Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis
Abstract
1. Introduction
2. Osteoarthritis Molecular Pathways
2.1. Reactive Oxygen Species
2.2. Cellular Apoptosis
2.3. Pro-Inflammatory Signaling
2.4. Anti-Inflammatory Signaling
3. Physical Exercise as a Modulator of Osteoarthritis Molecular Pathways
3.1. Chondroprotective Role of Physical Exercise
3.2. Anti-Inflammatory and Anti-Apoptotic Role of Physical Exercise on Murine Models
3.3. Beneficial Effects of Physical Exercise on Osteoarthritis Patients
3.4. Physical Exercise as an Antioxidant Intervention
3.5. Challenges and Potential Controversies
4. Nutrigenomic: Role of Nutraceuticals on Osteoarthritis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Agel, J.; Akesson, K.; Amadio, P.C.; Anderson, M.; Badley, E.; Balint, G.; Bellamy, N.; Bigos, S.; Bishop, N.; Bivans, B.; et al. The Burden of Musculoskeletal Conditions at the Start of the New Millennium; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.P.; Roughley, P.J. Cartilage in normal and osteoarthritis conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef]
- Wang, M.N.; Liu, L.; Zhao, L.P.; Yuan, F.; Fu, Y.B.; Xu, X.B.; Li, B. Research of inflammatory factors and signaling pathways in knee osteoarthritis. Zhongguo Gu Shang 2020, 33, 388–392. [Google Scholar] [PubMed]
- Hong, J.I.; Park, I.Y.; Kim, H.A. Understanding the molecular mechanisms underlying the pathogenesis of arthritis pain using animal models. Int. J. Mol. Sci. 2020, 21, 533. [Google Scholar] [CrossRef] [PubMed]
- Al-Modawi, R.N.; Brinchmann, J.E.; Karlsen, T.A. Multi-pathway Protective Effects of MicroRNAs on Human Chondrocytes in an In Vitro Model of Osteoarthritis. Mol. Ther. Nucleic Acids 2019. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Kraus, V.B.; Karsdal, M.A. Osteoarthritis: Current Molecular Biomarkers and the Way Forward. Calcif. Tissue Int. 2020, 1–10. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y. A Molecular Cascade Underlying Articular Cartilage Degeneration. Curr. Drug Targets 2020. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 1–19. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.M.; Jette, A.M. The contribution of osteoarthritis to functional limitations and disability. Clin. Geriatr. Med. 2010, 26, 387–399. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, F.; Moretti, A.; de Sire, A.; Migliore, A.; Brandi, M.L.; Piscitelli, P. Early osteoarthritis: How to define, diagnose, and manage. A systematic review. Eur. Geriatr. Med. 2017. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014. [Google Scholar] [CrossRef]
- Santilli, V.; Mangone, M.; Paoloni, M.; Agostini, F.; Alviti, F.; Bernetti, A. Comment on “early efficacy of intra-articular HYADD® 4 (Hymovis®) injections for symptomatic knee osteoarthritis”. Joints 2018, 5, 79. [Google Scholar] [CrossRef]
- Rabini, A.; De Sire, A.; Marzetti, E.; Gimigliano, R.; Ferriero, G.; Piazzini, D.B.; Iolascon, G.; Gimigliano, F. Effects of focal muscle vibration on physical functioning in patients with knee osteoarthritis: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 513–520. [Google Scholar]
- De Sire, A.; Stagno, D.; Minetto, M.A.; Cisari, C.; Baricich, A.; Invernizzi, M. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J. Back Musculoskelet. Rehabil. 2020. [Google Scholar] [CrossRef]
- El-Hakeim, E.H.; Elawamy, A.; Kamel, E.Z.; Goma, S.H.; Gamal, R.M.; Ghandour, A.M.; Osman, A.M.; Morsy, K.M. Fluoroscopic guided radiofrequency of genicular nerves for pain alleviation in chronic knee osteoarthritis: A single-blind randomized controlled trial. Pain Physician 2018. [Google Scholar] [CrossRef]
- Roato, I.; Ferracini, R. Is the adipose-derived mesenchymal stem cell therapy effective for treatment of knee osteoarthritis? Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Southworth, T.M.; Naveen, N.B.; Tauro, T.M.; Leong, N.L.; Cole, B.J. The Use of Platelet-Rich Plasma in Symptomatic Knee Osteoarthritis. J. Knee Surg. 2019. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef]
- Meiyappan, K.P.; Cote, M.P.; Bozic, K.J.; Halawi, M.J. Adherence to the American Academy of Orthopaedic Surgeons Clinical Practice Guidelines for Nonoperative Management of Knee Osteoarthritis. J. Arthroplast. 2020. [Google Scholar] [CrossRef]
- Mazor, M.; Best, T.M.; Cesaro, A.; Lespessailles, E.; Toumi, H. Osteoarthritis biomarker responses and cartilage adaptation to exercise: A review of animal and human models. Scand. J. Med. Sci. Sport. 2019, 29, 1072–1082. [Google Scholar] [CrossRef]
- de Sire, A.; de Sire, R.; Petito, V.; Masi, L.; Cisari, C.; Gasbarrini, A.; Scaldaferri, F.; Invernizzi, M. Gut–joint axis: The role of physical exercise on gut microbiota modulation in older people with osteoarthritis. Nutrients 2020, 12, 574. [Google Scholar] [CrossRef]
- Pérez-Lozano, M.L.; Cesaro, A.; Mazor, M.; Esteve, E.; Berteina-Raboin, S.; Best, T.M.; Lespessailles, E.; Toumi, H. Emerging natural-product-based treatments for the management of osteoarthritis. Antioxidants 2021, 10, 265. [Google Scholar] [CrossRef]
- Aghamohammadi, D.; Dolatkhah, N.; Bakhtiari, F.; Eslamian, F.; Hashemian, M. Nutraceutical supplements in management of pain and disability in osteoarthritis: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Deligiannidou, G.E.; Papadopoulos, R.E.; Kontogiorgis, C.; Detsi, A.; Bezirtzoglou, E.; Constantinides, T. Unraveling natural products’ role in osteoarthritis management—An overview. Antioxidants 2020, 9, 348. [Google Scholar] [CrossRef]
- Lee, H.; Zhao, X.; Son, Y.O.; Yang, S. Therapeutic single compounds for osteoarthritis treatment. Pharmaceuticals 2021, 14, 131. [Google Scholar] [CrossRef]
- Blazek, A.D.; Nam, J.; Gupta, R.; Pradhan, M.; Perera, P.; Weisleder, N.L.; Hewett, T.E.; Chaudhari, A.M.; Lee, B.S.; Leblebicioglu, B.; et al. Exercise-driven metabolic pathways in healthy cartilage. Osteoarthr. Cartil. 2016. [Google Scholar] [CrossRef]
- Hui, W.; Young, D.A.; Rowan, A.D.; Xu, X.; Cawston, T.E.; Proctor, C.J. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. Rheum. Dis. 2016. [Google Scholar] [CrossRef]
- Li, D.; Xie, G.; Wang, W. Reactive oxygen species: The 2-edged sword of osteoarthritis. Am. J. Med. Sci. 2012, 344, 486–490. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Mao, X.; Fu, P.; Wang, L.; Xiang, C. Mitochondria: Potential Targets for Osteoarthritis. Front. Med. 2020, 7, 7. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.P.L. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Regan, E.A.; Bowler, R.P.; Crapo, J.D. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthr. Cartil. 2008. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The protective role of glutathione in osteoarthritis. J. Clin. Orthop. Trauma 2021, 15, 145–151. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, C.; Ni, L.; Huang, C.; Chen, D.; Shi, K.; Jin, H.; Zhang, K.; Li, Y.; Xie, L.; et al. Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020. [Google Scholar] [CrossRef]
- Malemud, C.J. The PI3K/Akt/PTEN/mTOR pathway: A fruitful target for inducing cell death in rheumatoid arthritis? Future Med. Chem. 2015, 7, 1137–1147. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Deng, X.; Huang, D.; Shao, Z.; Ma, K. Compression stress induces nucleus pulposus cell autophagy by inhibition of the PI3K/AKT/mTOR pathway and activation of the JNK pathway. Connect. Tissue Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Wang, S.; Wang, J. Peroxiredoxin 4 inhibits IL-1β-induced chondrocyte apoptosis via PI3K/AKT signaling. Biomed. Pharmacother. 2017. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Resveratrol targeting the Wnt signaling pathway: A focus on therapeutic activities. J. Cell. Physiol. 2020, 235, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, W.; Sigaux, J.; Modrowski, D.; Devignes, C.S.; Funck-Brentano, T.; Richette, P.; Ea, H.K.; Provot, S.; Cohen-Solal, M.; Haÿ, E. Interaction of HIF1α and β-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc. Natl. Acad. Sci. USA 2016. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zheng, W.; Chen, H.; Shao, X.; Lin, P.; Liu, X.; Li, X.; Ye, H. Glucosamine promotes chondrocyte proliferation via the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic. Biol. Med. 2017. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Liu, B.; Tian, Q.Y.; Wei, J.L.; Richbourgh, B.; Liu, C.J. Progranulin protects against osteoarthritis through interacting with TNF-α and β-Catenin signalling. Ann. Rheum. Dis. 2015. [Google Scholar] [CrossRef]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef]
- Imagawa, K.; de Andrés, M.C.; Hashimoto, K.; Pitt, D.; Itoi, E.; Goldring, M.B.; Roach, H.I.; Oreffo, R.O.C. The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-κB) inhibitor on primary human chondrocytes—Implications for osteoarthritis. Biochem. Biophys. Res. Commun. 2011. [Google Scholar] [CrossRef]
- Vincent, T.L. Mechanoflammation in osteoarthritis pathogenesis. Semin. Arthritis Rheum. 2019. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, Y.; Wang, W.; Wang, Z.; Tang, G.; Zhang, P.; He, Z.; Liu, Y.; Dai, S.M.; Shen, Q. HMGB1-LPS complex promotes transformation of osteoarthritis synovial fibroblasts to a rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Millerand, M.; Berenbaum, F.; Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37, 48–56. [Google Scholar] [PubMed]
- Sun, J.; Nan, G. The Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway as a Discovery Target in Stroke. J. Mol. Neurosci. 2016, 59, 90–98. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, A.; Ma, L.; Yu, H.; Zhang, L.; Meng, H.; Cui, Y.; Yu, F.; Yang, B. Docosahexenoic acid treatment ameliorates cartilage degeneration via a p38 MAPK-dependent mechanism. Int. J. Mol. Med. 2016. [Google Scholar] [CrossRef]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative stress and chronic inflammation in osteoarthritis: Can NRF2 counteract these partners in crime? Ann. N. Y. Acad. Sci. 2017, 1404, 114–135. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 Controls Postinduction Repression of the Nrf2-Mediated Antioxidant Response by Escorting Nuclear Export of Nrf2. Mol. Cell. Biol. 2007. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Jevsevar, D.S.; Brown, G.A.; Jones, D.L.; Matzkin, E.G.; Manner, P.A.; Mooar, P.; Schousboe, J.T.; Stovitz, S.; Sanders, J.O.; Bozic, K.J.; et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: Treatment of osteoarthritis of the knee, 2nd edition. J. Bone Jt. Surg. Am. 2013. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011. [Google Scholar] [CrossRef] [PubMed]
- Rausch Osthoff, A.K.; Niedermann, K.; Braun, J.; Adams, J.; Brodin, N.; Dagfinrud, H.; Duruoz, T.; Esbensen, B.A.; Günther, K.P.; Hurkmans, E.; et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 1251–1260. [Google Scholar] [CrossRef]
- Fransen, M. When is physiotherapy appropriate? Best Pract. Res. Clin. Rheumatol. 2004. [Google Scholar] [CrossRef]
- Rahmann, A. Exercise for people with hip or knee osteoarthritis: A comparison of land-based and aquatic interventions. Open Access J. Sport. Med. 2010. [Google Scholar] [CrossRef]
- Raposo, F.; Ramos, M.; Lúcia Cruz, A. Effects of exercise on knee osteoarthritis: A systematic review. Musculoskelet. Care 2021. [Google Scholar] [CrossRef]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Yamaguchi, S.; Nagai, M.; Kiyan, W.; Zhang, X.; Kuroki, H. Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthr. Cartil. 2016. [Google Scholar] [CrossRef]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Muniz Renno, A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Kong, Y.; Zhang, X.; Bai, L. The effects of different frequency treadmill exercise on lipoxin A4 and articular cartilage degeneration in an experimental model of monosodium iodoacetate-induced osteoarthritis in rats. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, L.; Yang, Y.; Wei, Y.; Zhang, X.; Gang, Y.; Lu, J.; Bai, L. The Therapeutic Effects of Treadmill Exercise on Osteoarthritis in Rats by Inhibiting the HDAC3/NF-KappaB Pathway in vivo and in vitro. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int. J. Mol. Sci. 2019, 20, 511. [Google Scholar] [CrossRef]
- Lu, J.; Feng, X.; Zhang, H.; Wei, Y.; Yang, Y.; Tian, Y.; Bai, L. Maresin-1 suppresses IL-1β-induced MMP-13 secretion by activating the PI3K/AKT pathway and inhibiting the NF-κB pathway in synovioblasts of an osteoarthritis rat model with treadmill exercise. Connect. Tissue Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Li, X.; Zhang, H.; Gang, Y.; Bai, L. Alterations of autophagy in knee cartilage by treatment with treadmill exercise in a rat osteoarthritis model. Int. J. Mol. Med. 2019, 43, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gou, J.; Zhang, H.; Lu, J.; Jin, Z.; Jia, S.; Bai, L. The anti-inflammatory effects of 15-HETE on osteoarthritis during treadmill exercise. Life Sci. 2021. [Google Scholar] [CrossRef]
- Galois, L.; Etienne, S.; Grossin, L.; Watrin-Pinzano, A.; Cournil-Henrionnet, C.; Loeuille, D.; Netter, P.; Mainard, D.; Gillet, P. Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: A pilot study. Osteoarthr. Cartil. 2004. [Google Scholar] [CrossRef]
- Nam, J.; Perera, P.; Liu, J.; Wu, L.C.; Rath, B.; Butterfield, T.A.; Agarwal, S. Transcriptome-wide gene regulation by gentle treadmill walking during the progression of monoiodoacetate-induced arthritis. Arthritis Rheum. 2011. [Google Scholar] [CrossRef]
- Bricca, A.; Juhl, C.B.; Steultjens, M.; Wirth, W.; Roos, E.M. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: A systematic review of randomised controlled trials. Br. J. Sports Med. 2019. [Google Scholar] [CrossRef]
- Roos, E.M.; Dahlberg, L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: A four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005. [Google Scholar] [CrossRef]
- Hawezi, Z.K.; Lammentausta, E.; Svensson, J.; Roos, E.M.; Dahlberg, L.E.; Tiderius, C.J. Regional dGEMRIC analysis in patients at risk of osteoar thritis provides additional information about activity related changes in car tilage structure. Acta Radiol. 2016. [Google Scholar] [CrossRef]
- Munukka, M.; Waller, B.; Häkkinen, A.; Nieminen, M.T.; Lammentausta, E.; Kujala, U.M.; Paloneva, J.; Kautiainen, H.; Kiviranta, I.; Heinonen, A. Physical Activity Is Related with Cartilage Quality in Women with Knee Osteoarthritis. Med. Sci. Sports Exerc. 2017. [Google Scholar] [CrossRef] [PubMed]
- Helmark, I.C.; Mikkelsen, U.R.; Børglum, J.; Rothe, A.; Petersen, M.C.H.; Andersen, O.; Langberg, H.; Kjaer, M. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: A randomized controlled trial. Arthritis Res. Ther. 2010. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.A.; Pollock, C.L.; Kraus, V.B.; Saxne, T.; Peters, S.; Huebner, J.L.; Sayre, E.C.; Cibere, J. Relationships amongst osteoarthritis biomarkers, dynamic knee joint load, and exercise: Results from a randomized controlled pilot study. BMC Musculoskelet. Disord. 2013. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Mihalko, S.L.; Legault, C.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Beavers, D.P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA J. Am. Med. Assoc. 2013. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Mottes, M.; Cheri, S.; Deiana, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Salvagno, G.L.; Lippi, G.; Valenti, M.T. Increased Gene Expression of RUNX2 and SOX9 in Mesenchymal Circulating Progenitors Is Associated with Autophagy during Physical Activity. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Hunter, C.J.; Imler, S.M.; Malaviya, P.; Nerem, R.M.; Levenston, M.E. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials 2002. [Google Scholar] [CrossRef]
- Khan, N.M.; Ahmad, I.; Haqqi, T.M. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic. Biol. Med. 2018, 116, 159–171. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef]
- Cifuentes, D.J.; Rocha, L.G.; Silva, L.A.; Brito, A.C.; Rueff-Barroso, C.R.; Porto, L.C.; Pinho, R.A. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthr. Cartil. 2010. [Google Scholar] [CrossRef]
- Germanou, E.I.; Chatzinikolaou, A.; Malliou, P.; Beneka, A.; Jamurtas, A.Z.; Bikos, C.; Tsoukas, D.; Theodorou, A.; Katrabasas, I.; Margonis, K.; et al. Oxidative stress and inflammatory responses following an acute bout of isokinetic exercise in obese women with knee osteoarthritis. Knee 2013. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy—A review. BMC Musculoskelet. Disord. 2016, 17, 1–13. [Google Scholar] [CrossRef]
- Song, J.S.; Hong, K.T.; Kim, N.M.; Jung, J.Y.; Park, H.S.; Chun, Y.S.; Kim, S.J. Cartilage regeneration in osteoarthritic knees treated with distal femoral osteotomy and intra-lesional implantation of allogenic human umbilical cord blood-derived mesenchymal stem cells: A report of two cases. Knee 2019. [Google Scholar] [CrossRef]
- Minas, T.; Ogura, T.; Bryant, T. Autologous chondrocyte implantation. JBJS Essent. Surg. Tech. 2016. [Google Scholar] [CrossRef]
- Schmidt, A.; Bierwirth, S.; Weber, S.; Platen, P.; Schinköthe, T.; Bloch, W. Short intensive exercise increases the migratory activity of mesenchymal stem cells. Br. J. Sports Med. 2009. [Google Scholar] [CrossRef]
- Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Dalle Carbonare, L.; Mottes, M. Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis. Cells 2019, 8, 742. [Google Scholar] [CrossRef]
- Smith, J.K. Exercise as an adjuvant to cartilage regeneration therapy. Int. J. Mol. Sci. 2020, 21, 9471. [Google Scholar] [CrossRef] [PubMed]
- Emmons, R.; Niemiro, G.M.; Owolabi, O.; De Lisio, M. Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J. Appl. Physiol. 2016. [Google Scholar] [CrossRef]
- Bourzac, C.; Bensidhoum, M.; Pallu, S.; Portier, H. Use of adult mesenchymal stromal cells in tissue repair: Impact of physical exercise. Am. J. Physiol. Cell Physiol. 2019, 317, C642–C654. [Google Scholar] [CrossRef] [PubMed]
- Ocarino, N.M.; Boeloni, J.N.; Goes, A.M.; Silva, J.F.; Marubayashi, U.; Serakides, R. Osteogenic differentiation of mesenchymal stem cells from osteopenic rats subjected to physical activity with and without nitric oxide synthase inhibition. Nitric Oxide Biol. Chem. 2008. [Google Scholar] [CrossRef] [PubMed]
- Hell, R.C.R.; Ocarino, N.M.; Boeloni, J.N.; Silva, J.F.; Goes, A.M.; Santos, R.L.; Serakides, R. Physical activity improves age-related decline in the osteogenic potential of rats’ bone marrow-derived mesenchymal stem cells. Acta Physiol. 2012, 205, 292–301. [Google Scholar] [CrossRef]
- Andersson, M.L.E.; Thorstensson, C.A.; Roos, E.M.; Petersson, I.F.; Heinegård, D.; Saxne, T. Serum levels of Cartilage Oligomeric Matrix Protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Musculoskelet. Disord. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Siebelt, M.; Groen, H.C.; Koelewijn, S.J.; de Blois, E.; Sandker, M.; Waarsing, J.H.; Müller, C.; van Osch, G.J.V.M.; de Jong, M.; Weinans, H. Increased physical activity severely induces osteoarthritic changes in knee joints with papain induced sulfate-glycosaminoglycan depleted cartilage. Arthritis Res. Ther. 2014, 16. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhou, P.; Zhang, Y. Abnormal expression of key genes and proteins in the canonical Wnt/β-catenin pathway of articular cartilage in a rat model of exercise-induced osteoarthritis. Mol. Med. Rep. 2016, 13, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Ortega, M.; Cruz, R.; Vega-López, M.A.; Cabrera-González, M.; Hernández-Hernández, J.M.; Lavalle-Montalvo, C.; Kouri, J.B. Exercise modulates the expression of IL-1β and IL-10 in the articular cartilage of normal and osteoarthritis-induced rats. Pathol. Res. Pract. 2015. [Google Scholar] [CrossRef]
- Coyle, C.H.; Henry, S.E.; Haleem, A.M.; O’Malley, M.J.; Chu, C.R. Serum CTXii Correlates with Articular Cartilage Degeneration After Anterior Cruciate Ligament Transection or Arthrotomy Followed by Standardized Exercise. Sports Health 2012. [Google Scholar] [CrossRef]
- Jayabalan, P.; Gustafson, J.; Sowa, G.A.; Piva, S.R.; Farrokhi, S. A Stimulus-Response Framework to Investigate the Influence of Continuous Versus Interval Walking Exercise on Select Serum Biomarkers in Knee Osteoarthritis. Am. J. Phys. Med. Rehabil. 2019. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Gimigliano, R.; Bianco, M.; de Sire, A.; Moretti, A.; Giusti, A.; Malavolta, N.; Migliaccio, S.; Migliore, A.; Napoli, N.; et al. Are dietary supplements and nutraceuticals effective for musculoskeletal health and cognitive function? A scoping review. J. Nutr. Heal. Aging 2017. [Google Scholar] [CrossRef]
- Cho, C.; Kang, L.J.; Jang, D.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Kim, C.S.; et al. Cirsium japonicum var. maackii and apigenin block Hif-2α-induced osteoarthritic cartilage destruction. J. Cell. Mol. Med. 2019. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Berberine and musculoskeletal disorders: The therapeutic potential and underlying molecular mechanisms. Phytomedicine 2020, 73, 152892. [Google Scholar] [CrossRef]
- Korotkyi, O.; Huet, A.; Dvorshchenko, K.; Kobyliak, N.; Falalyeyeva, T.; Ostapchenko, L. Probiotic Composition and Chondroitin Sulfate Regulate TLR-2/4-Mediated NF-κB Inflammatory Pathway and Cartilage Metabolism in Experimental Osteoarthritis. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4 /MyD88/NF-κB signal pathway. Drug Dev. Res. 2019. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The emerging role of curcumin in the modulation of TLR-4 signaling pathway: Focus on neuroprotective and anti-rheumatic properties. Int. J. Mol. Sci. 2020, 21, 2299. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xu, X.; Yi, P.; Hao, Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-κB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 2020. [Google Scholar] [CrossRef]
- Lee, H.; Jang, D.; Jeon, J.; Cho, C.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Park, C.H.; Shin, Y.S.; et al. Seomae mugwort and jaceosidin attenuate osteoarthritic cartilage damage by blocking IκB degradation in mice. J. Cell. Mol. Med. 2020, 24, 8126–8137. [Google Scholar] [CrossRef]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective effects of genistein against osteoarthritis induced joint inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ding, W.; Wu, N.; Jiang, S.; Li, W. Protective Effect of Genistein on Condylar Cartilage through Downregulating NF- B Expression in Experimentally Created Osteoarthritis Rats. Biomed Res. Int. 2019. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Q.; Guo, P.; Huang, Y.; Ye, Z.; Hu, J. Anti-chondrocyte apoptosis effect of genistein in treating inflammation-induced osteoarthritis. Mol. Med. Rep. 2020, 22, 2032–2042. [Google Scholar] [CrossRef]
- Lv, C.; Wang, L.; Zhu, X.; Lin, W.; Chen, X.; Huang, Z.; Huang, L.; Yang, S. Glucosamine promotes osteoblast proliferation by modulating autophagy via the mammalian target of rapamycin pathway. Biomed. Pharmacother. 2018. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, Z.; Li, Y.; Song, X.; Ma, T.; Liu, C.; Liu, L.; Yuan, R.; Wang, X.; Gao, L. L-theanine reduced the development of knee osteoarthritis in rats via its anti-inflammation and anti-matrix degradation actions: In vivo and in vitro study. Nutrients 2020, 12, 1988. [Google Scholar] [CrossRef] [PubMed]
- Luk, H.Y.; Appell, C.; Chyu, M.C.; Chen, C.H.; Wang, C.Y.; Yang, R.S.; Shen, C.L. Impacts of green tea on joint and skeletal muscle health: Prospects of translational nutrition. Antioxidants 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Liu, H.W.; Chan, Y.C.; Hu, S.H.; Liu, M.Y.; Chang, S.J. The green tea polyphenol epigallocatechin-3-gallate attenuates age-associated muscle loss via regulation of miR-486-5p and myostatin. Arch. Biochem. Biophys. 2020. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef]
- Varela-Eirín, M.; Carpintero-Fernández, P.; Sánchez-Temprano, A.; Varela-Vázquez, A.; Paíno, C.L.; Casado-Díaz, A.; Continente, A.C.; Mato, V.; Fonseca, E.; Kandouz, M.; et al. Senolytic activity of small molecular polyphenols from olive restores chondrocyte redifferentiation and promotes a pro-regenerative environment in osteoarthritis. Aging 2020. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yan, D.Y.; Wu, C.Y.; Xuan, J.W.; Jin, C.Q.; Hu, X.L.; Bao, G.D.; Bian, Y.J.; Hu, Z.C.; Shen, Z.H.; et al. Maslinic acid prevents IL-1β-induced inflammatory response in osteoarthritis via PI3K/AKT/NF-κB pathways. J. Cell. Physiol. 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Zhao, D.; Wang, C.; Zhang, Y.; Wang, Y.; Li, T. Therapeutic effect and mechanism of action of quercetin in a rat model of osteoarthritis. J. Int. Med. Res. 2019. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Yang, Y.; He, J.; Jiang, M.; Huang, Y.; Liu, X.; Liu, L.; Gu, H. Resveratrol exerts anti-osteoarthritic effect by inhibiting TLR4/NF-κB signaling pathway via the TLR4/Akt/FoxO1 axis in IL-1β-stimulated SW1353 cells. Drug Des. Devel. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, W.; Cui, Z.M.; Cui, S.Y.; Fan, J.B.; Zhu, X.H.; Liu, W. Resveratrol alleviates the interleukin-1β-induced chondrocytes injury through the NF-κB signaling pathway. J. Orthop. Surg. Res. 2020. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Feng, Y.; Liu, N.; Fu, Z.; Wu, J.; Li, T.; Chen, H.; Chen, J.; Chen, C.; et al. Natural ingredients-derived antioxidants attenuate H2O2-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via Keap1/Nrf2 pathway. Free Radic. Biol. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Jhun, J.; Ryu, J.; Kwon, J.Y.; Kim, S.Y.; Jung, K.A.; Cho, M.L.; Min, J.K. The anti-arthritis effect of sulforaphane, an activator of Nrf2, is associated with inhibition of both B cell differentiation and the production of inflammatory cytokines. PLoS ONE 2021. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Fu, X.; Liu, Y.; Ji, Y.; Shang, Z. Sulforaphane Inhibits Osteoclastogenesis via Suppression of the Autophagic Pathway. Molecules 2021, 26, 347. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. A wogonin-rich-fraction of Scutellaria baicalensis root extract exerts chondroprotective effects by suppressing IL-1β-induced activation of AP-1 in human OA chondrocytes. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Ammendolia, A.; Marotta, N.; Marinaro, C.; Demeco, A.; Mondardini, P.; Costantino, C. High Power Laser Therapy and Glucosamine sulfate in the treatment of knee osteoarthritis: A single blinded randomized controlled trial. Acta Biomed. l’Ateneo Parm. 2021. [Google Scholar] [CrossRef]
- Zhang, C.; Sheng, J.; Li, G.; Zhao, L.; Wang, Y.; Yang, W.; Yao, X.; Sun, L.; Zhang, Z.; Cui, R. Effects of berberine and its derivatives on cancer: A systems pharmacology review. Front. Pharmacol. 2020, 10, 1461. [Google Scholar] [CrossRef]
- Hou, C.Y.; Tain, Y.L.; Yu, H.R.; Huang, L.T. The effects of resveratrol in the treatment of metabolic syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
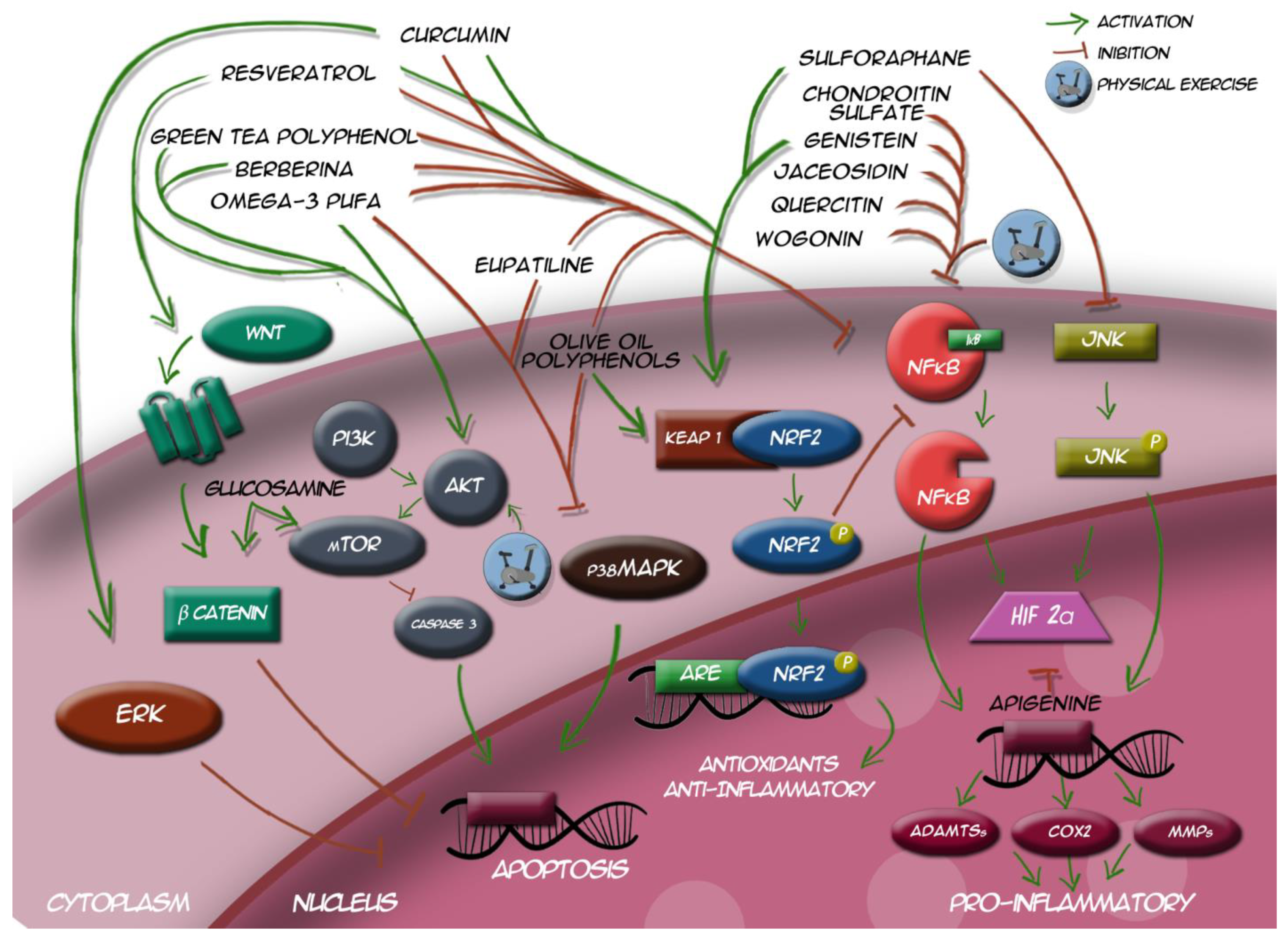
| Nutraceutical | OA Pathways Involved | Modulation Action | Main Findings | Journal | Authors | Year |
|---|---|---|---|---|---|---|
| Apigenin | ┤ HIF 2α | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Apigenin blocks osteoarthritis development as Hif-2α inhibitor. | Journal of Cellular and Molecular Medicine | Cho et al. [110] | 2019 |
| Berberine | → PI3K/AKT ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Berberine activates PI3K/Akt and NF-κB pathways. | Phytomedicine | Wong et al. [111] | 2019 |
| Chondroitin Sulfate | ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Separate administration of chondroitin sulfate raised expression of Comp and reduced TLRs, and NF-κB expressions in cartilage. | Probiotics and Antimicrobial Proteins | Korotkyi et al. [112] | 2021 |
| Curcumin | → ERK1/2 | ↓ Chondrocyte Apoptosis | Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. | Nutrients | Li et al. [52] | 2017 |
| ┤NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Curcumin reduces inflammation in knee osteoarthritis through blocking TLR4/MyD88/NF-κB signal pathway. | Drug Development Research | Zhang et al. [113] | 2018 | |
| → Nrf2/HO-1 | ↑ GPX1,3,4 ↑ SOD1 ↑ CAT ↑ GST | Curcumin inhibits chondrocytes inflammation through the Nrf2/ARE signaling pathway, thereby exerting cartilage protective effects. | Cell Stress and Chaperones | Jiang et al. [114] | 2020 | |
| ┤ TLR4/NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Curcumin improve neuroinflammatory process by reducing microglia/macrophage activation and neuronal apoptosis through a mechanism involving the TLR4/NF-κB signaling pathway in microglia/macrophages. | International Journal of Molecular Sciences | Panaro et al. [115] | 2020 | |
| ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Curcumin reduces expression of NF-κB and ROCK1. | Journal of Cellular and Molecular Medicine | Qiu et al. [116] | 2020 | |
| Eupatilin | ┤ NF-κB ┤ JNK ┤ p38MAPK | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Eupatilin suppressed expression of MMPs, ADAMTSs in chondrocytes by reducing JNK phosphorylation and NF-κB and MAPK signaling. | Pharmaceuticals | Lee et al. [117] | 2021 |
| Genistein | → Nrf2/HO-1 | ↑ GPX1,3,4 ↑ SOD1 ↑ CAT ↑ GST | Genistein downregulates MMPs, ADAMTSs via NF-κB signaling pathway by blocking IκB degradation and activating Keap1/Nrf2 pathway. | Nutrients | Liu et al. [118] | 2019 |
| ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | BioMed Research International | Yaun et al. [119] | 2019 | ||
| Molecular Medicine Report | Zou et al. [120] | 2020 | ||||
| Glucosamine | → mTOR | ↓ Chondrocyte Apoptosis | Glucosamine promotes osteoblast proliferation by modulating autophagy via the mTOR pathway. | Biomedicine & Pharmacotherapy | Lv et al. [121] | 2018 |
| → Wnt/β-catenin | ↓ Chondrocyte Apoptosis | GlcN increases β-catenin nuclear translocation, thus promoting chondrocyte proliferation. | International Journal of Molecular Medicine | Ma et al. [49] | 2018 | |
| Green tea polyphenol | ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | L-theanine inhibits upregulation of MMPs, as well as inhibiting NF-κB | Nutrients | Bai et al. [122] | 2020 |
| Green tea catechins increase NF-κB inhibitors expression | Antioxidants | Luk et al. [123] | 2020 | |||
| →PI3K/AKT | ↓ FOX-O1 | Epigallocatechin-3-gallate modulating AKT-FoxO1 via upregulating miR-486-5p. | Archives of Biochemistry and Biophysics | Chang et al. [124] | 2020 | |
| Jaceosidin | ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Jaceosidin attenuates cartilage destruction by suppressing MMPs, ADAMTSs and the NFκB signaling pathway by blocking IκB degradation. | Journal of Cellular and Molecular Medicine | Lee et al. [117] | 2019 |
| Omega-3 PUFA | ┤ p38MAPK | ↓ Chondrocyte Apoptosis | PUFA inactivates of p38MAPK | International Journal of Molecular Medicine | Wang et al. [58] | 2016 |
| → PI3K/AKT ┤ NF-κB | ↓ MMPs | PUFA metabolite suppresses MMP-13 secretion by activating PI3K/AKT pathway directly, while inhibiting NF-κB pathway. | Connective Tissue Research | Lu et al. [74] | 2020 | |
| OOP | ┤ NF-κB ┤ p38MAPK | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | OOPs inhibited IL-1β-induced expression of inflammatory mediators through suppressing NF-κB and MAPK activation in chondrocytes. | Food & Function | Feng et al. [125] | 2017 |
| → Nrf2/HO-1 ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | OOPs can activate Nrf-2 signaling and the blockage of NF-κB nuclear translocation | Cells | Serrelli et al. [126] | 2020 | |
| ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | OOPs reduce the inflammatory and catabolic factors mediated by NF-κB (IL-1ß, IL-6, COX-2 and MMP-3 | Aging | Varela-Eirín et al. [127] | 2020 | |
| ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Mechanistically, OOPs exhibited an anti-inflammatory effect by inactivating the PI3K/AKT/NF-κB pathway. | Journal of Cellular Physiology | Chen et al. [128] | 2021 | |
| Quercitin | ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Quercetin inhibits IL-1b and TNF-a production via TLR-4/NF-κB pathway. | Journal of International Medical Research | Zhang et al. [129] | 2019 |
| Resveratrol | →Wnt/β-catenin | ↓ Chondrocyte Apoptosis | Rev increased osteoblastogenesis and bone formation through stimulation of Wnt signaling pathway. | Journal of Cell Physiology | Ashrafizadeh et al. [47] | 2020 |
| →Nrf2/HO-1 | ↑ GPX1,3,4 ↑ SOD1 ↑ CAT ↑ GST | Res modulates the Nrf2 activation by inhibiting Keap1, Nrf2 gene expression, changing the upstream mediators of Nrf2, and potentiating the nuclear translocation of Nrf2. | Biomedicine & Pharmacotherapy | Farkhondeh et al. [130] | 2020 | |
| →PI3K/AKT | ↓ FOX-O1 | Resveratrol may exert anti-OA effect by enhancing the self-limiting mechanism of inflammation through TLR4/Akt/FoxO1 axis. | Drug Design, Development and Therapy | Xu et al. [131] | 2020 | |
| ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Resveratrol alleviates the interleukin-1β-induced chondrocytes injury through the NF-κB signaling pathway. | Journal of Orthopaedic Surgery and Research | Yi et al. [132] | 2020 | |
| Sulforaphane | → Nrf2/HO-1 | ↑ GPX1,3,4 ↑ SOD1 ↑ CAT ↑ GST | Sulforaphane ameliorates oxidative stress suppressing inflammatory cytokines and activating Keap1/Nrf2 pathway. | Free Radical Biology and Medicine | Yang et al. [133] | 2020 |
| Sulforaphane inhibits the production of inflammatory cytokines. | PLoS ONE | Moon et al. [134] | 2021 | |||
| ┤ JNK | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Sulforaphane inhibits osteoclastogenesis by suppressing autophagy modulating JNK pathway. | Molecules | Lou et al. [135] | 2021 | |
| Wogonin | ┤ NF-κB | ↓ ADAMTS-4 ↓ COX-2 ↓ MMP | Wogonin downregulates NF-κB pathway and genes involved in inflammatory-response. | Nature: Scientific Report | Khan et al. [136] | 2017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sire, A.; Marotta, N.; Marinaro, C.; Curci, C.; Invernizzi, M.; Ammendolia, A. Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5722. https://doi.org/10.3390/ijms22115722
de Sire A, Marotta N, Marinaro C, Curci C, Invernizzi M, Ammendolia A. Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis. International Journal of Molecular Sciences. 2021; 22(11):5722. https://doi.org/10.3390/ijms22115722
Chicago/Turabian Stylede Sire, Alessandro, Nicola Marotta, Cinzia Marinaro, Claudio Curci, Marco Invernizzi, and Antonio Ammendolia. 2021. "Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis" International Journal of Molecular Sciences 22, no. 11: 5722. https://doi.org/10.3390/ijms22115722
APA Stylede Sire, A., Marotta, N., Marinaro, C., Curci, C., Invernizzi, M., & Ammendolia, A. (2021). Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis. International Journal of Molecular Sciences, 22(11), 5722. https://doi.org/10.3390/ijms22115722










