Abstract
Sarcopenia is defined as the involuntary loss of skeletal muscle mass and function with aging and is associated with several adverse health outcomes. Recently, the disruption of regular circadian rhythms, due to shift work or nocturnal lifestyle, is emerging as a novel deleterious factor for the development of sarcopenia. The underlying mechanisms responsible for circadian disruption-induced sarcopenia include molecular circadian clock and mitochondrial function associated with the regulation of circadian rhythms. Exercise is a potent modulator of skeletal muscle metabolism and is considered to be a crucial preventative and therapeutic intervention strategy for sarcopenia. Moreover, emerging evidence shows that exercise, acting as a zeitgeber (time cue) of the skeletal muscle clock, can be an efficacious tool for re-setting the clock in sarcopenia. In this review, we provide the evidence of the impact of circadian disruption on skeletal muscle loss resulting in sarcopenia. Furthermore, we highlight the importance of exercise timing (i.e., scheduled physical activity) as a novel therapeutic strategy to target circadian disruption in skeletal muscle.
1. Introduction
Skeletal muscle, which represents the largest organ of the human body, comprises approximately 40% of the total body mass and contains 50%‒75% of all body proteins. The main function of skeletal muscle is to maintain posture and produce movement that controls locomotion. Aside from this, skeletal muscle plays a central role in whole-body protein metabolism by serving as the main reservoir for amino acids in the absence of nutrient intake, allowing the maintenance of protein synthesis in other tissues of the body [1]. Overall, the functions of skeletal muscle are critical for systemic health, and reduced muscle mass and functions can lead to the development of many chronic diseases, including sarcopenia.
Sarcopenia, a progressive and generalized skeletal muscle disorder involving the loss of skeletal muscle mass and function [2] has long been associated with older adults [3,4] and can lead to adverse health outcomes, including physical disability, morbidity, and mortality [5,6,7,8]. However, in the past decade, circadian rhythm disruption, due to shift work or nocturnal lifestyle, has emerged as a prominent factor influencing the development of sarcopenia in addition to aging. In fact, a recent epidemiological study has suggested that shift workers who undertake variable rotating day and night shifts have an increased risk of sarcopenia [9]. Sleep deficiency (decreased sleep quality and/or sleep loss) or nocturnal lifestyle, which can contribute to circadian disruption, might also be involved in the development of sarcopenia [10,11]. Considering the significant increase in the number of shift workers worldwide [12,13,14] and individuals with a nocturnal lifestyle owing to an increasing 24-h culture, the association between circadian disruption and the prevalence of sarcopenia is an important area for future investigation.
Exercise is well-characterized as the major preventative and therapeutic strategy of sarcopenia that can attenuate and even reverse the loss of muscle mass and strength [15,16]. Previous studies have indicated that a balanced exercise program (at least 3 times/week), including resistance and endurance exercise training, could have positive effects on sarcopenia parameters and physical function. However, it remains unclear whether there is a specific timing of the day for exercise to trigger optimal training effects. Recently, emerging evidence has showed that exercise modulates the molecular circadian clocks in skeletal muscle [17,18] and exercise performance is variable throughout the day [19]. These findings showed a crosstalk between circadian rhythm and exercise, and it could be hypothesized that exercise timing (scheduled exercise) can assist in re-setting the clock and maximizing the beneficial effects of exercise associated with sarcopenia.
The aim of this review is to highlight the current literature regarding the potential mechanisms of exercise as a novel viable strategy, targeting circadian disruption associated with sarcopenia. Thus, we summarized the effect of circadian disruption on skeletal muscle disorder resulting in sarcopenia, along with the potential underlying mechanisms, while demonstrating when and why engaging in exercise might act as a therapeutic intervention to alleviate circadian disruption-induced sarcopenia.
2. Sarcopenia and Circadian Disruption
Increasing evidence indicates a possible association between circadian disruption and sarcopenia. Approximately 15‒30% of the global work population is engaged in shift work [12,13,14], and these workers have an increased risk of circadian disruption [20]. A prospective cohort study in South Korea showed a significant 1.7-fold increased risk in sarcopenia prevalence in shift workers than in those who had never experienced shift work [9]. In particular, they reported that irregularly scheduled shift work was more strongly associated with a higher prevalence of sarcopenia compared to regularly scheduled work. Indirect disruption of circadian rhythm due to sleep problems (duration, quality, and timing) or social jet lag can also have similar deleterious effects on skeletal muscle health in humans. Recent population- and laboratory-based studies have reported a U-shaped association between sleep duration and the prevalence of sarcopenia [21,22,23,24,25]. Furthermore, population- or hospital-based studies have found associations between components of sarcopenia and obstructive sleep apnea and decreased sleep quality [11,26]. More recently, a systematic meta-analysis concluded that sleep quality can predict the risk of developing sarcopenia [27]. Interestingly, later sleep timing, which can induce circadian misalignment as well as adversely affect sleep quality, has been shown to be associated with sarcopenia in middle-aged individuals [11]. Interaction between biological and societal clocks can lead to a chronic form of jetlag, depending on chronotype and social situation (i.e., social jet lag). Wittmann et al. have indicated that late chronotype is typically associated with a greater degree of misalignment between social and circadian time than other chronotypes [28]. Indeed, Yu et al. reported that the prevalence of sarcopenia is 6.7% higher in middle-aged Korean individuals with an evening chronotype rather than a morning chronotype [10]. Taken together, these findings indicated that the disruption of circadian rhythms is also related with an increased risk of developing sarcopenia in addition to aging. An overview of studies on the association between sarcopenia and circadian disruption is provided in Table 1.

Table 1.
Sarcopenia and circadian disruption in humans.
3. Potential Mechanisms underlying Circadian Disruption Associated with Sarcopenia
3.1. Molecular Circadian Clock, Central and Peripheral Clocks, and Circadian Rhythm
The circadian system is governed by a central clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus and peripheral clocks situated in other organs and tissues throughout the body, such as skeletal muscle. The central circadian clock is readjusted mainly by light inputs, the most important being time cue (i.e., Zeitgeber) [29]. Peripheral organs, such as skeletal muscle, have their own clocks, which are synchronized not only by the central clock [29] but also by an individual’s behaviors, including feeding or exercise [18,30]. Both central and peripheral clocks consist of a transcriptional-translational feedback loop (TTFL) known as the molecular clock. This feedback loop is mainly mediated by various activators, such as Clock (circadian locomotor output cycles kaput), Bmal1 (brain and muscle Arnt-like protein-1), and their target genes, Per1, Per2, Cry1, and Cry2, which constitute negative repressor complexes that interact with Clock and Bmal1 to inhibit Per and Cry gene transcription [31]. In addition to the main TTFL, Rev-erbs (nuclear receptor) and RORs (RAR-related orphan receptor) genes, which are the part of the core clock, link the second feedback loop by acting as the repressor and activator of Bmal1 an Clock transcription, respectively [32,33]. As a result, the circadian rhythms produced by these molecular clocks oscillate over a period of approximately 24 h.
3.2. Mechanistic Pathways Connecting Circadian Disruption, Mitochondrial Dysfunction, and Sarcopenia
Although a direct relationship between circadian disruption and sarcopenia has not yet been elucidated in humans, several animal studies with mutations in clock genes have demonstrated that disruption of circadian rhythms can be detrimental to skeletal muscle health. Among clock genes, Bmal1 and Clock are the key genes involved in the regulation of circadian rhythm. Global (whole-body) Bmal1 knockout (KO) mice exhibited reduced muscle weight, body weight, and lifespan with disrupted circadian rhythms [34,35] and severe sarcopenia at 40 weeks of age [35]. Furthermore, core clock genes (Bmal1 and Clock) mutant mice showed reductions in muscle force with mitochondrial dysfunction [36]. It should be noted, however, that these results are in contrast with those of the studies on a muscle-specific Bmal1 KO model [37,38], showing no differences in muscle weight, muscle force, and life span between muscle-specific Bmal1 KO and wild-type mice. Although it is also possible that muscle clock affects lipid metabolism to coincide with muscle protein turnover [39,40], skeletal muscle clock seems not to simply contribute to the progression of muscle atrophy [38]. Together, these results suggest that disruption or weakening of the circadian rhythm via deletion of the central clock gene, not by the muscle-specific clock gene, might play an important role in the pathogenesis of sarcopenia.
Recent evidence has also suggested that mitochondria might play a critical role in circadian disruption-related sarcopenia. In skeletal muscles, mitochondria are an important and essential organelle involved in metabolic regulation and production of adenosine triphosphate (ATP) for muscle contractibility and plasticity. Many studies have revealed that mitochondria play a key role in the pathogenesis of sarcopenia [41,42,43], and mitochondrial dysfunction, along with aging, has been extensively studied as a contributor to sarcopenia [44,45]. However, regulation of mitochondrial function via the circadian system has also gained interest as emerging evidence indicates that circadian disruption is associated with mitochondrial dysfunction [46,47]. The importance of circadian rhythms for mitochondrial processes is made evident by the genetic disruption of the molecular clock. For example, circadian mutant mice (Bmal1 and Clock) displayed reduced muscle mitochondrial volume, respiratory function, and peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α) level with subsequent reduction in contractile muscle force [36]. Similarly, Clock mutant mice (Clock △19) exhibited decreased mitochondrial function including decrease in levels of PGC-1α, mitochondrial transcription factor-A (Tfam) protein, and mitochondria content with reduced exercise capacity [48]. In particular, PGC-1α is often referred to as the master regulator of mitochondrial biogenesis, and thus, these findings suggest that reduced PGC-1α in circadian mutant mice might contribute to decrease in mitochondrial content and respiration [36,48]. Moreover, Liu et al. found that mice with depletion of PGC-1α in skeletal muscle showed reduced clock gene expression and disrupted circadian rhythm [49]. They found that PGC-1α can stimulate the expression of clock genes, such as Bmal1 and Rev-erbα via coactivation of the ROR family, which is part of the core clock of the second feedback loop. In turn, these studies suggest that PGC-1α likely plays a pivotal role in the circadian TTFL, involved ultimately in mitochondrial functioning. Together, these findings indicate that decreases in the protein content and expressions of clock genes and PGC-1α might help in elucidating the effects of circadian disruption on mitochondrial dysfunction, leading to sarcopenia.
In addition to circadian clock-mediated regulation, mitochondrial function in skeletal muscle exhibits an intrinsic circadian rhythm [46,47]. A recent study in human skeletal muscle biopsy samples demonstrated that Bmal1 exhibited significant variation over time with peak expression at around midnight (23:00 h), whereas Per2 exhibited a trough expression at midnight, and that mitochondrial function (e.g., mitochondrial oxidative capacity) also displays circadian rhythm and peaks in the late afternoon [46]. Interestingly, the same study found time-dependent variations in proteins involved in mitochondrial dynamics; the levels of mitochondrial fission 1 (Fis1; fission mediator) displayed significant time-dependent differences similar to those of rhythm in mitochondrial oxidative capacity, whereas levels of PTEN-induced kinase 1 (PINK-1; a marker of mitophagy) showed an opposite rhythm as compared to Fis1. However, no rhythm was observed in the mitochondrial content (e.g., mitochondrial DNA) and mitochondrial biogenesis (e.g., PGC-1α) in human skeletal muscle. Taken together, these data indicate that skeletal muscle mitochondria are influenced either by the circadian clock or the intrinsic circadian rhythms, which suggests association between the circadian system and mitochondrial metabolism. Disturbances in the rhythm of the mitochondrial function of skeletal muscle could play a critical role in the pathology of skeletal muscle.
4. Novel Aspects of Exercise as a Zeitgeber for Re-setting the Clock against Sarcopenia
4.1. Exercise, Molecular Circadian Clock Gene, and Circadian Synchronization
As previously stated, skeletal muscles have their own circadian rhythm and can be entrained by physical activity for skeletal muscle [18,29] and light for the SCN. With respect to association between exercise and muscle clock genes, several studies have demonstrated that exercise, such as wheel running or forced treadmill running, in nocturnal rodents alters the expression of core clock genes in skeletal muscle [50] and could entrain circadian rhythms [51,52,53]. In addition, scheduled exercise training resulted in a significant shift in clock gene expression in the skeletal muscle of mice [18], supporting that exercise might be an external time cue (zeitgeber) for the clock in skeletal muscle.
Zambon et al. also examined the effect of a single bout of exercise on gene regulation in human muscle biopsy samples [17]. Their results showed that three core circadian clock genes, Bmal1, Cry1, and Per2, were upregulated by resistance exercise in one leg compared with the non-exercise leg. Human studies also have established that exercise has significant circadian phase-shifting effects (Table 2) [54,55,56,57,58,59,60,61,62] and can easily entrain an individual to a shifted light-dark and sleep/wake schedule. In particular, several studies showed that morning exercise accelerated phase advances, while evening exercise accelerated phase delays in the circadian rhythm [54,56,57,59], highlighting the importance of exercise timing in differentiating between phase-delaying and phase-advancing effects. With respect to the phase-shifting effect of exercise, especially in shift work, Eastman et al. examined whether timed exercise during consecutive night shifts could phase delay the circadian rhythm of humans to align with a daytime sleep schedule [55]. In this experiment, the participants cycled for 15 min every hour during the first three out of eight consecutive night shifts. As a result, exercise produced large phase delays in core temperature rhythm regardless of their chronotypes (morningness or eveningness), suggesting that exercise can be a powerful determinant of phase shift. Moreover, Youngstedt et al. revealed that early morning exercise with moderate intensity (65‒75% heart rate reserve at 4:10‒5:40 am) following late evening bright light (22:10‒23:40 pm) can have an additive circadian phase-shifting effect [61]. Interestingly, recent work by Thomas et al. indicated that individuals with late chronotypes, experiencing severe circadian misalignment, may benefit from phase advances with 5 days of morning or evening exercise, whereas evening exercise may exacerbate circadian misalignment in early chronotypes [62]. Together, these findings suggest that exercise, and more importantly, the timing of exercise could act as a circadian time cue by changing the phase of circadian clock, and thereby, personalized exercise timing prescription could alleviate circadian disruption.

Table 2.
Circadian phase-shifting effects of exercise in humans.
4.2. Impacts of the Scheduled Exercise (Exercise Timing) on Mitochondrial Function and Muscle Performance
In addition to the circadian phase-shifting effects of exercise, timing of exercise (i.e., circadian time of exercise) can contribute to the time-point of peak physical performance. Previous research has revealed that there are diurnal variations in skeletal muscular performance [19,63] and oxidative capacity [46,47]. Indeed, studies have consistently reported that skeletal muscle torque, strength, and power in humans increased in the late afternoon, between 16:00 and 18:00 pm, compared with in morning [19,63]. In addition, mitochondrial oxidative capacity in human skeletal muscle has been shown to exhibit circadian changes, with a peak in the late evening (23:00 pm) [46,47]. Previous studies have reported that the peak aerobic performance is also seen later in the day [64,65,66], which might be partly responsible for the diurnal fluctuations in response to mitochondrial function.
Evidence from our group and other studies has shown that both acute and chronic exercises lead to increased mitochondrial function of skeletal muscle in both animals and humans [67,68]. A recent study by our group revealed that a single bout of exercise improved mitochondrial function, including mitochondrial O2 respiration and Ca2+ retention capacity, in skeletal muscles of rats [68]. However, it has not been completely resolved whether any specific time of exercise with a concomitant peak in mitochondrial function increases the effect of exercise in terms of muscle function. It is clear that the time of exercise training (i.e., morning or evening exercise) in human has different outcomes on muscle mass and muscle performance [69,70,71,72]. A recent study from our group showed that evening exercise (19:00 h) in the cardiac muscles of rats, and not morning exercise (07:00 h), increases PGC-1α mRNA levels, suggesting that mitochondrial biogenesis could be influenced by circadian time of exercise [73]. However, there is still very little information regarding the effect of circadian exercise on skeletal muscle mitochondria. Further studies will be needed to investigate whether a specific time of exercise could maximize both skeletal muscle mitochondrial function and overall exercise performance.
5. Conclusions
Disruption of circadian rhythm is one of the critical factors that leads to sarcopenia in various individuals, especially in people with shift work, social jetlag, and sleep disorder [9,10,11,21]. Although a direct relationship between circadian disruption and sarcopenia has not been identified in humans, several studies in different mouse models of clock gene deficiency have demonstrated that circadian disruption associated with mitochondrial dysfunction might contribute to the pathology of skeletal muscle [36]. Indeed, mitochondria play a central role in the progression of sarcopenia [42,43], and emerging evidence has revealed that mitochondrial function in skeletal muscle has intrinsic circadian rhythms [46,47], possibly involved in the molecular mechanisms of sarcopenia. Taken together, there is ample evidence to highlight the important role of circadian rhythm in the skeletal muscle system, suggesting restoring the circadian rhythm as a possible therapeutic intervention in prevalent sarcopenia.
Exercise has been suggested to be one of the most effective strategies against the loss of muscle function and muscle mass [15]. Consideration of the circadian phase-shifting effects of exercise might help in rendering the circadian exercise intervention more effective in re-setting the clock and optimizing the beneficial effects of exercise associated with sarcopenia. In particular, personalized exercise timing could be prescribed as an effective tool to prevent and treat sarcopenia in various individuals who work shifts or have a nocturnal lifestyle. As a consequence, exercise, and more importantly scheduled exercise, might be useful in preventing and treating circadian disruption-related sarcopenia (Figure 1). Further research should elucidate whether a specific time of exercise would improve skeletal muscle function and the mechanisms through which scheduled exercise could optimize skeletal muscle function, particularly the mechanisms involving mitochondrial function.
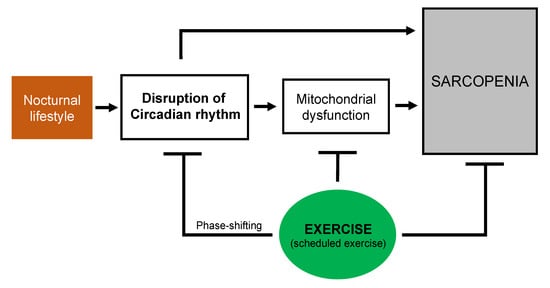
Figure 1.
Schematic overview of the potential beneficial effects of exercise on circadian disruption and mitochondrial dysfunction associated with sarcopenia.
Author Contributions
Y.C. and H.-B.K. contributed to the conception and design of the present review. J.C., J.-W.H., M.-H.N., E.-J.C., E.C., D.-H.P., and J.-H.K. conducted the critical discussion. Y.C. and H.-B.K. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF- 2019S1A5C2A03082727).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kamel, H.K. Sarcopenia and aging. Nutr. Rev. 2003, 61, 157–167. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Sayer, A.A.; Syddall, H.E.; Martin, H.J.; Dennison, E.M.; Roberts, H.C.; Cooper, C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing 2006, 35, 409–415. [Google Scholar] [CrossRef]
- Gale, C.R.; Martyn, C.N.; Cooper, C.; Sayer, A.A. Grip strength, body composition, and mortality. Int. J. Epidemiol. 2007, 36, 228–235. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Fusco, D.; Mastropaolo, S.; Quattrociocchi, D.; Proia, A.; Tosato, M.; Bernabei, R.; Onder, G. Sarcopenia and mortality among older nursing home residents. J. Am. Med. Dir. Assoc. 2012, 13, 121–126. [Google Scholar] [CrossRef]
- Choi, Y.I.; Park, D.K.; Chung, J.W.; Kim, K.O.; Kwon, K.A.; Kim, Y.J. Circadian rhythm disruption is associated with an increased risk of sarcopenia: A nationwide population-based study in Korea. Sci. Rep. 2019, 9, 12015. [Google Scholar] [CrossRef]
- Yu, J.H.; Yun, C.H.; Ahn, J.H.; Suh, S.; Cho, H.J.; Lee, S.K.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Choi, K.M.; et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J. Clin. Endocrinol. Metab. 2015, 100, 1494–1502. [Google Scholar] [CrossRef]
- Lucassen, E.A.; de Mutsert, R.; le Cessie, S.; Appelman-Dijkstra, N.M.; Rosendaal, F.R.; van Heemst, D.; den Heijer, M.; Biermasz, N.R.; NEO Study Group. Poor sleep quality and later sleep timing are risk factors for osteopenia and sarcopenia in middle-aged men and women: The NEO study. PLoS ONE 2017, 12, e0176685. [Google Scholar] [CrossRef]
- Alterman, T.; Luckhaupt, S.E.; Dahlhamer, J.M.; Ward, B.W.; Calvert, G.M. Prevalence rates of work organization characteristics among workers in the U.S.: Data from the 2010 National Health Interview Survey. Am. J. Ind. Med. 2013, 56, 647–659. [Google Scholar] [CrossRef]
- Parent-Thirion, A.; Biletta, I.; Cabrita, J.; Llave Vargas, O.; Vermeylen, G.; Wilczynska, A. 6th European Working Conditions Survey: Overview Report; 2017 Update; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Cheng, P.; Drake, C.L. Psychological impact of shift work. Curr. Sleep Med. Rep. 2018, 4, 104–109. [Google Scholar] [CrossRef]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Zambon, A.C.; McDearmon, E.L.; Salomonis, N.; Vranizan, K.M.; Johansen, K.L.; Adey, D.; Takahashi, J.S.; Schambelan, M.; Conklin, B.R. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 2003, 4, R61. [Google Scholar] [CrossRef]
- Wolff, G.; Esser, K.A. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med. Sci. Sports Exerc. 2012, 44, 1663–1670. [Google Scholar] [CrossRef]
- Facer-Childs, E.; Brandstaetter, R. The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr. Biol. 2015, 25, 518–522. [Google Scholar] [CrossRef]
- Nagano, M.; Adachi, A.; Nakahama, K.; Nakamura, T.; Tamada, M.; Meyer-Bernstein, E.; Sehgal, A.; Shigeyoshi, Y. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J. Neurosci. 2003, 23, 6141–6151. [Google Scholar] [CrossRef]
- Fex, A.; Barbat-Artigas, S.; Dupontgand, S.; Filion, M.E.; Karelis, A.D.; Aubertin-Leheudre, M. Relationship between long sleep duration and functional capacities in postmenopausal women. J. Clin. Sleep Med. 2012, 8, 309–313. [Google Scholar] [CrossRef]
- Chien, M.Y.; Wang, L.Y.; Chen, H.C. The relationship of sleep duration with obesity and sarcopenia in community-dwelling older adults. Gerontology 2015, 61, 399–406. [Google Scholar] [CrossRef]
- Buchmann, N.; Spira, D.; Norman, K.; Demuth, I.; Eckardt, R.; Steinhagen-Thiessen, E. Sleep, muscle mass and muscle function in older people. Dtsch. Arztebl. Int. 2016, 113, 253–260. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Jang, S.Y.; Park, E.C.; Cho, A.R.; Shim, J.Y.; Linton, J.A. Long sleep duration is associated with sarcopenia in Korean adults based on data from the 2008–2011 KNHANES. J. Clin. Sleep Med. 2017, 13, 1097–1104. [Google Scholar] [CrossRef]
- Hu, X.Y.; Jiang, J.J.; Wang, H.Z.; Zhang, L.; Dong, B.R.; Yang, M. Association between sleep duration and sarcopenia among community-dwelling older adults A cross-sectional study. Medicine 2017, 96, e6268. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanizawa, K.; Tachikawa, R.; Murase, K.; Minami, T.; Inouchi, M.; Handa, T.; Oga, T.; Hirai, T.; Chin, K. Associations of obstructive sleep apnea with truncal skeletal muscle mass and density. Sci. Rep. 2018, 8, 6550. [Google Scholar] [CrossRef]
- Rubio-Arias, J.A.; Rodriguez-Fernandez, R.; Andreu, L.; Martinez-Aranda, L.M.; Martinez-Rodriguez, A.; Ramos-Campo, D.J. Effect of sleep quality on the prevalence of sarcopenia in older adults: A systematic review with meta-Analysis. J. Clin. Med. 2019, 8, 2156. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Takahashi, J.S.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; Fitzgerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef]
- Andrews, J.L.; Zhang, X.; McCarthy, J.J.; McDearmon, E.L.; Hornberger, T.A.; Russell, B.; Campbell, K.S.; Arbogast, S.; Reid, M.B.; Walker, J.R.; et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc. Natl. Acad. Sci. USA 2010, 107, 19090–19095. [Google Scholar] [CrossRef]
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Bienso, R.S.; Tagliazucchi, G.M.; Patel, V.R.; Forcato, M.; Paz, M.I.; Gudiksen, A.; Solagna, F.; et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41. [Google Scholar] [CrossRef]
- Nakao, R.; Shimba, S.; Oishi, K. Muscle Bmal1 is dispensable for the progress of neurogenic muscle atrophy in mice. J. Circadian Rhythm. 2016, 14, 1–7. [Google Scholar] [CrossRef]
- Leong, I. Muscle circadian clock regulates lipid storage. Nat. Rev. Endocrinol. 2018, 14, 563. [Google Scholar] [CrossRef]
- Dyar, K.A.; Hubert, M.J.; Mir, A.A.; Ciciliot, S.; Lutter, D.; Greulich, F.; Quagliarini, F.; Kleinert, M.; Fischer, K.; Eichmann, T.O.; et al. Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol. 2018, 16, e2005886. [Google Scholar] [CrossRef]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef]
- Broskey, N.T.; Greggio, C.; Boss, A.; Boutant, M.; Dwyer, A.; Schlueter, L.; Hans, D.; Gremion, G.; Kreis, R.; Boesch, C.; et al. Skeletal muscle mitochondria in the elderly: Effects of physical fitness and exercise training. J. Clin. Endocrinol. Metab. 2014, 99, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A. Mitophagy as a new therapeutic target for sarcopenia. Acta Physiol. 2019, 225, e13219. [Google Scholar] [CrossRef]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Van Moorsel, D.; Hansen, J.; Havekes, B.; Scheer, F.; Jorgensen, J.A.; Hoeks, J.; Schrauwen-Hinderling, V.B.; Duez, H.; Lefebvre, P.; Schaper, N.C.; et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol. Metab. 2016, 5, 635–645. [Google Scholar] [CrossRef]
- De Goede, P.; Wefers, J.; Brombacher, E.C.; Schrauwen, P.; Kalsbeek, A. Circadian rhythms in mitochondrial respiration. J. Mol. Endocrinol. 2018, 60, R115–R130. [Google Scholar] [CrossRef]
- Pastore, S.; Hood, D.A. Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. J. Appl. Physiol. (1985) 2013, 114, 1076–1084. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Liu, T.; Borjigin, J.; Lin, J.D. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 2007, 447, 477–481. [Google Scholar] [CrossRef]
- Saracino, P.G.; Rossetti, M.L.; Steiner, J.L.; Gordon, B.S. Hormonal regulation of core clock gene expression in skeletal muscle following acute aerobic exercise. Biochem. Biophys. Res. Commun. 2019, 508, 871–876. [Google Scholar] [CrossRef]
- Reebs, S.G.; Mrosovsky, N. Effects of induced wheel running on the circadian activity rhythms of Syrian hamsters: Entrainment and phase response curve. J. Biol. Rhythm. 1989, 4, 39–48. [Google Scholar] [CrossRef]
- Edgar, D.M.; Dement, W.C. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am. J. Physiol. 1991, 261, R928–R933. [Google Scholar] [CrossRef]
- Marchant, E.G.; Mistlberger, R.E. Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol. Behav. 1996, 60, 657–663. [Google Scholar] [CrossRef]
- Van Reeth, O.; Sturis, J.; Byrne, M.M.; Blackman, J.D.; L’Hermite-Baleriaux, M.; Leproult, R.; Oliner, C.; Refetoff, S.; Turek, F.W.; Van Cauter, E. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am. J. Physiol. 1994, 266, E964–E974. [Google Scholar] [CrossRef]
- Eastman, C.I.; Hoese, E.K.; Youngstedt, S.D.; Liu, L. Phase-shifting human circadian rhythms with exercise during the night shift. Physiol. Behav. 1995, 58, 1287–1291. [Google Scholar] [CrossRef]
- Buxton, O.M.; Frank, S.A.; L’Hermite-Baleriaux, M.; Leproult, R.; Turek, F.W.; Van Cauter, E. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am. J. Physiol. 1997, 273, E536–E542. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hashimoto, S.; Masubuchi, S.; Honma, S.; Honma, K.I. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R197–R205. [Google Scholar] [CrossRef]
- Baehr, E.K.; Eastman, C.I.; Revelle, W.; Olson, S.H.; Wolfe, L.F.; Zee, P.C. Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1542–R1550. [Google Scholar] [CrossRef]
- Buxton, O.M.; Lee, C.W.; L’Hermite-Baleriaux, M.; Turek, F.W.; Van Cauter, E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R714–R724. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Hashimoto, S.; Takasu, N.N.; Tanahashi, Y.; Nishide, S.Y.; Honma, S.; Honma, K. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1112–R1121. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Kline, C.E.; Elliott, J.A.; Zielinski, M.R.; Devlin, T.M.; Moore, T.A. Circadian phase-shifting effects of bright light, exercise, and bright light + exercise. J. Circadian Rhythm. 2016, 14, 2. [Google Scholar] [CrossRef]
- Thomas, J.M.; Kern, P.A.; Bush, H.M.; McQuerry, K.J.; Black, W.S.; Clasey, J.L.; Pendergast, J.S. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Reilly, T. Circadian variation in sports performance. Sports Med. 1996, 21, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.T.; O’Brien, M. Proceedings: Diurnal variation in athletic performance. J. Physiol. 1974, 236, 51P. [Google Scholar] [PubMed]
- Reilly, T.; Baxter, C. Influence of time of day on reactions to cycling at a fixed high intensity. Br. J. Sports Med. 1983, 17, 128–130. [Google Scholar] [CrossRef]
- Hill, D.W.; Borden, D.O.; Darnaby, K.M.; Hendricks, D.N.; Hill, C.M. Effect of time of day on aerobic and anaerobic responses to high-intensity exercise. Can. J. Sport Sci. 1992, 17, 316–319. [Google Scholar]
- Lundby, C.; Jacobs, R.A. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, J.H.; Seo, D.Y.; Han, J.; Jung, S.J.; Kwak, H.B. Effects of acute exercise on mitochondrial function, dynamics, and mitophagy in rat cardiac and skeletal muscles. Int. Neurourol. J. 2019, 23, S22–S31. [Google Scholar] [CrossRef]
- Sedliak, M.; Finni, T.; Peltonen, J.; Hakkinen, K. Effect of time-of-day-specific strength training on maximum strength and EMG activity of the leg extensors in men. J. Sports Sci. 2008, 26, 1005–1014. [Google Scholar] [CrossRef]
- Sedliak, M.; Finni, T.; Cheng, S.; Lind, M.; Hakkinen, K. Effect of time-of-day-specific strength training on muscular hypertrophy in men. J. Strength Cond. Res. 2009, 23, 2451–2457. [Google Scholar] [CrossRef]
- Chtourou, H.; Souissi, N. The effect of training at a specific time of day: A review. J. Strength Cond. Res. 2012, 26, 1984–2005. [Google Scholar] [CrossRef]
- Kuusmaa, M.; Schumann, M.; Sedliak, M.; Kraemer, W.J.; Newton, R.U.; Malinen, J.P.; Nyman, K.; Hakkinen, A.; Hakkinen, K. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl. Physiol. Nutr. Metab. 2016, 41, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Yoon, C.S.; Dizon, L.A.; Lee, S.R.; Youm, J.B.; Yang, W.S.; Kwak, H.B.; Ko, T.H.; Kim, H.K.; Han, J.; et al. Circadian modulation of the cardiac proteome underpins differential adaptation to morning and evening exercise training: An LC-MS/MS analysis. Pflug. Arch. 2020, 472, 259–269. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).