Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders—An Introduction to the OBERON Project
Abstract
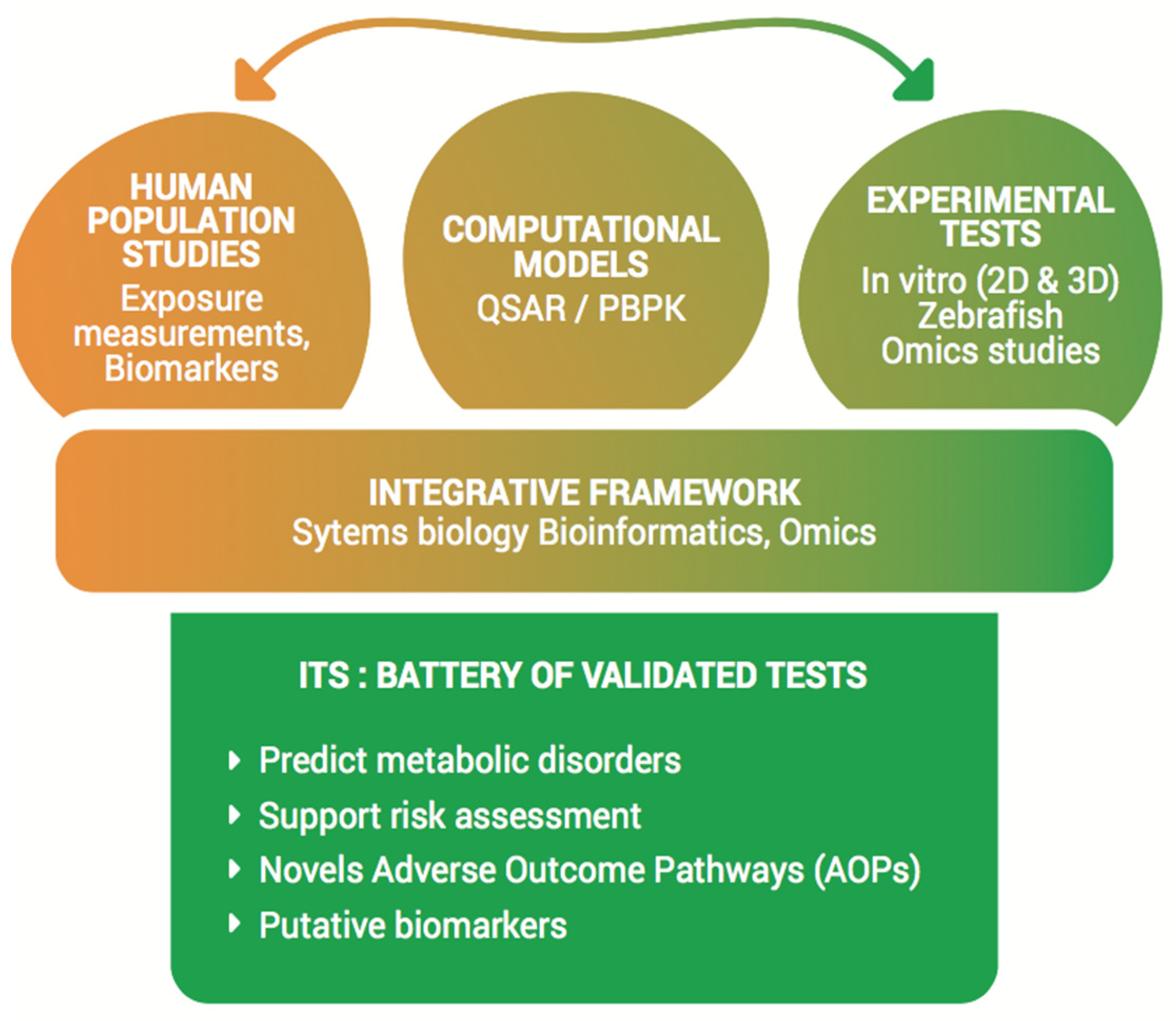
1. Introduction
2. The EU Initiative
3. The OBERON Project
3.1. Objective 1: Integration of Epidemiology and Human Biomonitoring Studies with ED Test Systems for Metabolic Disorders
3.2. Objective 2: Development of Whole Organism Test Systems to Identify EDs Implicated in Metabolic Disorders
- Receptor tests for obesogens. Obesogens are a specific class of EDs that promote obesity by altering adipocyte tissue development, lipid homeostasis, and hormonal physiology. It was recently demonstrated that obesogens may exert some of their biological effects via a nuclear receptor (NR)-dependent pathway, providing a direct molecular link between environmental ED exposure, endocrine-disrupting effects, and adipose tissue development [16]. Obesogens increase obesity through a variety of potential mechanisms, including the activation of peroxisome proliferator-activated receptor gamma (PPARγ), a key regulator of adipogenesis [17,18]. Assessing the activation of NR by EDs is certainly relevant in a screening strategy. Pathways important for adipogenesis and lipid metabolism are conserved between mammals and teleost fish [19]. In OBERON, a cell line stably transfected with zebrafish PPAR subtypes and luciferase as a reporter gene will be used in order to quantify the biological activity of substances towards zebrafish PPAR subtypes. These data will be compared with already published data, e.g., the literature, databases such as ToxCast, to identify potential similarities or differences between zebrafish and humans.
- The zebrafish obesogenic test. Activation of PPARγ is not sufficient to classify a compound as an obesogen. We therefore need a complementary whole-organism mechanism-based assay for screening substances acting as potential obesogens. The semitransparent zebrafish larvae with well-developed white adipose tissue offer a unique opportunity for studying the effects of chemicals on adipocyte biology and whole-organism adiposity in a vertebrate model [20]. The use of integrative methods is of prime importance for screening substances interfering with adipogenesis. We developed a simple short-term in vivo assay called the zebrafish obesogenic test (ZOT) and used it to examine the effects of diet, drugs, and environmental contaminants, singly or in combination, on white adipose tissue (WAT) dynamics in zebrafish larvae [20]. The ZOT was proven to be very useful in characterizing obesogenic or anti-obesogenic substances including EDs [21] and their mechanism of action [22].
- The zebrafish liver disease test. Non-alcoholic fatty liver disease (NAFLD) is a subset of liver disease beginning with simple steatosis which can evolve into steatohepatitis. NAFLD constitutes a public health priority as it can ultimately progress towards more severe and irreversible stage such as cirrhosis or hepatocellular carcinoma. NAFLD is prevalent in countries that consume a western diet. However, its actual pathogenesis remains elusive and, in this context, exposure to xenobiotics, especially EDs, has been suspected [23]. Zebrafish larva liver displays similarities to human models in the assessment of chemicals effects on steatosis and its transition to steatohepatitis [24]. OBERON is involved in the optimization of NAFLD test methods using zebrafish larva in order to screen potential EDs for their involvement in liver steatosis and its transition to steatohepatitis.
3.3. Objective 3: Development of Human-Relevant In Vitro Test Systems to Identify EDs Involved in Metabolic Disorders
- The liver system. The human hepatocellular carcinoma cell line HepaRG (HepaRG) cell line is constituted of human hepatic progenitor cells which, under appropriate culture conditions, are able to give rise to two different types of cells, one expressing differentiated hepatocytes functions and the other biliary cells [27]. These two types of cells maintain significant liver functions, such as detection of xenobiotics and subsequent induction of xenobiotics-metabolizing enzymes including cytochrome P450 (CYP450) and transport activities. HepaRG cells can be grown in 2D, either in the absence or in the presence of inflammatory cells, which represents a model system to study inflammatory metabolic diseases. They can also be grown in 3D conditions, leading to organoids which better mimic in vivo conditions. The OBERON project will also use the human hepatocellular carcinoma cell line HepG2 (HepG2) cell line or the immortalized human hepatocytes (MIHA) cells, a model of immortalized human hepatocytes which have been successfully applied both in cytotoxicity and certain mechanistic studies, covering a wide range of function of liver cells. Primary cells (hepatocytes and pre-adipocytes) will also be used in order to strengthen data generated from closely related differentiated cell lines.
- The pancreas system. We will use the murine pancreatic β and α-cell lines (mouse insulinoma 6 (MIN6), alphaTC1 Clone 9 (αTC1.9)) and a human pancreatic β-cell line (EndoC-βH1). Experiments will be performed first in the murine secreting cell models, which have been previously demonstrated to be valuable tools for toxicological studies and screening purposes, and then in the human model which displays an enhanced β-cell phenotype and genomic stability over 100 passages [28].
- The adipose tissue. We will use a) human multipotent adipose-derived stem cells (hMADS), a mesenchymal stem cell line from human adipose tissue of young male and female donors which is also able to convert into functional brown-like adipocytes or b) human pre-adipocyte Simpson–Golabi–Behmel syndrome (SGBS) cell line which originates from adipose tissue and is able to proliferate for up to 50 generations with retained capacity for adipogenic differentiation. So far, these cells have been used for a number of studies on adipose differentiation, adipocyte glucose uptake, lipolysis, apoptosis, regulation of expression of adipokines, and protein translocation [29]. The cells could be efficiently differentiated in the presence of PPARγ agonists (including some EDs) and the absence of serum. Importantly, many of the cell lines used in our studies can differentiate in vitro. This will allow us the opportunity not only to address metabolic dysfunctions in differentiated cells but also to assess the capacity of EDs to alter cell differentiation, a setting that could mimic the developmental effects observed in epidemiological studies and that could be mediated by epigenetic regulation.
3.4. Objective 4: Providing Computational Models to Help Prioritization of EDs
- Quantitative structure-activity relationship (QSAR) models have a valuable predictive potential in the field of endocrine disruption because of the crucial role played by the molecular interactions between nuclear receptors and chemicals in defining and triggering the initiating events of endocrine disruption [33,34]. From a practical and regulatory point of view, the relevance of structure-activity approaches in identifying chemicals associated with a disrupting hazard has been especially and extensively established for the estrogen receptor for a wide range of chemical classes, proving the reliability of QSAR models as predictive tools for endocrine disruption in priority setting contexts [35]. In OBERON, existing QSAR models will be optimized based on new experimental data generated in the project (Objectives 2 and 3) and from literature to characterize ED induced effects on metabolism and obesity. Novel QSAR models will also be established based on (1) priorities identified by AOP network investigations (Objective 5), and (2) events already identified, such as the thyroid function and nuclear receptors. The integration of multiple models will also be explored in a weight of evidence strategy. All QSAR models will be implemented within the VEGA platform (https://www.vegahub.eu/).
- Physiologically based pharmacokinetic (PBPK) models are continuously gaining ground in regulatory toxicology, describing in quantitative terms, the absorption, metabolism, distribution, and elimination (ADME) processes in the human body, with a focus on the effective dose at the expected target site. The need for the widespread use of PBPK models development is amplified by the continuously increasing scientific and regulatory interest about aggregate and cumulative exposure; PBPK models translate external exposures from multiple routes into internal exposure metrics, addressing the effects of exposure route in the overall bioavailability. These models also address increased vulnerability to EDs during the critical developmental windows of susceptibility, such as embryonic and fetal stages, and childhood [36]. This vulnerability could be due to the epigenetic remodeling occurring during such periods, highlighting the string relevance of epigenetic regulations. It could also be due to the developmental physiology and exploratory behaviors of children, but also to the immaturity of their xenobiotic detoxifying processes or different adiposity during the early stages of development. Within OBERON, we will develop and apply human PBPK models for EDs that cover the whole life of an individual including prenatal life. QSAR models will be developed to support the parametrization of the PBPK models for some ADME processes, e.g., partitioning into the tissues, metabolic clearance, and placental transfers. PBPK models will be used to reconstruct complex exposures for the various EDs from biomonitoring data (Objective 1), allowing us to estimate the internal dose of real-life EDs exposures both on the organism level and in target tissues. This is of particular importance considering the complex toxicokinetic and toxicodynamic behavior of EDs that influences their bioavailability and toxic potency. This approach will also allow reconstructing the exposure in-between biomarker measurement time points, especially at critical periods. The knowledge of the internal exposures in the target tissues will be critical for extrapolating effects observed in the in vitro systems (Objective 3) to humans [37,38]. Dedicated toxicokinetic models for the in vitro systems used in OBERON will be built in order to provide cellular concentrations to be used for dose-response or systems biology modeling and integration within AOPs (Objective 5).
3.5. Objective 5: Establishment of an ITS and Capture of Mechanistic Effects of EDs on Metabolic Disorders
- Metabolomics, including lipidomics analysis, will be carried out in human, in vivo (zebrafish) and in vitro samples. Metabolomics represents an effective approach for determining the activation of multiple AOPs and expanding knowledge on these AOPs relevant to complex exposures. This includes the identification of previously undefined key events by measuring changes in endogenous metabolites involved in a wide variety of biochemical pathways and associating these changes with exposure to combinations of xenobiotics. Existing protocols for human plasma, in vivo, and cellular model extracts will be used, with state-of-the-art equipment that includes gas chromatography-high resolution mass spectrometry (GC-HRMS) and liquid chromatography-high resolution mass spectrometry (LC-HRMS) with complementary instrumentation as well as nuclear magnetic resonance (NMR). These multiple platforms will allow us to capture an extended array of endogenous metabolites and to accurately define the related metabolic pathways. Data processing and analysis will be done using R packages and available databases.
4. State of the Project and Expected Outcomes
- -
- Delivering an innovative screening battery based on new and robust tests combining experimental and computational strategies for ED-related metabolic disorders assessment, to support current OECD tiered approaches.
- -
- Providing a validated tiered IATA (i.e., a decision tree) ready for submission to JRC with the aim to reduce the use of animal testing in industries and regulatory agencies.
- -
- Improving mechanistic toxicological knowledge and integrating them into AOPs with the aim of improving risk assessment frameworks for human health effects. This will feed into the AOP-wiki and the OECD AOP approach.
- -
- Providing putative effect markers for metabolic disorders such as new evidence on the potential metabolic and metabolomic effects of EDs in human populations.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3R | Replacement, reduction, and refinement |
| αTC1.9 | AlphaTC1 Clone 9 |
| ADME | Absorption, distribution, metabolism and elimination |
| AO | Adverse outcome |
| AOP | Adverse outcome pathway |
| BMI | Body mass index |
| BPA | Bisphenol A |
| BPF | Bisphenol F |
| BPS | Bisphenol S |
| CYP450 | Cytochrome P450 |
| DDE | Dichlorodiphenyldichloroethylene |
| DEHP | Di-(2-ethylhexyl)phthalate |
| DG | Directorate-general |
| ED | Endocrine disruptor |
| ECHA | European chemical agency |
| EFSA | European food safety authority |
| EPAA | European partnership for alternative approaches to animal testing |
| EndoC-βH1 | Human pancreatic β cell line EndoC-βH1 |
| EU | European Union |
| GC-HRMS | Gas chromatography-high resolution mass spectrometry |
| HepaRG | Human hepatocellular carcinoma cell line HepaRG |
| HepG2 | Human hepatocellular carcinoma cell line HepG2 |
| hMADS | Human multipotent adipose-derived stem |
| IATA | Integrated approach for testing and assessment |
| IQ | Intelligence quotient |
| ITS | Integrated testing strategy |
| JRC | Joint Research Center |
| KE | Key event |
| KER | Key event relationship |
| LC-HRMS | Liquid chromatography-high resolution mass spectrometry |
| MIE | Molecular initiating event |
| MIHA | Immortalized Human Hepatocytes |
| MIN6 | Mouse insulinoma 6 |
| NAFLD | Non-alcoholic fatty liver disease |
| NMR | Nuclear magnetic resonance |
| NR | Nuclear receptor |
| NRC | National Research Center |
| OECD | Organization for economic co-operation and development |
| PBPK | Physiologically based pharmacokinetic |
| PK | Pharmacokinetic |
| PoT | Pathway of toxicity |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| qPCR | Quantitative polymerase chain reaction |
| QSAR | Quantitative structure activity relationships |
| REACH | Registration, evaluation and authorization of chemicals |
| SGBS | Simpson-Golabi-Behmel syndrome |
| US | United Sates |
| WAT | White adipose tissue |
| ZOT | Zebrafish obesogenic test |
References
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; DiGangi, J.; Bellanger, M.; Hauser, R.; Legler, J.; et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J. Clin. Endocrinol. Metab. 2015, 100, 1245–1255. [Google Scholar] [CrossRef]
- Legler, J.; Fletcher, T.; Govarts, E.; Porta, M.; Blumberg, B.; Heindel, J.J.; Trasande, L. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1278–1288. [Google Scholar] [CrossRef]
- Gibb, S. Toxicity testing in the 21st century: A vision and a strategy. Reprod. Toxicol. 2008, 1, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Vrijheid, M.; Casas, M.; Gascon, M.; Valvi, D.; Nieuwenhuijsen, M. Environmental pollutants and child health-A review of recent concerns. Int. J. Hyg. Environ. Health 2016, 219, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Casas, M.; Basagaña, X.; Sakhi, A.K.; Haug, L.S.; Philippat, C.; Granum, B.; Manzano-Salgado, C.B.; Brochot, C.; Zeman, F.; de Bont, J.; et al. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ. Int. 2018, 121, 561–573. [Google Scholar] [CrossRef]
- Vernet, C.; Philippat, C.; Agier, L.; Calafat, A.M.; Ye, X.; Lyon-Caen, S.; Hainaut, P.; Siroux, V.; Schisterman, E.F.; Slama, R. An Empirical Validation of the Within-subject Biospecimens Pooling Approach to Minimize Exposure Misclassification in Biomarker-based Studies. Epidemiology 2019, 30, 756–767. [Google Scholar] [CrossRef]
- Guxens, M.; Ballester, F.; Espada, M.; Fernández, M.F.; Grimalt, J.O.; Ibarluzea, J.; Olea, N.; Rebagliato, M.; Tardón, A.; Torrent, M.; et al. Cohort Profile: The INMA—INfancia y Medio Ambiente—(Environment and Childhood) Project. Int. J. Epidemiol. 2012, 41, 930–940. [Google Scholar] [CrossRef]
- Vrijheid, M.; Slama, R.; Robinson, O.; Chatzi, L.; Coen, M.; van den Hazel, P.; Thomsen, C.; Wright, J.; Athersuch, T.J.; Avellana, N.; et al. The human early-life exposome (HELIX): Project rationale and design. Environ. Health Perspect. 2014, 122, 535–544. [Google Scholar] [CrossRef]
- Chevrier, C.; Limon, G.; Monfort, C.; Rouget, F.; Garlantézec, R.; Petit, C.; Durand, G.; Cordier, S. Urinary biomarkers of prenatal atrazine exposure and adverse birth outcomes in the PELAGIE birth cohort. Environ. Health Perspect. 2011, 119, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Lyon-Caen, S.; Siroux, V.; Lepeule, J.; Lorimier, P.; Hainaut, P.; Mossuz, P.; Quentin, J.; Supernant, K.; Meary, D.; Chaperot, L.; et al. Deciphering the Impact of Early-Life Exposures to Highly Variable Environmental Factors on Foetal and Child Health: Design of SEPAGES Couple-Child Cohort. Int. J. Environ. Res. Public Health 2019, 16, 3888. [Google Scholar] [CrossRef] [PubMed]
- Misso, G.; Zarone, M.R.; Grimaldi, A.; Di Martino, M.T.; Lombardi, A.; Kawasaki, H.; Stiuso, P.; Tassone, P.; Tagliaferri, P.; Caraglia, M. Non Coding RNAs: A New Avenue for the Self-Tailoring of Blood Cancer Treatment. Curr. Drug Targets 2017, 18, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Saydmohammed, M.; Tsang, M. High-Throughput Automated Chemical Screens in Zebrafish. Methods Mol. Biol. 2018, 1683, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.A.; Rennekamp, A.J.; Peterson, R.T. Chemical Screening in Zebrafish. Methods Mol. Biol. 2016, 1451, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.; Blumberg, B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res. C Embryo Today Rev. 2011, 93, 34–50. [Google Scholar] [CrossRef]
- Janesick, A.; Blumberg, B. Minireview: PPARγ as the target of obesogens. J. Steroid Biochem. Mol. Biol. 2011, 127, 4–8. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Babin, P.J.; Gibbons, G.F. The evolution of plasma cholesterol: Direct utility or a “spandrel” of hepatic lipid metabolism? Prog. Lipid Res. 2009, 48, 73–91. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Ouadah, N.; Babin, P.J. Zebrafish obesogenic test: A tool for screening molecules that target adiposity. J. Lipid Res. 2011, 52, 1765–1772. [Google Scholar] [CrossRef]
- Lutfi, E.; Babin, P.J.; Gutiérrez, J.; Capilla, E.; Navarro, I. Caffeic acid and hydroxytyrosol have anti-obesogenic properties in zebrafish and rainbow trout models. PLoS ONE 2017, 12, e0178833. [Google Scholar] [CrossRef] [PubMed]
- Ouadah-Boussouf, N.; Babin, P.J. Pharmacological evaluation of the mechanisms involved in increased adiposity in zebrafish triggered by the environmental contaminant tributyltin. Toxicol. Appl. Pharmacol. 2016, 294, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Foulds, C.E.; Treviño, L.S.; York, B.; Walker, C.L. Endocrine-disrupting chemicals and fatty liver disease. Nat. Rev. Endocrinol. 2017, 13, 445–457. [Google Scholar] [CrossRef]
- Bucher, S.; Tête, A.; Podechard, N.; Liamin, M.; Le Guillou, D.; Chevanne, M.; Coulouarn, C.; Imran, M.; Gallais, I.; Fernier, M.; et al. Co-exposure to benzo[a]pyrene and ethanol induces a pathological progression of liver steatosis in vitro and in vivo. Sci. Rep. 2018, 8, 5963. [Google Scholar] [CrossRef]
- Burden, N.; Chapman, K.; Sewell, F.; Robinson, V. Pioneering better science through the 3Rs: An introduction to the national centre for the replacement, refinement, and reduction of animals in research (NC3Rs). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 198–208. [Google Scholar]
- Benam, K.H.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K.-J.; Karalis, K.; Kim, H.J.; MacQueen, L.; Mahmoodian, R.; et al. Engineered in vitro disease models. Annu. Rev. Pathol. 2015, 10, 195–262. [Google Scholar] [CrossRef]
- Anthérieu, S.; Chesné, C.; Li, R.; Guguen-Guillouzo, C.; Guillouzo, A. Optimization of the HepaRG cell model for drug metabolism and toxicity studies. Toxicol. In Vitro 2012, 26, 1278–1285. [Google Scholar] [CrossRef]
- Ravassard, P.; Hazhouz, Y.; Pechberty, S.; Bricout-Neveu, E.; Armanet, M.; Czernichow, P.; Scharfmann, R. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J. Clin. Investig. 2011, 121, 3589–3597. [Google Scholar] [CrossRef]
- Rodriguez, A.-M.; Elabd, C.; Amri, E.-Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128. [Google Scholar] [CrossRef]
- Daston, G.; Knight, D.J.; Schwarz, M.; Gocht, T.; Thomas, R.S.; Mahony, C.; Whelan, M. SEURAT: Safety Evaluation Ultimately Replacing Animal Testing--recommendations for future research in the field of predictive toxicology. Arch. Toxicol. 2015, 89, 15–23. [Google Scholar] [CrossRef]
- Basketter, D.A.; Clewell, H.; Kimber, I.; Rossi, A.; Blaauboer, B.; Burrier, R.; Daneshian, M.; Eskes, C.; Goldberg, A.; Hasiwa, N.; et al. A roadmap for the development of alternative (non-animal) methods for systemic toxicity testing. ALTEX 2012, 29, 3–91. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Hasiwa, N.; Rovida, C.; Daneshian, M.; Basketter, D.; Kimber, I.; Clewell, H.; Gocht, T.; Goldberg, A.; Busquet, F.; et al. Consensus report on the future of animal-free systemic toxicity testing. ALTEX 2014, 31, 341–356. [Google Scholar] [CrossRef]
- le Maire, A.; Bourguet, W.; Balaguer, P. A structural view of nuclear hormone receptor: Endocrine disruptor interactions. Cell. Mol. Life Sci. 2010, 67, 1219–1237. [Google Scholar] [CrossRef]
- Gadaleta, D.; Manganelli, S.; Roncaglioni, A.; Toma, C.; Benfenati, E.; Mombelli, E. QSAR Modeling of ToxCast Assays Relevant to the Molecular Initiating Events of AOPs Leading to Hepatic Steatosis. J. Chem. Inf. Model. 2018, 58, 1501–1517. [Google Scholar] [CrossRef]
- Bhhatarai, B.; Wilson, D.M.; Price, P.S.; Marty, S.; Parks, A.K.; Carney, E. Evaluation of OASIS QSAR Models Using ToxCastTM in Vitro Estrogen and Androgen Receptor Binding Data and Application in an Integrated Endocrine Screening Approach. Environ. Health Perspect. 2016, 124, 1453–1461. [Google Scholar] [CrossRef]
- Brochot, C.; Casas, M.; Manzano-Salgado, C.; Zeman, F.A.; Schettgen, T.; Vrijheid, M.; Bois, F.Y. Prediction of maternal and foetal exposures to perfluoroalkyl compounds in a Spanish birth cohort using toxicokinetic modelling. Toxicol. Appl. Pharmacol. 2019, 379, 114640. [Google Scholar] [CrossRef]
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A.; et al. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects-2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef] [PubMed]
- Bessems, J.G.; Loizou, G.; Krishnan, K.; Clewell, H.J.; Bernasconi, C.; Bois, F.; Coecke, S.; Collnot, E.-M.; Diembeck, W.; Farcal, L.R.; et al. PBTK modelling platforms and parameter estimation tools to enable animal-free risk assessment: Recommendations from a joint EPAA--EURL ECVAM ADME workshop. Regul. Toxicol. Pharmacol. 2014, 68, 119–139. [Google Scholar] [CrossRef]
- Manrai, A.K.; Cui, Y.; Bushel, P.R.; Hall, M.; Karakitsios, S.; Mattingly, C.J.; Ritchie, M.; Schmitt, C.; Sarigiannis, D.A.; Thomas, D.C.; et al. Informatics and Data Analytics to Support Exposome-Based Discovery for Public Health. Annu. Rev. Public Health 2017, 38, 279–294. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Zhang, Q.; Carmichael, P.L.; Boekelheide, K.; Andersen, M.E. Toxicity testing in the 21 century: Defining new risk assessment approaches based on perturbation of intracellular toxicity pathways. PLoS ONE 2011, 6, e20887. [Google Scholar] [CrossRef] [PubMed]
- Vitkina, T.I.; Yankova, V.I.; Gvozdenko, T.A.; Kuznetsov, V.L.; Krasnikov, D.V.; Nazarenko, A.V.; Chaika, V.V.; Smagin, S.V.; Tsatsakis, A.Μ.; Engin, A.B.; et al. The impact of multi-walled carbon nanotubes with different amount of metallic impurities on immunometabolic parameters in healthy volunteers. Food Chem. Toxicol. 2016, 87, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D. 4.6 Toxicogenomics and biology-based modeling framework for health risk assessment. Hum. Exp. Toxicol. 2009, 28, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B.; Langefeld, C.D.; Olivier, M.; Cox, L.A. Integrated Omics: Tools, Advances, and Future Approaches. J. Mol. Endocrinol. 2019, 62, R21–R45. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.; Noyes, P.D.; Casey, W.M.; Dix, D.J. Application of Adverse Outcome Pathways to U.S. EPA’s Endocrine Disruptor Screening Program. Environ. Health Perspect. 2017, 125, 096001. [Google Scholar] [CrossRef]
- Carvaillo, J.-C.; Barouki, R.; Coumoul, X.; Audouze, K. Linking Bisphenol S to Adverse Outcome Pathways Using a Combined Text Mining and Systems Biology Approach. Environ. Health Perspect. 2019, 127, 47005. [Google Scholar] [CrossRef]
- Rugard, M.; Coumoul, X.; Carvaillo, J.-C.; Barouki, R.; Audouze, K. Deciphering Adverse Outcome Pathway Network Linked to Bisphenol F Using Text Mining and Systems Toxicology Approaches. Toxicol. Sci. 2020, 173, 32–40. [Google Scholar] [CrossRef]
- Leist, M.; Ghallab, A.; Graepel, R.; Marchan, R.; Hassan, R.; Bennekou, S.H.; Limonciel, A.; Vinken, M.; Schildknecht, S.; Waldmann, T.; et al. Adverse outcome pathways: Opportunities, limitations and open questions. Arch. Toxicol. 2017, 91, 3477–3505. [Google Scholar] [CrossRef]
- Audouze, K.; Juncker, A.S.; Roque, F.J.S.S.A.; Krysiak-Baltyn, K.; Weinhold, N.; Taboureau, O.; Jensen, T.S.; Brunak, S. Deciphering diseases and biological targets for environmental chemicals using toxicogenomics networks. PLoS Comput. Biol. 2010, 6, e1000788. [Google Scholar] [CrossRef]
- Audouze, K.; Brunak, S.; Grandjean, P. A computational approach to chemical etiologies of diabetes. Sci. Rep. 2013, 3, 2712. [Google Scholar] [CrossRef]
- Aguayo-Orozco, A.; Audouze, K.; Siggaard, T.; Barouki, R.; Brunak, S.; Taboureau, O. sAOP: Linking chemical stressors to Adverse Outcomes Pathway Networks. Bioinformatics 2019, 35, 5391–5392. [Google Scholar] [CrossRef]
- Wu, Q.; Achebouche, R.; Audouze, K. Computational systems biology as an animal-free approach to characterize toxicological effects of persistent organic pollutants. ALTEX 2020, 37, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Foran, C.M.; Rycroft, T.; Keisler, J.; Perkins, E.J.; Linkov, I.; Garcia-Reyero, N. A modular approach for assembly of quantitative adverse outcome pathways. ALTEX 2019, 36, 353–362. [Google Scholar] [CrossRef] [PubMed]
| Chemical Name | Acronym | CAS Number |
|---|---|---|
| Bisphenol A | BPA | 80-05-7 |
| Bisphenol S | BPS | 80-09-1 |
| Bisphenol F | BPF | 620-92-8 |
| Di(2-ethylhexyl) phthalate | DEHP | 117-81-7 |
| Dibutyl phthalate | DBP | 84-74-2 |
| Perfluorooctanesulfonic acid | PFOS | 1763-23-1 |
| Perfluorooctanoic acid | PFOA | 335-67-1 |
| Cadmium | Cd | 7440-43-9 |
| Dichlorodiphenyldichloroethylene | DDE | 72-55-9 |
| Butyl-paraben | 94-26-8 |
| Cohort Name | Full Name and Key References | Country | Enrollment Period | No of Children at Birth |
|---|---|---|---|---|
| INMA | Environment and Childhood [9] | Spain | 2004–2007 | 2000 |
| HELIX | The human early-life exposome [10] | UK, Norway, Lithuania, France, Greece, Spain | 1999–2010 | 1300 |
| Pélagie | Endocrine disruptors: longitudinal study on pregnancy abnormalities, infertility, and childhood [11] | France | 2002–2006 | 500 |
| SEPAGES | Assessment of air pollution exposure during pregnancy and effect on health [12] | France | 2014–2017 | 500 |
| EXHES | European exposure and health examination survey (ISBN 978-3-319-89321-1) | Greece | 2017–2019 | 300 |
| ELSPAC | The European longitudinal study of pregnancy and childhood [13] | Czech Republic | 1991–1992 | 300 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audouze, K.; Sarigiannis, D.; Alonso-Magdalena, P.; Brochot, C.; Casas, M.; Vrijheid, M.; Babin, P.J.; Karakitsios, S.; Coumoul, X.; Barouki, R. Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders—An Introduction to the OBERON Project. Int. J. Mol. Sci. 2020, 21, 2988. https://doi.org/10.3390/ijms21082988
Audouze K, Sarigiannis D, Alonso-Magdalena P, Brochot C, Casas M, Vrijheid M, Babin PJ, Karakitsios S, Coumoul X, Barouki R. Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders—An Introduction to the OBERON Project. International Journal of Molecular Sciences. 2020; 21(8):2988. https://doi.org/10.3390/ijms21082988
Chicago/Turabian StyleAudouze, Karine, Denis Sarigiannis, Paloma Alonso-Magdalena, Celine Brochot, Maribel Casas, Martine Vrijheid, Patrick J. Babin, Spyros Karakitsios, Xavier Coumoul, and Robert Barouki. 2020. "Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders—An Introduction to the OBERON Project" International Journal of Molecular Sciences 21, no. 8: 2988. https://doi.org/10.3390/ijms21082988
APA StyleAudouze, K., Sarigiannis, D., Alonso-Magdalena, P., Brochot, C., Casas, M., Vrijheid, M., Babin, P. J., Karakitsios, S., Coumoul, X., & Barouki, R. (2020). Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders—An Introduction to the OBERON Project. International Journal of Molecular Sciences, 21(8), 2988. https://doi.org/10.3390/ijms21082988









