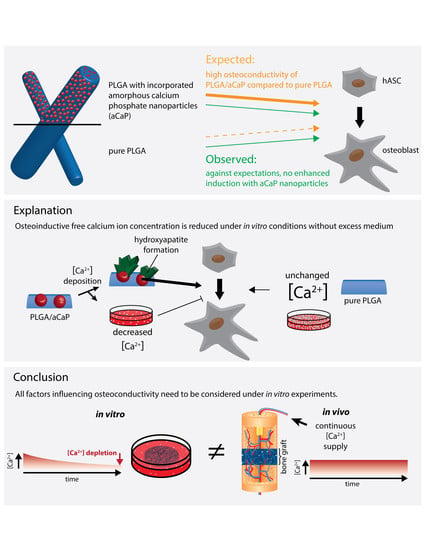
Directing Stem Cell Commitment by Amorphous Calcium Phosphate Nanoparticles Incorporated in PLGA: Relevance of the Free Calcium Ion Concentration
Abstract
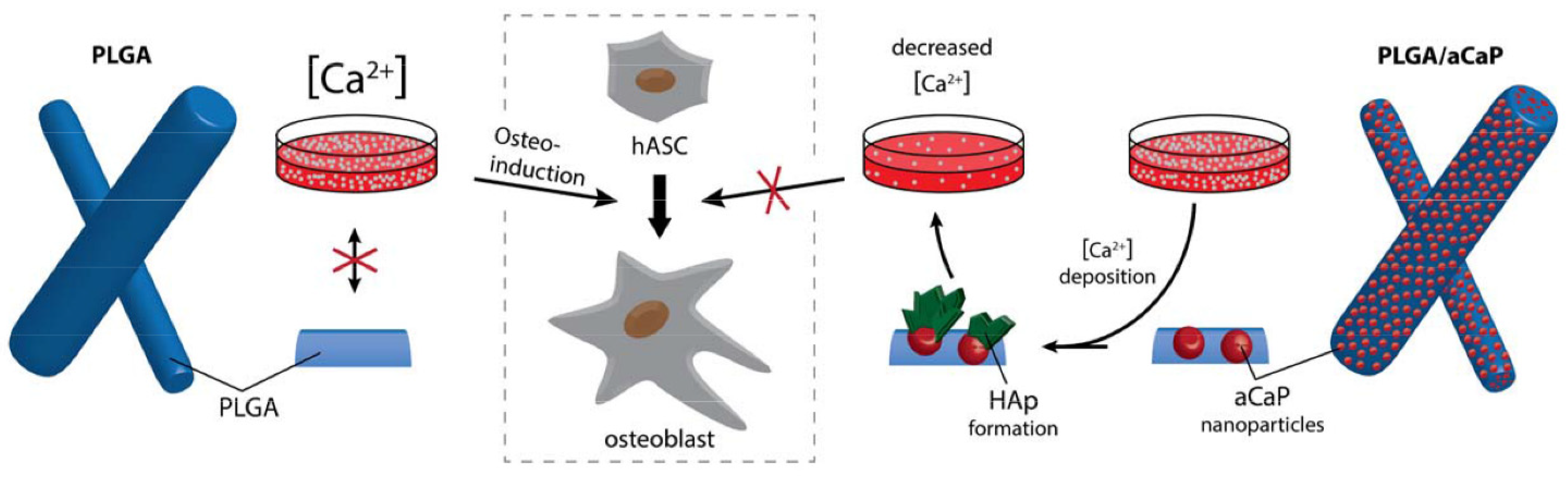
1. Introduction
- (i)
- aCaP nanoparticles in PLGA evoke the osteogenic commitment of ASCs in basal medium;
- (ii)
- osteogenic commitment—as evoked by aCaP nanoparticles—is enhanced in osteogenic induction medium;
- (iii)
- 3D fiber meshes of PLGA/aCaP evoke osteogenic commitment even to a greater extent than do corresponding 2D flat films of PLGA/aCaP.
2. Results
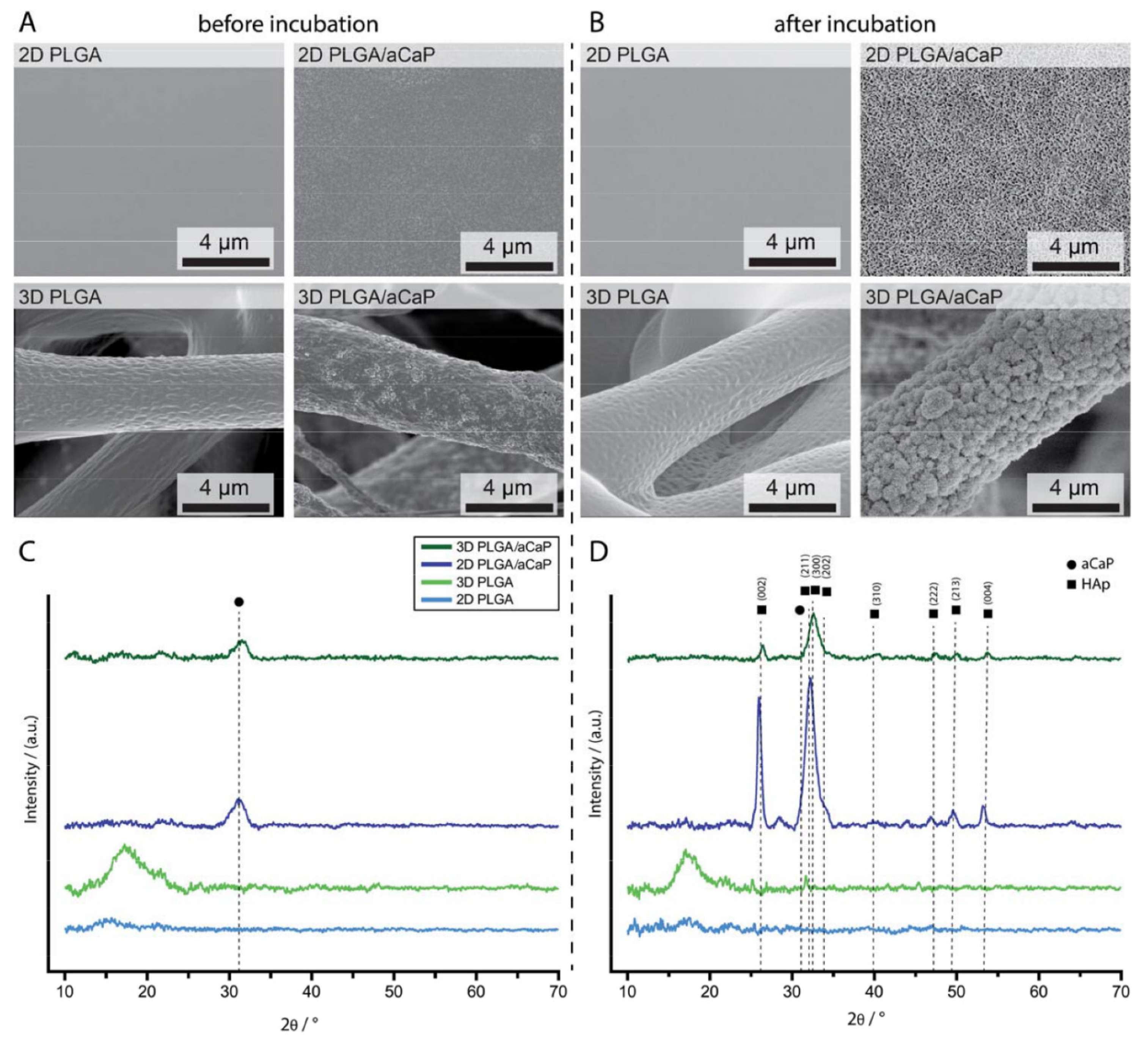
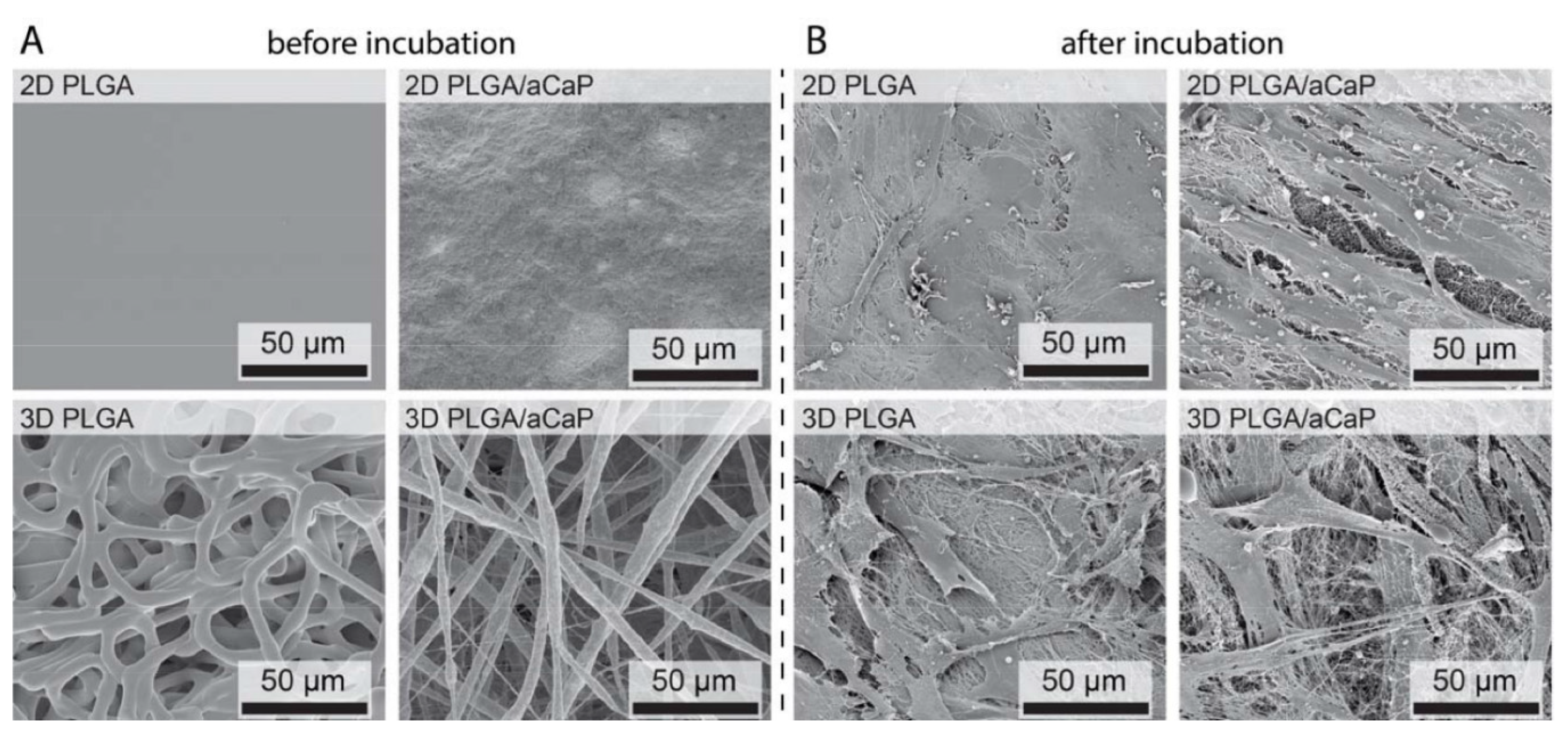
2.1. Material Characterization
2.2. Seeding Adipose-Derived Stem Cells and Gene Expression Analysis
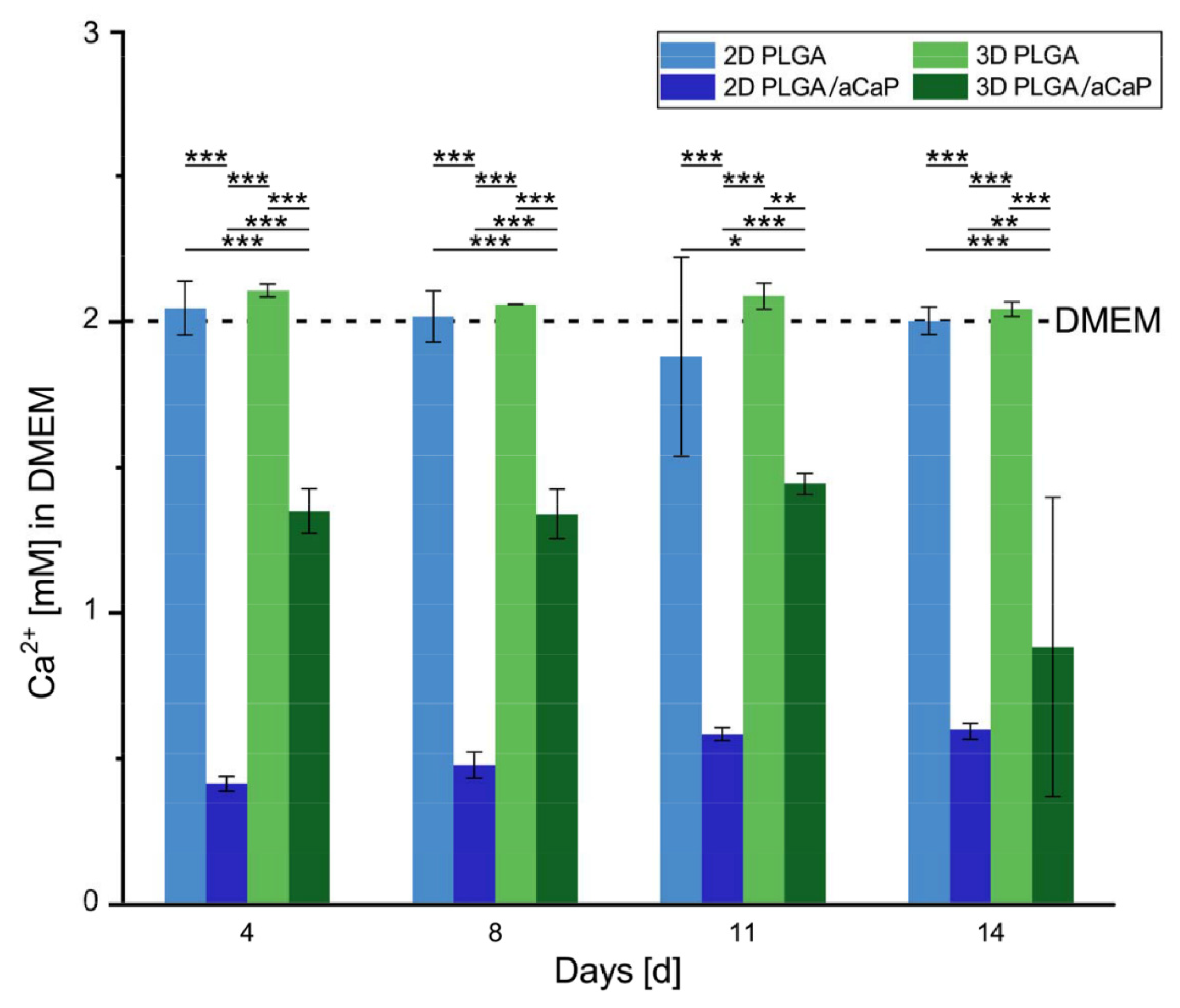
2.3. Free Calcium Ion Concentration
2.4. Providing Excess Culture Medium
2.5. Mineralization
3. Discussion
4. Materials and Methods
4.1. Scaffolds
4.2. Non-Cellular Scaffold Incubation
4.3. Cell Isolation
4.4. Multilineage Cell Differentiation
4.5. Tissue Engineered Constructs
4.6. Gene Expression
4.7. Micro-CT
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aCaP | Amorphous calcium phosphate |
| ASCs | Adipose-derived stem cells |
| DMEM | Dulbecco’s modified essential medium |
| OS | Osteogenic induction medium |
| PLGA | Poly(lactic-co-glycolic acid) |
| Ca2+ | Calcium ions |
References
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, M.; Yamada, T.; Taniyama, T.; Masaoka, T.; Xuetao, W.; Yoshii, T.; Horie, M.; Yasuda, H.; Uemura, T.; Okawa, A.; et al. Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLoS ONE 2015, 10, e0116462. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.; Belton, D.J.; Kaplan, D.L.; Perry, C.C. Quantifying the efficiency of hydroxyapatite mineralising peptides. Sci. Rep. 2017, 7, 7681. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Fan, Y.; Li, X. The use of bioactive peptides to modify materials for bone tissue repair. Regen. Biomater. 2017, 4, 191–206. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, L.; Bai, Y.; Hu, M.; Mo, J.; He, H.; Lou, A.; Yang, B.; Zhao, H.; Guo, Y.; et al. Effect of the co-culture of human bone marrow mesenchymal stromal cells with human umbilical vein endothelial cells in vitro. J. Recept. Signal. Transduct. Res. 2016, 36, 221–224. [Google Scholar] [CrossRef]
- Schneider, I.; Baumgartner, W.; Gröninger, O.; Stark, W.J.; Märsmann, S.; Calcagni, M.; Cinelli, P.; Wolint, P.; Buschmann, J. 3D microtissue–derived human stem cells seeded on electrospun nanocomposites under shear stress: Modulation of gene expression. J. Mech. Behav. Biomed. Mater. 2019, 102, 103481. [Google Scholar] [CrossRef]
- Heo, J.; Nam, H.; Hwang, D.; Cho, S.J.; Jung, S.-Y.; Cho, D.-W.; Shim, J.-H.; Lim, G. Enhanced cellular distribution and infiltration in a wet electrospun three-dimensional fibrous scaffold using eccentric rotation-based hydrodynamic conditions. Sens. Actuators B Chem. 2016, 226, 357–363. [Google Scholar] [CrossRef]
- Xu, Z.C.; Zhang, Q.; Li, H. Elastic large muscular vessel wall engineered with bone marrow-derived cells under pulsatile stimulation in a bioreactor. Mol. Med. Report. 2015, 12, 6005–6012. [Google Scholar] [CrossRef][Green Version]
- Baumgartner, W.; Schneider, I.; Hess, S.C.; Stark, W.J.; Märsmann, S.; Brunelli, M.; Calcagni, M.; Cinelli, P.; Buschmann, J. Cyclic uniaxial compression of human stem cells seeded on a bone biomimetic nanocomposite decreases anti-osteogenic commitment evoked by shear stress. J. Mech. Behav. Biomed. Mater. 2018, 83, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, H.; Lee, K.Y. Effect of spacer arm length between adhesion ligand and alginate hydrogel on stem cell differentiation. Carbohydr. Polym. 2016, 139, 82–89. [Google Scholar] [CrossRef]
- Li, Y.; Kilian, K.A. Bridging the Gap: From 2D Cell Culture to 3D Microengineered Extracellular Matrices. Adv. Healthc. Mater. 2015, 4, 2780–2796. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Wagner, H.D. The material bone: Structure mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Li, Y.; Aparicio, C. Discerning the Subfibrillar Structure of Mineralized Collagen Fibrils: A Model for the Ultrastructure of Bone. PLoS ONE 2013, 8, e76782. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Shekaran, A.; Garcia, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. Part. A 2011, 96A, 261–272. [Google Scholar] [CrossRef]
- Bongio, M.; Lopa, S.; Gilardi, M.; Bersini, S.; Moretti, M. A 3D vascularized bone remodeling model combining osteoblasts and osteoclasts in a CaP nanoparticle-enriched matrix. Nanomedicine 2016, 11, 1073–1091. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Song, J.L.; Ji, P.; Zhang, X.H.; Li, X.M.; Xu, X.; Wang, M.K.; Zhang, S.Q.; Deng, Y.; Deng, F.; et al. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 3499–3515. [Google Scholar] [CrossRef] [PubMed]
- Ramezanifard, R.; Seyedjafari, E.; Ardeshirylajimi, A.; Soleimani, M. Biomimetic scaffolds containing nanofibers coated with willemite nanoparticles for improvement of stem cell osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Chang, Q.; Xu, L.; Li, G.L.; Yang, G.Z.; Ding, X.; Wang, X.S.; Cui, D.X.; Jiang, X.Q. Graphene Oxide-copper nanocomposite-coated porous cap scaffold for vascularized bone regeneration via activation of Hif-1. Adv. Healthc. Mater. 2016, 5, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, X.; Ruan, J.; Arola, D.D.; Ji, C.; Weir, M.D.; Oates, T.W.; Chang, X.; Zhang, K.; Xu, H.H.K. Bonding durability, antibacterial activity and biofilm pH of novel adhesive containing antibacterial monomer and nanoparticles of amorphous calcium phosphate. J. Dent. 2019, 81, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bisso, S.; Mura, S.; Castagner, B.; Couvreur, P.; Leroux, J.-C. Dual delivery of nucleic acids and PEGylated-bisphosphonates via calcium phosphate nanoparticles. Eur. J. Pharm. Biopharm. 2019, 142, 142–152. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Jiang, J.; Cui, N.; Xue, X.; Wang, T.; Wang, X.; He, Y.; Wang, D. Doxorubicin-loaded carbon dots lipid-coated calcium phosphate nanoparticles for visual targeted delivery and therapy of tumor. Int. J. Nanomed. 2020, 15, 433–444. [Google Scholar] [CrossRef]
- Xing, Q.; Qian, Z.C.; Kannan, B.; Tahtinen, M.; Zhao, F. Osteogenic differentiation evaluation of an engineered extracellular matrix based tissue sheet for potential periosteum replacement. ACS Appl. Mater. Interfaces 2015, 7, 23239–23247. [Google Scholar] [CrossRef]
- Linh, N.T.B.; Lee, K.H.; Lee, B.T. Functional nanofiber mat of polyvinyl alcohol/gelatin containing nanoparticles of biphasic calcium phosphate for bone regeneration in rat calvaria defects. J. Biomed. Mater. Res. Part. A 2013, 101A, 2412–2423. [Google Scholar] [CrossRef]
- Baumgartner, W.; Welti, M.; Hild, N.; Hess, S.C.; Stark, W.J.; Meier Buergisser, G.; Giovanoli, P.; Buschmann, J. Tissue mechanics of piled critical size biomimetic and biominerizable nanocomposites: Formation of bioreactor-induced stem cell gradients under perfusion and compression. J. Mech. Behav. Biomed. Mater. 2015, 47, 124–134. [Google Scholar] [CrossRef]
- Gao, S.P.; Calcagni, M.; Welti, M.; Hemmi, S.; Hild, N.; Stark, W.J.; Meier Buergisser, G.; Wanner, G.A.; Cinelli, P.; Buschmann, J. Proliferation of ASC-derived endothelial cells in a 3D electrospun mesh: Impact of bone-biomimetic nanocomposite and co-culture with ASC-derived osteoblasts. Injury 2014, 45, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Härter, L.; Gao, S.; Hemmi, S.; Welti, M.; Hild, N.; Schneider, O.D.; Stark, W.J.; Lindenblatt, N.; Werner, C.M.L.; et al. Tissue engineered bone grafts based on biomimetic nanocomposite PLGA/amorphous calcium phosphate scaffold and human adipose-derived stem cells. Injury 2012, 43, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.C.; Stark, W.J.; Mohn, D.; Cohrs, N.; Marsmann, S.; Calcagni, M.; Cinelli, P.; Buschmann, J. Gene expression in human adipose-derived stem cells: Comparison of 2D films, 3D electrospun meshes or co-cultured scaffolds with two-way paracrine effects. Eur. Cell Mater. 2017, 34, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, S.J.; Paschalis, E.P.; Betts, F.; Mendelsohn, R.; Boskey, A.L. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: New correlations between X-ray diffraction and infrared data. Calcif. Tissue Int. 1996, 58, 9–16. [Google Scholar] [CrossRef]
- Puvvada, N.; Panigrahi, P.K.; Pathak, A. Room temperature synthesis of highly hemocompatible hydroxyapatite, study of their physical properties and spectroscopic correlation of particle size. Nanoscale 2010, 2, 2631–2638. [Google Scholar] [CrossRef]
- Guneta, V.; Tan, N.S.; Chan, S.K.J.; Tanavde, V.; Lim, T.C.; Wong, T.C.M.; Choong, C. Comparative study of adipose-derived stem cells and bone marrow-derived stem cells in similar microenvironmental conditions. Exp. Cell Res. 2016, 348, 155–164. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C 2019, 98, 30–41. [Google Scholar] [CrossRef]
- Schneider, O.D.; Weber, F.; Brunner, T.J.; Loher, S.; Ehrbar, M.; Schmidlin, P.R.; Stark, W.J. In vivo and in vitro evaluation of flexible, cottonwool-like nanocomposites as bone substitute material for complex defects. Acta Biomater. 2009, 5, 1775–1784. [Google Scholar] [CrossRef]
- Schneider, O.D.; Mohn, D.; Fuhrer, R.; Klein, K.; Kämpf, K.; Nuss, K.M.R.; Sidler, M.; Zlinsky, K.; von Rechenberg, B.; Stark, W.J. Biocompatibility and bone formation of flexible, cotton wool-like PLGA/calcium phosphate nanocomposites in sheep. Open Orthop. J. 2011, 5, 63–71. [Google Scholar] [CrossRef]
- Chai, Y.C.; Roberts, S.J.; Schrooten, J.; Luyten, F.P. Probing the osteoinductive effect of calcium phosphate by using an In Vitro biomimetic model. Tissue Eng. Part. A 2011, 17, 1083–1097. [Google Scholar] [CrossRef]
- Rubin, J.; Rubin, C.; Jacobs, C.R. Molecular pathways mediating mechanical signaling in bone. Gene 2006, 367, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Ottolini, D.; Calì, T.; Carafoli, E. Calcium in health and disease. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 81–137. [Google Scholar]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic. Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-j.; Su, Y. Nuclear matrix-targeting of the osteogenic factor Runx2 is essential for its recognition and activation of the alkaline phosphatase gene. Biochim. Et Biophys. Acta-General Subj. 2013, 1830, 2839–2852. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Matsumoto, D.; Eto, H.; Inoue, K.; Aoi, N.; Kato, H.; Araki, J.; Yoshimura, K. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009, 18, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; González, A.; Planell, J.A.; Engel, E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2010, 393, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Loher, S.; Stark, W.J.; Maciejewski, M.; Baiker, A.; Pratsinis, S.E.; Reichardt, D.; Maspero, F.; Krumeich, F.; Gunther, D. Fluoro-apatite and calcium phosphate nanoparticles by flame synthesis. Chem. Mater. 2005, 17, 36–42. [Google Scholar] [CrossRef]
- Schneider, O.D.; Loher, S.; Brunner, T.J.; Uebersax, L.; Simonet, M.; Grass, R.N.; Merkle, H.P.; Stark, W.J. Cotton wool-like nanocomposite biomaterials prepared by electrospinning: In vitro bioactivity and osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2008, 84B, 350–362. [Google Scholar] [CrossRef]
- Buschmann, J.; Gao, S.; Härter, L.; Hemmi, S.; Welti, M.; Werner, C.M.L.; Calcagni, M.; Cinelli, P.; Wanner, G.A. Yield and proliferation rate of adipose-derived stem cells as a function of age, BMI and harvest site: Increasing the yield by using adherent and supernatant fractions? Cytotherapy 2013, 15, 1098–1105. [Google Scholar] [CrossRef]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Darwiche, S.E.; Kettenberger, U.; Pioletti, D.P. The role of energy dissipation of polymeric scaffolds in the mechanobiological modulation of chondrogenic expression. Biomaterials 2014, 35, 1890–1897. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gröninger, O.; Hess, S.; Mohn, D.; Schneider, E.; Stark, W.; Märsmann, S.; Wolint, P.; Calcagni, M.; Cinelli, P.; Buschmann, J. Directing Stem Cell Commitment by Amorphous Calcium Phosphate Nanoparticles Incorporated in PLGA: Relevance of the Free Calcium Ion Concentration. Int. J. Mol. Sci. 2020, 21, 2627. https://doi.org/10.3390/ijms21072627
Gröninger O, Hess S, Mohn D, Schneider E, Stark W, Märsmann S, Wolint P, Calcagni M, Cinelli P, Buschmann J. Directing Stem Cell Commitment by Amorphous Calcium Phosphate Nanoparticles Incorporated in PLGA: Relevance of the Free Calcium Ion Concentration. International Journal of Molecular Sciences. 2020; 21(7):2627. https://doi.org/10.3390/ijms21072627
Chicago/Turabian StyleGröninger, Olivier, Samuel Hess, Dirk Mohn, Elia Schneider, Wendelin Stark, Sonja Märsmann, Petra Wolint, Maurizio Calcagni, Paolo Cinelli, and Johanna Buschmann. 2020. "Directing Stem Cell Commitment by Amorphous Calcium Phosphate Nanoparticles Incorporated in PLGA: Relevance of the Free Calcium Ion Concentration" International Journal of Molecular Sciences 21, no. 7: 2627. https://doi.org/10.3390/ijms21072627
APA StyleGröninger, O., Hess, S., Mohn, D., Schneider, E., Stark, W., Märsmann, S., Wolint, P., Calcagni, M., Cinelli, P., & Buschmann, J. (2020). Directing Stem Cell Commitment by Amorphous Calcium Phosphate Nanoparticles Incorporated in PLGA: Relevance of the Free Calcium Ion Concentration. International Journal of Molecular Sciences, 21(7), 2627. https://doi.org/10.3390/ijms21072627