Effects of Strontium-Doped β-Tricalcium Scaffold on Longitudinal Nuclear Factor-Kappa Beta and Vascular Endothelial Growth Factor Receptor-2 Promoter Activities during Healing in a Murine Critical-Size Bone Defect Model
Abstract
1. Introduction
2. Results
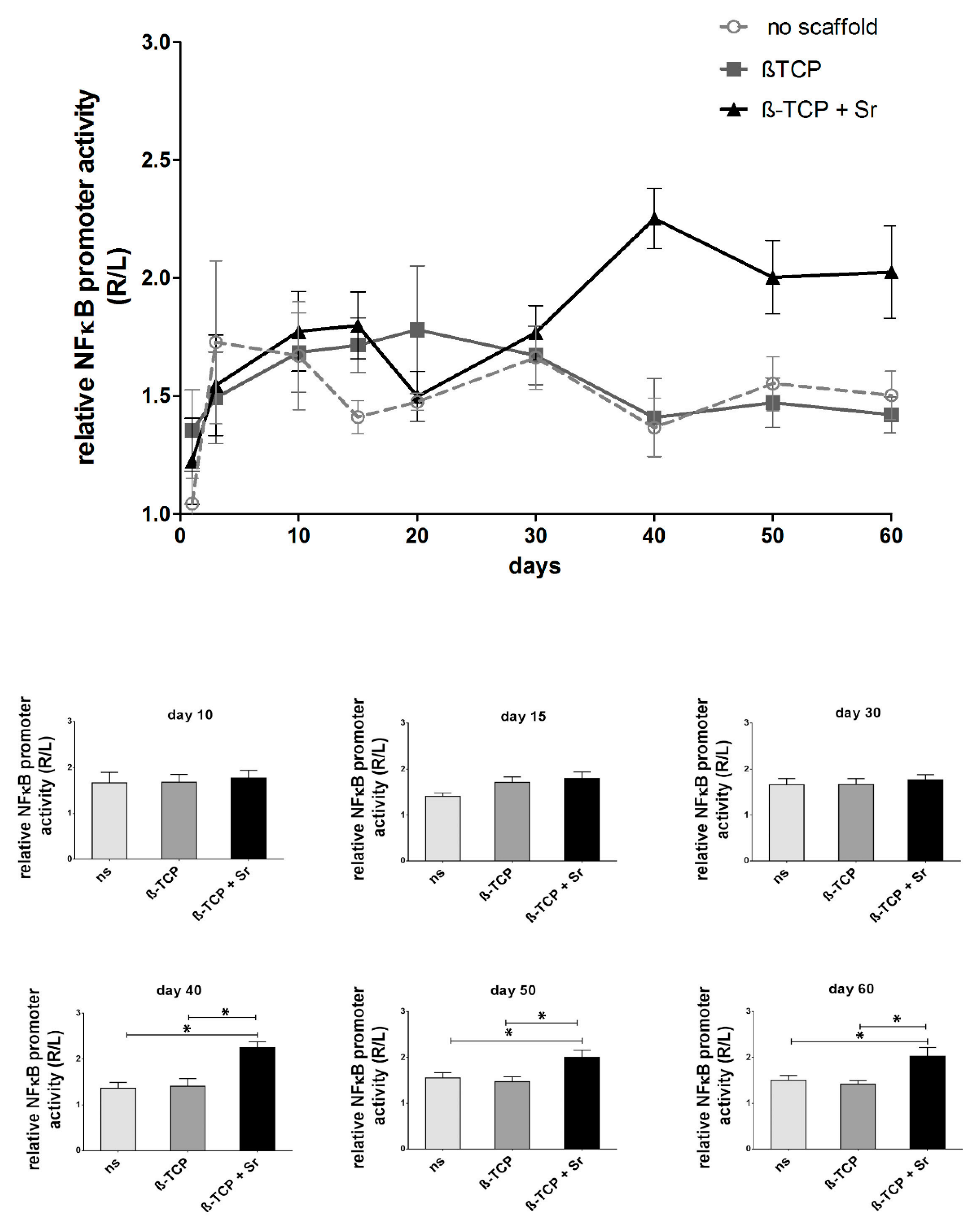
2.1. Longitudinal Monitoring of NF-κB Activity
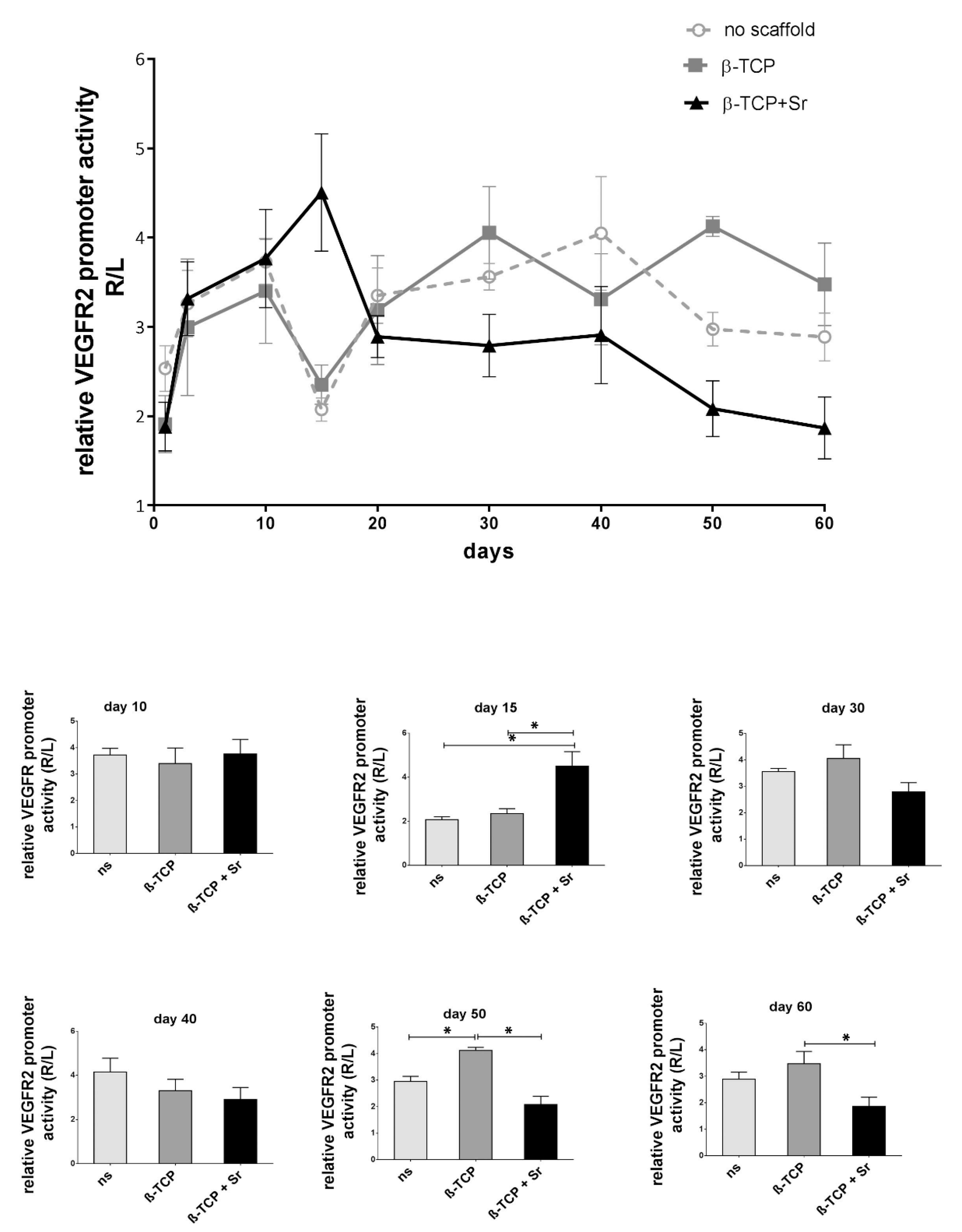
2.2. Longitudinal Monitoring of VEGFR-2 Activity
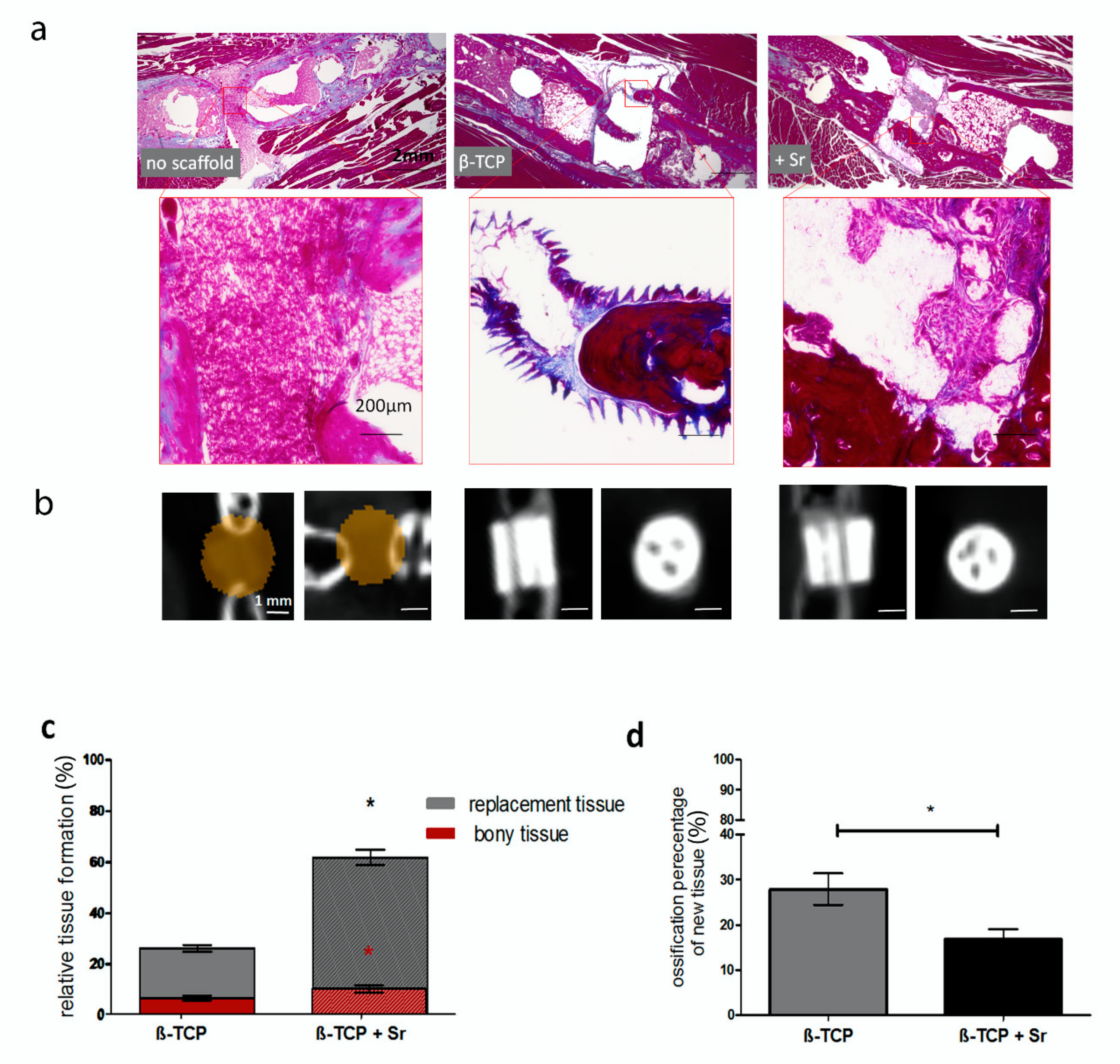
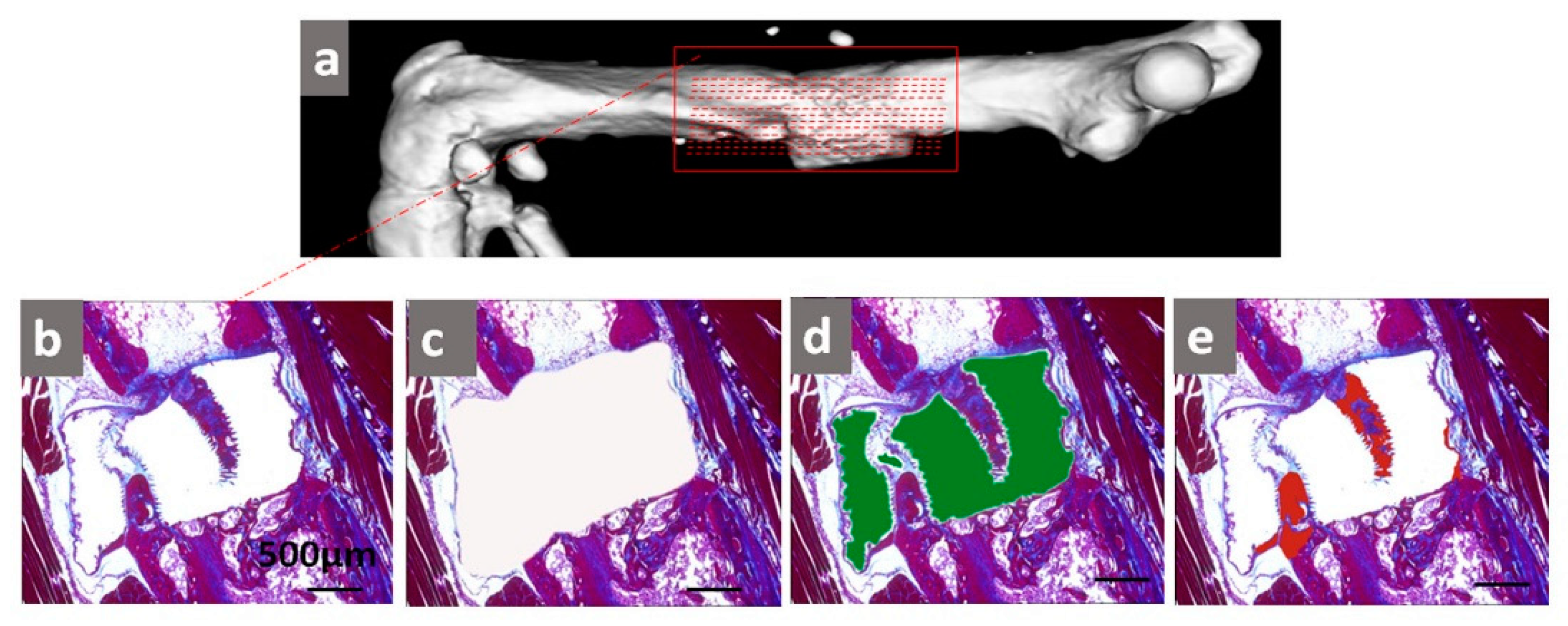
2.3. Histological Analysis of Tissue Formation
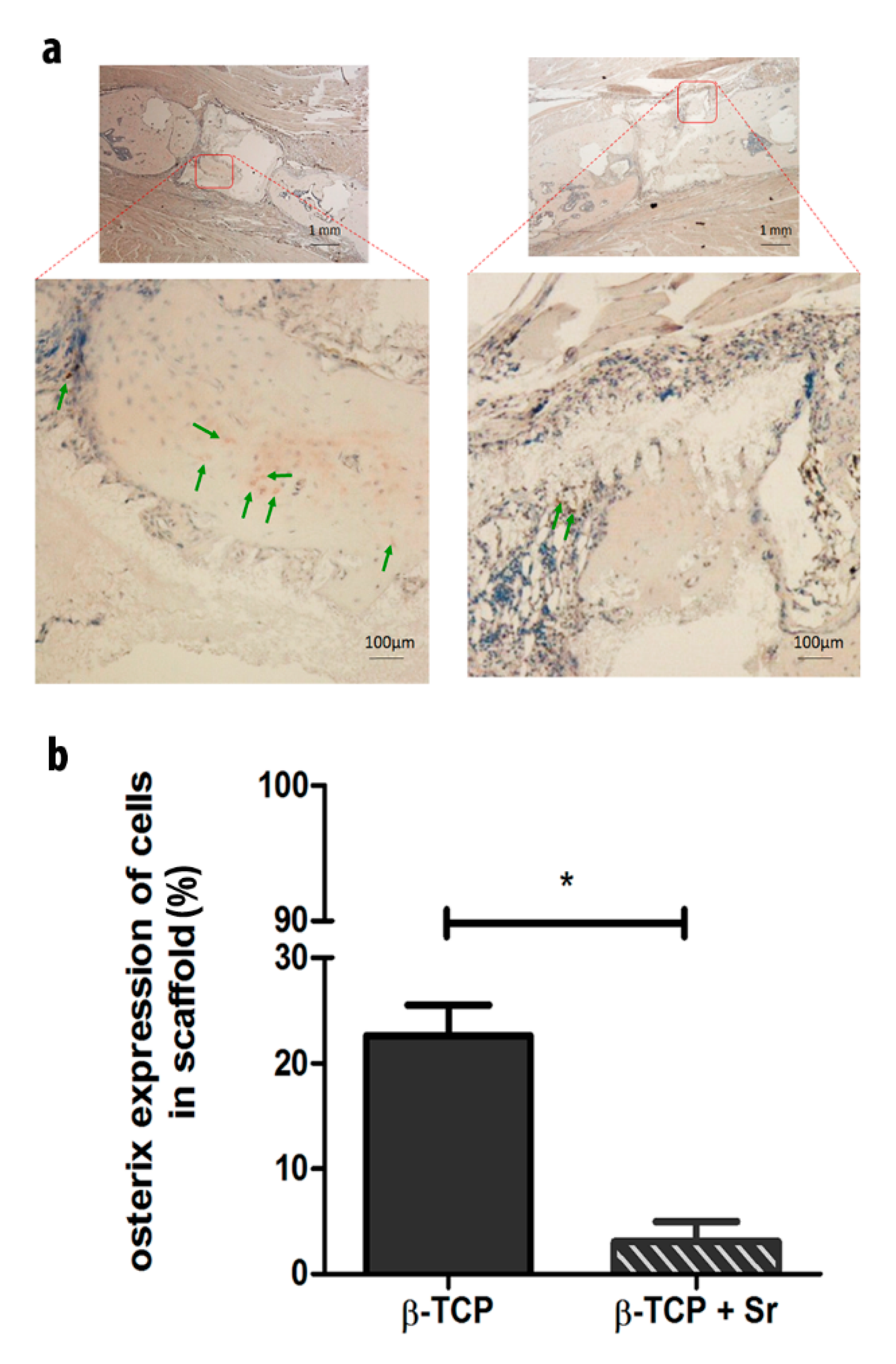
2.4. Relative Protein Expression of Osterix (Osx) in Scaffolds
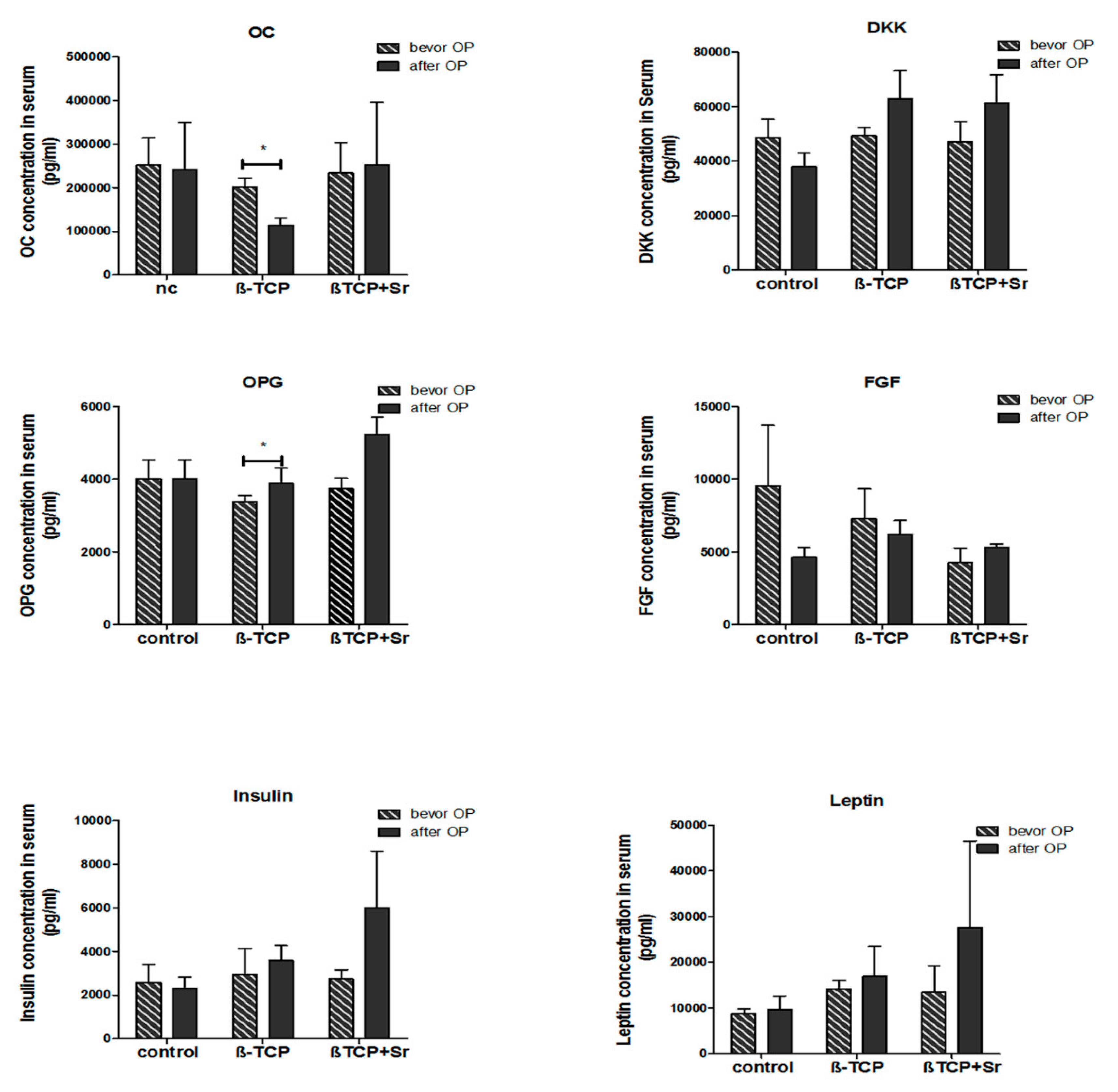
2.5. Quantification of Bone-Marker Levels in Serum
3. Discussion
3.1. Use of Transgenic Mice
3.2. Longitudinal NF-κB Activity during Fracture Healing
3.3. Longitudinal VEGFR-2 Activity during Fracture Healing
3.4. Bone and Tissue Regeneration
3.5. Feasible Size of Femur Defect in Mice
3.6. Strontium-Doped β-TCP Scaffold
3.7. Limitations
4. Materials and Methods
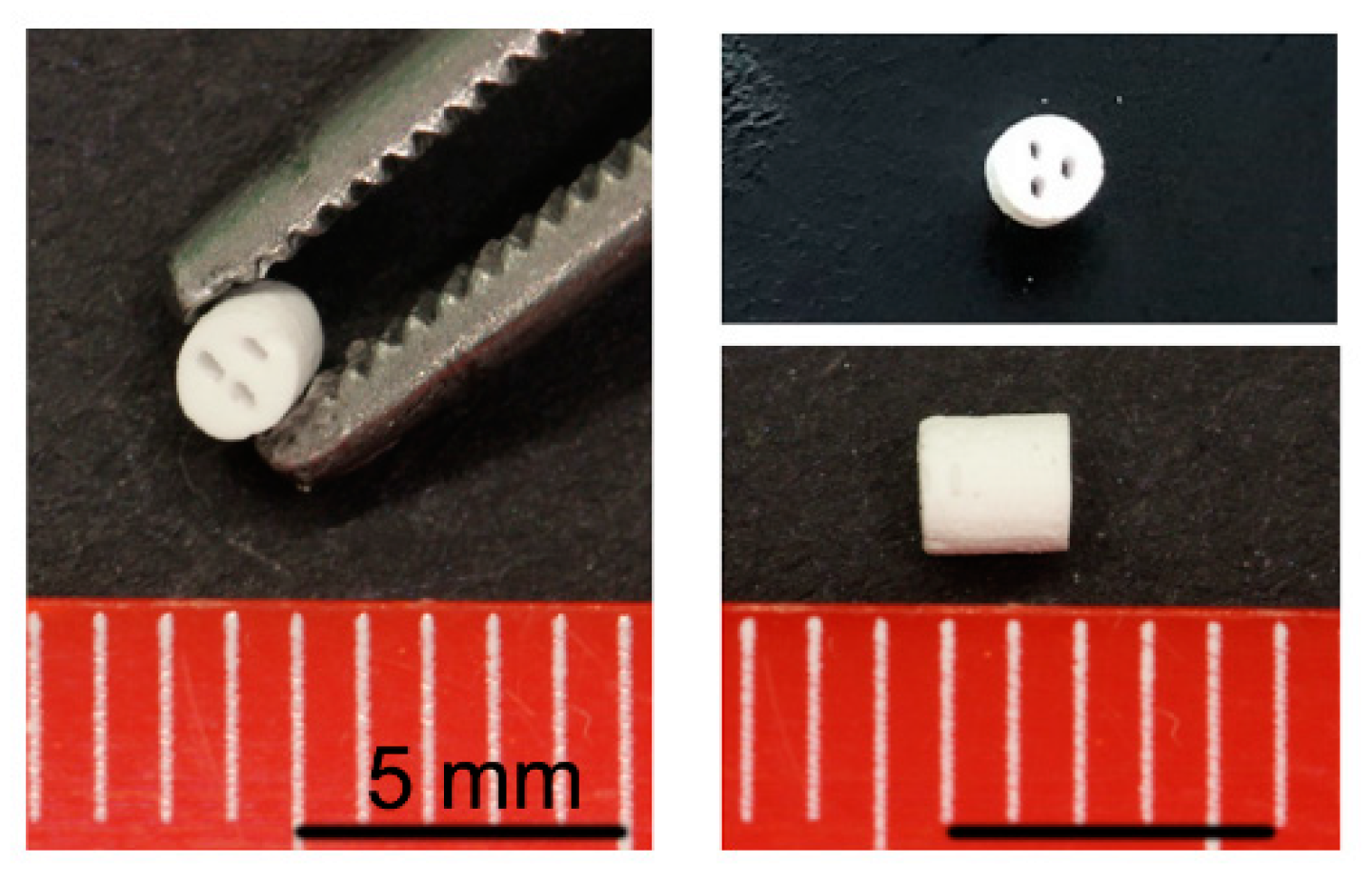
4.1. Scaffold Manufacturing
4.2. Transgenic Animals
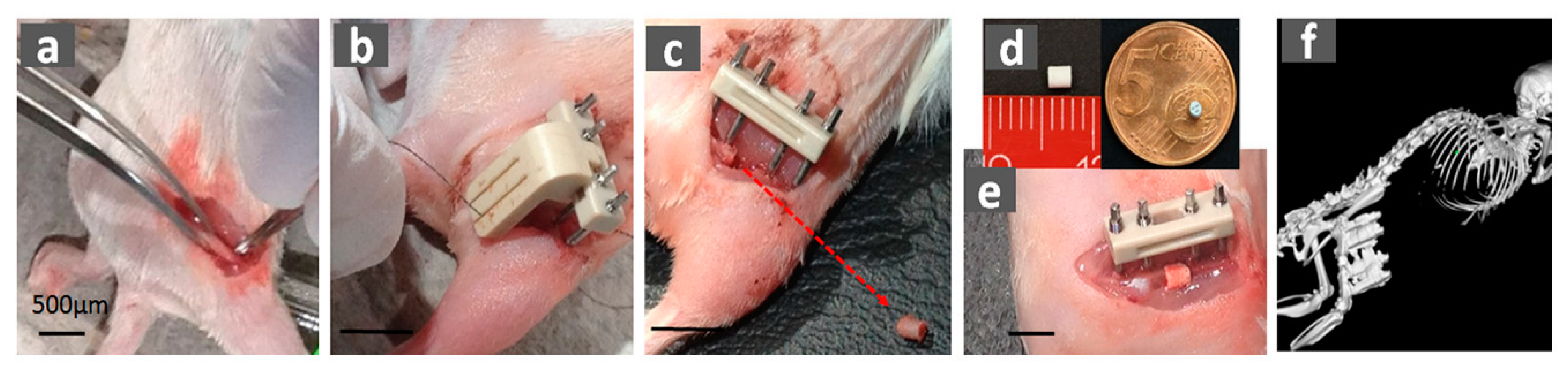
4.3. Critical-Size Fracture Model (Large Bone Defect)
4.4. In-Vivo Luminescent Imaging
4.5. Micro-CT Imaging
4.6. Histological Staining
4.7. AZAN Staining
4.8. Immunohistochemical Staining Analysis
4.9. Quantification of Serum Protein Level
4.10. Statistical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| β-TCP | β-tricalcium phosphate |
| CCD | Charge Coupled Devices |
| FGF | Fibroblast growth factor |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| LBD | Large bone defects |
| luc | Luciferase |
| µCT | Micro-computed tomography |
| mL | Milliliter |
| NF-κB | Nuclear factor “kappa-light-chain-enhancer” of activated B-cells |
| OC | Osteocalcin |
| OPG | Osteoprotegerin |
| Osx | Osterix |
| PDGF | Platelet-derived growth factor |
| pg | Picogram |
| ROI | Region of interest |
| Sr | Strontium |
| TNF-α | Tumor necrosis factor-alpha |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Kubo, Y.; Wruck, C.J.; Fragoulis, A.; Drescher, W.; Pape, H.C.; Lichte, P.; Fischer, H.; Tohidnezhad, M.; Hildebrand, F.; Pufe, T.; et al. Role of Nrf2 in Fracture Healing: Clinical Aspects of Oxidative Stress. Calcif. Tissue Int. 2019, 105, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Lippross, S.; Beckmann, R.; Streubesand, N.; Ayub, F.; Tohidnezhad, M.; Campbell, G.; Kan, Y.W.; Horst, F.; Sonmez, T.T.; Varoga, D.; et al. Nrf2 deficiency impairs fracture healing in mice. Calcif. Tissue Int. 2014, 95, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F.; et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2014, 8, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Brown, K.D.; Claudio, E.; Siebenlist, U. The roles of the classical and alternative nuclear factor-kappaB pathways: Potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, 212. [Google Scholar] [CrossRef]
- Ai-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef]
- Reumann, M.K.; Nair, T.; Strachna, O.; Boskey, A.L.; Mayer-Kuckuk, P. Production of VEGF receptor 1 and 2 mRNA and protein during endochondral bone repair is differential and healing phase specific. J. Appl. Physiol. (1985) 2010, 109, 1930–1938. [Google Scholar] [CrossRef]
- Contag, P.R.; Olomu, I.N.; Stevenson, D.K.; Contag, C.H. Bioluminescent indicators in living mammals. Nat. Med. 1998, 4, 245–247. [Google Scholar] [CrossRef]
- Zhang, N.; Fang, Z.; Contag, P.R.; Purchio, A.F.; West, D.B. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood 2004, 103, 617–626. [Google Scholar] [CrossRef]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J. Bone Joint Surg. Am. 2002, 84, 454–464. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. 3), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Bergmann, C.; Telle, R.; Fischer, H. Calcium phosphate scaffolds mimicking the gradient architecture of native long bones. J. Biomed. Mater. Res. A 2014, 102, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Metsger, D.S.; Driskell, T.D.; Paulsrud, J.R. Tricalcium phosphate ceramic--a resorbable bone implant: Review and current status. J. Am. Dent. Assoc. 1982, 105, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shi, Y.; Ye, F.; Bu, H. Osteoinduction of calcium phosphate biomaterials in small animals. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1254–1260. [Google Scholar] [CrossRef]
- Coathup, M.J.; Hing, K.A.; Samizadeh, S.; Chan, O.; Fang, Y.S.; Campion, C.; Buckland, T.; Blunn, G.W. Effect of increased strut porosity of calcium phosphate bone graft substitute biomaterials on osteoinduction. J. Biomed. Mater. Res. A 2012, 100, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yang, Z.; Li, Y.; Zhang, X.; De Bruijn, J.D.; De Groot, K. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci. Mater. Med. 1998, 9, 723–726. [Google Scholar] [CrossRef]
- Hanke, A.; Baumlein, M.; Lang, S.; Gueorguiev, B.; Nerlich, M.; Perren, T.; Rillmann, P.; Ryf, C.; Miclau, T.; Loibl, M. Long-term radiographic appearance of calcium-phosphate synthetic bone grafts after surgical treatment of tibial plateau fractures. Injury 2017, 48, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Montufar, E.B.; Casas-Luna, M.; Horynova, M.; Tkachenko, S.; Fohlerova, Z.; Diaz-de-la-Torre, S.; Dvorak, K.; Celko, L.; Kaiser, J. High strength, biodegradable and cytocompatible alpha tricalcium phosphate-iron composites for temporal reduction of bone fractures. Acta Biomater. 2018, 70, 293–303. [Google Scholar] [CrossRef]
- Ikumi, A.; Funayama, T.; Tsukanishi, T.; Noguchi, H.; Yamazaki, M. Novel Unidirectional Porous beta-Tricalcium Phosphate Used as a Bone Substitute after Excision of Benign Bone Tumors of the Hand: A Case Series. J. Hand Surg. Asian Pac. Vol. 2018, 23, 424–429. [Google Scholar] [CrossRef]
- Ishida, H.; Haniu, H.; Takeuchi, A.; Ueda, K.; Sano, M.; Tanaka, M.; Takizawa, T.; Sobajima, A.; Kamanaka, T.; Saito, N. In Vitro and In Vivo Evaluation of Starfish Bone-Derived beta-Tricalcium Phosphate as a Bone Substitute Material. Materials (Basel) 2019, 12, 1881. [Google Scholar] [CrossRef]
- Querido, W.; Rossi, A.L.; Farina, M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron 2016, 80, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Dimai, H.P. [Strontium ranelate: A novel concept for the treatment of osteoporosis]. Wien. Klin. Wochenschr. 2005, 117, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Fogelman, I.; Blake, G.M. Strontium ranelate for the treatment of osteoporosis. BMJ 2005, 330, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P. New strategies for osteoporosis patients previously managed with strontium ranelate. Ther. Adv. Musculoskelet. Dis. 2014, 6, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, A.H.; Torres, A.L.; Vasconcelos, D.P.; Ribeiro-Machado, C.; Barbosa, J.N.; Barbosa, M.A.; Barrias, C.C.; Ribeiro, C.C. Osteogenic, anti-osteoclastogenic and immunomodulatory properties of a strontium-releasing hybrid scaffold for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1289–1303. [Google Scholar] [CrossRef]
- Wei, L.; Jiang, Y.; Zhou, W.; Liu, S.; Liu, Y.; Rausch-Fan, X.; Liu, Z. Strontium ion attenuates lipopolysaccharide-stimulated proinflammatory cytokine expression and lipopolysaccharide-inhibited early osteogenic differentiation of human periodontal ligament cells. J. Periodont. Res. 2018, 53, 999–1008. [Google Scholar] [CrossRef]
- Bonnarens, F.; Einhorn, T.A. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res. 1984, 2, 97–101. [Google Scholar] [CrossRef]
- Wehrle, E.; Tourolle Ne Betts, D.C.; Kuhn, G.A.; Scheuren, A.C.; Hofmann, S.; Muller, R. Evaluation of longitudinal time-lapsed in vivo micro-CT for monitoring fracture healing in mouse femur defect models. Sci. Rep. 2019, 9, 17445. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Muller-Graf, F.; Matthys, R.; Abaei, A.; Jonas, R.; Gebhard, F.; Rasche, V.; Ignatius, A. In Vivo Evaluation of Fracture Callus Development During Bone Healing in Mice Using an MRI-compatible Osteosynthesis Device for the Mouse Femur. J. Vis. Exp. 2017, 129, e56679. [Google Scholar] [CrossRef]
- Morgan, E.F.; Mason, Z.D.; Chien, K.B.; Pfeiffer, A.J.; Barnes, G.L.; Einhorn, T.A.; Gerstenfeld, L.C. Micro-computed tomography assessment of fracture healing: Relationships among callus structure, composition, and mechanical function. Bone 2009, 44, 335–344. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Muller-Graf, F.; Matthys, R.; Hagele, Y.; Fischer, V.; Jonas, R.; Abaei, A.; Gebhard, F.; Rasche, V.; Ignatius, A. Evaluation of high-resolution In Vivo MRI for longitudinal analysis of endochondral fracture healing in mice. PLoS ONE 2017, 12, e0174283. [Google Scholar] [CrossRef] [PubMed]
- Quade, M.; Schumacher, M.; Bernhardt, A.; Lode, A.; Kampschulte, M.; Voss, A.; Simon, P.; Uckermann, O.; Kirsch, M.; Gelinsky, M. Strontium-modification of porous scaffolds from mineralized collagen for potential use in bone defect therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, W.; Liu, Z.; Ma, S.; Sun, Y.; Wu, X.; Zhang, X.; Gao, P. Application of a Strontium-Loaded, Phase-Transited Lysozyme Coating to a Titanium Surface to Enhance Osteogenesis and Osteoimmunomodulation. Med. Sci. Monit. 2019, 25, 2658–2671. [Google Scholar] [CrossRef]
- Andrew, J.G.; Andrew, S.M.; Freemont, A.J.; Marsh, D.R. Inflammatory cells in normal human fracture healing. Acta Orthop. Scand. 1994, 65, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.J. Fracture healing perspectives. Clin. Orthop. Relat. Res. 1985, 200, 100–113. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Mao, J. Bone tissue engineering and regeneration: From discovery to the clinic--an overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef]
- Waters, R.V.; Gamradt, S.C.; Asnis, P.; Vickery, B.H.; Avnur, Z.; Hill, E.; Bostrom, M. Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop. Scand. 2000, 71, 316–321. [Google Scholar] [CrossRef]
- Klement, M.R.; Nickel, B.T.; Bala, A.; Penrose, C.T.; Zura, R.D.; Garrigues, G.E. Glenohumeral arthritis as a risk factor for proximal humerus nonunion. Injury 2016, 47 (Suppl. 7), S36–S39. [Google Scholar] [CrossRef]
- Zura, R.; Braid-Forbes, M.J.; Jeray, K.; Mehta, S.; Einhorn, T.A.; Watson, J.T.; Della Rocca, G.J.; Forbes, K.; Steen, R.G. Bone fracture nonunion rate decreases with increasing age: A prospective inception cohort study. Bone 2017, 95, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, M.; Henke, T.; Claes, L.; Augat, P. Revascularisation during fracture healing with soft tissue injury. Arch. Orthop. Trauma Surg. 2008, 128, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Keramaris, N.C.; Calori, G.M.; Nikolaou, V.S.; Schemitsch, E.H.; Giannoudis, P.V. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury 2008, 39 (Suppl. 2), S45–S57. [Google Scholar] [CrossRef]
- Pufe, T.; Wildemann, B.; Petersen, W.; Mentlein, R.; Raschke, M.; Schmidmaier, G. Quantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: A study in the rat. Cell Tissue Res. 2002, 309, 387–392. [Google Scholar] [CrossRef]
- Takebe, H.; Shalehin, N.; Hosoya, A.; Shimo, T.; Irie, K. Sonic Hedgehog Regulates Bone Fracture Healing. Int. J. Mol. Sci. 2020, 21, 677. [Google Scholar] [CrossRef]
- Peng, S.; Liu, X.S.; Wang, T.; Li, Z.; Zhou, G.; Luk, K.D.; Guo, X.E.; Lu, W.W. In vivo anabolic effect of strontium on trabecular bone was associated with increased osteoblastogenesis of bone marrow stromal cells. J. Orthop. Res. 2010, 28, 1208–1214. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Z.; Chang, H.; Wang, Y.; Xiang, H.; Zhang, X.; Yu, B. Strontiumcontaining alphacalcium sulfate hemihydrate promotes bone repair via the TGFbeta/Smad signaling pathway. Mol. Med. Rep. 2019, 20, 3555–3564. [Google Scholar]
- Manassero, M.; Decambron, A.; Huu Thong, B.T.; Viateau, V.; Bensidhoum, M.; Petite, H. Establishment of a Segmental Femoral Critical-size Defect Model in Mice Stabilized by Plate Osteosynthesis. J. Vis. Exp. 2016, 116, e52940. [Google Scholar] [CrossRef]
- Srouji, S.; Ben-David, D.; Kohler, T.; Muller, R.; Zussman, E.; Livne, E. A model for tissue engineering applications: Femoral critical size defect in immunodeficient mice. Tissue Eng. Part C Methods 2011, 17, 597–606. [Google Scholar] [CrossRef]
- Westberg, S.M.; Awker, A.; Torkelson, C.J. Use of Strontium Chloride for the Treatment of Osteoporosis: A Case Report. Altern. Ther. Health Med. 2016, 22, 66–70. [Google Scholar] [PubMed]
- Wohl, G.R.; Chettle, D.R.; Pejovic-Milic, A.; Druchok, C.; Webber, C.E.; Adachi, J.D.; Beattie, K.A. Accumulation of bone strontium measured by in vivo XRF in rats supplemented with strontium citrate and strontium ranelate. Bone 2013, 52, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Hott, M.; Modrowski, D.; De Pollak, C.; Guillemain, J.; Deloffre, P.; Tsouderos, Y. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J. Bone Miner. Res. 1993, 8, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Babu, S.S.; Vs, H.K.; Varma, H.K.; John, A. Osteogenic efficacy of strontium hydroxyapatite micro-granules in osteoporotic rat model. J. Biomater. Appl. 2016, 31, 499–509. [Google Scholar] [CrossRef]
- Meininger, S.; Moseke, C.; Spatz, K.; Marz, E.; Blum, C.; Ewald, A.; Vorndran, E. Effect of strontium substitution on the material properties and osteogenic potential of 3D powder printed magnesium phosphate scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1145–1158. [Google Scholar] [CrossRef]
- Scalera, F.; Palazzo, B.; Barca, A.; Gervaso, F. Sintering of magnesium-strontium doped hydroxyapatite nanocrystals: Towards the production of 3D biomimetic bone scaffolds. J. Biomed. Mater. Res. A 2020, 108, 633–644. [Google Scholar] [CrossRef]
- Tian, M.; Chen, F.; Song, W.; Song, Y.; Chen, Y.; Wan, C.; Yu, X.; Zhang, X. In vivo study of porous strontium-doped calcium polyphosphate scaffolds for bone substitute applications. J. Mater. Sci. Mater. Med. 2009, 20, 1505–1512. [Google Scholar] [CrossRef]
- Aveic, S.; Davtalab, R.; Vogt, M.; Weber, M.; Buttler, P.; Tonini, G.P.; Fischer, H. Calcium phosphate scaffolds with defined interconnecting channel structure provide a mimetic 3D niche for bone marrow metastasized tumor cell growth. Acta Biomater. 2019, 88, 527–539. [Google Scholar] [CrossRef]
- Lan Levengood, S.K.; Polak, S.J.; Poellmann, M.J.; Hoelzle, D.J.; Maki, A.J.; Clark, S.G.; Wheeler, M.B.; Wagoner Johnson, A.J. The effect of BMP-2 on micro- and macroscale osteointegration of biphasic calcium phosphate scaffolds with multiscale porosity. Acta Biomater. 2010, 6, 3283–3291. [Google Scholar] [CrossRef]
- Fet, N.; Alizai, P.H.; Fragoulis, A.; Wruck, C.; Pufe, T.; Tolba, R.H.; Neumann, U.P.; Klinge, U. In vivo characterisation of the inflammatory reaction following mesh implantation in transgenic mice models. Langenbecks Arch. Surg. 2014, 399, 579–588. [Google Scholar] [CrossRef]
- Gremse, F.; Doleschel, D.; Zafarnia, S.; Babler, A.; Jahnen-Dechent, W.; Lammers, T.; Lederle, W.; Kiessling, F. Hybrid microCT-FMT imaging and image analysis. J. Vis. Exp. 2015, 100, e52770. [Google Scholar]
- Gremse, F.; Stark, M.; Ehling, J.; Menzel, J.R.; Lammers, T.; Kiessling, F. Imalytics Preclinical: Interactive Analysis of Biomedical Volume Data. Theranostics 2016, 6, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Mulisch, M.; Welsch, U. Romeis - Mikroskopische Technik; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohidnezhad, M.; Kubo, Y.; Lichte, P.; Heigl, T.; Roch, D.; Barahmand Pour, N.; Bergmann, C.; Sönmez, T.T.; Hock, J.V.P.; Fragoulis, A.; et al. Effects of Strontium-Doped β-Tricalcium Scaffold on Longitudinal Nuclear Factor-Kappa Beta and Vascular Endothelial Growth Factor Receptor-2 Promoter Activities during Healing in a Murine Critical-Size Bone Defect Model. Int. J. Mol. Sci. 2020, 21, 3208. https://doi.org/10.3390/ijms21093208
Tohidnezhad M, Kubo Y, Lichte P, Heigl T, Roch D, Barahmand Pour N, Bergmann C, Sönmez TT, Hock JVP, Fragoulis A, et al. Effects of Strontium-Doped β-Tricalcium Scaffold on Longitudinal Nuclear Factor-Kappa Beta and Vascular Endothelial Growth Factor Receptor-2 Promoter Activities during Healing in a Murine Critical-Size Bone Defect Model. International Journal of Molecular Sciences. 2020; 21(9):3208. https://doi.org/10.3390/ijms21093208
Chicago/Turabian StyleTohidnezhad, Mersedeh, Yusuke Kubo, Philipp Lichte, Tobias Heigl, Diana Roch, Nazanin Barahmand Pour, Christian Bergmann, Tolga Taha Sönmez, Jennifer Vanessa Phi Hock, Athanassios Fragoulis, and et al. 2020. "Effects of Strontium-Doped β-Tricalcium Scaffold on Longitudinal Nuclear Factor-Kappa Beta and Vascular Endothelial Growth Factor Receptor-2 Promoter Activities during Healing in a Murine Critical-Size Bone Defect Model" International Journal of Molecular Sciences 21, no. 9: 3208. https://doi.org/10.3390/ijms21093208
APA StyleTohidnezhad, M., Kubo, Y., Lichte, P., Heigl, T., Roch, D., Barahmand Pour, N., Bergmann, C., Sönmez, T. T., Hock, J. V. P., Fragoulis, A., Gremse, F., Rosenhain, S., Slowik, A., Bienert, M., Kweider, N., Wruck, C. J., Jahr, H., Hildebrand, F., Pape, H. C., ... Pufe, T. (2020). Effects of Strontium-Doped β-Tricalcium Scaffold on Longitudinal Nuclear Factor-Kappa Beta and Vascular Endothelial Growth Factor Receptor-2 Promoter Activities during Healing in a Murine Critical-Size Bone Defect Model. International Journal of Molecular Sciences, 21(9), 3208. https://doi.org/10.3390/ijms21093208







