Resveratrol in Cancer Patients: From Bench to Bedside
Abstract
1. Introduction
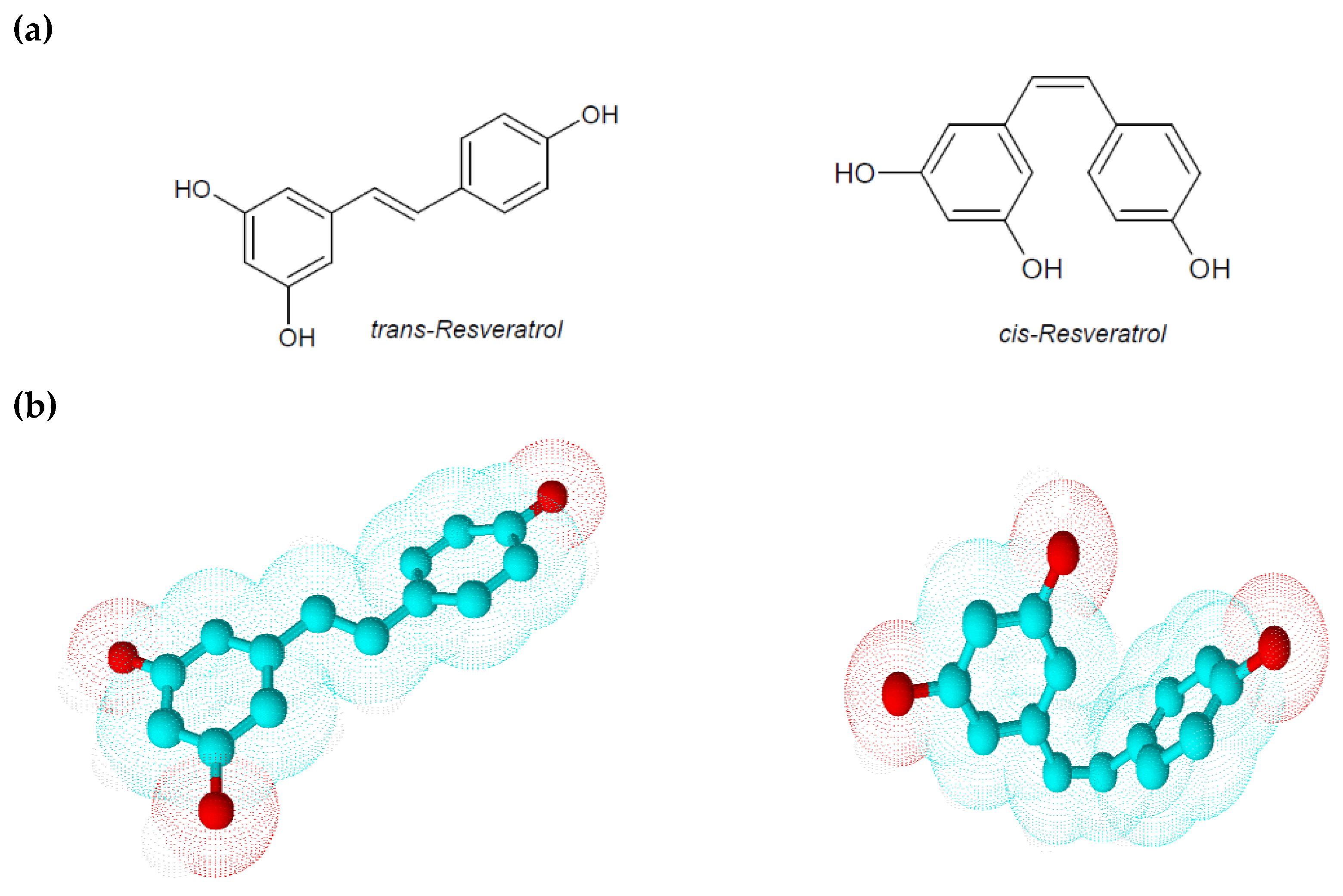
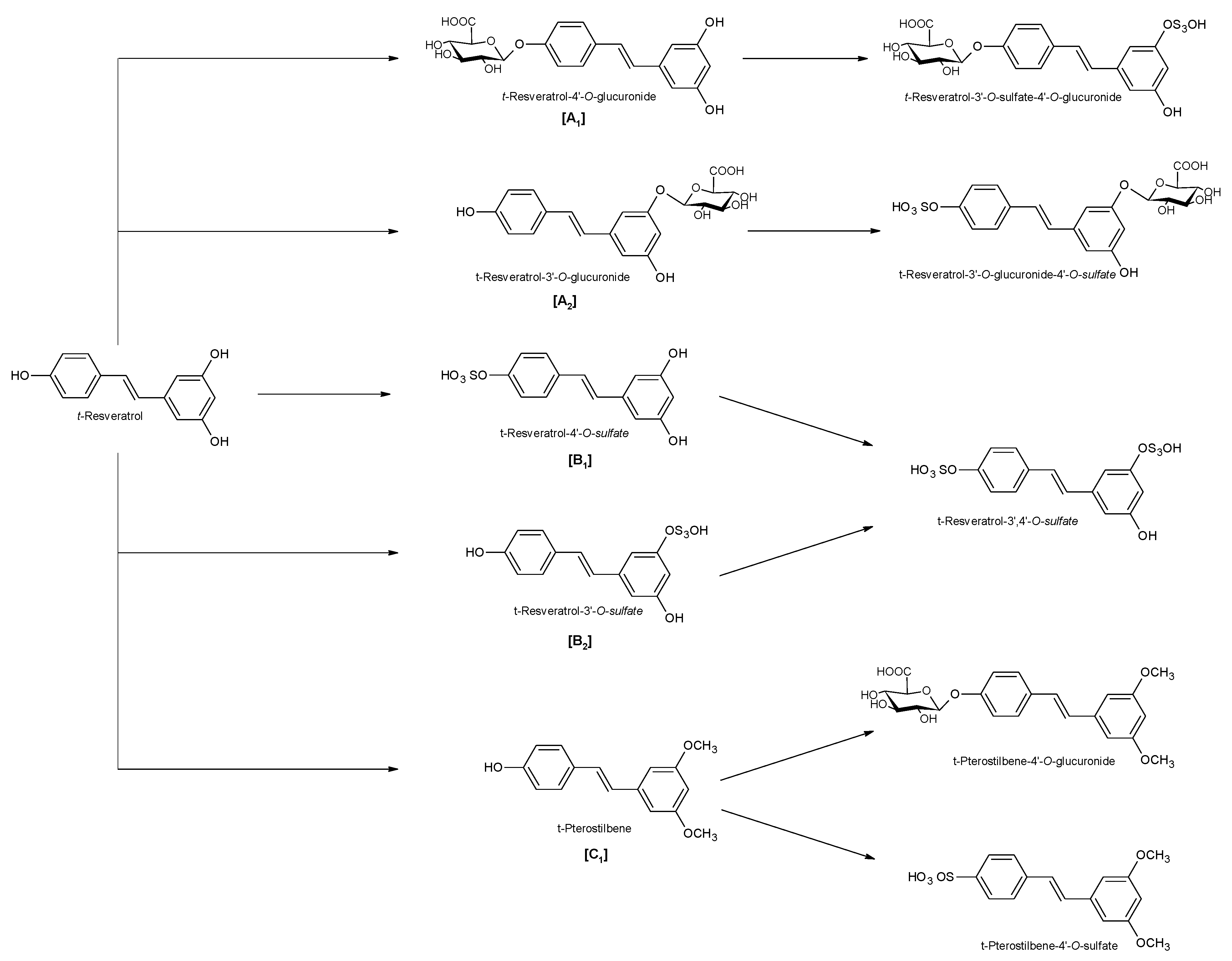
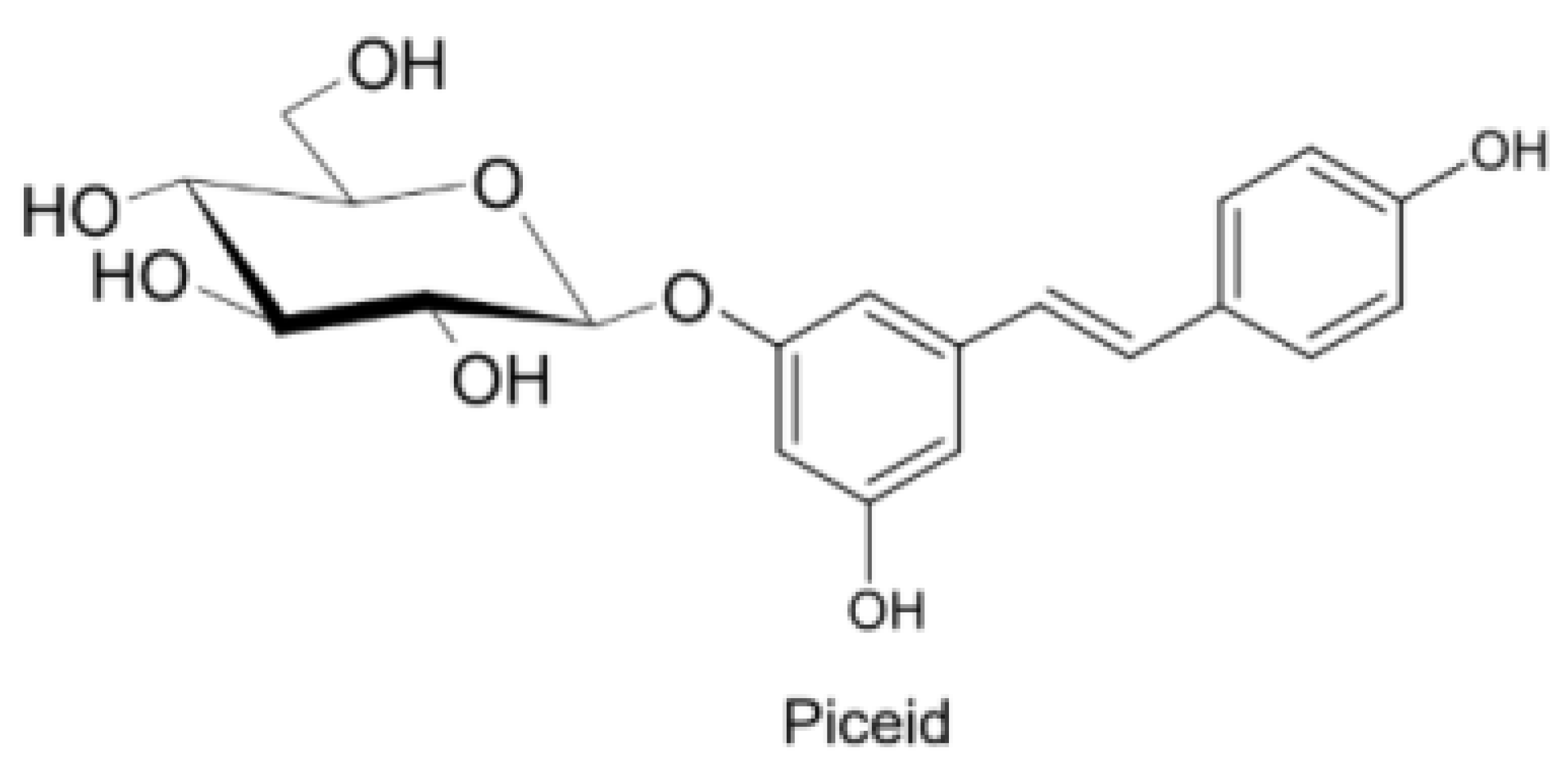
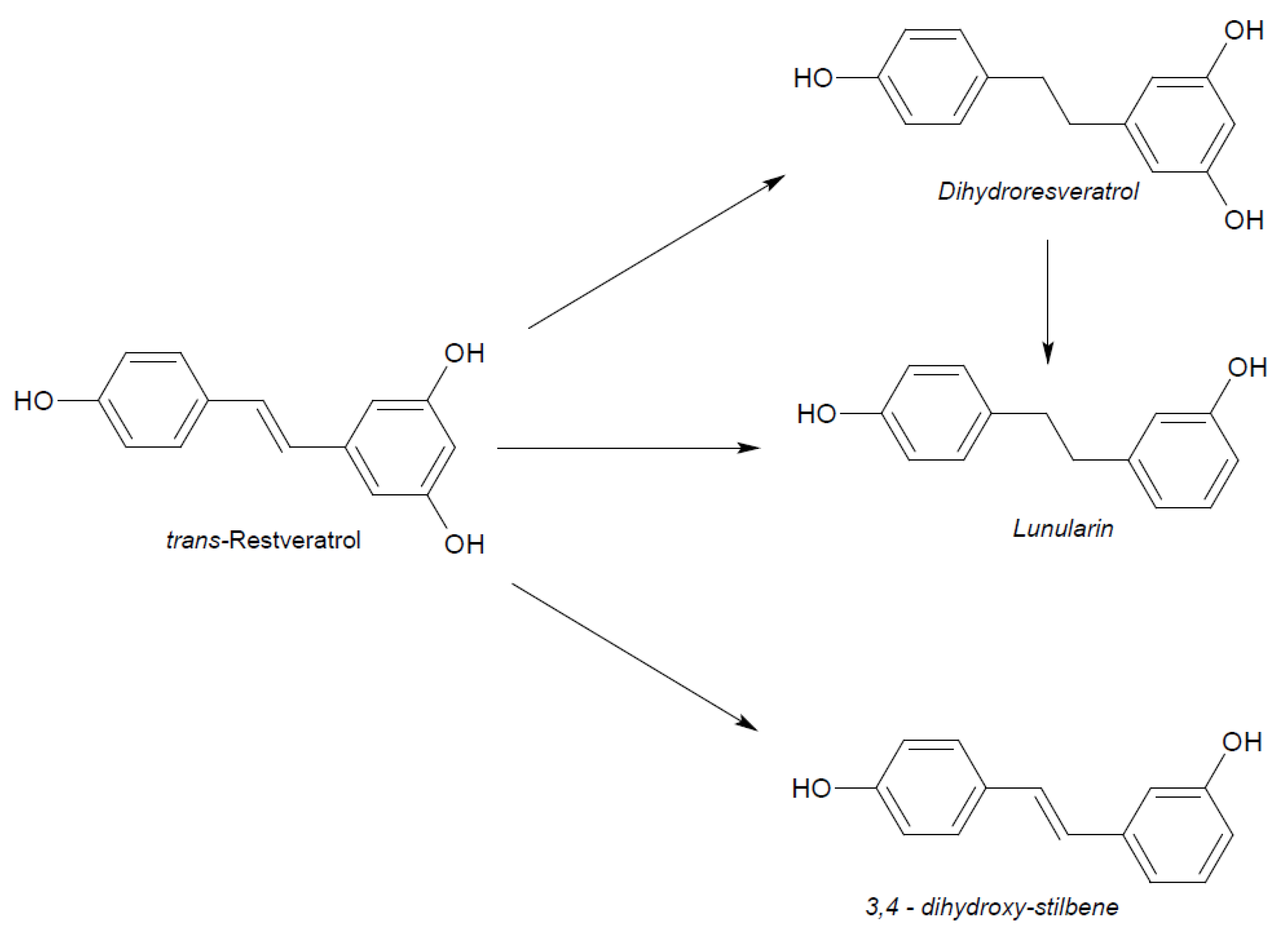
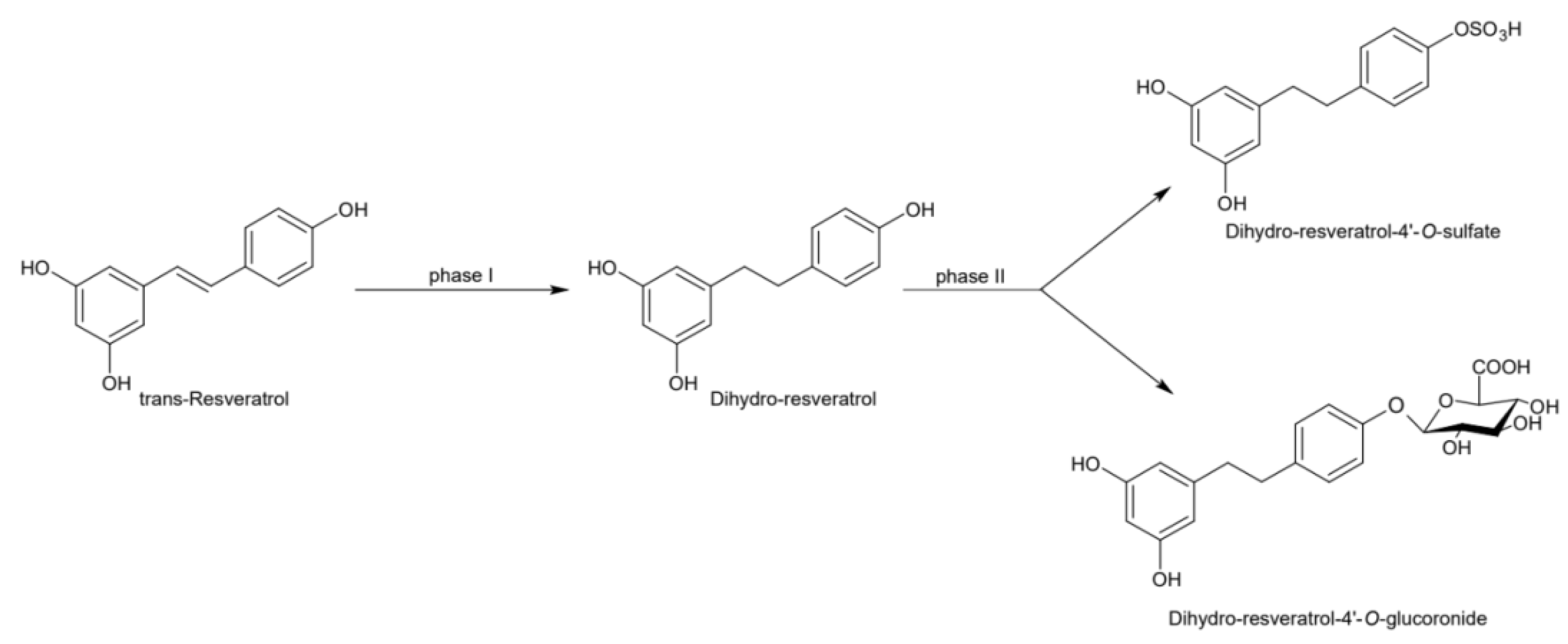
2. Physico-Chemical and Pharmacokinetic Properties of Resveratrol
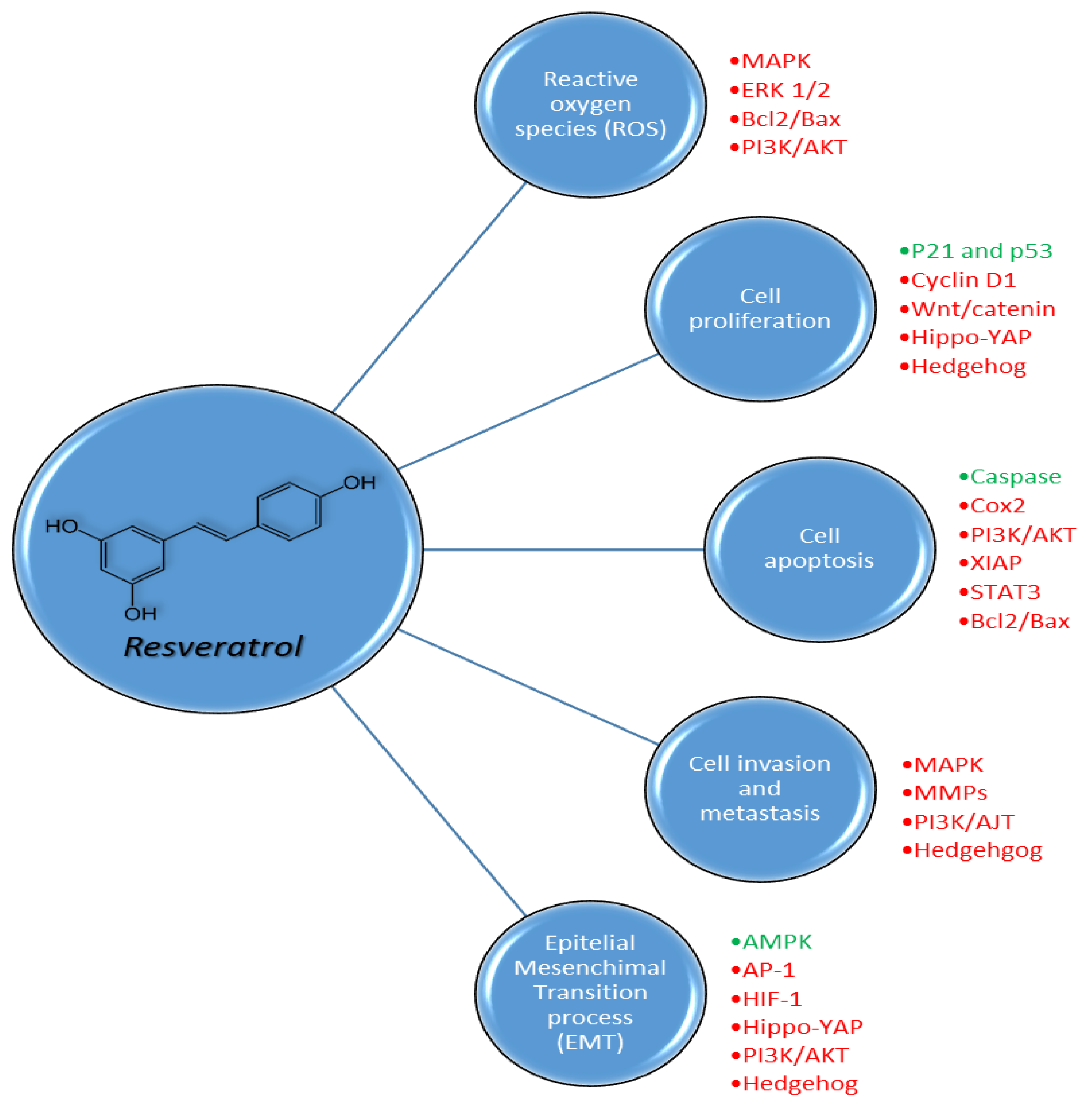
3. Pharmacodynamic Properties of Resveratrol
4. Resveratrol in Cardio-Oncology
5. Metabolic Effect and Cancer–Drug Interactions
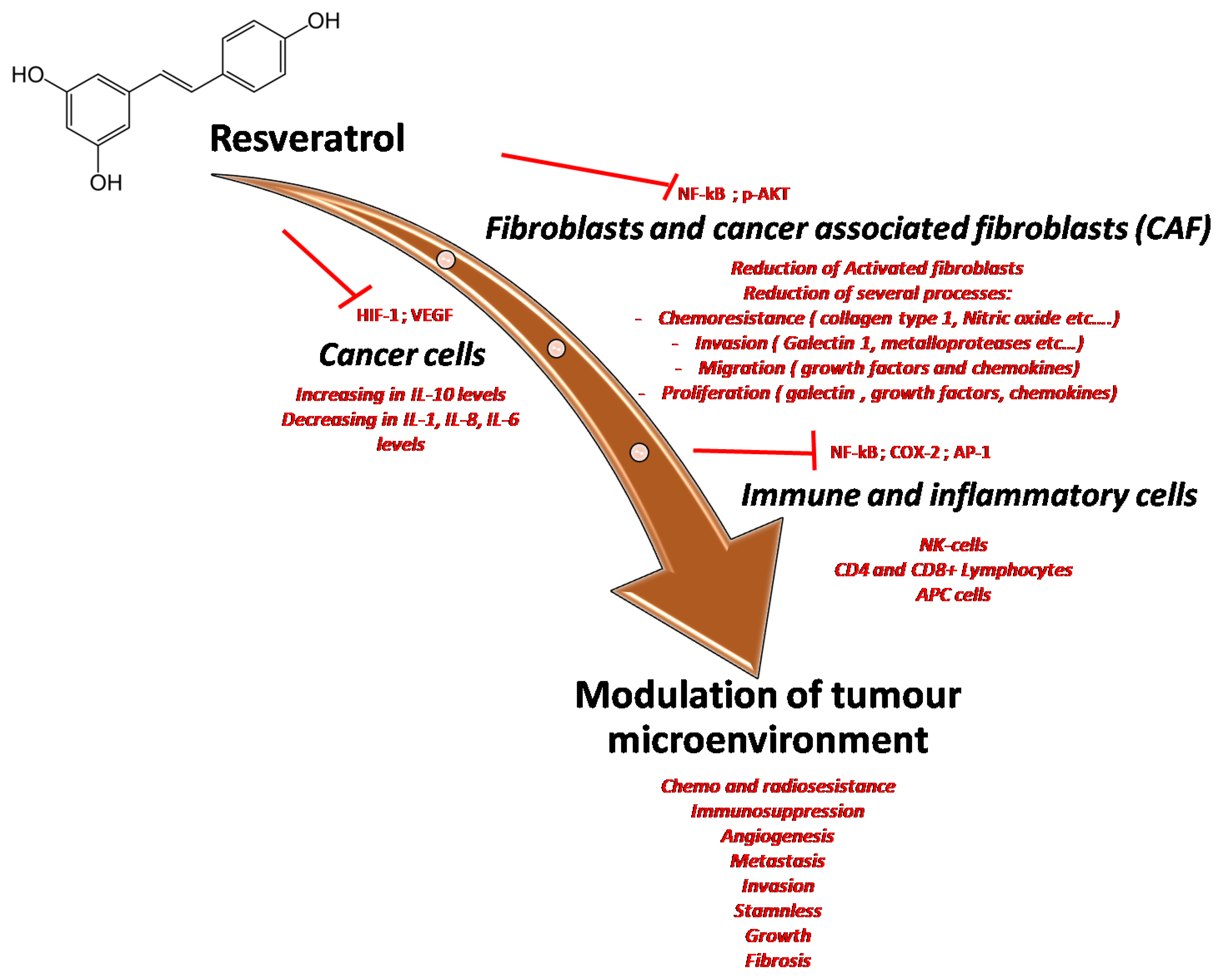
6. Resveratrol and Cancer Evidence
7. Conclusion and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef]
- Wang, F.; Chatterjee, S. Dominant Carbons in trans- and cis-Resveratrol Isomerization. J. Phys. Chem. B 2017, 121, 4745–4755. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.; Russo, N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: A quantum chemical and computational kinetics study. J. Org. Chem. 2012, 77, 3868–3877. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef]
- O’Neil, M.J. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th ed.; Royal Society of Chemistry: London, UK, 2013; ISBN 978-1-84973-670-1. [Google Scholar]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. J. Chem. Soc. Japan 1939, 60, 1090–1100. [Google Scholar]
- Arichi, H.; Kimura, Y.; Okuda, H.; Baba, K.; Kozawa, M.; Arichi, S. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem. Pharm. Bull. (Tokyo) 1982, 30, 1766–1770. [Google Scholar] [CrossRef]
- Azorín-Ortuño, M.; Yañéz-Gascón, M.J.; Pallarés, F.J.; Vallejo, F.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F.; Espín, J.C. Pharmacokinetic study of trans-resveratrol in adult pigs. J. Agric. Food Chem. 2010, 58, 11165–11171. [Google Scholar] [CrossRef]
- Scott, E.; Steward, W.P.; Gescher, A.J.; Brown, K. Resveratrol in human cancer chemoprevention--choosing the “right” dose. Mol. Nutr. Food Res. 2012, 56, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. Biol. Fate Chem. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Angelini, M.; Grass, L.; Diamandis, E.P.; Goldberg, D.M. Absorption of trans-resveratrol in rats. Methods Enzymol. 2001, 335, 145–154. [Google Scholar] [PubMed]
- Abd El-Mohsen, M.; Bayele, H.; Kuhnle, G.; Gibson, G.; Debnam, E.; Kaila Srai, S.; Rice-Evans, C.; Spencer, J.P.E. Distribution of [3H] trans-resveratrol in rat tissues following oral administration. Br. J. Nutr. 2006, 96, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Vitrac, X.; Decendit, A.; Ennamany, R.; Krisa, S.; Mérillon, J.-M. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J. Agric. Food Chem. 2005, 53, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Henry-Vitrac, C.; Desmoulière, A.; Girard, D.; Mérillon, J.-M.; Krisa, S. Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur. J. Nutr. 2006, 45, 376–382. [Google Scholar] [CrossRef]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef]
- Vitrac, X.; Desmoulière, A.; Brouillaud, B.; Krisa, S.; Deffieux, G.; Barthe, N.; Rosenbaum, J.; Mérillon, J.M. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003, 72, 2219–2233. [Google Scholar] [CrossRef]
- Menet, M.-C.; Baron, S.; Taghi, M.; Diestra, R.; Dargère, D.; Laprévote, O.; Nivet-Antoine, V.; Beaudeux, J.-L.; Bédarida, T.; Cottart, C.-H. Distribution of trans-resveratrol and its metabolites after acute or sustained administration in mouse heart, brain, and liver. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Su, M.; Dong, C.; Wan, J.; Zhou, M. Pharmacokinetics, tissue distribution and excretion study of trans-resveratrol-3-O-glucoside and its two metabolites in rats. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 58, 152882. [Google Scholar] [CrossRef]
- Boocock, D.J.; Patel, K.R.; Faust, G.E.S.; Normolle, D.P.; Marczylo, T.H.; Crowell, J.A.; Brenner, D.E.; Booth, T.D.; Gescher, A.; Steward, W.P. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 848, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Juan, M.E.; Maijó, M.; Planas, J.M. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J. Pharm. Biomed. Anal. 2010, 51, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Maliakal, P.; Lu, H.; Lee, M.-J.; Yang, C.S. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J. Agric. Food Chem. 2004, 52, 935–942. [Google Scholar] [CrossRef]
- Aumont, V.; Krisa, S.; Battaglia, E.; Netter, P.; Richard, T.; Mérillon, J.M.; Magdalou, J.; Sabolovic, N. Regioselective and stereospecific glucuronidation of trans- and cis-resveratrol in human. Arch. Biochem. Biophys. 2001, 393, 281–289. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef]
- la Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Wenzel, E.; Soldo, T.; Erbersdobler, H.; Somoza, V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol. Nutr. Food Res. 2005, 49, 482–494. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Marier, J.-F.; Vachon, P.; Gritsas, A.; Zhang, J.; Moreau, J.-P.; Ducharme, M.P. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 2002, 302, 369–373. [Google Scholar] [CrossRef]
- Chen, W.; Yeo, S.C.M.; Elhennawy, M.G.A.A.; Xiang, X.; Lin, H.-S. Determination of naturally occurring resveratrol analog trans-4,4′-dihydroxystilbene in rat plasma by liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study. Anal. Bioanal. Chem. 2015, 407, 5793–5801. [Google Scholar] [CrossRef]
- Jannin, B.; Menzel, M.; Berlot, J.-P.; Delmas, D.; Lançon, A.; Latruffe, N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: Plasmatic protein binding and cell uptake. Biochem. Pharmacol. 2004, 68, 1113–1118. [Google Scholar] [CrossRef]
- Nair, M.S. Spectroscopic study on the interaction of resveratrol and pterostilbene with human serum albumin. J. Photochem. Photobiol. B 2015, 149, 58–67. [Google Scholar] [CrossRef]
- N’ soukpoe-Kossi, C.N.; St-Louis, C.; Beauregard, M.; Subirade, M.; Carpentier, R.; Hotchandani, S.; Tajmir-Riahi, H.A. Resveratrol binding to human serum albumin. J. Biomol. Struct. Dyn. 2006, 24, 277–283. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef]
- Wang, D.; Hang, T.; Wu, C.; Liu, W. Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 829, 97–106. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Mulla, D.; Praznik, W.; Viernstein, H.; Mueller, M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess Biosyst. Eng. 2016, 39, 1879–1885. [Google Scholar] [CrossRef]
- Qiu, Z.; Yu, J.; Dai, Y.; Yang, Y.; Lu, X.; Xu, J.; Qin, Z.; Huang, F.; Li, N. A simple LC-MS/MS method facilitated by salting-out assisted liquid-liquid extraction to simultaneously determine trans-resveratrol and its glucuronide and sulfate conjugates in rat plasma and its application to pharmacokinetic assay. Biomed. Chromatogr. BMC 2017, 31, e4001. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.-L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Kinsella, J.E. Inhibition of human LDL oxidation by resveratrol. Lancet Lond. Engl. 1993, 341, 1103–1104. [Google Scholar] [CrossRef]
- Miyagi, Y.; Miwa, K.; Inoue, H. Inhibition of human low-density lipoprotein oxidation by flavonoids in red wine and grape juice. Am. J. Cardiol. 1997, 80, 1627–1631. [Google Scholar] [CrossRef]
- Belguendouz, L.; Frémont, L.; Gozzelino, M.T. Interaction of transresveratrol with plasma lipoproteins. Biochem. Pharmacol. 1998, 55, 811–816. [Google Scholar] [CrossRef]
- Asadi, S.; Rahimi, Z.; Saidijam, M.; Shabab, N.; Goodarzi, M.T. Effects of Resveratrol on FOXO1 and FOXO3a Genes Expression in Adipose Tissue, Serum Insulin, Insulin Resistance and Serum SOD Activity in Type 2 Diabetic Rats. Int. J. Mol. Cell. Med. 2018, 7, 176–184. [Google Scholar]
- St John, S.E.; Jensen, K.C.; Kang, S.; Chen, Y.; Calamini, B.; Mesecar, A.D.; Lipton, M.A. Design, synthesis, biological and structural evaluation of functionalized resveratrol analogues as inhibitors of quinone reductase 2. Bioorg. Med. Chem. 2013, 21, 6022–6037. [Google Scholar] [CrossRef]
- Belguendouz, L.; Fremont, L.; Linard, A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem. Pharmacol. 1997, 53, 1347–1355. [Google Scholar] [CrossRef]
- Low, I.C.C.; Chen, Z.X.; Pervaiz, S. Bcl-2 modulates resveratrol-induced ROS production by regulating mitochondrial respiration in tumor cells. Antioxid. Redox Signal. 2010, 13, 807–819. [Google Scholar] [CrossRef]
- Jung, K.-H.; Lee, J.H.; Thien Quach, C.H.; Paik, J.-Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.-H.; Lee, K.-H. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1α activation. J. Nucl. Med. 2013, 54, 2161–2167. [Google Scholar] [CrossRef]
- Cao, L.; Chen, X.; Xiao, X.; Ma, Q.; Li, W. Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. Int. J. Oncol. 2016, 49, 735–743. [Google Scholar] [CrossRef]
- Kong, Q.; Ren, X.; Hu, R.; Yin, X.; Jiang, G.; Pan, Y. Isolation and purification of two antioxidant isomers of resveratrol dimer from the wine grape by counter-current chromatography. J. Sep. Sci. 2016, 39, 2374–2379. [Google Scholar] [CrossRef]
- Li, W.; Cao, L.; Chen, X.; Lei, J.; Ma, Q. Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol. Rep. 2016, 35, 1718–1726. [Google Scholar] [CrossRef]
- Yar, A.S.; Menevse, S.; Alp, E.; Helvacioglu, F.; Take, G. The effects of resveratrol on cyclooxygenase-1 and cyclooxygenase-2 mRNA and protein levels in diabetic rat kidneys. Mol. Biol. Rep. 2010, 37, 2323–2331. [Google Scholar] [CrossRef]
- Kutil, Z.; Temml, V.; Maghradze, D.; Pribylova, M.; Dvorakova, M.; Schuster, D.; Vanek, T.; Landa, P. Impact of wines and wine constituents on cyclooxygenase-1, cyclooxygenase-2, and 5-lipoxygenase catalytic activity. Mediat. Inflamm. 2014, 2014, 178931. [Google Scholar] [CrossRef]
- Shen, M.Y.; Hsiao, G.; Liu, C.L.; Fong, T.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. Inhibitory mechanisms of resveratrol in platelet activation: Pivotal roles of p38 MAPK and NO/cyclic GMP. Br. J. Haematol. 2007, 139, 475–485. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Kimura, Y.; Okuda, H.; Arichi, S. Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim. Biophys. Acta 1985, 834, 275–278. [Google Scholar]
- Yang, J.; Wang, N.; Li, J.; Zhang, J.; Feng, P. Effects of resveratrol on NO secretion stimulated by insulin and its dependence on SIRT1 in high glucose cultured endothelial cells. Endocrine 2010, 37, 365–372. [Google Scholar] [CrossRef]
- Huang, J.-P.; Hsu, S.-C.; Li, D.-E.; Chen, K.-H.; Kuo, C.-Y.; Hung, L.-M. Resveratrol Mitigates High-Fat Diet-Induced Vascular Dysfunction by Activating the Akt/eNOS/NO and Sirt1/ER Pathway. J. Cardiovasc. Pharmacol. 2018, 72, 231–241. [Google Scholar] [CrossRef]
- Wiciński, M.; Malinowski, B.; Węclewicz, M.M.; Grześk, E.; Grześk, G. Resveratrol Increases Serum BDNF Concentrations and Reduces Vascular Smooth Muscle Cells Contractility via a NOS-3-Independent Mechanism. BioMed. Res. Int. 2017, 2017, 9202954. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Basile, G.; Agodi, A. Resveratrol Modulates SIRT1 and DNMT Functions and Restores LINE-1 Methylation Levels in ARPE-19 Cells under Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2018, 19, 2118. [Google Scholar] [CrossRef]
- Della-Morte, D.; Dave, K.R.; DeFazio, R.A.; Bao, Y.C.; Raval, A.P.; Perez-Pinzon, M.A. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 2009, 159, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Rivera, L.; Morón, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef]
- Beaudoin, M.-S.; Snook, L.A.; Arkell, A.M.; Simpson, J.A.; Holloway, G.P.; Wright, D.C. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R542–R551. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Penumathsa, S.V.; Koneru, S.; Juhasz, B.; Zhan, L.; Otani, H.; Bagchi, D.; Das, D.K.; Maulik, N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic. Biol. Med. 2007, 43, 720–729. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. Baltim. 2000, 164, 6509–6519. [Google Scholar] [CrossRef]
- Estrov, Z.; Shishodia, S.; Faderl, S.; Harris, D.; Van, Q.; Kantarjian, H.M.; Talpaz, M.; Aggarwal, B.B. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 2003, 102, 987–995. [Google Scholar] [CrossRef]
- Kanemaki, T.; Kitade, H.; Kaibori, M.; Sakitani, K.; Hiramatsu, Y.; Kamiyama, Y.; Ito, S.; Okumura, T. Interleukin 1beta and interleukin 6, but not tumor necrosis factor alpha, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatol. Baltim. 1998, 27, 1296–1303. [Google Scholar] [CrossRef]
- Haslbeck, K.-M.; Schleicher, E.; Bierhaus, A.; Nawroth, P.; Haslbeck, M.; Neundörfer, B.; Heuss, D. The AGE/RAGE/NF-(kappa)B pathway may contribute to the pathogenesis of polyneuropathy in impaired glucose tolerance (IGT). Exp. Clin. Endocrinol. Diabetes 2005, 113, 288–291. [Google Scholar] [CrossRef]
- Li, W.; Ma, J.; Ma, Q.; Li, B.; Han, L.; Liu, J.; Xu, Q.; Duan, W.; Yu, S.; Wang, F.; et al. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr. Med. Chem. 2013, 20, 4185–4194. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, X.; Chen, K.; Sun, L.; Gao, L.; Zhou, C.; Lei, M.; Duan, W.; Wang, Z.; Ma, Q.; et al. YAP Inhibition by Resveratrol via Activation of AMPK Enhances the Sensitivity of Pancreatic Cancer Cells to Gemcitabine. Nutrients 2016, 8, 546. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Huang, H.; Lin, H.; Zhang, X.; Li, J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway. Oncol. Rep. 2012, 27, 2050–2056. [Google Scholar]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D.; Niu, H.; Zhu, G.; Xu, Y.; Ye, D.; Li, J.; Zhang, Q. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 19. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Conjugated Physiological Resveratrol Metabolites Induce Senescence in Breast Cancer Cells: Role of p53/p21 and p16/Rb Pathways, and ABC Transporters. Mol. Nutr. Food Res. 2019, 63, e1900629. [Google Scholar] [CrossRef]
- Chatterjee, B.; Ghosh, K.; Kanade, S.R. Resveratrol modulates epigenetic regulators of promoter histone methylation and acetylation that restores BRCA1, p53, p21CIP1 in human breast cancer cell lines. BioFactors Oxf. Engl. 2019, 45, 818–829. [Google Scholar] [CrossRef]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef]
- Mo, W.; Xu, X.; Xu, L.; Wang, F.; Ke, A.; Wang, X.; Guo, C. Resveratrol inhibits proliferation and induces apoptosis through the hedgehog signaling pathway in pancreatic cancer cell. Pancreatology 2011, 11, 601–609. [Google Scholar] [CrossRef]
- Cilibrasi, C.; Riva, G.; Romano, G.; Cadamuro, M.; Bazzoni, R.; Butta, V.; Paoletta, L.; Dalprà, L.; Strazzabosco, M.; Lavitrano, M.; et al. Resveratrol Impairs Glioma Stem Cells Proliferation and Motility by Modulating the Wnt Signaling Pathway. PLoS ONE 2017, 12, e0169854. [Google Scholar] [CrossRef]
- Kim, C.-W.; Hwang, K.-A.; Choi, K.-C. Anti-metastatic potential of resveratrol and its metabolites by the inhibition of epithelial-mesenchymal transition, migration, and invasion of malignant cancer cells. Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 1787–1796. [Google Scholar] [CrossRef]
- Garvin, S.; Ollinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef]
- Bishayee, A.; Waghray, A.; Barnes, K.F.; Mbimba, T.; Bhatia, D.; Chatterjee, M.; Darvesh, A.S. Suppression of the inflammatory cascade is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. Pharm. Res. 2010, 27, 1080–1091. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Berretta, M.; Della Pepa, C.; Tralongo, P.; Fulvi, A.; Martellotta, F.; Lleshi, A.; Nasti, G.; Fisichella, R.; Romano, C.; De Divitiis, C.; et al. Use of Complementary and Alternative Medicine (CAM) in cancer patients: An Italian multicenter survey. Oncotarget 2017, 8, 24401–24414. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, X.; Fu, J.; Wang, X. Resveratrol increase myocardial Nrf2 expression in type 2 diabetic rats and alleviate myocardial ischemia/reperfusion injury (MIRI). Ann. Palliat. Med. 2019, 8, 565–575. [Google Scholar] [CrossRef]
- Yoshida, Y.; Shioi, T.; Izumi, T. Resveratrol ameliorates experimental autoimmune myocarditis. Circ. J. Off. J. Jpn. Circ. Soc. 2007, 71, 397–404. [Google Scholar] [CrossRef]
- Guan, P.; Sun, Z.-M.; Wang, N.; Zhou, J.; Luo, L.-F.; Zhao, Y.-S.; Ji, E.-S. Resveratrol prevents chronic intermittent hypoxia-induced cardiac hypertrophy by targeting the PI3K/AKT/mTOR pathway. Life Sci. 2019, 233, 116748. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Chen, C.; Zou, L.-X.; Lin, Q.-Y.; Yan, X.; Bi, H.-L.; Xie, X.; Wang, S.; Wang, Q.-S.; Zhang, Y.-L.; Li, H.-H. Resveratrol as a new inhibitor of immunoproteasome prevents PTEN degradation and attenuates cardiac hypertrophy after pressure overload. Redox Biol. 2019, 20, 390–401. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, M.; Chen, K.; Chen, B.; Zhao, Y.; Gong, H.; Zhao, X.; Qi, R. Suppression of TLR4 activation by resveratrol is associated with STAT3 and Akt inhibition in oxidized low-density lipoprotein-activated platelets. Eur. J. Pharmacol. 2018, 836, 1–10. [Google Scholar] [CrossRef]
- Cinoku, I.I.; Mavragani, C.P.; Moutsopoulos, H.M. Atherosclerosis: Beyond the lipid storage hypothesis. The role of autoimmunity. Eur. J. Clin. Investig. 2020, 50, e13195. [Google Scholar] [CrossRef]
- Bitorina, A.V.; Oligschlaeger, Y.; Shiri-Sverdlov, R.; Theys, J. Low profile high value target: The role of OxLDL in cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158518. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, S.; Zhu, X.; Yuan, X.; Zhan, Y.; Li, Y.; Wang, W. Resveratrol Induces Endothelial Progenitor Cells Angiogenesis via MiR-542-3p by Targeting Angiopoietin-2 and Involves in Recanalization of Venous Thrombosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7675–7683. [Google Scholar] [CrossRef]
- Maurea, N.; Riva, L. Venous thromboembolism and atrial fibrillation in patients with cancer. G. Ital. Cardiol. 2006 2018, 19, 3S–6S. [Google Scholar]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef]
- Tolva, V.; Mazzola, S.; Zerbi, P.; Casana, R.; Albertini, M.; Calvillo, L.; Selmin, F.; Cilurzo, F. A successful experimental model for intimal hyperplasia prevention using a resveratrol-delivering balloon. J. Vasc. Surg. 2016, 63, 788–794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kao, H.-H.; Kao, L.-T.; Li, I.-H.; Pan, K.-T.; Shih, J.-H.; Chou, Y.-C.; Wu, S.-T. Androgen Deprivation Therapy Use Increases the Risk of Heart Failure in Patients with Prostate Cancer: A Population-Based Cohort Study. J. Clin. Pharmacol. 2019, 59, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Rossetti, S.; Cavaliere, C.; Di Palo, R.; Lamantia, E.; Castaldo, L.; Nocerino, F.; Ametrano, G.; Cappuccio, F.; Malzone, G.; et al. Correction: Metabolic syndrome, endocrine disruptors and prostate cancer associations: Biochemical and pathophysiological evidences. Oncotarget 2017, 8, 62816. [Google Scholar] [CrossRef]
- Mele, D.; Tocchetti, C.G.; Pagliaro, P.; Madonna, R.; Novo, G.; Pepe, A.; Zito, C.; Maurea, N.; Spallarossa, P. Pathophysiology of anthracycline cardiotoxicity. J. Cardiovasc. Med. Hagerstown Md 2016, 17 (Suppl. S1), S3–S11. [Google Scholar] [CrossRef]
- Spallarossa, P.; Maurea, N.; Cadeddu, C.; Madonna, R.; Mele, D.; Monte, I.; Novo, G.; Pagliaro, P.; Pepe, A.; Tocchetti, C.G.; et al. A recommended practical approach to the management of anthracycline-based chemotherapy cardiotoxicity: An opinion paper of the working group on drug cardiotoxicity and cardioprotection, Italian Society of Cardiology. J. Cardiovasc. Med. Hagerstown 2016, 17 (Suppl. S1), S84–S92. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10, 1304. [Google Scholar] [CrossRef]
- Quagliariello, V.; Coppola, C.; Mita, D.G.; Piscopo, G.; Iaffaioli, R.V.; Botti, G.; Maurea, N. Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environ. Toxicol. Pharmacol. 2019, 69, 1–8. [Google Scholar] [CrossRef]
- Gu, J.; Fan, Y.-Q.; Zhang, H.-L.; Pan, J.-A.; Yu, J.-Y.; Zhang, J.-F.; Wang, C.-Q. Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion. Biochem. Pharmacol. 2018, 150, 202–213. [Google Scholar] [CrossRef]
- Arafa, M.H.; Mohammad, N.S.; Atteia, H.H.; Abd-Elaziz, H.R. Protective effect of resveratrol against doxorubicin-induced cardiac toxicity and fibrosis in male experimental rats. J. Physiol. Biochem. 2014, 70, 701–711. [Google Scholar] [CrossRef]
- Gordish, K.L.; Beierwaltes, W.H. Chronic resveratrol reverses a mild angiotensin II-induced pressor effect in a rat model. Integr. Blood Press. Control 2016, 9, 23–31. [Google Scholar] [PubMed]
- Wong, R.H.X.; Howe, P.R.C.; Buckley, J.D.; Coates, A.M.; Kunz, I.; Berry, N.M. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. NMCD 2011, 21, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Urpi-Sarda, M.; Lamuela-Raventós, R.M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Arós, F.; Fitó, M.; Lapetra, J.; Estruch, R.; Andres-Lacueva, C.; et al. High urinary levels of resveratrol metabolites are associated with a reduction in the prevalence of cardiovascular risk factors in high-risk patients. Pharmacol. Res. 2012, 65, 615–620. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef]
- Guthrie, A.R.; Chow, H.-H.S.; Martinez, J.A. Effects of resveratrol on drug- and carcinogen-metabolizing enzymes, implications for cancer prevention. Pharmacol. Res. Perspect. 2017, 5, e00294. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Bitterman, J.L.; Chung, J.H. Metabolic effects of resveratrol: Addressing the controversies. Cell. Mol. Life Sci. CMLS 2015, 72, 1473–1488. [Google Scholar] [CrossRef]
- Detampel, P.; Beck, M.; Krähenbühl, S.; Huwyler, J. Drug interaction potential of resveratrol. Drug Metab. Rev. 2012, 44, 253–265. [Google Scholar] [CrossRef]
- Orsini, F.; Verotta, L.; Klimo, K.; Gerhäuser, C. Synthesis of Resveratrol Derivatives and In Vitro Screening for Potential Cancer Chemopreventive Activities. Arch. Pharm. 2016, 349, 414–427. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Garland, L.L.; Hsu, C.-H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef]
- Chang, T.K.H.; Chen, J.; Yu, C.-T. In vitro inhibition of rat CYP1A1 and CYP1A2 by piceatannol, a hydroxylated metabolite of trans-resveratrol. Drug Metab. Lett. 2007, 1, 13–16. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Hurh, Y.-J.; Na, H.-K.; Kim, J.-H.; Chun, Y.-J.; Kim, D.-H.; Kang, K.-S.; Cho, M.-H.; Surh, Y.-J. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis 2004, 25, 2005–2013. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.-H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef]
- Chiba, T.; Kimura, Y.; Suzuki, S.; Tatefuji, T.; Umegaki, K. Trans-Resveratrol Enhances the Anticoagulant Activity of Warfarin in a Mouse Model. J. Atheroscler. Thromb. 2016, 23, 1099–1110. [Google Scholar] [CrossRef]
- Zhan, Y.-Y.; Liang, B.-Q.; Li, X.-Y.; Gu, E.-M.; Dai, D.-P.; Cai, J.-P.; Hu, G.-X. The effect of resveratrol on pharmacokinetics of aripiprazole in vivo and in vitro. Xenobiotica Fate Foreign Compd. Biol. Syst. 2016, 46, 439–444. [Google Scholar] [CrossRef]
- Bedada, S.K.; Nearati, P. Effect of resveratrol on the pharmacokinetics of carbamazepine in healthy human volunteers. Phytother. Res. PTR 2015, 29, 701–706. [Google Scholar] [CrossRef]
- McIlwain, C.C.; Townsend, D.M.; Tew, K.D. Glutathione S-transferase polymorphisms: Cancer incidence and therapy. Oncogene 2006, 25, 1639–1648. [Google Scholar] [CrossRef]
- Cavalieri, E.; Rogan, E. The molecular etiology and prevention of estrogen-initiated cancers: Ockham’s Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol. Aspects Med. 2014, 36, 1–55. [Google Scholar] [CrossRef]
- Yager, J.D. Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention—A review. Steroids 2015, 99, 56–60. [Google Scholar] [CrossRef]
- Yu, C.; Shin, Y.G.; Kosmeder, J.W.; Pezzuto, J.M.; van Breemen, R.B. Liquid chromatography/tandem mass spectrometric determination of inhibition of human cytochrome P450 isozymes by resveratrol and resveratrol-3-sulfate. Rapid Commun. Mass Spectrom. 2003, 17, 307–313. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 2 March 2020).
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA: Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Lin, J.-N.; Lin, V.C.-H.; Rau, K.-M.; Shieh, P.-C.; Kuo, D.-H.; Shieh, J.-C.; Chen, W.-J.; Tsai, S.-C.; Way, T.-D. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J. Agric. Food Chem. 2010, 58, 1584–1592. [Google Scholar] [CrossRef]
- Vervandier-Fasseur, D.; Latruffe, N. The Potential Use of Resveratrol for Cancer Prevention. Mol. Basel Switz. 2019, 24, 4506. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Honari, M.; Shafabakhsh, R.; Reiter, R.J.; Mirzaei, H.; Asemi, Z. Resveratrol is a promising agent for colorectal cancer prevention and treatment: Focus on molecular mechanisms. Cancer Cell Int. 2019, 19, 180. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Albuquerque, R.V.; Malcher, N.S.; Amado, L.L.; Coleman, M.D.; Dos Santos, D.C.; Borges, R.S.; Valente, S.A.S.; Valente, V.C.; Monteiro, M.C. In Vitro Protective Effect and Antioxidant Mechanism of Resveratrol Induced by Dapsone Hydroxylamine in Human Cells. PLoS ONE 2015, 10, e0134768. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Lu, Q.Y.; Zhang, Z.F.; Brown, J.; Le, A.D. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol. Cancer Ther. 2005, 4, 1465–1474. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, Y.-C.; Sun, C.-K.; Chen, Q.-M. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol. Rep. 2016, 35, 2499–2515. [Google Scholar] [CrossRef]
- Han, Y.; Jo, H.; Cho, J.H.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol as a Tumor-Suppressive Nutraceutical Modulating Tumor Microenvironment and Malignant Behaviors of Cancer. Int. J. Mol. Sci. 2019, 20, 925. [Google Scholar] [CrossRef]
- Luo, J.-L.; Maeda, S.; Hsu, L.-C.; Yagita, H.; Karin, M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef]
- Sharma, S.; Chopra, K.; Kulkarni, S.K.; Agrewala, J.N. Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin. Exp. Immunol. 2007, 147, 155–163. [Google Scholar] [CrossRef]
- Baek, S.H.; Ko, J.-H.; Lee, H.; Jung, J.; Kong, M.; Lee, J.; Lee, J.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine Int. J. Phytother. Phytopharm. 2016, 23, 566–577. [Google Scholar] [CrossRef]
- Di Francia, R.; Berretta, M.; Benincasa, G.; D’Avino, A.; Facchini, S.; Costagliola, D.; Rossi, P. Pharmacogenetic-Based Interactions between Nutraceuticals and Angiogenesis Inhibitors. Cells 2019, 8, 522. [Google Scholar] [CrossRef]
- Majeed, I.; Rana, A.; Rafique, M.; Anwar, A.W.; Mahmood, F. Time delay barriers in diagnosis and treatment of cancer. WCRJ 2018, 5, e1118. [Google Scholar]
- D’Andrea, F.; Ceccarelli, M.; Rullo, E.V.; Facciolà, A.; D’Aleo, F.; Cacopardo, B.; Iacobello, C.; Costa, A.; Altavilla, G.; Pellicanò, G.F.; et al. Cancer screening in HIV-infected patients: Early diagnosis in a high-risk population. WCRJ 2018, 5, e1130. [Google Scholar]
| Effect | Pathway | Reference |
|---|---|---|
| Antioxidant | Copper-chelant | [44,45,49] |
| Activation p38-MAPK | [52] | |
| Inhibition QR2 | [48] | |
| Up-regulation SOD | [47] | |
| Anti-Inflammatory | Inhibition COX1 | [55,56,58] |
| Inhibition COX2 | [55,56,58] | |
| Blocked TxA2 | [57,58] | |
| Blocked MAPK | [57,58] | |
| Activation Sirt-1→↑TLR4/NF-κB/STAT; ↓TNF-α/IL-6/IL-1β | [63,64] | |
| Vasodilatation | Activation K+ channel Ca2+→↑eNOS, ↑eNOS | [60,61,62] |
| Metabolic Disorder (CVD/T2DM/Obesity) | Activation Sirt-1→ Activation PGC-1α→↓plasma levels glucose & glycated | [68,69,70] |
| Activation AMPK | [68,69,70] | |
| Cancer | Inhibition Sirt-1/PTEN/PI3K/AKT | [75,76,77,78,79,80] |
| Upregulation p21/p53 | [81,82,83] | |
| Inhibition AMPK/YAP | [54,84,85,86] | |
| Inhibition NF-κB/STAT3 | [78] | |
| Downregulation HIF-1α | [54] |
| Metabolic Enzyme (Direct Effect) | Documented Interaction. Plasma Levels: (H) Higher (L) Lower Dosage | Potential Cancer Drug Interaction | Pharmacogenomic Test | Final Consideration [Reference] |
|---|---|---|---|---|
| CYP1A1 (Inhibition) | Testosterone # Caffeine (in human) Phenacetin | Bendamustine | CYP1A2*F 5′UTR -163C>A rs7625551 | [123] |
| CYP1B1 (Inhibition) | (L) Cathecol estrogens (anti-breast cancer activity of resveratrol) # | ND | ND | 1 g/day for 12 weeks had a favorable effect in post-menopausal women [124] |
| CYP2B6 (Inhibition) | ND | Cyclophosphamide | ND | [127] |
| CYP2C9 (Inhibition) | (H) Warfarin # | ND | CYP2C9*2 430C>T R144C. rs1799853 CYP2c9*3 1075A>C I359L rs1057910 | 1 g daily resveratrol inhibited CYP2C9 by 2.71-fold [126] |
| CYP2D6 (Low inhibition) | (L) dextromethorphan $ | Tamoxifen | CYP2D6*3 2459delA frameshift rs35742686 CYP2D6*4 1846G>A splicing rs3892097 CYP2D6*10 100C>T P35S rs1065852 CYP2D6*XN copy number variation | Probable low activation of tamoxifen in the active metabolite endoxifen [122] |
| CYP2C19 (Moderate Inhibition) | (H) Pantoprazole $ | ND | CYP2C19*17 -806C>T 5′UTR rs12248560 | [121] |
| CYP3A4 (Inhibition) | (H) Nicardipine $ (H) diltiazem $ (H) Carbamazepine $ | Imatinib Docetaxel | CYP3A4*22 15389C>T 5”UTR rs35599367 | [125,128] |
| GST (Induction) | (L) nitrosamines and polycyclic aromatic Hydrocarbon # | (H) platin derivates | GSTP1 Iso105Val | [129] |
| NQO1 (Induction) | (L) Estrogens by inactivation by catechol-O-methyl transferase # | ND | ND | [130,131] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berretta, M.; Bignucolo, A.; Di Francia, R.; Comello, F.; Facchini, G.; Ceccarelli, M.; Iaffaioli, R.V.; Quagliariello, V.; Maurea, N. Resveratrol in Cancer Patients: From Bench to Bedside. Int. J. Mol. Sci. 2020, 21, 2945. https://doi.org/10.3390/ijms21082945
Berretta M, Bignucolo A, Di Francia R, Comello F, Facchini G, Ceccarelli M, Iaffaioli RV, Quagliariello V, Maurea N. Resveratrol in Cancer Patients: From Bench to Bedside. International Journal of Molecular Sciences. 2020; 21(8):2945. https://doi.org/10.3390/ijms21082945
Chicago/Turabian StyleBerretta, Massimiliano, Alessia Bignucolo, Raffaele Di Francia, Francesco Comello, Gaetano Facchini, Manuela Ceccarelli, Rosario Vincenzo Iaffaioli, Vincenzo Quagliariello, and Nicola Maurea. 2020. "Resveratrol in Cancer Patients: From Bench to Bedside" International Journal of Molecular Sciences 21, no. 8: 2945. https://doi.org/10.3390/ijms21082945
APA StyleBerretta, M., Bignucolo, A., Di Francia, R., Comello, F., Facchini, G., Ceccarelli, M., Iaffaioli, R. V., Quagliariello, V., & Maurea, N. (2020). Resveratrol in Cancer Patients: From Bench to Bedside. International Journal of Molecular Sciences, 21(8), 2945. https://doi.org/10.3390/ijms21082945







