Recombinant Human Plasma Gelsolin Stimulates Phagocytosis while Diminishing Excessive Inflammatory Responses in Mice with Pseudomonas aeruginosa Sepsis
Abstract
1. Introduction
2. Results
2.1. pGSN-800CW Synthesis and Physicochemical Evaluation of Dye-Labeled pGSN
2.2. The Biodistribution of IRDye® 800CW-Labeled pGSN in Healthy and PA Xen5-Infected Mice
2.3. Administration of pGSN Decreases the Inflammatory Response in Septic Mice
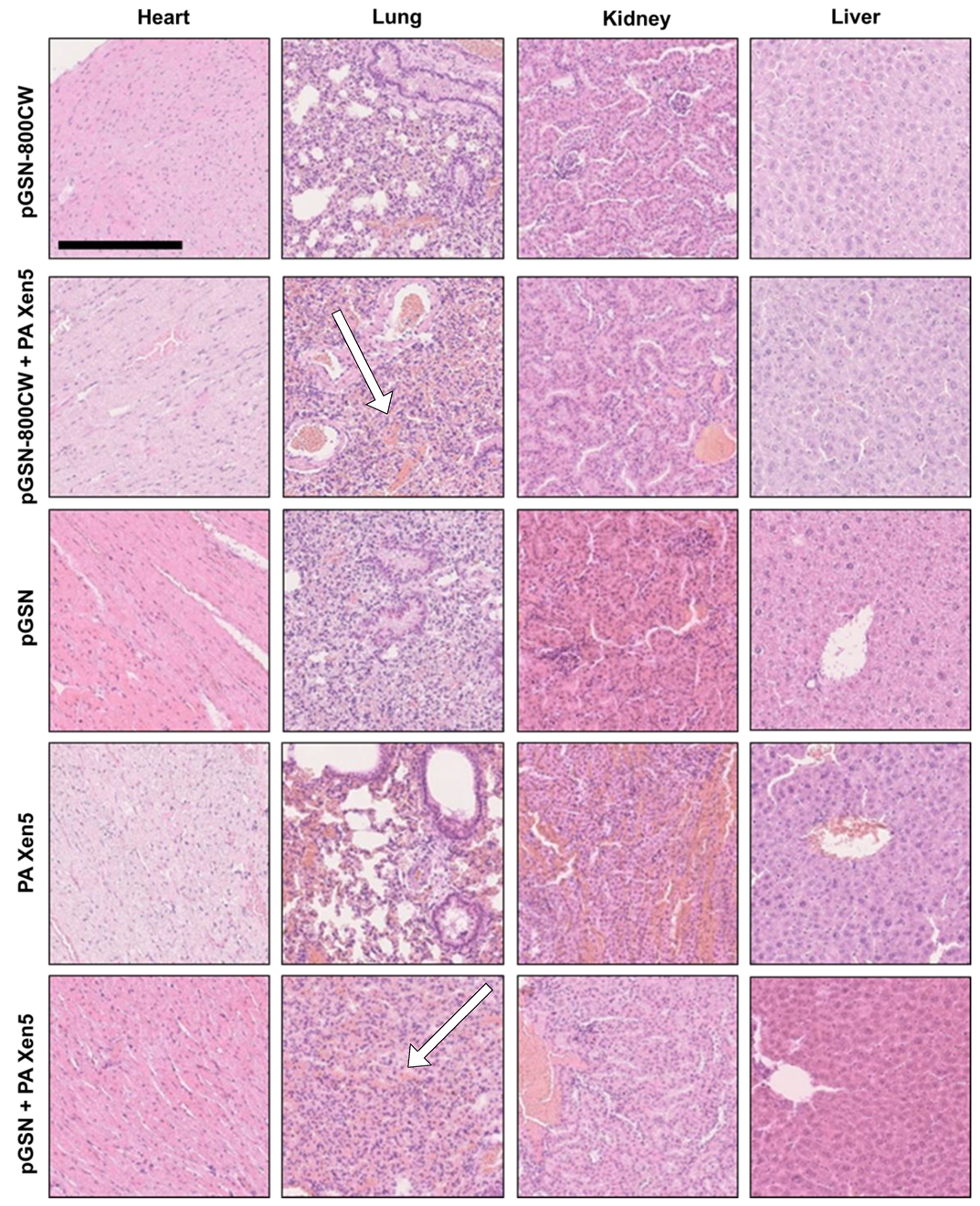
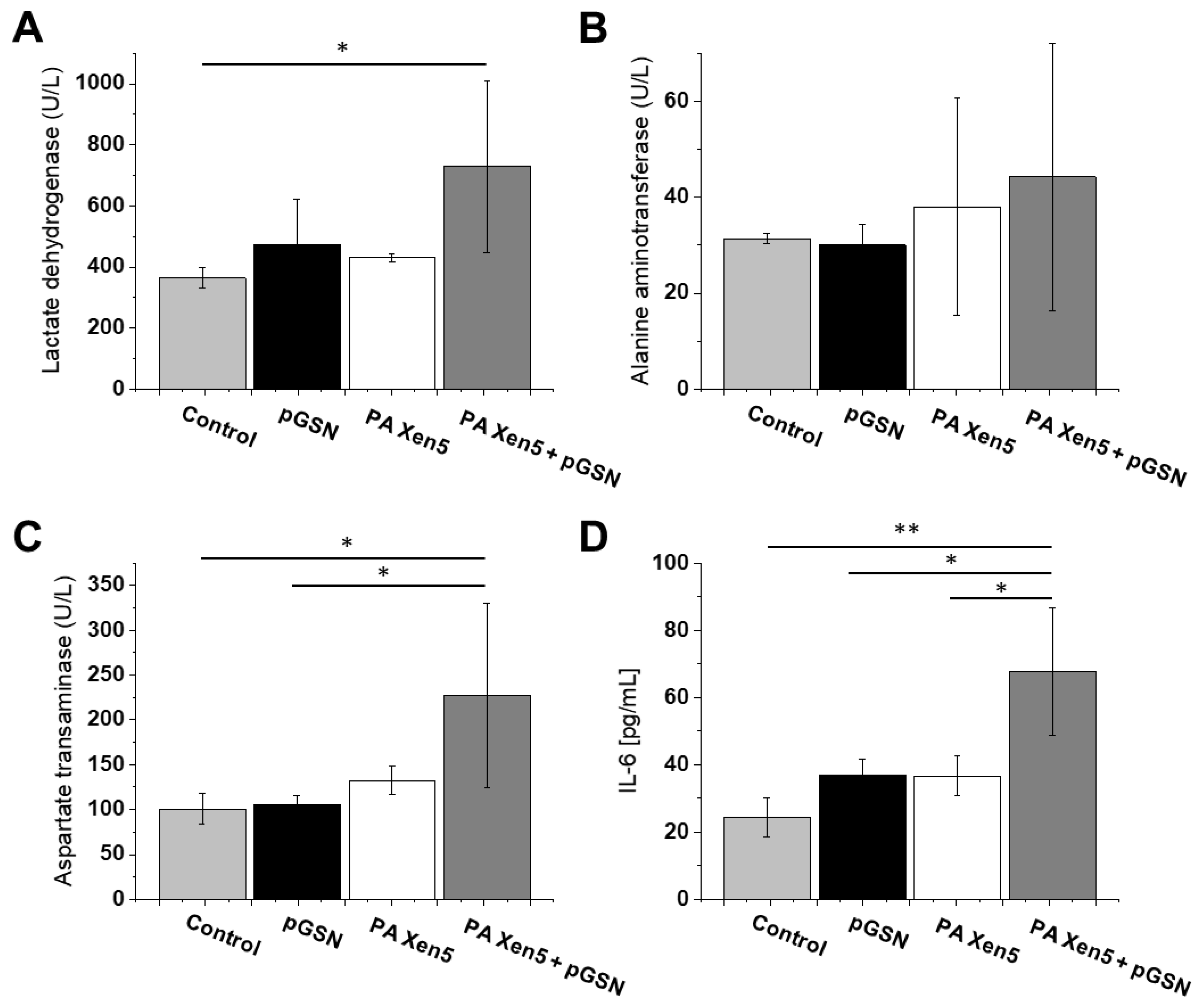
2.4. Histopathological and Biochemical Analysis
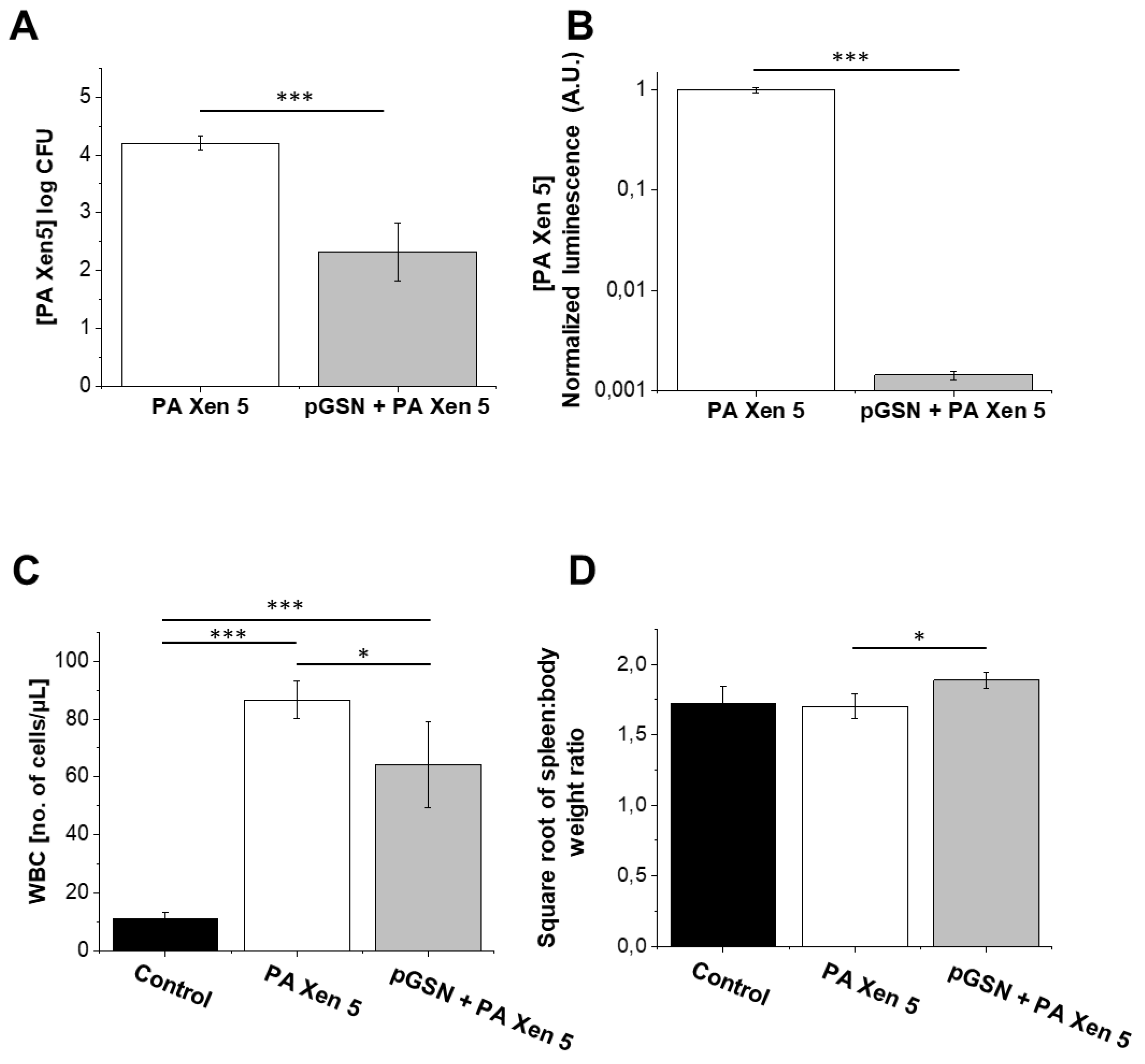
2.5. Bactericidal Activities of Exogenously Administrated pGSN
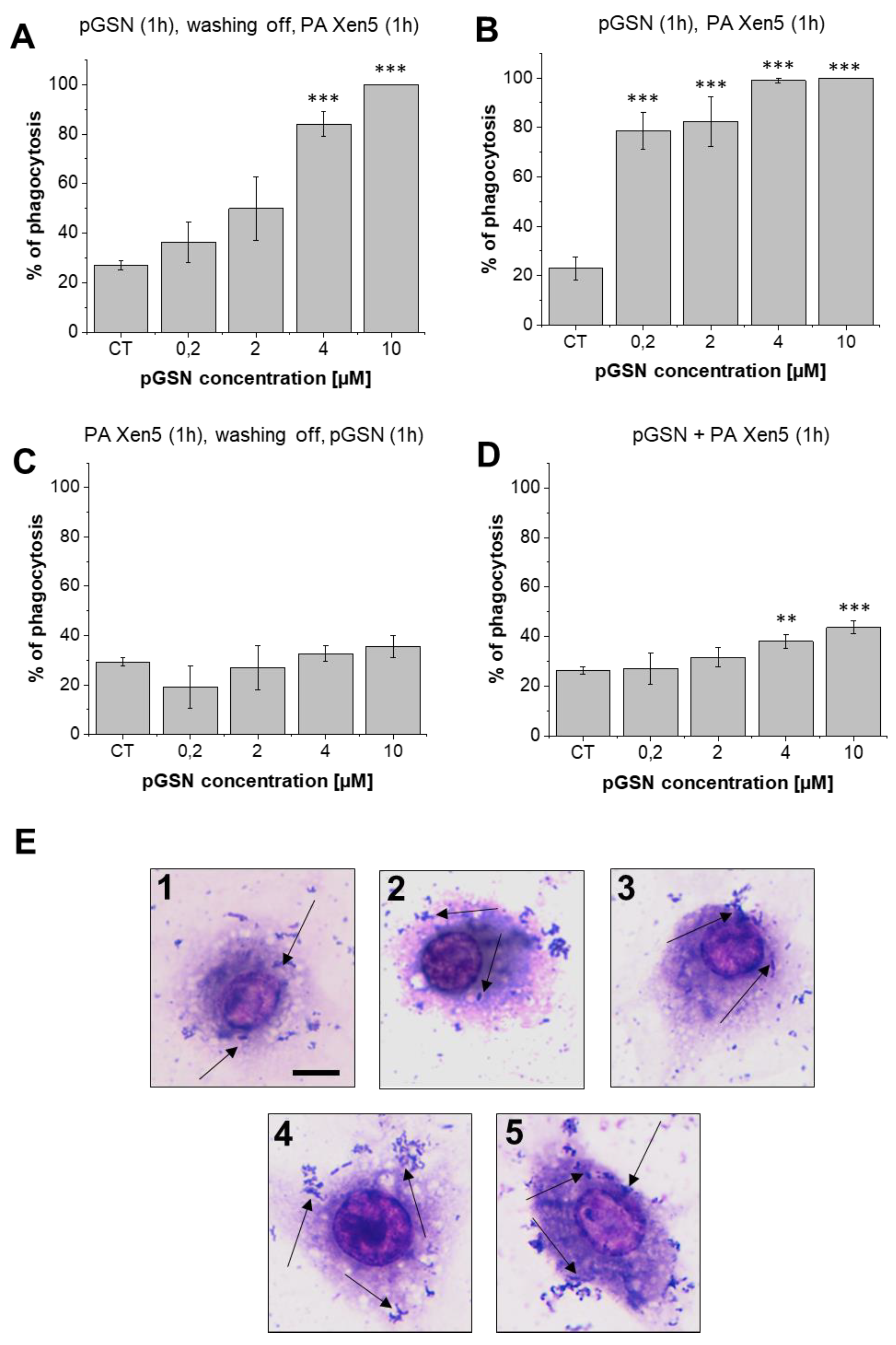
2.6. pGSN Increases Phagocytosis of PA Xen5 by Macrophages
3. Discussion
4. Materials and Methods
4.1. Labeling of pGSN with IRDye® 800CW
4.2. Induction of Sepsis due to Peritonitis
4.3. Biodistribution of pGSN-800CW
4.4. Evaluation of Anti-Inflammatory and Bactericidal Effects of pGSN Administration. Histopathological Analysis
4.5. Analysis of Phagocytosis Using In Vitro Settings
4.6. Biochemical Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DG-800CW | IRDye 800CW-labelled 2-deoxyglucose |
| GSN | gelsolin |
| pGSN | plasma gelsolin |
| pGSN-800CW | IRDye 800CW-labelled plasma gelsolin |
| Pa Xen5 | Pseudomonas aeruginosa Xen5 strain |
References
- Lee, P.S.; Patel, S.R.; Christiani, D.C.; Bajwa, E.; Stossel, T.P.; Waxman, A.B. Plasma gelsolin depletion and circulating actin in sepsis: A pilot study. PLoS ONE 2008, 3, e3712. [Google Scholar] [CrossRef] [PubMed]
- Li-ChunHsieh, K.; Schob, S.; Zeller, M.W.; Pulli, B.; Ali, M.; Wang, C.; Chiou, T.T.; Tsang, Y.M.; Lee, P.S.; Stossel, T.P.; et al. Gelsolin decreases actin toxicity and inflammation in murine multiple sclerosis. J. Neuroimmunol. 2015, 287, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Kulakowska, A.; Byfield, F.J.; Zendzian-Piotrowska, M.; Baranowski, M.; Marzec, M.; Winer, J.P.; Ciccarelli, N.J.; Górski, J.; Drozdowski, W.; et al. Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. Am. J. Physiol. Cell Physiol. 2010, 299, C1516–C1523. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Georges, P.C.; Espinassous, Q.; Funaki, M.; Pastore, J.J.; Chaby, R.; Janmey, P.A. Inactivation of endotoxin by human plasma gelsolin. Biochemistry 2005, 44, 9590–9597. [Google Scholar] [CrossRef]
- Piktel, E.; Levental, I.; Durnas, B.; Janmey, P.A.; Bucki, R. Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target. Int. J. Mol. Sci. 2018, 19, 2516. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Scherpereel, A.; Solomides, C.C.; Muzykantov, V.R.; Machtay, M.; Albelda, S.M.; DiNubile, M.J. Changes in plasma gelsolin concentration during acute oxidant lung injury in mice. Lung 2002, 180, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Xianhui, L.; Pinglian, L.; Xiaojuan, W.; Wei, C.; Yong, Y.; Feng, R.; Peng, S.; Gang, X. The association between plasma gelsolin level and prognosis of burn patients. Burns 2014, 40, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, B.; Chen, Q.; Wu, S.; Lv, C.; Xie, G.; Jin, Y.; Fang, X. Time course of plasma gelsolin concentrations during severe sepsis in critically ill surgical patients. Crit. Care 2008, 12, R106. [Google Scholar] [CrossRef]
- Marrocco, C.; Rinalducci, S.; Mohamadkhani, A.; D’Amici, G.M.; Zolla, L. Plasma gelsolin protein: A candidate biomarker for hepatitis B-associated liver cirrhosis identified by proteomic approach. Blood Transfus. 2010, 8, S105–S112. [Google Scholar] [CrossRef]
- Stalmach, A.; Johnsson, H.; McInnes, I.B.; Husi, H.; Klein, J.; Dakna, M.; Mullen, W.; Mischak, H.; Porter, D. Identification of urinary peptide biomarkers associated with rheumatoid arthritis. PLoS ONE 2014, 9, e104625. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Li, W.H.; Meng, H.X.; Fan, Y.Z.; Li, W.J.; Ji, Y.T.; Zhao, H.; Zhang, L.; Jin, X.M.; et al. The value of decreased plasma gelsolin levels in patients with systemic lupus erythematosus and rheumatoid arthritis in diagnosis and disease activity evaluation. Lupus 2013, 22, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Waxman, A.B.; Cotich, K.L.; Chung, S.W.; Perrella, M.A.; Stossel, T.P. Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit. Care Med. 2007, 35, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Levental, I.; Kulakowska, A.; Janmey, P.A. Plasma gelsolin: Function, prognostic value, and potential therapeutic use. Curr. Protein. Pept. Sci. 2008, 9, 541–551. [Google Scholar] [CrossRef]
- Cohen, T.S.; Bucki, R.; Byfield, F.J.; Ciccarelli, N.J.; Rosenberg, B.; DiNubile, M.J.; Janmey, P.A.; Margulies, S.S. Therapeutic potential of plasma gelsolin administration in a rat model of sepsis. Cytokine 2011, 54, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.V.; Draney, D.; Sevick-Muraca, E.M.; Olive, D.M. Single-dose intravenous toxicity study of IRDye 800CW in Sprague-Dawley rats. Mol. Imaging Biol. 2010, 12, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.L.; Volcheck, W.; Sevick-Muraca, E.; Simpson, M.A.; Olive, D.M. Characterization and performance of a near-infrared 2-deoxyglucose optical imaging agent for mouse cancer models. Anal. Biochem. 2009, 384, 254–262. [Google Scholar] [CrossRef]
- Nanda, J.S.; Lorsch, J.R. Labeling a protein with fluorophores using NHS ester derivitization. Methods Enzymol. 2014, 536, 87–94. [Google Scholar] [CrossRef]
- Mounzer, K.C.; Moncure, M.; Smith, Y.R.; Dinubile, M.J. Relationship of admission plasma gelsolin levels to clinical outcomes in patients after major trauma. Am. J. Respir. Crit. Care Med. 1999, 160, 1673–1681. [Google Scholar] [CrossRef]
- Osborn, T.M.; Verdrengh, M.; Stossel, T.P.; Tarkowski, A.; Bokarewa, M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R117. [Google Scholar] [CrossRef]
- Lee, P.S.; Sampath, K.; Karumanchi, S.A.; Tamez, H.; Bhan, I.; Isakova, T.; Gutierrez, O.M.; Wolf, M.; Chang, Y.; Stossel, T.P.; et al. Plasma gelsolin and circulating actin correlate with hemodialysis mortality. J. Am. Soc. Nephrol. 2009, 20, 1140–1148. [Google Scholar] [CrossRef]
- Mochizuki, T.; Tsukamoto, E.; Kuge, Y.; Kanegae, K.; Zhao, S.; Hikosaka, K.; Hosokawa, M.; Kohanawa, M.; Tamaki, N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J. Nucl. Med. 2001, 42, 1551–1555. [Google Scholar] [PubMed]
- Wittmann, J.; Dieckow, J.; Schröder, H.; Hampel, U.; Garreis, F.; Jacobi, C.; Milczarek, A.; Hsieh, K.L.; Pulli, B.; Chen, J.W.; et al. Plasma gelsolin promotes re-epithelialization. Sci. Rep. 2018, 8, 13140. [Google Scholar] [CrossRef] [PubMed]
- Witke, W.; Sharpe, A.H.; Hartwig, J.H.; Azuma, T.; Stossel, T.P.; Kwiatkowski, D.J. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell 1995, 81, 41–51. [Google Scholar] [CrossRef]
- Lind, S.E.; Smith, D.B.; Janmey, P.A.; Stossel, T.P. Role of plasma gelsolin and the vitamin D-binding protein in clearing actin from the circulation. J. Clin. Investig. 1986, 78, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Serrander, L.; Skarman, P.; Rasmussen, B.; Witke, W.; Lew, D.P.; Krause, K.H.; Stendahl, O.; Nüsse, O. Selective inhibition of IgG-mediated phagocytosis in gelsolin-deficient murine neutrophils. J. Immunol. 2000, 165, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; Glogauer, M.; Kapus, A.; Kwiatkowski, D.J.; McCulloch, C.A. Gelsolin mediates collagen phagocytosis through a rac-dependent step. Mol. Biol. Cell 2004, 15, 588–599. [Google Scholar] [CrossRef]
- Jutras, I.; Desjardins, M. Phagocytosis: At the crossroads of innate and adaptive immunity. Annu. Rev. Cell Dev. Biol. 2005, 21, 511–527. [Google Scholar] [CrossRef]
- Kitchens, R.L.; Thompson, P.A. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J. Endotoxin. Res. 2005, 11, 225–229. [Google Scholar] [CrossRef]
- Lamping, N.; Dettmer, R.; Schröder, N.W.; Pfeil, D.; Hallatschek, W.; Burger, R.; Schumann, R.R. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J. Clin. Investig. 1998, 101, 2065–2071. [Google Scholar] [CrossRef]
- Jack, R.S.; Fan, X.; Bernheiden, M.; Rune, G.; Ehlers, M.; Weber, A.; Kirsch, G.; Mentel, R.; Fürll, B.; Freudenberg, M.; et al. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature 1997, 389, 742–745. [Google Scholar] [CrossRef]
- Schiff, D.E.; Kline, L.; Soldau, K.; Lee, J.D.; Pugin, J.; Tobias, P.S.; Ulevitch, R.J. Phagocytosis of gram-negative bacteria by a unique CD14-dependent mechanism. J. Leukoc. Biol. 1997, 62, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Su, G.L.; Schmidt, C.; Aminlari, A.; Steinstraesser, L.; Alarcon, W.H.; Zhang, H.Y.; Wang, S.C. Lipopolysaccharide-binding protein accelerates and augments Escherichia coli phagocytosis by alveolar macrophages. J. Surg. Res. 2000, 94, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Niemirowicz, K.; Wnorowska, U.; Byfield, F.J.; Piktel, E.; Wątek, M.; Janmey, P.A.; Savage, P.B. Bactericidal activity of ceragenin CSA-13 in cell culture and an animal model of peritoneal infection. Antimicrob. Agents Chemother. 2015. [Google Scholar] [CrossRef] [PubMed]
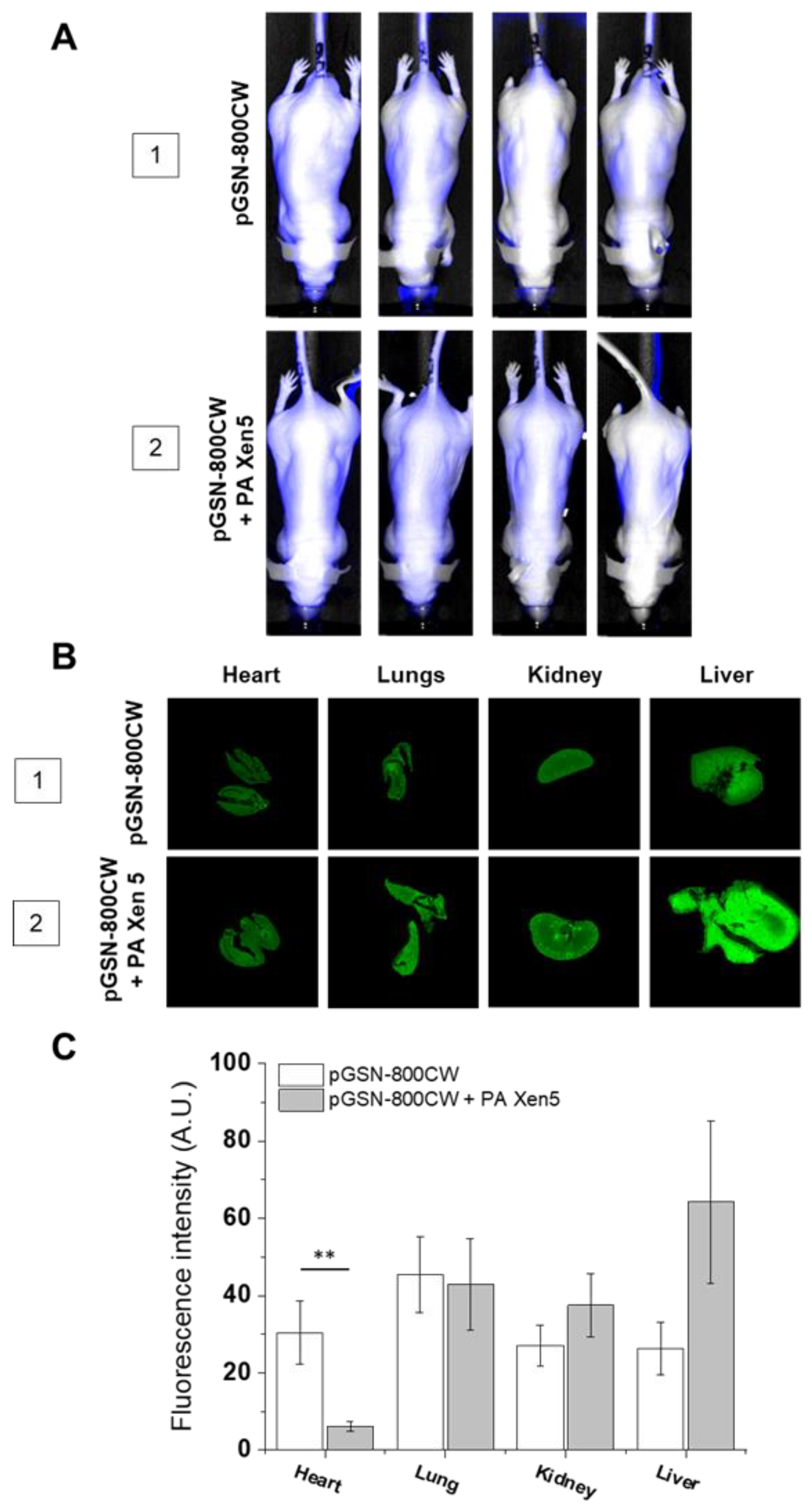
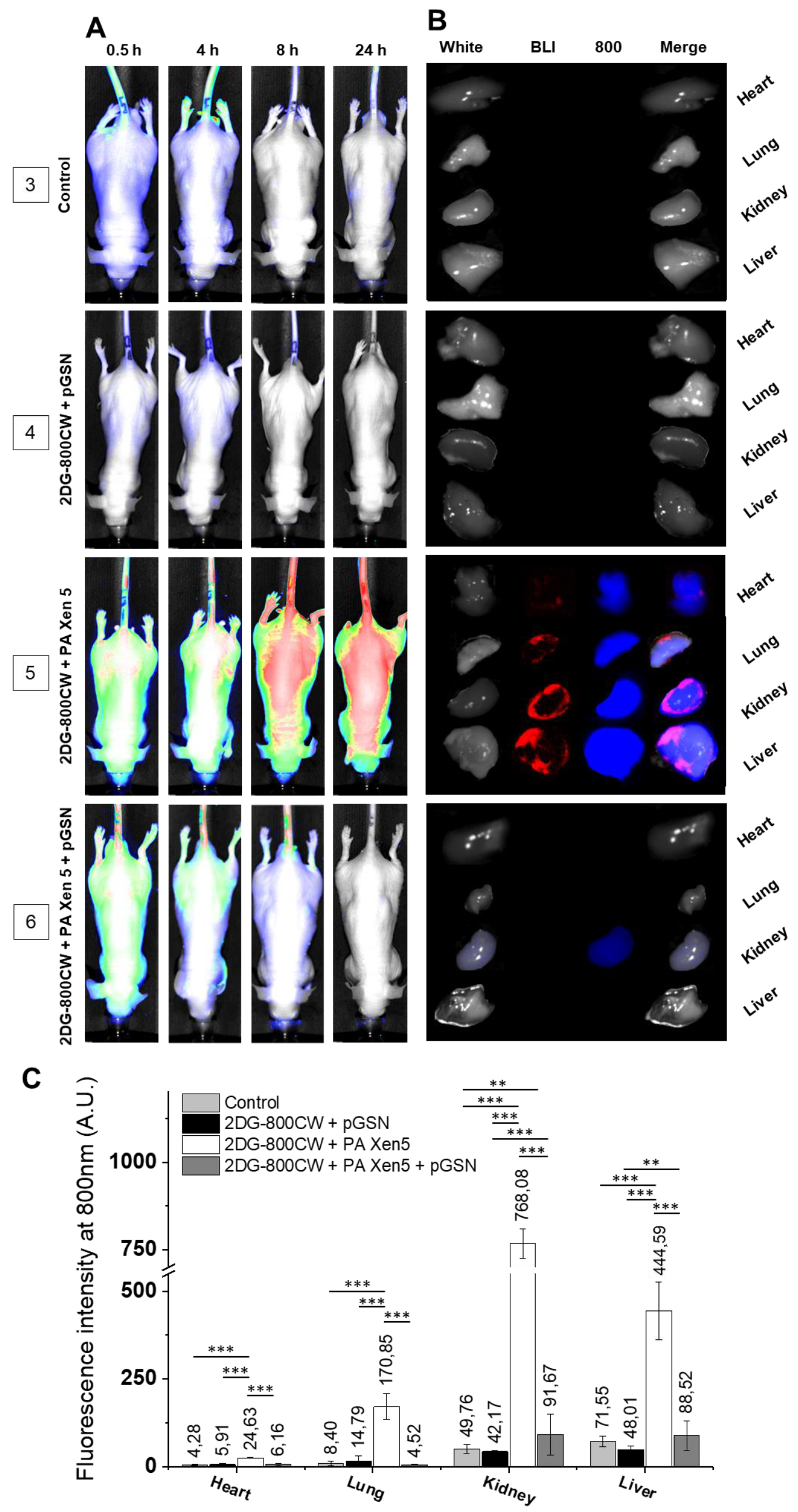
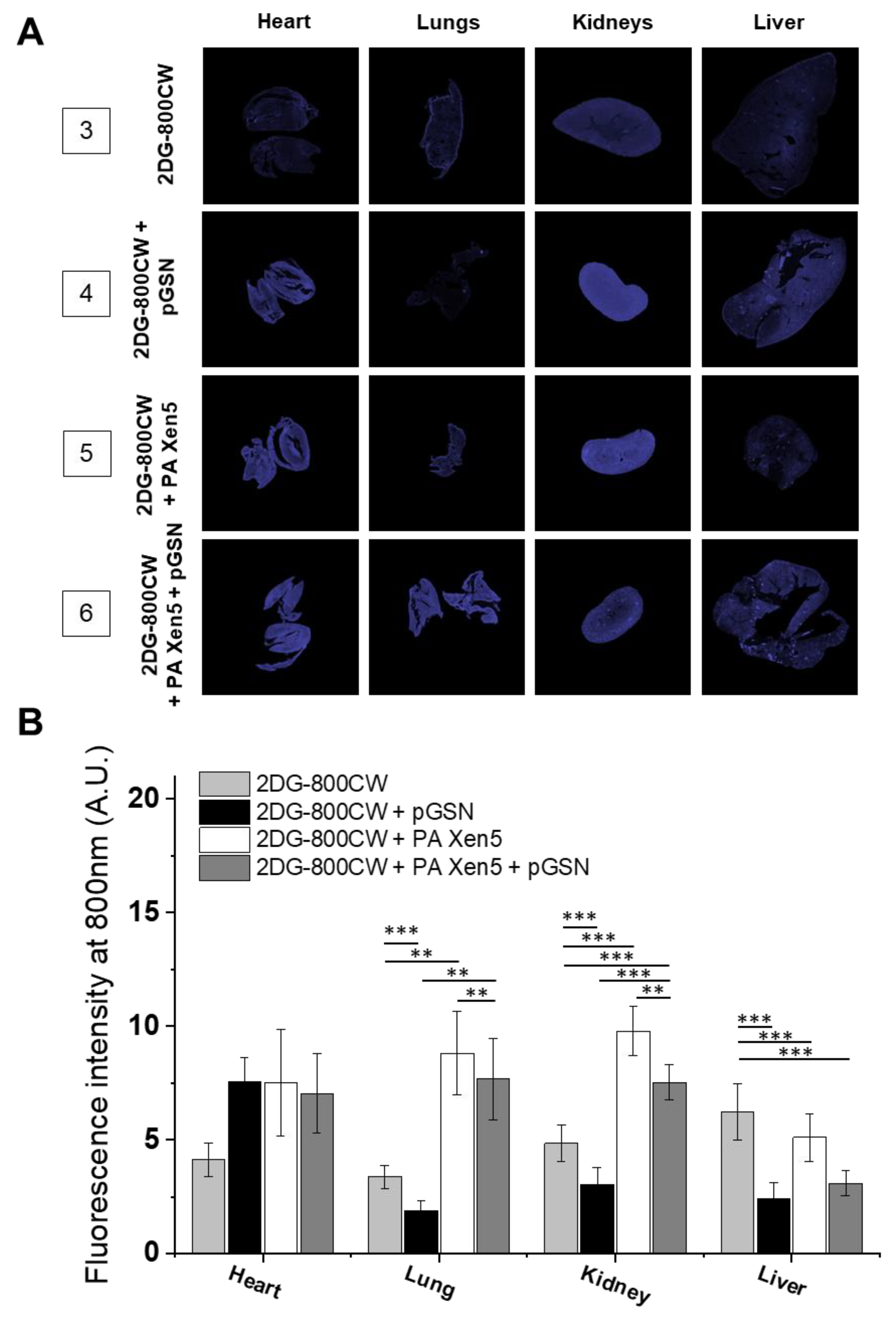
| No. | Description | No. of Animals | 0 h | 8 h | 24 h | |
|---|---|---|---|---|---|---|
| Biodistribution | 1 | pGSN-800CW | n = 5 | Sterile saline | pGSN-800CW (40 mg/kg) | Collection of blood, peritoneal fluid and organs for analysis |
| 2 | pGSN-800CW + PA Xen5 | n = 5 | PA Xen5 inoculum (6 × 105 CFU/mL) | pGSN-800CW (40 mg/kg) | ||
| Anti-inflammatory | 3 | Control | n = 5 | Sterile saline | 2DG-800 CW (10 µM) | |
| 4 | 2DG-800CW + pGSN | n = 5 | Sterile saline | 2DG-800 CW (10 µM) + pGSN (40 mg/kg) | ||
| 5 | 2DG-800CW + PA Xen5 | n = 5 | PA Xen5 inoculum (6 × 105 CFU/mL) | 2DG-800 CW (10 µM) | ||
| 6 | 2DG-800CW + PA Xen5 + pGSN | n = 5 | PA Xen5 inoculum (6 × 105 CFU/mL) | 2DG-800 CW + pGSN (40 mg/kg |
| Heart | Lungs | Kidneys | Liver | |
|---|---|---|---|---|
| pGSN-800CW | no change | slight atelectasis, few interstitial lymphocytes | no change | no change |
| pGSN-800CW + PA Xen5 | no change | atelectasis *, hyperemia in the intestinal tissue | no change | slight hyperaemia, hepatic cell degradation, few lymphocytes in the liver sinuses |
| pGSN | no change | slight atelectasis, few interstitial lymphocytes | slight hyperaemia, few interstitial lymphocytes | hepatic cells degradation, few interstitial lymphocytes |
| PA Xen5 | no change | slight atelectasis, hyperaemia * | hyperaemia * | hyperaemia * |
| pGSN + PA Xen5 | no change | atelectatic changes *, hyperemia in the intestinal tissue | slight hyperaemia, few interstitial lymphocytes | slight hyperaemia |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piktel, E.; Wnorowska, U.; Cieśluk, M.; Deptuła, P.; Prasad, S.V.; Król, G.; Durnaś, B.; Namiot, A.; Markiewicz, K.H.; Niemirowicz-Laskowska, K.; et al. Recombinant Human Plasma Gelsolin Stimulates Phagocytosis while Diminishing Excessive Inflammatory Responses in Mice with Pseudomonas aeruginosa Sepsis. Int. J. Mol. Sci. 2020, 21, 2551. https://doi.org/10.3390/ijms21072551
Piktel E, Wnorowska U, Cieśluk M, Deptuła P, Prasad SV, Król G, Durnaś B, Namiot A, Markiewicz KH, Niemirowicz-Laskowska K, et al. Recombinant Human Plasma Gelsolin Stimulates Phagocytosis while Diminishing Excessive Inflammatory Responses in Mice with Pseudomonas aeruginosa Sepsis. International Journal of Molecular Sciences. 2020; 21(7):2551. https://doi.org/10.3390/ijms21072551
Chicago/Turabian StylePiktel, Ewelina, Urszula Wnorowska, Mateusz Cieśluk, Piotr Deptuła, Suhanya V. Prasad, Grzegorz Król, Bonita Durnaś, Andrzej Namiot, Karolina H. Markiewicz, Katarzyna Niemirowicz-Laskowska, and et al. 2020. "Recombinant Human Plasma Gelsolin Stimulates Phagocytosis while Diminishing Excessive Inflammatory Responses in Mice with Pseudomonas aeruginosa Sepsis" International Journal of Molecular Sciences 21, no. 7: 2551. https://doi.org/10.3390/ijms21072551
APA StylePiktel, E., Wnorowska, U., Cieśluk, M., Deptuła, P., Prasad, S. V., Król, G., Durnaś, B., Namiot, A., Markiewicz, K. H., Niemirowicz-Laskowska, K., Wilczewska, A. Z., Janmey, P. A., Reszeć, J., & Bucki, R. (2020). Recombinant Human Plasma Gelsolin Stimulates Phagocytosis while Diminishing Excessive Inflammatory Responses in Mice with Pseudomonas aeruginosa Sepsis. International Journal of Molecular Sciences, 21(7), 2551. https://doi.org/10.3390/ijms21072551








