Synergistic Combinations of Curcumin, Sulforaphane, and Dihydrocaffeic Acid against Human Colon Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Validation of Dose-Effect Data of Curcumin, Sulforaphane, Dihydrocaffeic Acid and Their Constant-Ratio Combinations
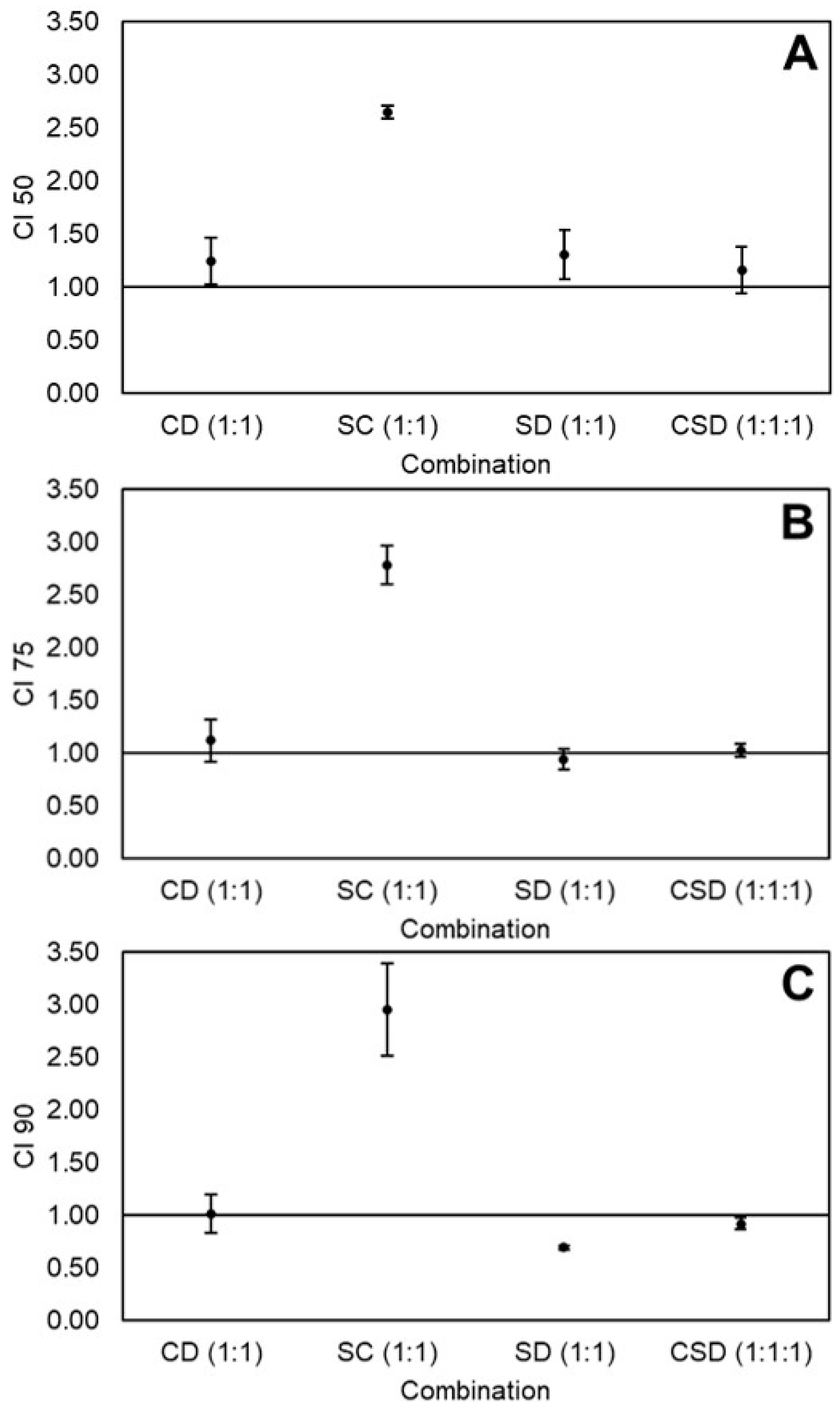
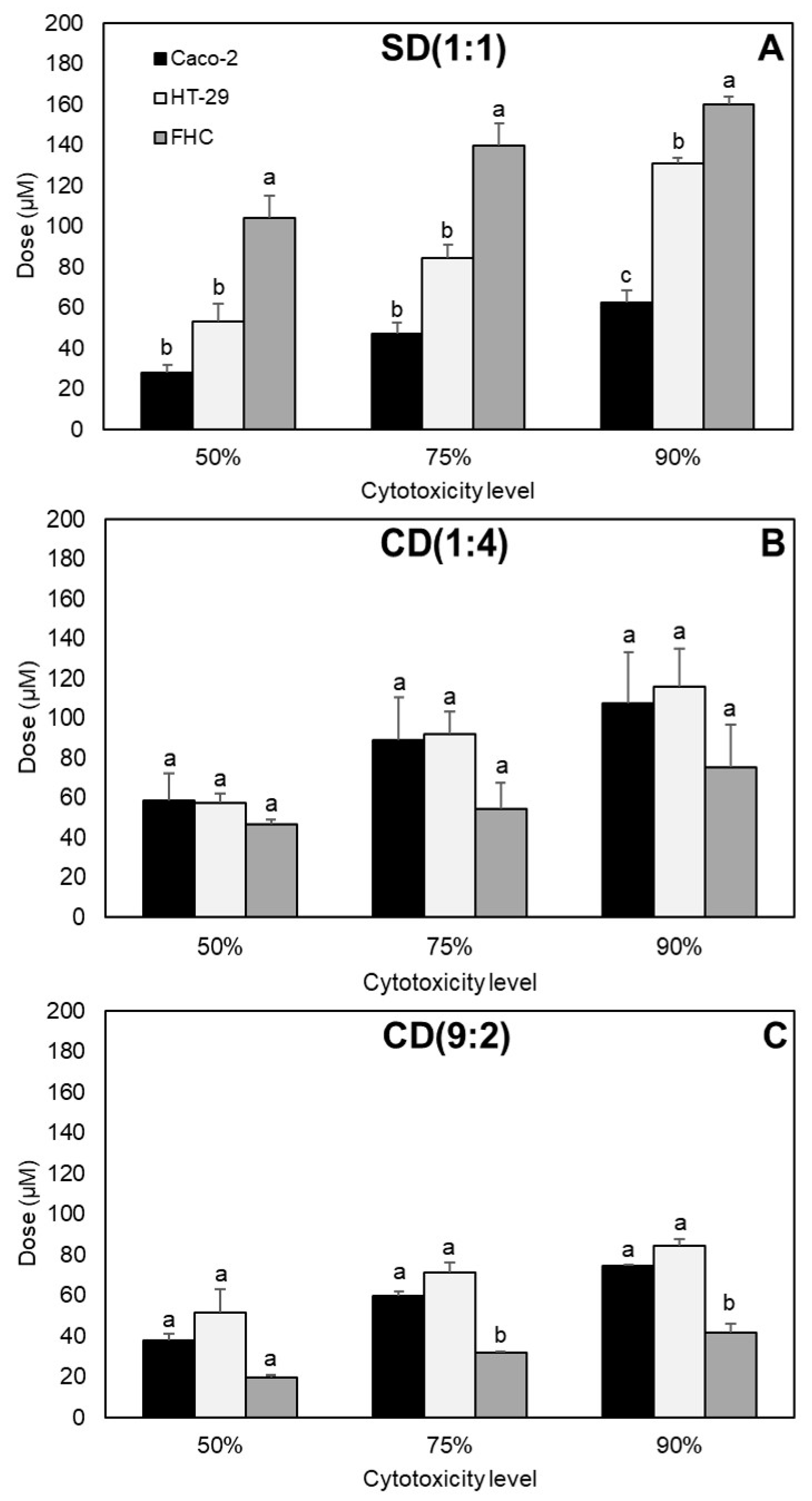
2.2. Effects of Equimolar Combinations of Curcumin, Sulforaphane, and Dihydrocaffeic Acid on the Viability of HT-29 Colon Cancer Cells
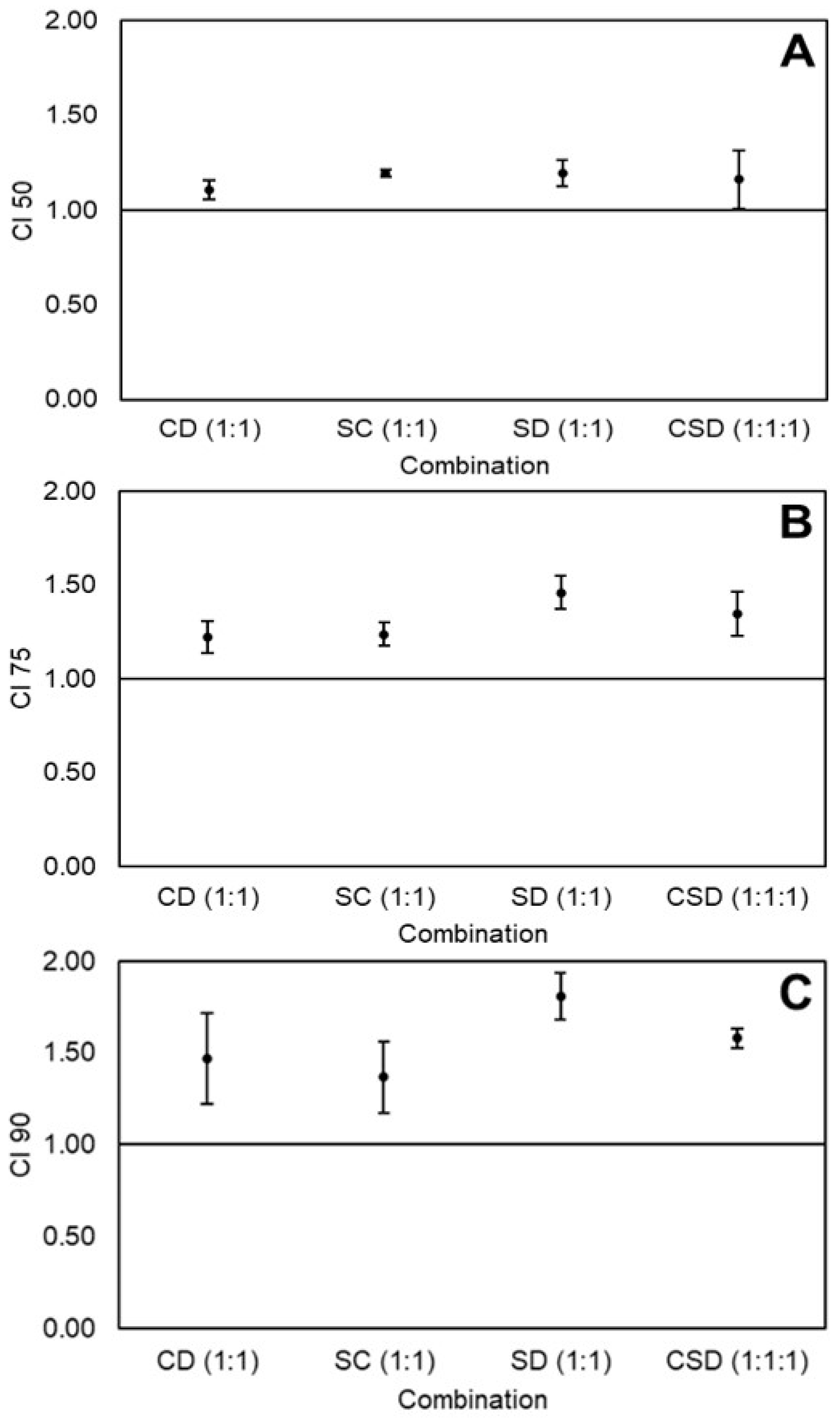
2.3. Effects of Equimolar Combinations of Curcumin, Sulforaphane, and Dihydrocaffeic Acid on the Viability of Caco-2 Colon Cancer Cells
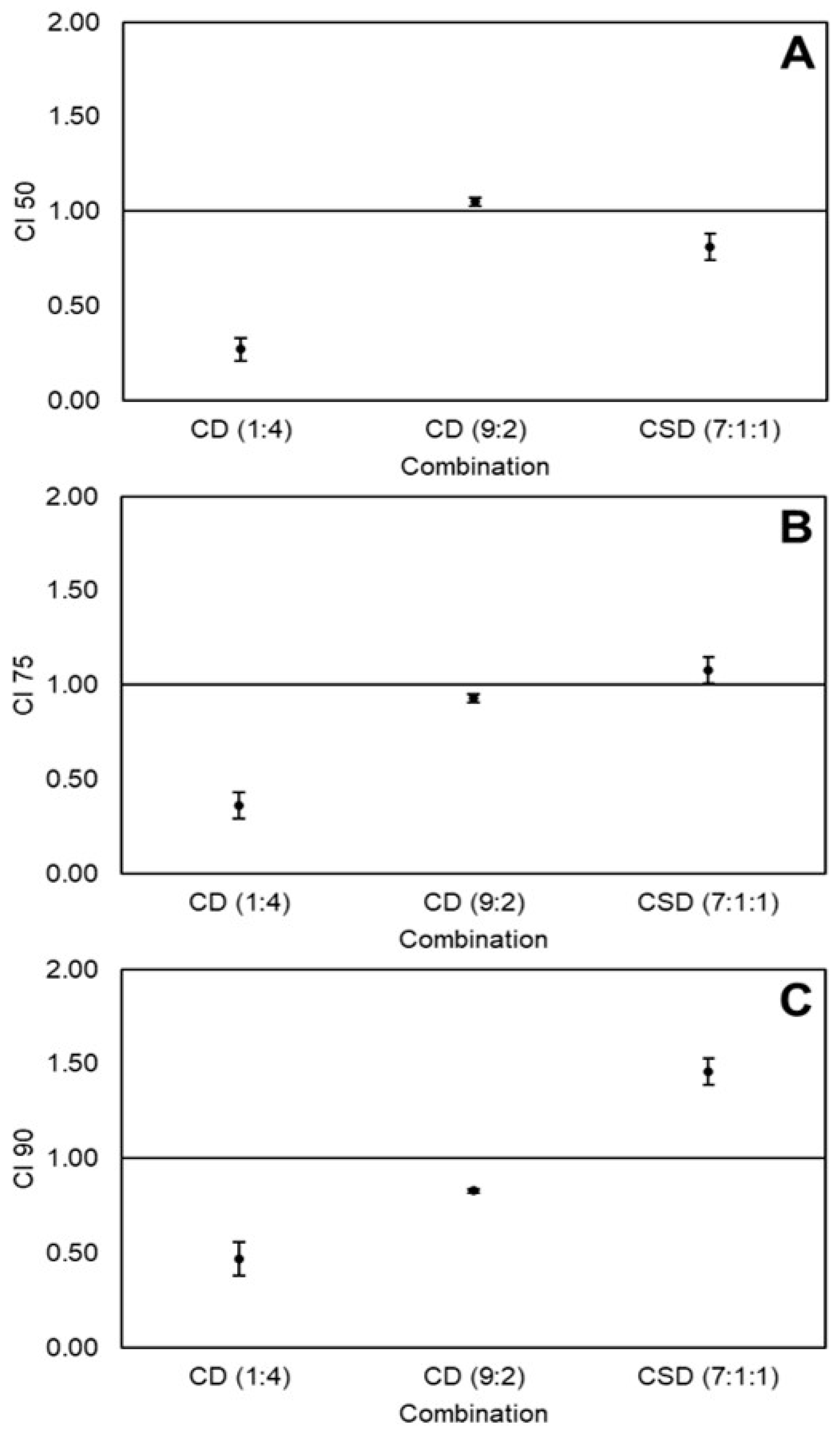
2.4. Effects of Non-Equimolar Combinations of Curcumin, Sulforaphane, and Dihydrocaffeic Acid on the Viability of Caco-2 Cancer Cells
2.5. Determination of Best Combination
3. Discussion
3.1. Some General Aspects about Cell Cycle and Apoptosis
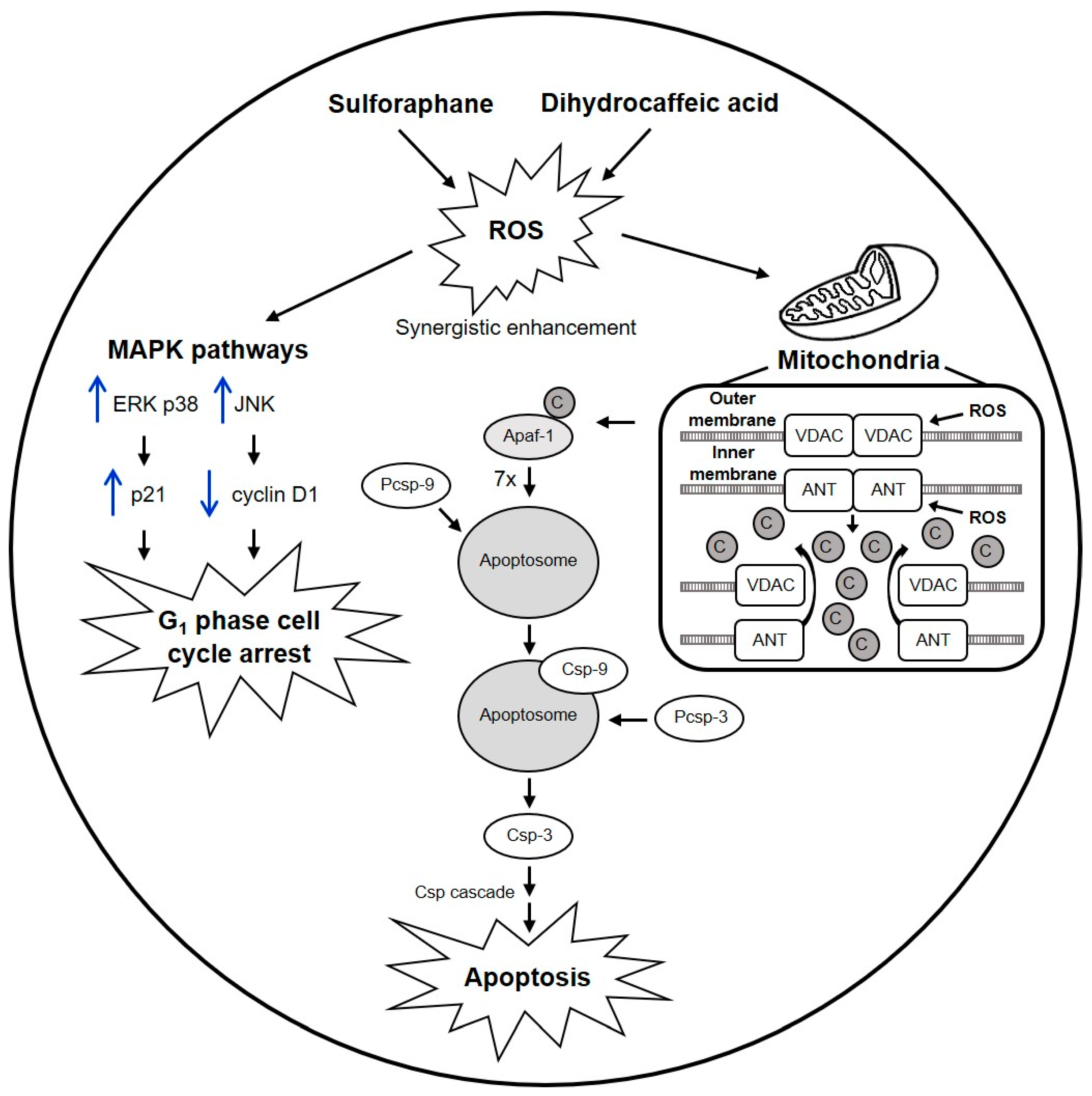
3.2. Mechanisms of Sulforaphane and Dihydrocaffeic Acid against Colon Cancer Cells, and Proposed Model of SD(1:1) Synergy against HT-29
3.3. Mode of Action of Curcumin against Colon Cancer Cells, and Proposed Mechanisms of CD(1:4) and CD(9:2) Synergy against Caco-2
3.4. Different Effects of Same Combinations between Colon Cancer Cell Lines
3.5. Different Effects of Varying Doses and Proportions of the Same Nutraceuticals in Colon Cancer Cells
3.6. Selectivity of Synergistic Combinations between Healthy and Colon Cancer Cells
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Combination Studies
4.4. Cell Viability Assay
4.5. Determination of Best Combination
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACF | Aberrant crypt foci |
| AIF | Apoptosis inducing factor |
| ANT | Adenine nucleotide translocator |
| Anti–Anti | Antibiotic–antimycotic |
| Apaf-1 | Apoptosis protease activating factor-1 |
| C | Curcumin |
| CC50,75,90 | Cytotoxic concentration to reduce cell viability by 50%, 75%, and 90%, respectively |
| CI | Combination index |
| CDK | Cyclin-dependent kinase |
| COX-2 | Cyclooxygenase-2 |
| D | Dihydrocaffeic acid |
| DMEM/F12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| DMSO | Dimethyl sulfoxide |
| DRI | Dose-reduction index |
| EGF | Epidermal growth factor |
| ERK | Extracellular-regulated kinase |
| FBS | Fetal bovine serum |
| FHC | Fetal human colon |
| JNK | c-jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| ROS | Reactive oxygen species |
| S | Sulforaphane |
| Smac | Second mitochondria derived activator of caspase |
| VDAC | Voltage-dependent anion channel |
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 16 January 2020).
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci. Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Montgomery, A.; Adeyeni, T.; San, K.K.; Heuertz, R.M.; Ezekiel, U.R. Curcumin sensitizes silymarin to exert synergistic anticancer activity in colon cancer cells. J. Cancer 2016, 7, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; George, J. Combinatorial strategies employing nutraceuticals for cancer development. Ann. N. Y. Acad. Sci. 2011, 1229, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Shukla, Y. Combinatorial approaches utilizing nutraceuticals in cancer chemoprevention and therapy: A complementary shift with promising acuity. In Genomics, Proteomics and Metabolomics in Nutraceuticals and Functional Foods, 2nd ed.; Bagchi, D., Swaroop, A., Bagchi, M., Eds.; Wiley-Blackwell: Oxford, UK, 2015; pp. 185–216. [Google Scholar]
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. β-Catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002, 21, 8414–8427. [Google Scholar] [CrossRef]
- Su, C.C.; Chen, G.W.; Lin, J.G.; Wu, L.T.; Chung, J.G. Curcumin inhibits cell migration of human colon cancer Colo 205 cells through the inhibition of nuclear factor kappa B /p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res. 2006, 26, 1281–1288. [Google Scholar]
- Singh, N.; Shrivastav, A.; Sharma, R.K. Curcumin induces caspase and calpain-dependent apoptosis in HT29 human colon cancer cells. Mol. Med. Rep. 2009, 2, 627–631. [Google Scholar]
- Villegas, I.; Sánchez-Fidalgo, S.; de la Lastra, C.A. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Mol. Nutr. Food Res. 2011, 55, 259–267. [Google Scholar] [CrossRef]
- Bounaama, A.; Djerdjouri, B.; Laroche-Clary, A.; Le Morvan, V.; Robert, J. Short curcumin treatment modulates oxidative stress, arginase activity, aberrant crypt foci, and TGF-β1 and HES-1 transcripts in 1,2-dimethylhydrazine-colon carcinogenesis in mice. Toxicology 2012, 302, 308–317. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Andělová, H.; Rudolf, E.; Červinka, M. In vitro antiproliferative effects of sulforaphane on human colon cancer cell line SW620. Acta Medica (Hradec Králové) 2007, 50, 171–176. [Google Scholar] [CrossRef]
- Kim, D.H.; Sung, B.; Kang, Y.J.; Hwang, S.Y.; Kim, M.J.; Yoon, J.H.; Im, E.; Kim, N.D. Sulforaphane inhibits hypoxia-induced HIF-1α and VEGF expression and migration of human colon cancer cells. Int. J. Oncol. 2015, 47, 2226–2232. [Google Scholar] [CrossRef]
- Zeng, H.; Trujillo, O.N.; Moyer, M.P.; Botnen, J.H. Prolonged sulforaphane treatment activates survival signaling in nontumorigenic NCM460 colon cells but apoptotic signaling in tumorigenic HCT116 colon cells. Nutr. Cancer 2011, 63, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Tang, W.Y.; Hsu, C.W.; Tsai, Y.T.; Wu, J.F.; Lin, C.W.; Cheng, Y.M.; Hsu, Y.C. Apoptosis induction in primary human colorectal cancer cell lines and retarded tumor growth in SCID mice by sulforaphane. Evid. Based Complement. Alternat. Med. 2012, 2012, 415231. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.L.; Conaway, C.C.; Rao, C.V.; Reddy, B.S. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis 2000, 21, 2287–2291. [Google Scholar] [CrossRef]
- Egner, P.A.; Chen, J.G.; Wang, J.B.; Wu, Y.; Sun, Y.; Lu, J.H.; Zhu, J.; Zhang, Y.H.; Chen, Y.S.; Friesen, M.D.; et al. Bioavailability of sulforaphane from two broccoli sprout beverages: Results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev. Res. 2011, 4, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.; van den Berg, R.; Vaes, W.H. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Hou, N.; Liu, N.; Han, J.; Yan, Y.; Li, J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anti-Cancer Drugs 2017, 28, 59–65. [Google Scholar] [CrossRef]
- Matsunaga, K.; Katayama, M.; Sakata, K.; Kuno, T.; Yoshida, K.; Yamada, Y.; Hirose, Y.; Yoshimi, N.; Mori, H. Inhibitory effects of chlorogenic acid on azoxymethane-induced colon carcinogenesis in male F344 rats. Asian Pac. J. Cancer Prev. 2002, 3, 163–166. [Google Scholar]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ekbatan, S.S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of polyphenols in a dynamic multistage gastrointestinal model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Paz de Peña, M.; Cid, C.; Crozier, A. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013, 39, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Villela-Castrejón, J.; Serna-Saldívar, S.O.; Jacobo-Velázquez, D.A. Anticancer potential of dihydrocaffeic acid: A chlorogenic acid metabolite. CyTA J. Food 2020, 18, 245–248. [Google Scholar] [CrossRef]
- Pappa, G.; Strathmann, J.; Löwinger, M.; Bartsch, H.; Gerhäuser, C. Quantitative combination effects between sulforaphane and 3,3′-diindolylmethane on proliferation of human colon cancer cells in vitro. Carcinogenesis 2007, 28, 1471–1477. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yogosawa, S.; Izutani, Y.; Watanabe, H.; Otsuji, E.; Sakai, T. A combination of indole-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol. Cancer 2009, 8, 100. [Google Scholar] [CrossRef]
- De Maria, S.; Scognamiglio, I.; Lombardi, A.; Amodio, N.; Caraglia, M.; Cartenì, M.; Ravagnan, G.; Stiuso, P. Polydatin, a natural precursor of resveratrol, induces cell cycle arrest and differentiation of human colorectal Caco-2 cell. J. Transl. Med. 2013, 11, 264. [Google Scholar] [CrossRef]
- Xu, G.; Ren, G.; Xu, X.; Yuan, H.; Wang, Z.; Kang, L.; Yu, W.; Tian, K. Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food Chem. Toxicol. 2010, 58, 390–395. [Google Scholar] [CrossRef]
- Jin, G.; Yang, Y.; Liu, K.; Zhao, J.; Chen, X.; Liu, H.; Bai, R.; Li, X.; Jiang, Y.; Zhang, X.; et al. Combination curcumin and (−)-epigallocatechin-3-gallate inhibits colorectal carcinoma microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway. Oncogenesis 2017, 6, e384. [Google Scholar] [CrossRef]
- Majumdar, A.P.N.; Banerjee, S.; Nautiyal, J.; Patel, B.B.; Patel, V.; Du, J.; Yu, Y.; Elliot, A.A.; Levi, E.; Sarkar, F.H. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer 2009, 61, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Khor, T.O.; Hu, R.; Yu, S.; Nair, S.; Ho, C.T.; Reddy, B.S.; Huang, M.T.; Newmark, H.L.; Kong, A.N.T. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007, 67, 9937–9944. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Li, X.; Chen, Y.; Zhou, X.; Garrett, S.H.; Guo, B. 3,3′-Diindolylmethane enhances the efficacy of butyrate in colon cancer prevention through down-regulation of survivin. Cancer Prev. Res. 2009, 2, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Doldán-Martelli, V.; Míguez, D.G. Synergistic interaction between selective drugs in cell populations models. PLoS ONE 2015, 10, e0117558. [Google Scholar] [CrossRef]
- Shen, G.; Xu, C.; Chen, C.; Hebbar, V.; Kong, A.N.T. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother. Pharmacol. 2006, 57, 317–327. [Google Scholar] [CrossRef]
- Gamet-Payrastre, L.; Li, P.; Lumeau, S.; Cassar, G.; Dupont, M.A.; Chevolleau, S.; Gasc, N.; Tulliez, J.; Tercé, F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000, 60, 1426–1433. [Google Scholar]
- Parnaud, G.; Li, P.; Cassar, G.; Rouimi, P.; Tulliez, J.; Combaret, L.; Gamet-Payrastre, L. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr. Cancer 2004, 48, 198–206. [Google Scholar] [CrossRef]
- Dabrowska, C.; Li, M.; Fan, Y. Apoptotic caspases in promoting cancer: Implications from their roles in development and tissue homeostasis. In Apoptosis in Cancer Pathogenesis and Anti-cancer Therapy: New Perspectives and Opportunities, 1st ed.; Gregory, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 89–112. [Google Scholar]
- Hu, Q.; Wu, D.; Chen, W.; Yan, Z.; Shi, Y. Proteolytic processing of the caspase-9 zymogen is required for apoptosome-mediated activation of caspase-9. J. Biol. Chem. 2013, 288, 15142–15147. [Google Scholar] [CrossRef]
- Rashmi, R.; Kumar, S.; Karunagaran, D. Human colon cancer cells lacking Bax resist curcumin-induced apoptosis and Bax requirement is dispensable with ectopic expression of Smac or downregulation of Bcl-XL. Carcinogenesis 2005, 26, 713–723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Debatin, K.M.; Poncet, D.; Kroemer, G. Chemotherapy: Targeting the mitochondrial cell death pathway. Oncogene 2002, 21, 8786–8803. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K. Growth inhibition by caffeic acid, one of the phenolic constituents of honey, in HCT 15 colon cancer cells. Sci. World J. 2012, 2012, 372345. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Farabegoli, F.; Iori, R.; Orlandi, M.; De Nicola, G.R.; Bagatta, M.; Angelino, D.; Gennari, L.; Ninfali, P. Vitexin-2-O-xyloside, raphasatin and (-)-epigallocatechin-3-gallate synergistically affect cell growth and apoptosis of colon cancer cells. Food Chem. 2013, 138, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sarriá, B.; Mateos, R.; Goya, L.; Bravo-Clemente, L. TNF-α-induced oxidative stress and endothelial dysfunction in EA.hy926 cells is prevented by mate and green coffee extracts, 5-caffeoylquinic acid and its microbial metabolite, dihydrocaffeic acid. Int. J. Food Sci. Nutr. 2019, 70, 267–284. [Google Scholar] [CrossRef]
- Collett, G.P.; Campbell, F.C. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2004, 25, 2183–2189. [Google Scholar] [CrossRef]
- Goel, A.; Boland, C.R.; Chauhan, D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Wang, S.; Sun, D.; Chen, J.; Li, Y.; Han, W.; Yang, X.; Gao, H.Q. Effects of phytochemicals sulforaphane on uridine diphosphate-glucuronosyltransferase expression as well as cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. Chin. J. Physiol. 2012, 55, 134–144. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
| Cell Line | Nutraceutical/Combination (Proportions) | r |
|---|---|---|
| HT-29 | C | 0.979 ± 0.003 |
| S | 0.989 ± 0.002 | |
| D | 0.980 ± 0.010 | |
| CD(1:1) | 0.989 ± 0.004 | |
| SC(1:1) | 0.970 ± 0.008 | |
| SD(1:1) | 0.984 ± 0.014 | |
| CSD(1:1:1) | 0.981 ± 0.002 | |
| Caco-2 | C | 0.997 ± 0.001 |
| S | 0.971 ± 0.026 | |
| D | 0.982 ± 0.009 | |
| CD(1:4) | 0.952 ± 0.004 | |
| CD(9:2) | 0.996 ± 0.001 | |
| CSD(7:1:1) | 0.986 ± 0.002 |
| Cytotoxicity Level | 50% | 75% | 90% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | C | S | D | C | S | D | C | S | D |
| Individual dose (µM) | 31.6 ± 4.2b | 41.3 ± 14.6b | 188.4 ± 9.4a | 60.6 ± 6.0b | 81.9 ± 29.4b | 389.5 ± 34.7a | 116.3 ± 7.5b | 162.2 ± 59.1b | 806.2 ± 103.5a |
| Combination dose (µM) | |||||||||
| CD(1:1) | 33.9 ± 9.6b | 33.9 ± 9.6b | 59.0 ± 14.6b | 59.0 ± 14.6b | 102.6 ± 21.9b | 102.6 ± 21.9b | |||
| SC(1:1) | 46.5 ± 10.0b | 46.5 ± 10.0b | 96.5 ± 26.8b | 96.5 ± 26.8b | 200.7 ± 68.2b | 200.7 ± 68.2b | |||
| SD(1:1) | 40.9 ± 4.7b | 40.9 ± 4.7b | 60.1 ± 11.5b | 60.1 ± 11.5b | 88.8 ± 23.5b | 88.8 ± 23.5b | |||
| CSD(1:1:1) | 19.4 ± 7.4b | 19.4 ± 7.4b | 19.4 ± 7.4b | 32.3 ± 8.1b | 32.3 ± 8.1b | 32.3 ± 8.1b | 55.0 ± 6.0b | 55.0 ± 6.0b | 55.0 ± 6.0b |
| DRI | |||||||||
| CD(1:1) | 1.00 ± 0.2b | 6.1 ± 2.0ab | 1.1 ± 0.2b | 7.2 ± 2.4ab | 1.2 ± 0.2b | 8.5 ± 2.8ab | |||
| SC(1:1) | 0.7 ± 0.1b | 0.9 ± 0.1b | 0.7 ± 0.1b | 0.8 ± 0.1b | 0.6 ± 0.2b | 0.80 ± 0.0b | |||
| SD(1:1) | 1.00 ± 0.2b | 4.7 ± 0.8ab | 1.3 ± 0.2b | 6.8 ± 1.9ab | 1.8 ± 0.2b | 10.1 ± 3.8ab | |||
| CSD(1:1:1) | 1.8 ± 0.5ab | 2.2 ± 0.1ab | 11.6 ± 4.9a | 2.00 ± 0.3b | 2.5 ± 0.3b | 13.2 ± 4.4a | 2.1 ± 0.1b | 2.9 ± 0.8b | 15.1 ± 3.5a |
| Combination | Doses (μM) | Proportions | Cytotoxicity Level (%) | CI |
|---|---|---|---|---|
| CD | 45:10 | 9:2 | 64 | 0.79 ± 0.01g |
| 35:20 | 7:4 | 15 | 1.41 ± 0.36efg | |
| 20:80 | 1:4 | 52 | 0.88 ± 0.02fg | |
| SC | 30:10 | 3:1 | 35 | 2.63 ± 0.09bc |
| 25:5 | 5:1 | 29 | 2.31 ± 0.15bcd | |
| 10:35 | 2:7 | 34 | 1.58 ± 0.15ef | |
| SD | 30:10 | 3:1 | 25 | 2.90 ± 0.19b |
| 15:10 | 3:2 | 5 | 2.80 ± 0.40b | |
| 15:80 | 3:16 | 44 | 1.66 ± 0.10de | |
| CSD | 35:5:5 | 7:1:1 | 62 | 0.90 ± 0.07fg |
| 15:5:80 | 3:1:16 | 5 | 3.70 ± 0.52a | |
| 5:25:5 | 1:5:1 | 38 | 2.06 ± 0.05cde |
| Cytotoxicity Level | 50% | 75% | 90% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | C | S | D | C | S | D | C | S | D |
| Individual dose (µM) | 65.8 ± 2.3b | 44.6 ± 2.6bc | 248.0 ± 11.7a | 94.6 ± 2.7b | 48.7 ± 2.5cd | 386.8 ± 3.6a | 135.9 ± 2.9bc | 53.2 ± 2.5cde | 605.3 ± 39.8a |
| Combination dose (µM) | |||||||||
| CD(1:1) | 8.8 ± 2.2e | 35.3 ± 8.8cde | 17.2 ± 3.6de | 68.7 ± 14.5bc | 33.5 ± 5.8de | 134.0 ± 23.2bc | |||
| SC(1:1) | 65.4 ± 3.6b | 14.5 ± 0.8de | 83.6 ± 3.7b | 18.6 ± 0.8de | 106.9 ± 3.5bcd | 23.8 ± 0.8de | |||
| CSD(1:1:1) | 42.7 ± 5.6bcd | 6.1 ± 0.8e | 6.1 ± 0.8e | 78.0 ± 7.9bc | 11.1 ± 1.1e | 11.1 ± 1.1e | 142.6 ± 10.1b | 20.4 ± 1.4e | 20.4 ± 1.4e |
| DRI | |||||||||
| CD(1:1) | 7.89 ± 1.71c | 7.41 ± 1.52c | 5.73 ± 1.06c | 5.91 ± 1.30c | 4.17 ± 0.63b | 4.71 ± 1.11b | |||
| SC(1:1) | 1.01 ± 0.02c | 17.07 ± 0.15b | 1.13 ± 0.02c | 20.87 ± 1.12b | 1.27 ± 0.01b | 25.56 ± 2.51a | |||
| CSD(1:1:1) | 1.56 ± 0.15c | 7.37 ± 0.55c | 41.07 ± 3.49a | 1.22 ± 0.09c | 4.39 ± 0.22c | 35.09 ± 3.88a | 0.96 ± 0.05b | 2.62 ± 0.06b | 30.00 ± 4.08a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Gálvez, J.; Villela-Castrejón, J.; Serna-Saldívar, S.O.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Synergistic Combinations of Curcumin, Sulforaphane, and Dihydrocaffeic Acid against Human Colon Cancer Cells. Int. J. Mol. Sci. 2020, 21, 3108. https://doi.org/10.3390/ijms21093108
Santana-Gálvez J, Villela-Castrejón J, Serna-Saldívar SO, Cisneros-Zevallos L, Jacobo-Velázquez DA. Synergistic Combinations of Curcumin, Sulforaphane, and Dihydrocaffeic Acid against Human Colon Cancer Cells. International Journal of Molecular Sciences. 2020; 21(9):3108. https://doi.org/10.3390/ijms21093108
Chicago/Turabian StyleSantana-Gálvez, Jesús, Javier Villela-Castrejón, Sergio O. Serna-Saldívar, Luis Cisneros-Zevallos, and Daniel A. Jacobo-Velázquez. 2020. "Synergistic Combinations of Curcumin, Sulforaphane, and Dihydrocaffeic Acid against Human Colon Cancer Cells" International Journal of Molecular Sciences 21, no. 9: 3108. https://doi.org/10.3390/ijms21093108
APA StyleSantana-Gálvez, J., Villela-Castrejón, J., Serna-Saldívar, S. O., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2020). Synergistic Combinations of Curcumin, Sulforaphane, and Dihydrocaffeic Acid against Human Colon Cancer Cells. International Journal of Molecular Sciences, 21(9), 3108. https://doi.org/10.3390/ijms21093108






