In Vivo Analysis of Optic Fissure Fusion in Zebrafish: Pioneer Cells, Basal Lamina, Hyaloid Vessels, and How Fissure Fusion is Affected by BMP
Abstract
1. Introduction
2. Results and Discussion
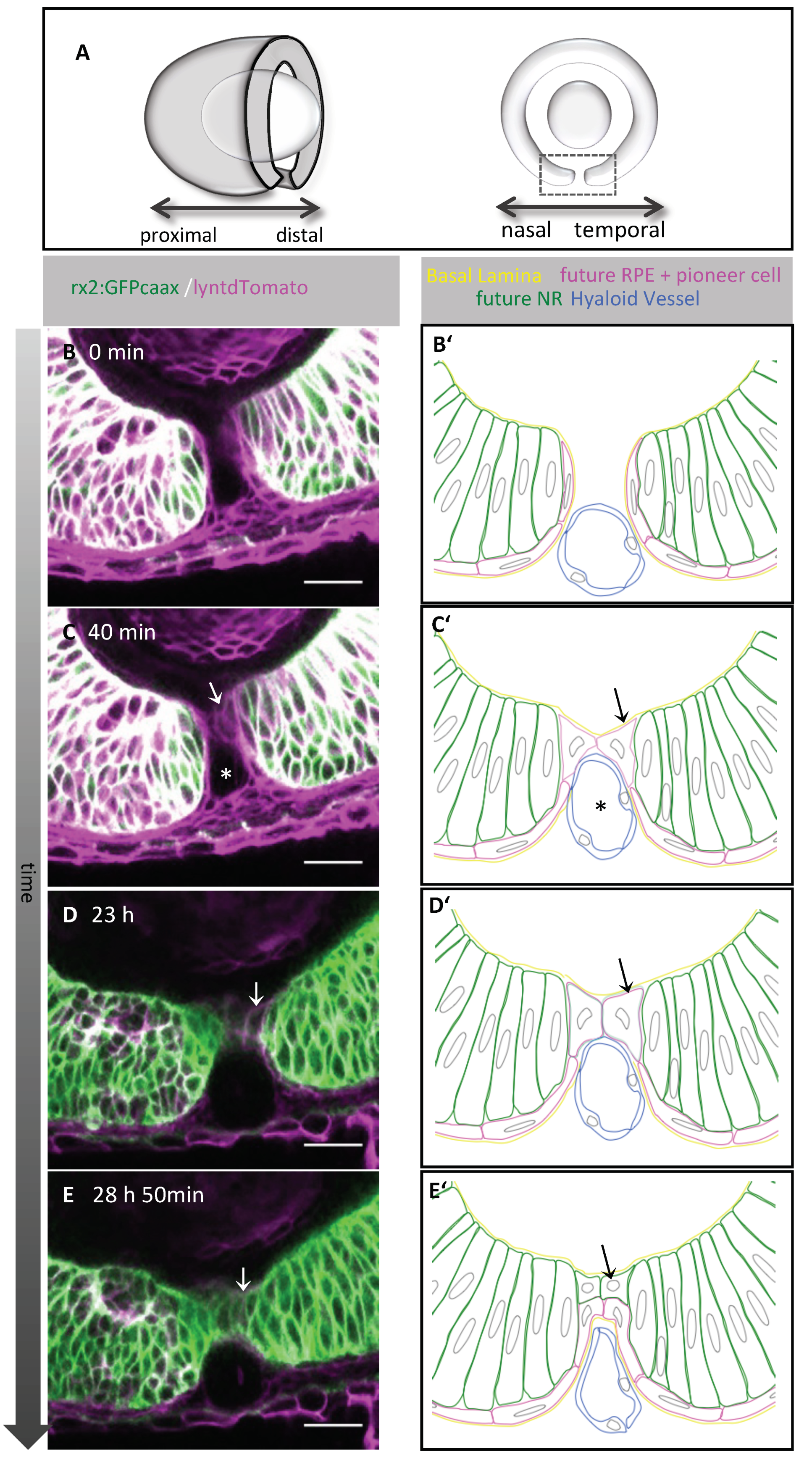
2.1. Pioneer Cells Establish the Contact between the Optic Fissure Margins
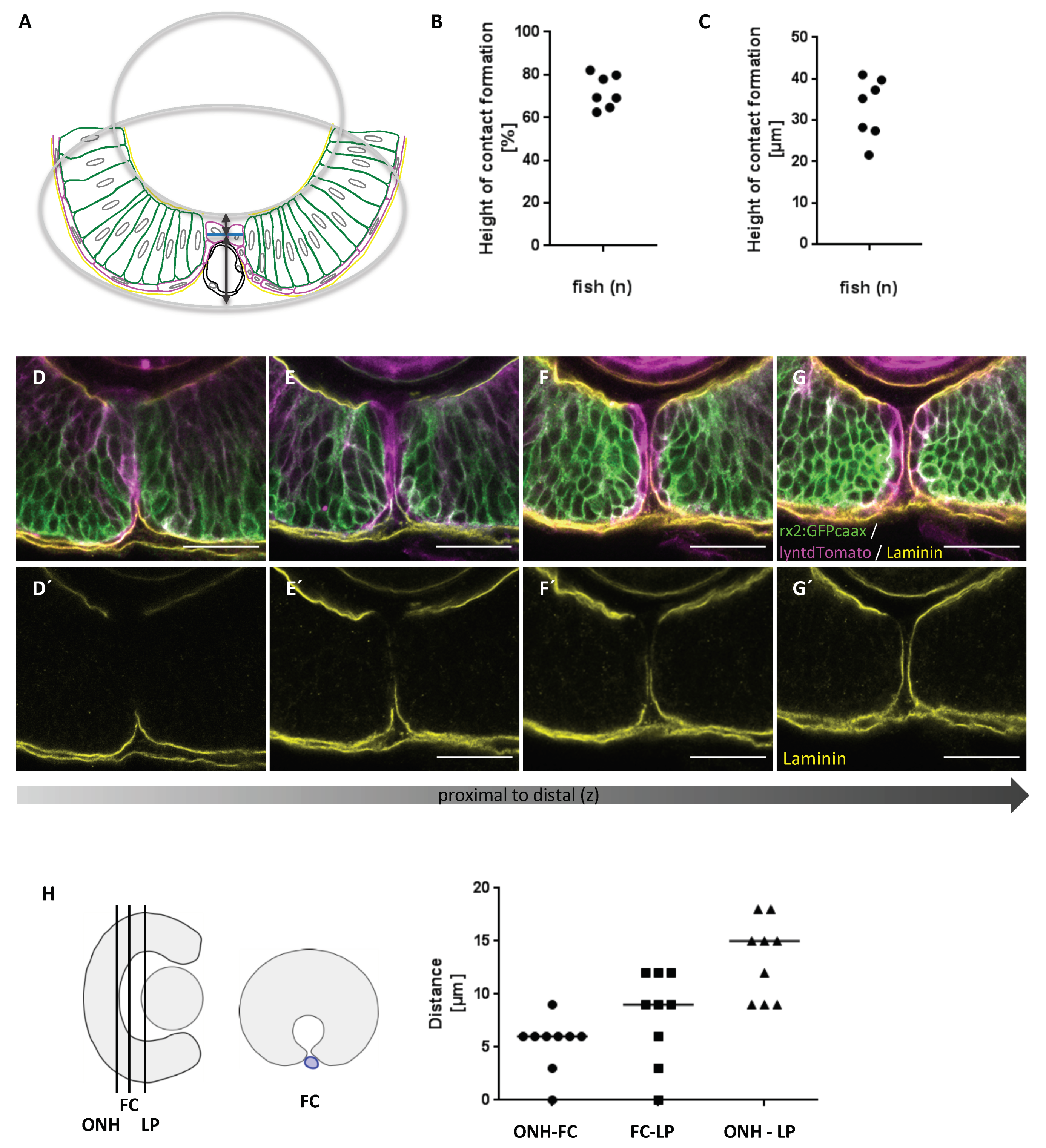
2.2. First Contact between Optic Fissure Margins Occurs Close to the Prospective Optic Nerve Head
2.3. Orientation and Characterization of Pioneer Cells
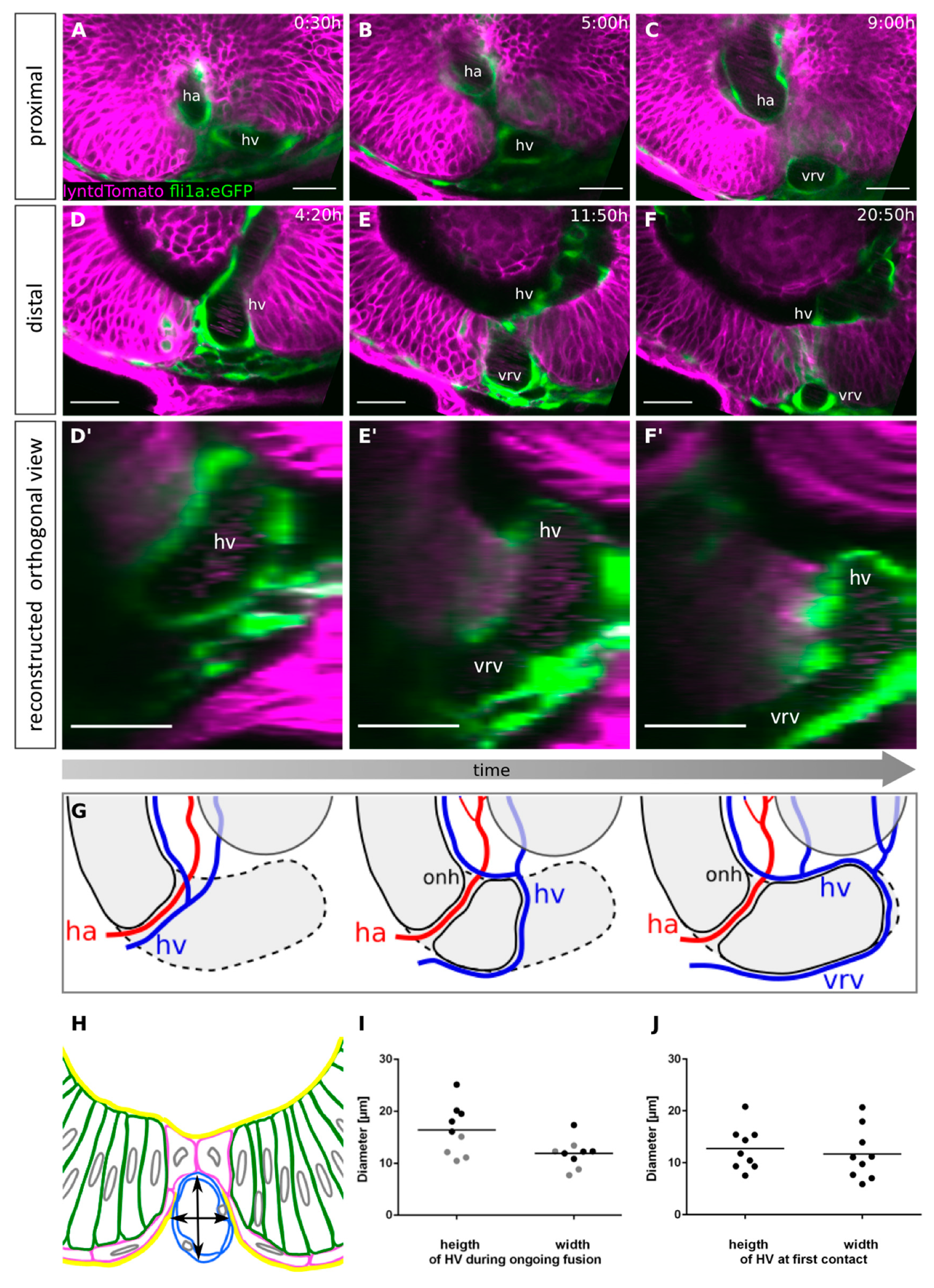
2.4. Pioneer Cells Initiate Optic Fissure Fusion between Two Hyaloid Vessels
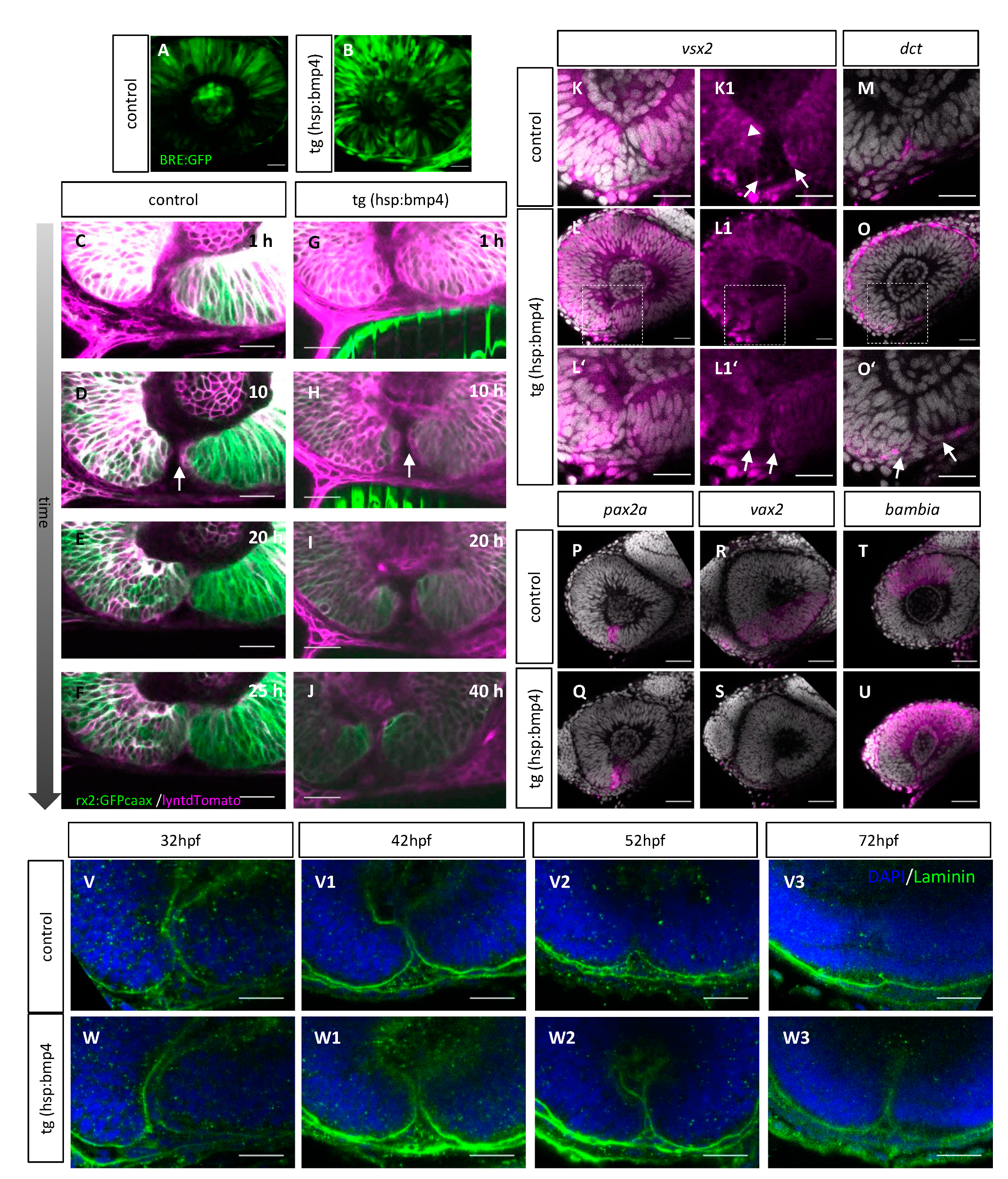
2.5. Late BMP Induction Does Not Alter Margin Architecture but Identity and Hampers Basal Lamina Dissolution
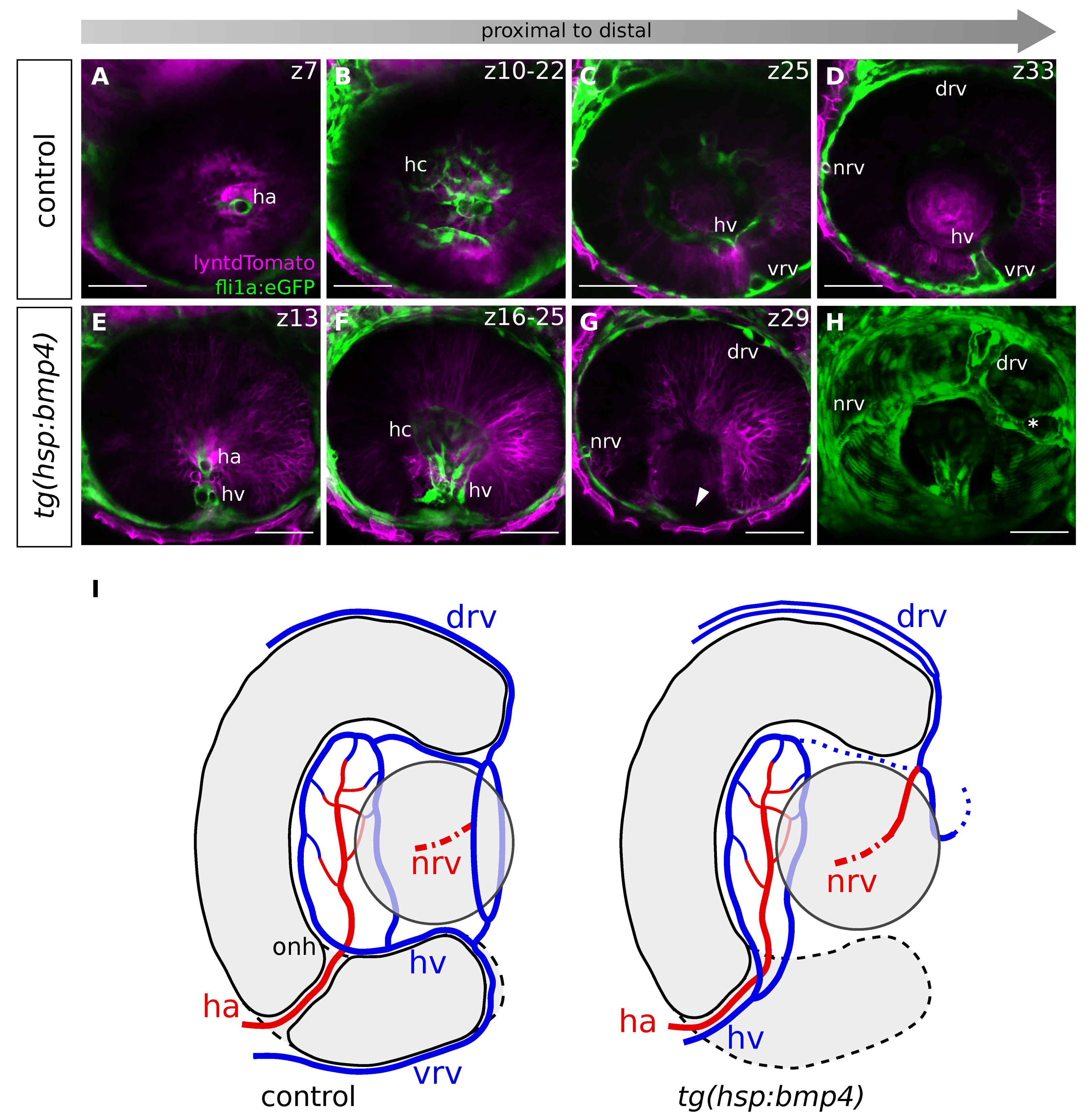
2.6. Late BMP Induction Results in Only Subtle Direct Changes of the POM and Hyaloid Vessel
2.7. BMP-Induced Failure of Optic Fissure Fusion Disrupts Development of Ventral Eye Vasculature
3. Materials and Methods
3.1. Heat Shock
3.2. Whole Mount Immunohistochemistry
3.3. Time-Lapse Imaging and Image Processing
3.4. Quantitative Analysis of the Height of Optic Fissure Fusion
3.5. Analysis of the Proximal-Distal Position of the First Contact (FC)
3.6. Analysis of the Size of the Hyaloid Vein
3.7. Whole Mount In Situ Hybridization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NR | Neuroretina |
| RPE | retinal pigmented epithelium |
| BMP | bone morphogenic protein |
| POM | periocular mesenchym |
| RA | retinoic acid |
| OF | optic fissure |
| BL | basal lamina |
| ECM | extracellular matrix |
| HA | hyaloid artery |
| HV | hyaloid vein |
| onh | optic nerve head |
| VRV | ventral radial vessel |
| DRV | dorsal radial vessel |
| NRV | nasal radial vessel |
| hc | hyaloid capillaries |
| LP | proximal border of the lens |
| FC | first contact |
| HS | heat shock |
| hpf | hours past fertilization |
| wmish | whole mount in situ hybridization |
References
- Eckert, P.; Knickmeyer, M.D.; Schütz, L.; Wittbrodt, J.; Heermann, S. Morphogenesis and axis specification occur in parallel during optic cup and optic fissure formation, differentially modulated by BMP and WNT. Open Biol. 2019, 9, 180179. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.L.; Lang, R.A. Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 2001, 17, 255–296. [Google Scholar] [CrossRef] [PubMed]
- Walls, G.L. The Vertebrate Eye and Its Adaptive Radiation; Cranbrook Institute of Science: Bloomfield Hills, MI, USA, 1942. [Google Scholar]
- Gage, P.J.; Rhoades, W.; Prucka, S.K.; Hjalt, T. Fate maps of neural crest and mesoderm in the mammalian eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4200–4208. [Google Scholar] [CrossRef] [PubMed]
- Onwochei, B.C.; Simon, J.W.; Bateman, J.B.; Couture, K.C.; Mir, E. Ocular colobomata. Surv. Ophthalmol. 2000, 45, 175–194. [Google Scholar] [CrossRef]
- ALSomiry, A.S.; Gregory-Evans, C.Y.; Gregory-Evans, K. An update on the genetics of ocular coloboma. Hum. Genet. 2019, 138, 865–880. [Google Scholar] [CrossRef]
- Graw, J. The genetic and molecular basis of congenital eye defects. Nat. Rev. Genet. 2003, 4, 876–888. [Google Scholar] [CrossRef]
- Gregory-Evans, C.Y.; Williams, M.J.; Halford, S.; Gregory-Evans, K. Ocular coloboma: A reassessment in the age of molecular neuroscience. J. Med. Genet. 2004, 41, 881–891. [Google Scholar] [CrossRef]
- Gregory-Evans, C.Y.; Wallace, V.A.; Gregory-Evans, K. Gene networks: Dissecting pathways in retinal development and disease. Prog. Retin. Eye Res. 2013, 33, 40–66. [Google Scholar] [CrossRef]
- Westenskow, P.; Piccolo, S.; Fuhrmann, S. Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development 2009, 136, 2505–2510. [Google Scholar] [CrossRef]
- Bankhead, E.J.; Colasanto, M.P.; Dyorich, K.M.; Jamrich, M.; Murtaugh, L.C.; Fuhrmann, S. Multiple requirements of the focal dermal hypoplasia gene porcupine during ocular morphogenesis. Am. J. Pathol. 2015, 185, 197–213. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.; Gaudenz, K.; Paulson, A.; Guo, F.; Trimble, R.; Xie, T.; Peak, A.; Deng, C.; Furuta, Y.; et al. Defective FGF signaling causes coloboma formation and disrupts retinal neurogenesis. Cell Res. 2012, 23, 254–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, Z.; Tao, C.; Li, H.; Ladher, R.; Gotoh, N.; Feng, G.S.; Zhang, X.; Wang, F. Deficient FGF signaling causes optic nerve dysgenesis and ocular coloboma. Development 2013, 140, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Matt, N.; Ghyselinck, N.B.; Pellerin, I.; Dupé, V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev. Biol. 2008, 320, 140–148. [Google Scholar] [CrossRef]
- Lupo, G.; Gestri, G.; O’Brien, M.; Denton, R.M.; Chandraratna, R.A.; Ley, S.V.; Wilson, S.W.; Harris, W.A. Retinoic acid receptor signaling regulates choroid fissure closure through independent mechanisms in the ventral optic cup and periocular mesenchyme. Proc. Natl. Acad. Sci. USA 2011, 108, 8698–8703. [Google Scholar] [CrossRef] [PubMed]
- Miesfeld, J.B.; Gestri, G.; Clark, B.S.; Flinn, M.A.; Poole, R.J.; Bader, J.R.; Link, B.A.; Besharse, J.C.; Wilson, S.W. Yap and Taz regulate retinal pigment epithelial cell fate. Development 2015, 142, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Willer, J.R.; Willer, G.B.; Smith, K.; Gregg, R.G.; Gross, J.M. Zebrafish blowout provides genetic evidence for Patched1-mediated negative regulation of Hedgehog signaling within the proximal optic vesicle of the vertebrate eye. Dev. Biol. 2008, 319, 10–22. [Google Scholar] [CrossRef]
- Knickmeyer, M.D.; Mateo, J.L.; Eckert, P.; Roussa, E.; Rahhal, B.; Zuniga, A.; Heermann, S.; Krieglstein, K.; Wittbrodt, J. TGFβ-facilitated optic fissure fusion and the role of bone morphogenetic protein antagonism. Open Biol. 2018, 8, 170314. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Filas, B.; Gunhaga, L.; Beebe, D.C. Negative and positive auto-regulation of BMP expression in early eye development. Dev. Biol. 2015, 407, 256–264. [Google Scholar] [CrossRef]
- Gestri, G.; Link, B.A.; Neuhauss, S.C.F. The visual system of zebrafish and its use to model human ocular diseases. Dev. Neurobiol. 2012, 72, 302–327. [Google Scholar] [CrossRef]
- Heermann, S.; Schütz, L.; Lemke, S.; Krieglstein, K.; Wittbrodt, J. Eye morphogenesis driven by epithelial flow into the optic cup facilitated by modulation of bone morphogenetic protein. Elife 2015, 4, e05216. [Google Scholar] [CrossRef]
- Gestri, G.; Bazin-Lopez, N.; Scholes, C.; Wilson, S.W. Cell Behaviors during Closure of the Choroid Fissure in the Developing Eye. Front. Cell Neurosci. 2018, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Lee, C.; Williams, A.M.; Angileri, K.; Lathrop, K.L.; Gross, J.M. The hyaloid vasculature facilitates basement membrane breakdown during choroid fissure closure in the zebrafish eye. Dev. Biol. 2016, 419, 262–272. [Google Scholar] [CrossRef] [PubMed]
- See, A.W.-M.; Clagett-Dame, M. The temporal requirement for vitamin A in the developing eye: Mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev. Biol. 2009, 325, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.; Weinstein, B.M.; Detrich, H.W.; Zon, L.I.; Fishman, M.C. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 1995, 121, 3141–3150. [Google Scholar] [PubMed]
- Thompson, M.A.; Ransom, D.G.; Pratt, S.J.; MacLennan, H.; Kieran, M.W.; Detrich, H.W., III; Oates, A.C.; Vail, B.; Huber, T.L.; Paw, B.; et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 1998, 197, 248–269. [Google Scholar] [CrossRef] [PubMed]
- Reischauer, S.; Stone, O.A.; Villasenor, A.; Chi, N.; Jin, S.W.; Martin, M.; Fiddes, I.; Lee, M.T.; Fukuda, N.; Marass, M.; et al. Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature 2016, 535, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Marass, M.; Beisaw, A.; Gerri, C.; Luzzani, F.; Fukuda, N.; Günther, S.; Stainier, D.Y.; Kuenne, C.; Reischauer, S. Genome-wide strategies reveal target genes of Npas4l associated with vascular development in zebrafish. Development 2019, 146, dev173427. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Stevens, C.B.; Sebbagh, M.; Weiss, O.; Frey, R.A.; Adamson, S.; Stenkamp, D.L.; Shelden, E.A.; Inbal, A. Abnormal retinal development in Cloche mutant zebrafish. Dev. Dyn. 2015, 244, 1439–1455. [Google Scholar] [CrossRef] [PubMed]
- Weiss, O.; Kaufman, R.; Michaeli, N.; Inbal, A. Abnormal vasculature interferes with optic fissure closure in lmo2 mutant zebrafish embryos. Dev. Biol. 2012. [Google Scholar] [CrossRef]
- Yamada, Y.; Pannell, R.; Forster, A.; Rabbitts, T.H. The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 320–324. [Google Scholar] [CrossRef]
- Hardy, H.; Prendergast, J.G.; Patel, A.; Dutta, S.; Trejo-Reveles, V.; Kroeger, H.; Rainger, J.; Yung, A.R.; Goodrich, L.V.; Brooks, B.; et al. Detailed analysis of chick optic fissure closure reveals Netrin-1 as an essential mediator of epithelial fusion. Elife 2019, 8, e43877. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Gómez-Pardo, E.; Gruss, P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development 1996, 122, 3381–3391. [Google Scholar] [PubMed]
- Piedade, W.P.; Veith, S.; Famulski, J.K. Ubiquitin-mediated proteasome degradation regulates optic fissure fusion. Biol. Open 2019, 8, bio044974. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Owen, N.; Toms, M.; Young, R.M.; Tracey-White, D.; Moosajee, M. Transcriptome profiling of zebrafish optic fissure fusion. Sci. Rep. 2019, 9, 1541. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, S.; Takabatake, T.; Takabatake, Y.; Muramatsu, T.; Takeshima, K. Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. Genesis 2002, 33, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Behesti, H.; Holt, J.K.L.; Sowden, J.C. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev. Biol. 2006, 6, 62. [Google Scholar] [CrossRef]
- French, C.R.; Erickson, T.; French, D.V.; Pilgrim, D.B.; Waskiewicz, A.J. Gdf6a is required for the initiation of dorsal-ventral retinal patterning and lens development. Dev. Biol. 2009, 333, 37–47. [Google Scholar] [CrossRef]
- Morcillo, J.; Martínez-Morales, J.R.; Trousse, F.; Fermin, Y.; Sowden, J.C.; Bovolenta, P. Proper patterning of the optic fissure requires the sequential activity of BMP7 and SHH. Development 2006, 133, 3179–3190. [Google Scholar] [CrossRef]
- Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 2010, 93, 61–84. [Google Scholar]
- Steinfeld, J.; Steinfeld, I.; Coronato, N.; Hampel, M.L.; Layer, P.G.; Araki, M.; Vogel-Höpker, A. RPE specification in the chick is mediated by surface ectoderm-derived BMP and Wnt signalling. Development 2013, 140, 4959–4969. [Google Scholar] [CrossRef]
- Hocking, J.C.; Famulski, J.K.; Yoon, K.H.; Widen, S.A.; Bernstein, C.S.; Koch, S.; Waskiewicz, A.J.; Weiss, O.; Agarwala, S.; Inbal, A.; et al. Morphogenetic defects underlie Superior Coloboma, a newly identified closure disorder of the dorsal eye. PLoS Genet. 2018, 14, e1007246. [Google Scholar] [CrossRef] [PubMed]
- Lahrouchi, N.; George, A.; Ratbi, I.; Schneider, R.; Elalaoui, S.C.; Moosa, S.; Adadi, N.; Bharti, S.; Sharma, R.; Onojafe, F.; et al. Homozygous frameshift mutations in FAT1 cause a syndrome characterized by colobomatous-microphthalmia, ptosis, nephropathy and syndactyly. Nat. Commun. 2019, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Goodman, J.; Anderson, K.V.; Niswander, L. Phactr4 regulates neural tube and optic fissure closure by controlling PP1-, Rb-, and E2F1-regulated cell-cycle progression. Dev. Cell 2007, 13, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Lee, H.; Park, E.; Park, S. Proper closure of the optic fissure requires ephrin A5-EphB2-JNK signaling. Development 2016, 143, 461–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozeki, H.; Ogura, Y.; Hirabayashi, Y.; Shimada, S. Apoptosis is associated with formation and persistence of the embryonic fissure. Curr. Eye Res. 2000, 20, 367–372. [Google Scholar] [CrossRef]
- Hero, I. Optic fissure closure in the normal cinnamon mouse. An ultrastructural study. Investig. Ophthalmol. Vis. Sci. 1990, 31, 197–216. [Google Scholar]
- Eckert, P.; Schütz, L.; Wittbrodt, J.; Heermann, S. Optic fissure margin morphogenesis sets the stage for consecutive optic fissure fusion, pioneered by a distinct subset of margin cells using a hyaloid vessel as scaffold. bioRxiv 2017. [Google Scholar] [CrossRef]
- Reinhardt, R.; Centanin, L.; Tavhelidse, T.; Inoue, D.; Wittbrodt, B.; Concordet, J.P.; Wittbrodt, J.; Martinez-Morales, J.R. Sox2, Tlx, Gli3, and Her9 converge on Rx2 to define retinal stem cells in vivo. EMBO J. 2015, 34, 1572–1588. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Tinevez, J.Y.; Preibisch, S.; Rueden, C.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bernstein, C.S.; Anderson, M.T.; Gohel, C.; Slater, K.; Gross, J.M.; Agarwala, S. The cellular bases of choroid fissure formation and closure. Dev. Biol. 2018, 440, 137–151. [Google Scholar] [CrossRef]
- Revenu, C.; Streichan, S.; Donà, E.; Lecaudey, V.; Hufnagel, L.; Gilmour, D. Quantitative cell polarity imaging defines leader-to-follower transitions during collective migration and the key role of microtubule-dependent adherens junction formation. Development 2014, 141, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Cechmanek, P.B.; McFarlane, S. Retinal pigment epithelium expansion around the neural retina occurs in two separate phases with distinct mechanisms. Dev. Dyn. 2017, 246, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.; Weiss, O.; Sebbagh, M.; Ravid, R.; Gibbs-Bar, L.; Yaniv, K.; Inbal, A. Development and origins of zebrafish ocular vasculature. BMC Dev. Biol. 2015, 15, 18. [Google Scholar] [CrossRef]
- Müller, I.I.; Knapik, E.W.; Hatzopoulos, A.K. Expression of the protein related to Dan and Cerberus gene--prdc--During eye, pharyngeal arch, somite, and swim bladder development in zebrafish. Dev. Dyn. 2006, 235, 2881–2888. [Google Scholar] [CrossRef]
- Collery, R.F.; Link, B.A. Dynamic smad-mediated BMP signaling revealed through transgenic zebrafish. Dev. Dyn. 2011, 240, 712–722. [Google Scholar] [CrossRef]
- Veien, E.S.; Rosenthal, J.S.; Kruse-Bend, R.C.; Chien, C.-B.; Dorsky, R.I. Canonical Wnt signaling is required for the maintenance of dorsal retinal identity. Development 2008, 135, 4101–4111. [Google Scholar] [CrossRef]
- Dutton, K.A.; Pauliny, A.; Lopes, S.S.; Elworthy, S.; Carney, T.J.; Rauch, J.; Kelsh, R.N.; Geisler, R.; Haffter, P. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 2001, 128, 4113–4125. [Google Scholar]
- Langenberg, T.; Kahana, A.; Wszalek, J.A.; Halloran, M.C. The eye organizes neural crest cell migration. Dev. Dyn. 2008, 237, 1645–1652. [Google Scholar] [CrossRef]
- Bryan, C.D.; Casey, M.A.; Pfeiffer, R.L.; Jones, B.W.; Kwan, K.M. Optic cup morphogenesis requires neural crest-mediated basement membrane assembly. Development 2020, 147, dev181420. [Google Scholar] [CrossRef]
- Cao, M.; Ouyang, J.; Liang, H.; Guo, J.; Lin, S.; Yang, S.; Chen, S.; Xie, T. Regional Gene Expression Profile Comparison Reveals the Unique Transcriptome of the Optic Fissure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5773–5784. [Google Scholar] [CrossRef] [PubMed]
- Weiss, O.; Kaufman, R.; Mishani, E.; Inbal, A. Ocular vessel patterning in zebrafish is indirectly regulated by Hedgehog signaling. Int. J. Dev. Biol. 2017, 61, 277–284. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Kwan, K.M.; Fujimoto, E.; Grabher, C.; Mangum, B.D.; Hardy, M.E.; Campbell, D.S.; Chien, C.B.; Parant, J.M.; Yost, H.J.; Kanki, J.P. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007, 236, 3088–3099. [Google Scholar] [CrossRef] [PubMed]
- Luisier, F.; Vonesch, C.; Blu, T.; Unser, M. Fast interscale wavelet denoising of Poisson-corrupted images. Signal Process. 2010, 90, 415–427. [Google Scholar] [CrossRef]
- Quiring, R.; Wittbrodt, B.; Henrich, T.; Ramialison, M.; Burgtorf, C.; Lehrach, H.; Wittbrodt, J. Large-scale expression screening by automated whole-mount in situ hybridization. Mech. Dev. 2004, 121, 971–976. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, P.; Knickmeyer, M.D.; Heermann, S. In Vivo Analysis of Optic Fissure Fusion in Zebrafish: Pioneer Cells, Basal Lamina, Hyaloid Vessels, and How Fissure Fusion is Affected by BMP. Int. J. Mol. Sci. 2020, 21, 2760. https://doi.org/10.3390/ijms21082760
Eckert P, Knickmeyer MD, Heermann S. In Vivo Analysis of Optic Fissure Fusion in Zebrafish: Pioneer Cells, Basal Lamina, Hyaloid Vessels, and How Fissure Fusion is Affected by BMP. International Journal of Molecular Sciences. 2020; 21(8):2760. https://doi.org/10.3390/ijms21082760
Chicago/Turabian StyleEckert, Priska, Max D. Knickmeyer, and Stephan Heermann. 2020. "In Vivo Analysis of Optic Fissure Fusion in Zebrafish: Pioneer Cells, Basal Lamina, Hyaloid Vessels, and How Fissure Fusion is Affected by BMP" International Journal of Molecular Sciences 21, no. 8: 2760. https://doi.org/10.3390/ijms21082760
APA StyleEckert, P., Knickmeyer, M. D., & Heermann, S. (2020). In Vivo Analysis of Optic Fissure Fusion in Zebrafish: Pioneer Cells, Basal Lamina, Hyaloid Vessels, and How Fissure Fusion is Affected by BMP. International Journal of Molecular Sciences, 21(8), 2760. https://doi.org/10.3390/ijms21082760



