Anti-Aging Effects of GDF11 on Skin
Abstract
1. Introduction
2. Skin and Cell Regeneration
3. Skin Aging
4. Cutaneous Wound Healing
5. Growth Factors and Regenerative Process
5.1. Epidermal Growth Factor Receptor (EGFR) and Its Ligands
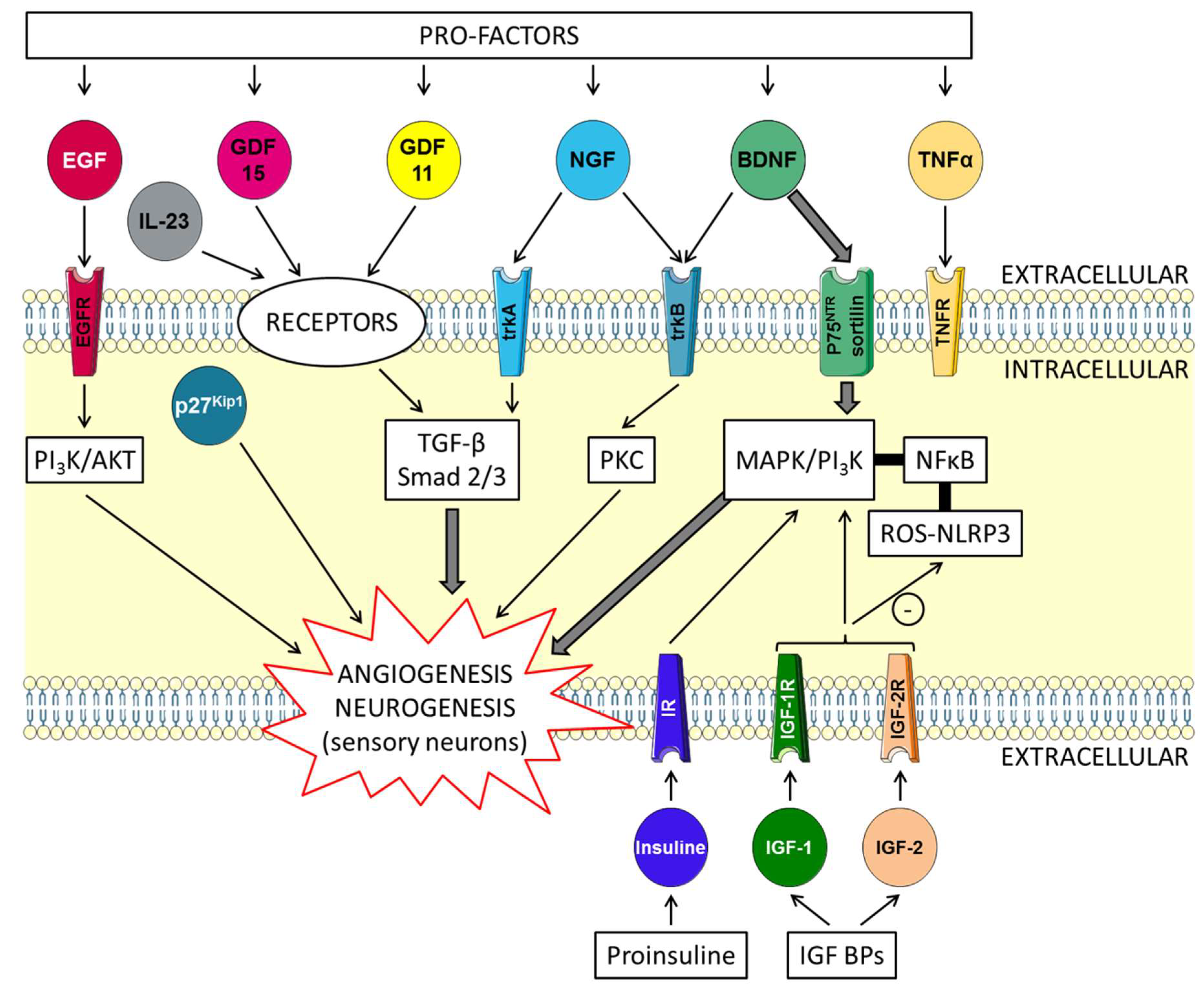
5.2. Brain-Derived Neurotrophic Factor (BDNF) and Insulin-Like Growth Factors (IGFs) Trophic Factors with “Yin and Yang” Effects on the Skin (Figure 1)
6. Potential Activity of Endogenous Factors on Skin Regeneration: Role of GDF11
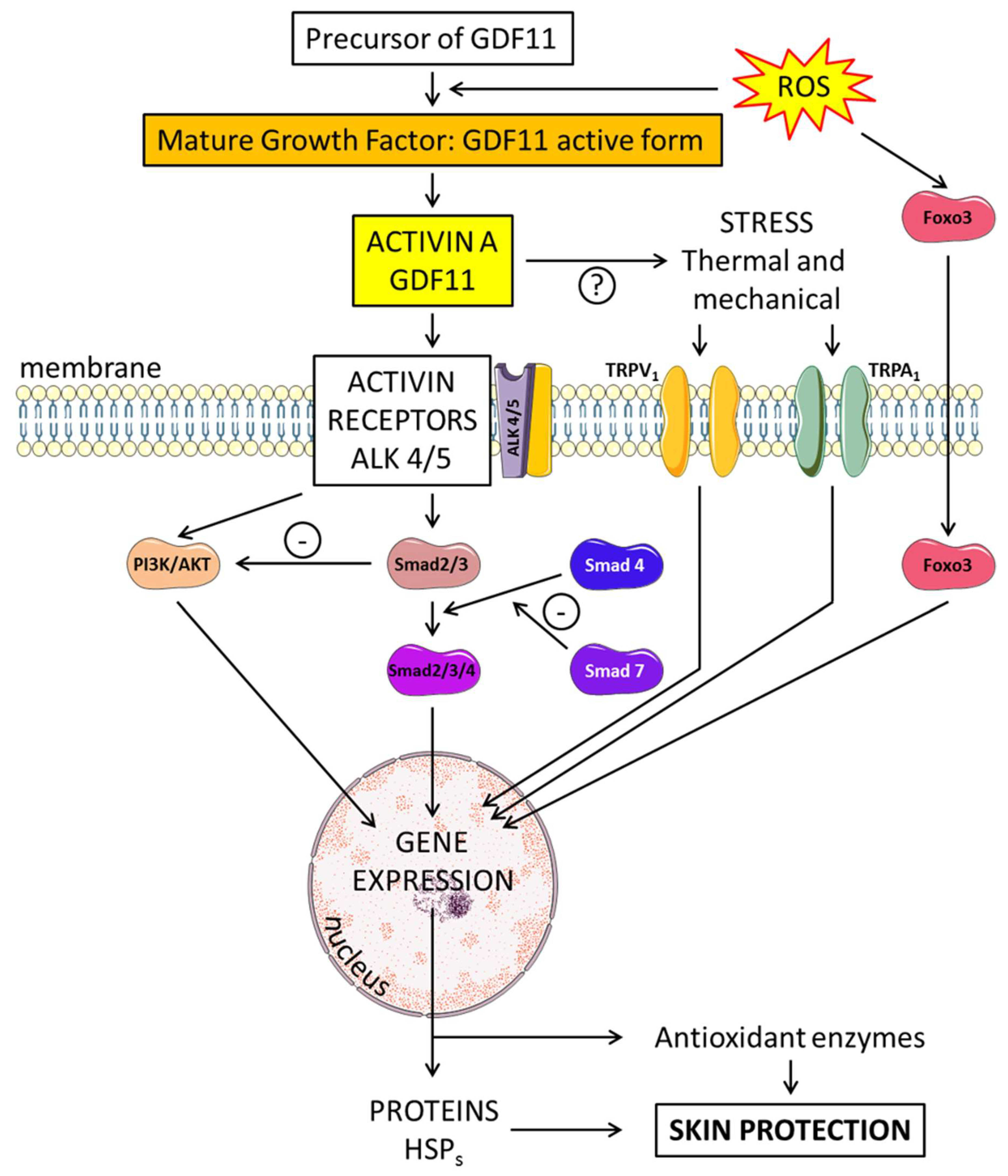
6.1. Structure and Formation of GDF11
6.2. Effects of GDF11 on Skin Regeneration
7. Conclusions and Future Directions
Funding
Conflicts of Interest
Abbreviations
| ACT | Activin |
| ALKR | Activin receptor |
| BDNF | Brain-derived neurotrophic factor |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| ESC | Epidermal stem cell |
| GDF | Growth differentiation factor |
| GF | Growth factor |
| HDAC | Histone deacetylase |
| IGR | Insulin-like growth receptor |
| IL | Interleukin |
| IR | Insulin receptor |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| MSC | Mesenchymal stem cell |
| NGF | Nerve growth factor |
| NLR | Nucleotide-binding oligomerization domain-like receptor |
| ROS | Reactive oxygen species |
| SC | Stem cell |
| TGF | Transforming growth factor |
| TNF | Tumor necrosis factor |
| TRPA | Transient receptor potential ankyrin |
| TRPV | Transient receptor potential vanilloid |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
References
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–399. [Google Scholar]
- Nystrom, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef]
- Koivisto, L.; Bi, J.; Hakkinen, L.; Larjava, H. Integrin alphavbeta6: Structure, function and role in health and disease. Int. J. Biochem. Cell Biol. 2018, 99, 186–196. [Google Scholar] [CrossRef]
- Senoo, M. Epidermal Stem Cells in Homeostasis and Wound Repair of the Skin. Adv. Wound Care 2013, 2, 273–282. [Google Scholar] [CrossRef]
- Nurkovic, J.; Volarevic, V.; Lako, M.; Armstrong, L.; Arsenijevic, N.; Stojkovic, M. Aging of Stem and Progenitor Cells: Mechanisms, Impact on Therapeutic Potential, and Rejuvenation. Rejuvenation Res. 2016, 19, 3–12. [Google Scholar] [CrossRef]
- Kodji, X.; Aubdool, A.A.; Brain, S.D. Evidence for physiological and pathological roles for sensory nerves in the microvasculature and skin. Curr. Res. Transl. Med. 2016, 64, 195–201. [Google Scholar] [CrossRef]
- Schwendinger-Schreck, J.; Wilson, S.R.; Bautista, D.M. Interactions between keratinocytes and somatosensory neurons in itch. Handb. Exp. Pharmacol. 2015, 226, 177–190. [Google Scholar]
- Poitras, T.; Chandrasekhar, A.; McCoy, L.; Komirishetty, P.; Areti, A.; Webber, C.A.; Zochodne, D.W. Selective Sensory Axon Reinnervation and TRPV1 Activation. Mol. Neurobiol. 2019, 56, 7144–7158. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Zhang, Y. Cell toxicity mechanism and biomarker. Clin. Transl. Med. 2018, 7, 34. [Google Scholar] [CrossRef]
- Kuk, M.U.; Kim, J.W.; Lee, Y.S.; Cho, K.A.; Park, J.T.; Park, S.C. Alleviation of Senescence via ATM Inhibition in Accelerated Aging Models. Mol. Cells 2019, 42, 210–217. [Google Scholar]
- Orioli, D.; Dellambra, E. Epigenetic Regulation of Skin Cells in Natural Aging and Premature Aging Diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Serra, M.B.; Barroso, W.A.; da Silva, N.N.; Silva, S.D.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflamm. 2017, 2017, 3406215. [Google Scholar] [CrossRef]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef]
- Wu, N.; Rollin, J.; Masse, I.; Lamartine, J.; Gidrol, X. p63 regulates human keratinocyte proliferation via MYC-regulated gene network and differentiation commitment through cell adhesion-related gene network. J. Biol. Chem. 2012, 287, 5627–5638. [Google Scholar] [CrossRef]
- Kretzschmar, K.; Clevers, H. Wnt/beta-catenin signaling in adult mammalian epithelial stem cells. Dev. Biol. 2017, 428, 273–282. [Google Scholar] [CrossRef]
- Gutowska-Owsiak, D.; Ogg, G.S. Cytokine regulation of the epidermal barrier. Clin. Exp. Allergy 2013, 43, 586–598. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Peng, H.; Zeng, K. Recent advances on the roles of epidermal growth factor receptor in psoriasis. Am. J. Transl. Res. 2019, 11, 520–528. [Google Scholar]
- Nanba, D.; Toki, F.; Barrandon, Y.; Higashiyama, S. Recent advances in the epidermal growth factor receptor/ligand system biology on skin homeostasis and keratinocyte stem cell regulation. J. Dermatol. Sci. 2013, 72, 81–86. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, C.; Gibson, A.M.; Bass, S.A.; Khurana Hershey, G.K. EGFR signaling blunts allergen-induced IL-6 production and Th17 responses in the skin and attenuates development and relapse of atopic dermatitis. J. Immunol. 2014, 192, 859–866. [Google Scholar] [CrossRef]
- Le, M.; Naridze, R.; Morrison, J.; Biggs, L.C.; Rhea, L.; Schutte, B.C.; Kaartinen, V.; Dunnwald, M. Transforming growth factor Beta 3 is required for excisional wound repair in vivo. PLoS ONE 2012, 7, e48040. [Google Scholar] [CrossRef]
- McGregor, C.E.; English, A.W. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front. Cell. Neurosci. 2018, 12, 522. [Google Scholar] [CrossRef]
- Lu, L.; Bai, X.; Cao, Y.; Luo, H.; Yang, X.; Kang, L.; Shi, M.J.; Fan, W.; Zhao, B.Q. Growth Differentiation Factor 11 Promotes Neurovascular Recovery After Stroke in Mice. Front. Cell. Neurosci. 2018, 12, 205. [Google Scholar] [CrossRef]
- Fernandez, T.L.; Van Lonkhuyzen, D.R.; Dawson, R.A.; Kimlin, M.G.; Upton, Z. Insulin-like growth factor-I and UVB photoprotection in human keratinocytes. Exp. Dermatol. 2015, 24, 235–238. [Google Scholar] [CrossRef]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef]
- Landi, F.; Aloe, L.; Russo, A.; Cesari, M.; Onder, G.; Bonini, S.; Carbonin, P.U.; Bernabei, R. Topical treatment of pressure ulcers with nerve growth factor: A randomized clinical trial. Ann. Intern. Med. 2003, 139, 635–641. [Google Scholar] [CrossRef]
- Generini, S.; Tuveri, M.A.; Matucci Cerinic, M.; Mastinu, F.; Manni, L.; Aloe, L. Topical application of nerve growth factor in human diabetic foot ulcers. A study of three cases. Exp. Clin. Endocrinol. Diabetes 2004, 112, 542–544. [Google Scholar] [CrossRef]
- Semenova, E.; Koegel, H.; Hasse, S.; Klatte, J.E.; Slonimsky, E.; Bilbao, D.; Paus, R.; Werner, S.; Rosenthal, N. Overexpression of mIGF-1 in keratinocytes improves wound healing and accelerates hair follicle formation and cycling in mice. Am. J. Pathol. 2008, 173, 1295–1310. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Growth and differentiation factor 11 (GDF11): Functions in the regulation of erythropoiesis and cardiac regeneration. Pharmacol. Ther. 2015, 156, 26–33. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Malka, G.; Vergely, C. Regenerative Capacity of Endogenous Factor: Growth Differentiation Factor 11; a New Approach of the Management of Age-Related Cardiovascular Events. Int. J. Mol. Sci. 2018, 19, 3998. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Steinhauser, M.L.; Jay, S.M.; Gannon, J.; Pancoast, J.R.; Yalamanchi, P.; Sinha, M.; Dall’Osso, C.; Khong, D.; Shadrach, J.L.; et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013, 153, 828–839. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Liu, D.; Liu, F.; Li, X.; Pan, L.; Pang, Y.; Chen, D. Role of growth differentiation factor 11 in development, physiology and disease. Oncotarget 2017, 8, 81604–81616. [Google Scholar] [CrossRef]
- Zhang, X.; Wharton, W.; Yuan, Z.; Tsai, S.C.; Olashaw, N.; Seto, E. Activation of the growth-differentiation factor 11 gene by the histone deacetylase (HDAC) inhibitor trichostatin A and repression by HDAC3. Mol. Cell. Biol. 2004, 24, 5106–5118. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.P. Gdf11 facilitates temporal progression of neurogenesis in the developing spinal cord. J. Neurosci. 2011, 31, 883–893. [Google Scholar] [CrossRef]
- Hara, T.; Miyazaki, M.; Hakuno, F.; Takahashi, S.; Chida, K. PKCeta promotes a proliferation to differentiation switch in keratinocytes via upregulation of p27Kip1 mRNA through suppression of JNK/c-Jun signaling under stress conditions. Cell Death Dis. 2011, 2, e157. [Google Scholar] [CrossRef]
- Granata, M.; Skarmoutsou, E.; Gangemi, P.; Mazzarino, M.C.; D’Amico, F. S100A7, Jab1, and p27(kip1) expression in psoriasis and S100A7 CRISPR-activated human keratinocyte cell line. J. Cell Biochem. 2019, 120, 3384–3392. [Google Scholar] [CrossRef]
- Wang, W.; Qu, R.; Wang, X.; Zhang, M.; Zhang, Y.; Chen, C.; Chen, X.; Qiu, C.; Li, J.; Pan, X.; et al. GDF11 Antagonizes Psoriasis-like Skin Inflammation via Suppression of NF-kappaB Signaling Pathway. Inflammation 2019, 42, 319–330. [Google Scholar] [CrossRef]
- Tito, A.; Barbulova, A.; Zappelli, C.; Leone, M.; Ruvo, M.; Mercurio, F.A.; Chambery, A.; Russo, R.; Colucci, M.G.; Apone, F. The Growth Differentiation Factor 11 is Involved in Skin Fibroblast Ageing and is Induced by a Preparation of Peptides and Sugars Derived from Plant Cell Cultures. Mol. Biotechnol. 2019, 61, 209–220. [Google Scholar] [CrossRef]
- Kim, Y.J.; Seo, D.H.; Lee, S.H.; Lee, S.H.; An, G.H.; Ahn, H.J.; Kwon, D.; Seo, K.W.; Kang, K.S. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem. Biophys. Rep. 2018, 16, 96–102. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Liu, L.; Qu, R.; Chen, X.; Qiu, C.; Li, J.; Hayball, J.; Liu, L.; Chen, J.; et al. GDF11 antagonizes TNF-alpha-induced inflammation and protects against the development of inflammatory arthritis in mice. FASEB J. 2019, 33, 3317–3329. [Google Scholar] [CrossRef]
- Moura, J.; da Silva, L.; Cruz, M.T.; Carvalho, E. Molecular and cellular mechanisms of bone morphogenetic proteins and activins in the skin: Potential benefits for wound healing. Arch. Dermatol. Res. 2013, 305, 557–569. [Google Scholar] [CrossRef]
- Idkowiak-Baldys, J.; Santhanam, U.; Buchanan, S.M.; Pfaff, K.L.; Rubin, L.L.; Lyga, J. Growth differentiation factor 11 (GDF11) has pronounced effects on skin biology. PLoS ONE 2019, 14, e0218035. [Google Scholar] [CrossRef]
- Chen, J.L.; Colgan, T.D.; Walton, K.L.; Gregorevic, P.; Harrison, C.A. The TGF-beta Signalling Network in Muscle Development, Adaptation and Disease. Adv. Exp. Med. Biol. 2016, 900, 97–131. [Google Scholar]
- Subramaniam, N.; Petrik, J.J.; Vickaryous, M.K. VEGF, FGF-2 and TGFbeta expression in the normal and regenerating epidermis of geckos: Implications for epidermal homeostasis and wound healing in reptiles. J. Anat. 2018, 232, 768–782. [Google Scholar] [CrossRef]
- Jacquemin, C.; Rambert, J.; Guillet, S.; Thiolat, D.; Boukhedouni, N.; Doutre, M.S.; Darrigade, A.S.; Ezzedine, K.; Blanco, P.; Taieb, A.; et al. Heat shock protein 70 potentiates interferon alpha production by plasmacytoid dendritic cells: Relevance for cutaneous lupus and vitiligo pathogenesis. Br. J. Dermatol. 2017, 177, 1367–1375. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochette, L.; Mazini, L.; Meloux, A.; Zeller, M.; Cottin, Y.; Vergely, C.; Malka, G. Anti-Aging Effects of GDF11 on Skin. Int. J. Mol. Sci. 2020, 21, 2598. https://doi.org/10.3390/ijms21072598
Rochette L, Mazini L, Meloux A, Zeller M, Cottin Y, Vergely C, Malka G. Anti-Aging Effects of GDF11 on Skin. International Journal of Molecular Sciences. 2020; 21(7):2598. https://doi.org/10.3390/ijms21072598
Chicago/Turabian StyleRochette, Luc, Loubna Mazini, Alexandre Meloux, Marianne Zeller, Yves Cottin, Catherine Vergely, and Gabriel Malka. 2020. "Anti-Aging Effects of GDF11 on Skin" International Journal of Molecular Sciences 21, no. 7: 2598. https://doi.org/10.3390/ijms21072598
APA StyleRochette, L., Mazini, L., Meloux, A., Zeller, M., Cottin, Y., Vergely, C., & Malka, G. (2020). Anti-Aging Effects of GDF11 on Skin. International Journal of Molecular Sciences, 21(7), 2598. https://doi.org/10.3390/ijms21072598




