
| 446.7/254.8 | −75 | −9 | −20 | −40 | −2 | ||
| Isorhamnetin 3-O-glucoside | 14.76 | 476.8/313.9 | −95 | −10 | −22 | −30 | −4 |
| 476.8/270.9 | −95 | −10 | −22 | −44 | −4 | ||
| Apigenin 7-O-glucoside (Apigetrin and Cosmosiin) | 14.91 | 430.7/267.7 | −70 | −9 | −20 | −38 | −4 |
| 430.7/116.9 | −70 | −9 | −20 | −84 | 0 | ||
| Naringenin 7-O-glucoside | 15.12 | 432.7/270.8 | −40 | −8.5 | −20 | −22 | −4 |
| 432.7/118.9 | −40 | −8.5 | −20 | −64 | 0 | ||
| Compound | LOD (ng/mL) | LOQ (ng/mL) | R2 | Linearity Range (ng/mL) |
|---|---|---|---|---|
| Phenolic acids | ||||
| 5-Caffeoylquinic acid (chlorogenic acid) | 70 | 150 | 0.9989 | 150–15,000 |
| Caffeic acid | 200 | 400 | 0.9991 | 400–20,000 |
| 4-Hydroxycinnamic acid (p-coumaric acid) | 50 | 200 | 0.9990 | 500–15,000 |
| Sinapic acid | 500 | 1000 | 0.9989 | 1000–30,000 |
| Ferulic acid | 1000 | 1500 | 0.9985 | 1830–35,000 |
| Salicylic acid | 300 | 500 | 0.9974 | 1500–15,000 |
| Flavonoid aglycones | ||||
| Naringenin | 150 | 300 | 0.9982 | 600–6000 |
| Taxifolin | 10 | 20 | 0.9984 | 40–10,000 |
| Luteolin | 5 | 15 | 0.9987 | 50–1500 |
| Eriodyctiol | 25 | 50 | 0.9985 | 100–6600 |
| Quercetin | 50 | 100 | 0.9982 | 100–6600 |
| Apigenin | 15 | 22 | 0.9970 | 100–4000 |
| Kaempferol | 30 | 50 | 0.9971 | 150–3000 |
| Isorhamnetin | 300 | 500 | 0.9990 | 1000–5000 |
| Flavonoid glycosides | ||||
| Isovitexin 4’-O-glucoside (Isosaponarin) | 100 | 250 | 0.9958 | 500–10,000 |
| Luteolin 3′,7′-diglucoside | 250 | 500 | 0.9980 | 1000–25,000 |
| Quercetin 3-O-rutinoside (Rutin) | 100 | 250 | 0.9980 | 2000–28,800 |
| Apigenin 6-C-glucoside (Isovitexin) | 100 | 250 | 0.9991 | 1500–50,000 |
| Apigenin 8-C-glucoside (Vitexin) | 100 | 200 | 0.9981 | 2000–50,000 |
| Luteolin 7-O-glucoside (Luteoloside) | 50 | 100 | 0.9982 | 200–25,000 |
| Quercetin 3-O-glucoside (Isoquercetin) | 100 | 250 | 0.9975 | 2500–50,000 |
| Kaempferol 3-O-rutinoside (Nicotiflorin) | 60 | 120 | 0.9957 | 150–60,000 |
| Isorhamnetin 3-O-rutinoside (Narcissoside) | 50 | 100 | 0.9981 | 150–25,000 |
| Kaempferol 3-O-glucoside (Astragalin) | 100 | 250 | 0.9974 | 1500–25,000 |
| Isorhamnetin 3-O-glucoside | 100 | 150 | 0.9969 | 2000–35,000 |
| Apigenin 7-O-glucoside (Apigetrin, Cosmosiin) | 100 | 250 | 0.9989 | 1500–25,000 |
| Naringenin 7-O-glucoside | 100 | 200 | 0.9990 | 250–25,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, K.; Pietrzak, W.; Klimek, K.; Miazga-Karska, M.; Firlej, A.; Flisiński, M.; Grzywa-Celińska, A. Flavonoid and Phenolic Acids Content and In Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. Int. J. Mol. Sci. 2021, 22, 6219. https://doi.org/10.3390/ijms22126219
Szewczyk K, Pietrzak W, Klimek K, Miazga-Karska M, Firlej A, Flisiński M, Grzywa-Celińska A. Flavonoid and Phenolic Acids Content and In Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. International Journal of Molecular Sciences. 2021; 22(12):6219. https://doi.org/10.3390/ijms22126219
Chicago/Turabian StyleSzewczyk, Katarzyna, Wioleta Pietrzak, Katarzyna Klimek, Małgorzata Miazga-Karska, Agnieszka Firlej, Marek Flisiński, and Anna Grzywa-Celińska. 2021. "Flavonoid and Phenolic Acids Content and In Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland" International Journal of Molecular Sciences 22, no. 12: 6219. https://doi.org/10.3390/ijms22126219
APA StyleSzewczyk, K., Pietrzak, W., Klimek, K., Miazga-Karska, M., Firlej, A., Flisiński, M., & Grzywa-Celińska, A. (2021). Flavonoid and Phenolic Acids Content and In Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. International Journal of Molecular Sciences, 22(12), 6219. https://doi.org/10.3390/ijms22126219







