FSH for the Treatment of Male Infertility
Abstract
1. Introduction
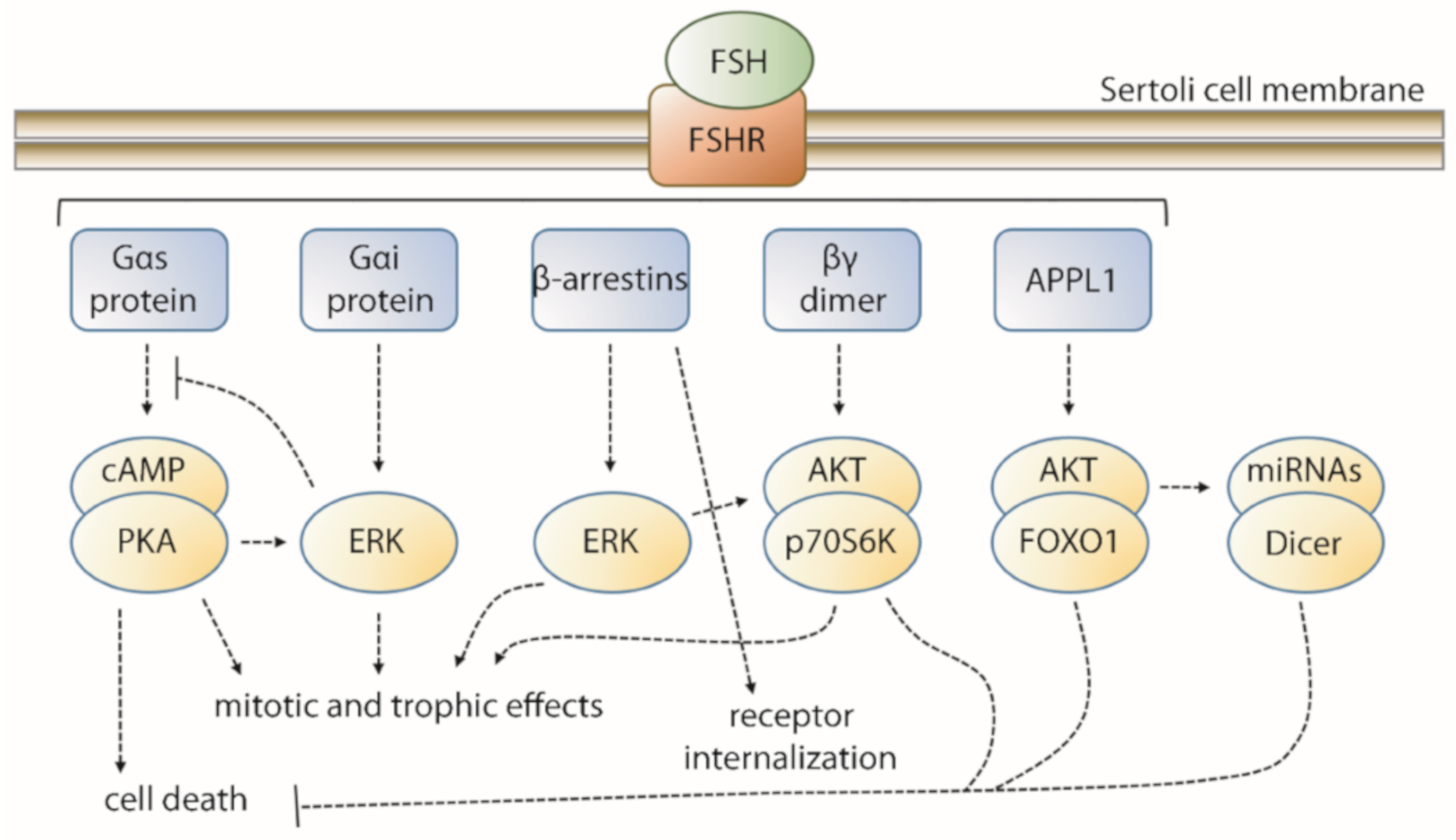
2. FSH-Induced Signaling Network
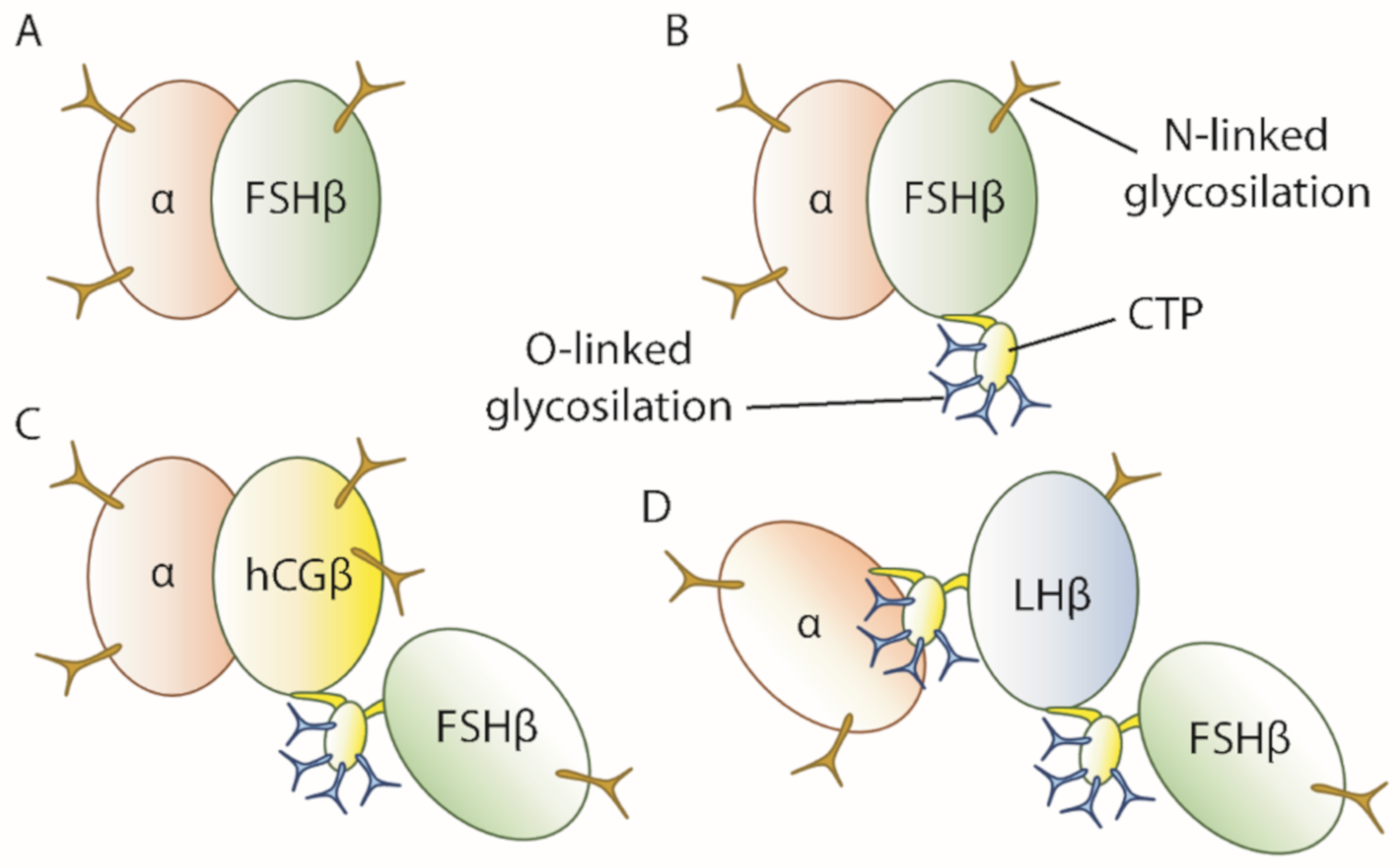
3. FSH in Therapy
4. FSH Use in Hypogonadotropic Hypogonadism: State-Of-The-Art
5. FSH Use in Idiopathic Infertility: State-Of-The-Art
6. Future Directions in the Control of FSHR Signaling
7. Future Clinical Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simoni, M.; Gromoll, J.; Nieschlag, E. The Follicle-Stimulating Hormone Receptor: Biochemistry, Molecular Biology, Physiology, and Pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [CrossRef]
- Santi, D.; Potì, F.; Simoni, M.; Casarini, L. Pharmacogenetics of G-protein-coupled receptors variants: FSH receptor and infertility treatment. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Behre, H.M. Clinical Use of FSH in Male Infertility. Front. Endocrinol. (Lausanne) 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Fischer, D.; Chen, X.; McKenna, S.D.; Liu, H.; Sriraman, V.; Yu, H.N.; Goutopoulos, A.; Arkinstall, S.; He, X. Evidence for Follicle-stimulating Hormone Receptor as a Functional Trimer. J. Biol. Chem. 2014, 289, 14273–14282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, H.; Chen, X.; Chen, P.-H.; Fischer, D.; Sriraman, V.; Yu, H.N.; Arkinstall, S.; He, X. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 12491–12496. [Google Scholar] [CrossRef]
- Brüser, A.; Schulz, A.; Rothemund, S.; Ricken, A.; Calebiro, D.; Kleinau, G.; Schöneberg, T. The Activation Mechanism of Glycoprotein Hormone Receptors with Implications in the Cause and Therapy of Endocrine Diseases. J. Biol. Chem. 2016, 291, 508–520. [Google Scholar] [CrossRef]
- Rasmussen, S.G.F.; Choi, H.-J.; Fung, J.J.; Pardon, E.; Casarosa, P.; Chae, P.S.; Devree, B.T.; Rosenbaum, D.M.; Thian, F.S.; Kobilka, T.S.; et al. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature 2011, 469, 175–180. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Zhang, C.; Lyons, J.A.; Holl, R.; Aragao, D.; Arlow, D.H.; Rasmussen, S.G.F.; Choi, H.-J.; Devree, B.T.; Sunahara, R.K.; et al. Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature 2011, 469, 236–240. [Google Scholar] [CrossRef]
- Manglik, A.; Kim, T.H.; Masureel, M.; Altenbach, C.; Yang, Z.; Hilger, D.; Lerch, M.T.; Kobilka, T.S.; Thian, F.S.; Hubbell, W.L.; et al. Structural Insights into the Dynamic Process of β2-Adrenergic Receptor Signaling. Cell 2015, 161, 1101–1111. [Google Scholar] [CrossRef]
- Nakamura, K.; Krupnick, J.G.; Benovic, J.L.; Ascoli, M. Signaling and Phosphorylation-impaired Mutants of the Rat Follitropin Receptor Reveal an Activation- and Phosphorylation-independent but Arrestin-dependent Pathway for Internalization. J. Biol. Chem. 1998, 273, 24346–24354. [Google Scholar] [CrossRef]
- Troispoux, C.; Guillou, F.; Elalouf, J.-M.; Firsov, D.; Iacovelli, L.; De Blasi, A.; Combarnous, Y.; Reiter, E. Involvement of G Protein-Coupled Receptor Kinases and Arrestins in Desensitization to Follicle-Stimulating Hormone Action. Mol. Endocrinol. 1999, 13, 1599–1614. [Google Scholar] [CrossRef] [PubMed]
- Lazari, M.F.; Liu, X.; Nakamura, K.; Benovic, J.L.; Ascoli, M. Role of G Protein-Coupled Receptor Kinases on the Agonist-Induced Phosphorylation and Internalization of the Follitropin Receptor. Mol. Endocrinol. 1999, 13, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Marion, S.; Robert, F.; Crepieux, P.; Martinat, N.; Troispoux, C.; Guillou, F.; Reiter, E. G protein-coupled receptor kinases and beta arrestins are relocalized and attenuate cyclic 3′,5′-adenosine monophosphate response to follicle-stimulating hormone in rat primary Sertoli cells. Biol. Reprod. 2002, 66, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, H.; Galet, C.; Ascoli, M. The association of arrestin-3 with the follitropin receptor depends on receptor activation and phosphorylation. Mol. Cell. Endocrinol. 2003, 204, 127–140. [Google Scholar] [CrossRef]
- Marion, S.; Kara, E.; Crepieux, P.; Piketty, V.; Martinat, N.; Guillou, F.; Reiter, E. G protein-coupled receptor kinase 2 and -arrestins are recruited to FSH receptor in stimulated rat primary Sertoli cells. J. Endocrinol. 2006, 190, 341–350. [Google Scholar] [CrossRef]
- Kara, E.; Crépieux, P.; Gauthier, C.; Martinat, N.; Piketty, V.; Guillou, F.; Reiter, E. A Phosphorylation Cluster of Five Serine and Threonine Residues in the C-Terminus of the Follicle-Stimulating Hormone Receptor Is Important for Desensitization But Not for β-Arrestin-Mediated ERK Activation. Mol. Endocrinol. 2006, 20, 3014–3026. [Google Scholar] [CrossRef]
- Laporte, S.A.; Miller, W.E.; Kim, K.-M.; Caron, M.G. beta-Arrestin/AP-2 interaction in G protein-coupled receptor internalization: Identification of a beta-arrestin binging site in beta 2-adaptin. J. Biol. Chem. 2002, 277, 9247–9254. [Google Scholar] [CrossRef]
- Zeleznik, A.J.; Saxena, D.; Little-Ihrig, L. Protein Kinase B Is Obligatory for Follicle-Stimulating Hormone-Induced Granulosa Cell Differentiation. Endocrinology 2003, 144, 3985–3994. [Google Scholar] [CrossRef]
- Majumdar, S.S.; Sarda, K.; Bhattacharya, I.; Plant, T.M. Insufficient androgen and FSH signaling may be responsible for the azoospermia of the infantile primate testes despite exposure to an adult-like hormonal milieu. Hum. Reprod. 2012, 27, 2515–2525. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Basu, S.; Pradhan, B.S.; Sarkar, H.; Nagarajan, P.; Majumdar, S.S. Testosterone augments FSH signaling by upregulating the expression and activity of FSH-Receptor in Pubertal Primate Sertoli cells. Mol. Cell. Endocrinol. 2019, 482, 70–80. [Google Scholar] [CrossRef]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular Mechanisms and Signaling Pathways Involved in Sertoli Cell Proliferation. Front. Endocrinol. (Lausanne) 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Aguirre, A.; Lira-Albarrán, S. Clinical Applications of Gonadotropins in the Male. Prog. Mol. Biol. Transl. Sci. 2016, 143, 121–174. [Google Scholar] [PubMed]
- Arey, B.J.; Stevis, P.E.; Deecher, D.C.; Shen, E.S.; Frail, D.E.; Negro-Vilar, A.; López, F.J. Induction of Promiscuous G Protein Coupling of the Follicle-Stimulating Hormone (FSH) Receptor: A Novel Mechanism for Transducing Pleiotropic Actions of FSH Isoforms. Mol. Endocrinol. 1997, 11, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Crépieux, P.; Marion, S.; Martinat, N.; Fafeur, V.; Vern, Y.L.; Kerboeuf, D.; Guillou, F.; Reiter, E. The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene 2001, 20, 4696–4709. [Google Scholar] [CrossRef]
- Crépieux, P.; Poupon, A.; Langonné-Gallay, N.; Reiter, E.; Delgado, J.; Schaefer, M.H.; Bourquard, T.; Serrano, L.; Kiel, C. A Comprehensive View of the β-Arrestinome. Front. Endocrinol. (Lausanne) 2017, 8, 32. [Google Scholar] [CrossRef]
- DeWire, S.M.; Ahn, S.; Lefkowitz, R.J.; Shenoy, S.K. β-Arrestins and Cell Signaling. Annu. Rev. Physiol. 2007, 69, 483–510. [Google Scholar] [CrossRef]
- Tréfier, A.; Musnier, A.; Landomiel, F.; Bourquard, T.; Boulo, T.; Ayoub, M.A.; León, K.; Bruneau, G.; Chevalier, M.; Durand, G.; et al. G protein–dependent signaling triggers a β-arrestin–scaffolded p70S6K/ rpS6 module that controls 5′TOP mRNA translation. FASEB J. 2018, 32, 1154–1169. [Google Scholar] [CrossRef]
- Musnier, A.; Heitzler, D.; Boulo, T.; Tesseraud, S.; Durand, G.; Lécureuil, C.; Guillou, H.; Poupon, A.; Reiter, E.; Crépieux, P. Developmental regulation of p70 S6 kinase by a G protein-coupled receptor dynamically modelized in primary cells. Cell. Mol. Life Sci. 2009, 66, 3487–3503. [Google Scholar] [CrossRef]
- Das, S.; Maizels, E.T.; DeManno, D.; St Clair, E.; Adam, S.A.; Hunzicker-Dunn, M. A stimulatory role of cyclic adenosine 3′,5′-monophosphate in follicle-stimulating hormone-activated mitogen-activated protein kinase signaling pathway in rat ovarian granulosa cells. Endocrinology 1996, 137, 967–974. [Google Scholar] [CrossRef]
- Kayampilly, P.P.; Menon, K.M.J. Inhibition of Extracellular Signal-Regulated Protein Kinase-2 Phosphorylation by Dihydrotestosterone Reduces Follicle-Stimulating Hormone-Mediated Cyclin D2 Messenger Ribonucleic Acid Expression in Rat Granulosa Cells. Endocrinology 2004, 145, 1786–1793. [Google Scholar] [CrossRef]
- Casarini, L.; Reiter, E.; Simoni, M. β-arrestins regulate gonadotropin receptor-mediated cell proliferation and apoptosis by controlling different FSHR or LHCGR intracellular signaling in the hGL5 cell line. Mol. Cell. Endocrinol. 2016, 437, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lécureuil, C.; Tesseraud, S.; Kara, E.; Martinat, N.; Sow, A.; Fontaine, I.; Gauthier, C.; Reiter, E.; Guillou, F.; Crépieux, P. Follicle-stimulating hormone activates p70 ribosomal protein S6 kinase by protein kinase A-mediated dephosphorylation of Thr 421/Ser 424 in primary Sertoli cells. Mol. Endocrinol. 2005, 19, 1812–1820. [Google Scholar] [CrossRef] [PubMed][Green Version]
- León, K.; Boulo, T.; Musnier, A.; Morales, J.; Gauthier, C.; Dupuy, L.; Heyne, S.; Backofen, R.; Poupon, A.; Cormier, P.; et al. Activation of a GPCR leads to eIF4G phosphorylation at the 5′ cap and to IRES-dependent translation. J. Mol. Endocrinol. 2014, 52, 373–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Musnier, A.; León, K.; Morales, J.; Reiter, E.; Boulo, T.; Costache, V.; Vourc’h, P.; Heitzler, D.; Oulhen, N.; Poupon, A.; et al. mRNA-selective translation induced by FSH in primary Sertoli cells. Mol. Endocrinol. 2012, 26, 669–680. [Google Scholar] [CrossRef][Green Version]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef]
- Herndon, M.K.; Law, N.C.; Donaubauer, E.M.; Kyriss, B.; Hunzicker-Dunn, M. Forkhead box O member FOXO1 regulates the majority of follicle-stimulating hormone responsive genes in ovarian granulosa cells. Mol. Cell. Endocrinol. 2016, 434, 116–126. [Google Scholar] [CrossRef]
- Park, Y.; Maizels, E.T.; Feiger, Z.J.; Alam, H.; Peters, C.A.; Woodruff, T.K.; Unterman, T.G.; Lee, E.J.; Jameson, J.L.; Hunzicker-Dunn, M. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J. Biol. Chem. 2005, 280, 9135–9148. [Google Scholar] [CrossRef]
- Papaioannou, M.D.; Pitetti, J.-L.; Ro, S.; Park, C.; Aubry, F.; Schaad, O.; Vejnar, C.E.; Kühne, F.; Descombes, P.; Zdobnov, E.M.; et al. Sertoli cell Dicer is essential for spermatogenesis in mice. Dev. Biol. 2009, 326, 250–259. [Google Scholar] [CrossRef]
- Gonzalez, G.; Behringer, R.R. Dicer is required for female reproductive tract development and fertility in the mouse. Mol. Reprod. Dev. 2009, 76, 678–688. [Google Scholar] [CrossRef]
- Nagaraja, A.K.; Andreu-Vieyra, C.; Franco, H.L.; Ma, L.; Chen, R.; Han, D.Y.; Zhu, H.; Agno, J.E.; Gunaratne, P.H.; DeMayo, F.J.; et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol. Endocrinol. 2008, 22, 2336–2352. [Google Scholar] [CrossRef]
- Hong, X.; Luense, L.J.; McGinnis, L.K.; Nothnick, W.B.; Christenson, L.K. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 2008, 149, 6207–6212. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Yang, Y.; Cheung, H.-H.; Chen, Z.-J.; Chan, W.-Y. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci. Rep. 2017, 7, 41304. [Google Scholar]
- Nicholls, P.K.; Harrison, C.A.; Walton, K.L.; McLachlan, R.I.; O’Donnell, L.; Stanton, P.G. Hormonal regulation of sertoli cell micro-RNAs at spermiation. Endocrinology 2011, 152, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Musnier, A.; Decourtye, J.; Boulo, T.; Lécureuil, C.; Guillou, H.; Valet, S.; Fouchécourt, S.; Pitetti, J.L.; Nef, S.; et al. FSH-stimulated PTEN activity accounts for the lack of FSH mitogenic effect in prepubertal rat Sertoli cells. Mol. Cell. Endocrinol. 2010, 315, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Simoni, M.; Brigante, G. Is polycystic ovary syndrome a sexual conflict? A review. Reprod. Biomed. Online 2016, 32, 350–361. [Google Scholar] [CrossRef]
- Casarini, L.; Santi, D.; Marino, M. Impact of gene polymorphisms of gonadotropins and their receptors on human reproductive success. Reproduction 2015, 150, R175–R184. [Google Scholar] [CrossRef]
- Casarini, L.; Brigante, G.; Simoni, M.; Santi, D. Clinical Applications of Gonadotropins in the Female: Assisted Reproduction and Beyond. Prog. Mol. Biol. Transl. Sci. 2016, 143, 85–119. [Google Scholar]
- Young, J.; Xu, C.; Papadakis, G.E.; Acierno, J.S.; Maione, L.; Hietamäki, J.; Raivio, T.; Pitteloud, N. Clinical Management of Congenital Hypogonadotropic Hypogonadism. Endocr. Rev. 2019, 40, 669–710. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Alam, V.; D’Hooghe, T.; Sunkara, S.K. The Development of Gonadotropins for Clinical Use in the Treatment of Infertility. Front. Endocrinol. (Lausanne) 2019, 10, 429. [Google Scholar] [CrossRef]
- Bassett, R.; Driebergen, R. Continued improvements in the quality and consistency of follitropin alfa, recombinant human FSH. Reprod. Biomed. Online 2005, 10, 169–177. [Google Scholar] [CrossRef]
- Santi, D.; Simoni, M. Biosimilar recombinant follicle stimulating hormones in infertility treatment. Expert Opin. Biol. Ther. 2014, 14, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Wide, L.; Eriksson, K. Dynamic changes in glycosylation and glycan composition of serum FSH and LH during natural ovarian stimulation. Ups. J. Med. Sci. 2013, 118, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Mastrangeli, R.; Satwekar, A.; Cutillo, F.; Ciampolillo, C.; Palinsky, W.; Longobardi, S. In-vivo biological activity and glycosylation analysis of a biosimilar recombinant human follicle-stimulating hormone product (Bemfola) compared with its reference medicinal product (GONAL-f). PLoS ONE 2017, 12, e0184139. [Google Scholar] [CrossRef] [PubMed]
- Butnev, V.Y.; Butnev, V.Y.; May, J.V.; Shuai, B.; Tran, P.; White, W.K.; Brown, A.; Smalter Hall, A.; Harvey, D.J.; Bousfield, G.R. Production, purification, and characterization of recombinant hFSH glycoforms for functional studies. Mol. Cell. Endocrinol. 2015, 405, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Aebi, M. Intracellular functions of N-linked glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Timossi, C.; Damián-Matsumura, P.; Dias, J.A. Role of Glycosylation in Function of Follicle-Stimulating Hormone. Endocrine 1999, 11, 205–216. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Timossi, C.; Barrios-de-Tomasi, J.; Maldonado, A.; Nayudu, P. Impact of Carbohydrate Heterogeneity in Function of Follicle-Stimulating Hormone: Studies Derived from in Vitro and in Vivo Models1. Biol. Reprod. 2003, 69, 379–389. [Google Scholar] [CrossRef]
- Bousfield, G.R.; May, J.V.; Davis, J.S.; Dias, J.A.; Kumar, T.R. In Vivo and In Vitro Impact of Carbohydrate Variation on Human Follicle-Stimulating Hormone Function. Front. Endocrinol. (Lausanne) 2018, 9, 216. [Google Scholar] [CrossRef]
- Campo, S.; Andreone, L.; Ambao, V.; Urrutia, M.; Calandra, R.S.; Rulli, S.B. Hormonal Regulation of Follicle-Stimulating Hormone Glycosylation in Males. Front. Endocrinol. (Lausanne) 2019, 10, 17. [Google Scholar] [CrossRef]
- Meher, B.R.; Dixit, A.; Bousfield, G.R.; Lushington, G.H. Glycosylation Effects on FSH-FSHR Interaction Dynamics: A Case Study of Different FSH Glycoforms by Molecular Dynamics Simulations. PLoS ONE 2015, 10, e0137897. [Google Scholar] [CrossRef]
- Núñez Miguel, R.; Sanders, J.; Furmaniak, J.; Rees Smith, B. Glycosylation pattern analysis of glycoprotein hormones and their receptors. J. Mol. Endocrinol. 2017, 58, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Hou, X.; Wang, C.; May, J.V.; Butnev, V.Y.; Bousfield, G.R.; Davis, J.S. Hypoglycosylated hFSH Has Greater Bioactivity Than Fully Glycosylated Recombinant hFSH in Human Granulosa Cells. J. Clin. Endocrinol. Metab. 2015, 100, E852–E860. [Google Scholar] [CrossRef] [PubMed]
- Arey, B.J.; López, F.J. Are circulating gonadotropin isoforms naturally occurring biased agonists? Basic and therapeutic implications. Rev. Endocr. Metab. Disord. 2011, 12, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Bousfield, G.R.; Butnev, V.Y.; Butnev, V.Y.; Hiromasa, Y.; Harvey, D.J.; May, J.V. Hypo-glycosylated human follicle-stimulating hormone (hFSH(21/18)) is much more active in vitro than fully-glycosylated hFSH (hFSH(24)). Mol. Cell. Endocrinol. 2014, 382, 989–997. [Google Scholar] [CrossRef]
- Wang, H.; May, J.; Butnev, V.; Shuai, B.; May, J.V.; Bousfield, G.R.; Kumar, T.R. Evaluation of in vivo bioactivities of recombinant hypo- (FSH 21/18 ) and fully- (FSH 24 ) glycosylated human FSH glycoforms in Fshb null mice. Mol. Cell. Endocrinol. 2016, 437, 224–236. [Google Scholar] [CrossRef]
- Timossi, C.; Damian-Matsumura, P.; Dominguez-Gonzalez, A.; Ulloa-Aguirre, A. A less acidic human follicle-stimulating hormone preparation induces tissue-type plasminogen activator enzyme activity earlier than a predominantly acidic analogue in phenobarbital-blocked pro-oestrous rats. Mol. Hum. Reprod. 1998, 4, 1032–1038. [Google Scholar] [CrossRef]
- Howles, C.M. Role of LH and FSH in ovarian function. Mol. Cell. Endocrinol. 2000, 161, 25–30. [Google Scholar] [CrossRef]
- Fares, F. The role of O-linked and N-linked oligosaccharides on the structure–function of glycoprotein hormones: Development of agonists and antagonists. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 560–567. [Google Scholar] [CrossRef]
- Anobile, C.J.; Talbot, J.A.; McCann, S.J.; Padmanabhan, V.; Robertson, W.R. Glycoform composition of serum gonadotrophins through the normal menstrual cycle and in the post-menopausal state. Mol. Hum. Reprod. 1998, 4, 631–639. [Google Scholar] [CrossRef]
- Selman, H.; Pacchiarotti, A.; El-Danasouri, I. Ovarian stimulation protocols based on follicle-stimulating hormone glycosylation pattern: Impact on oocyte quality and clinical outcome. Fertil. Steril. 2010, 94, 1782–1786. [Google Scholar] [CrossRef]
- Perlman, S.; van den Hazel, B.; Christiansen, J.; Gram-Nielsen, S.; Jeppesen, C.B.; Andersen, K.V.; Halkier, T.; Okkels, S.; Schambye, H.T. Glycosylation of an N-Terminal Extension Prolongs the Half-Life and Increases the in Vivo Activity of Follicle Stimulating Hormone. J. Clin. Endocrinol. Metab. 2003, 88, 3227–3235. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Turner, L.; Rushford, D.; McDonald, J.; Baker, H.W.; Conway, A.J. Efficacy and safety of recombinant human follicle stimulating hormone (Gonal-F) with urinary human chorionic gonadotrophin for induction of spermatogenesis and fertility in gonadotrophin-deficient men. Hum. Reprod. 1999, 14, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Goa, K.L.; Wagstaff, A.J. Follitropin Alpha in Infertility. BioDrugs 1998, 9, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Creus, S.; Chaia, Z.; Pellizzari, E.H.; Cigorraga, S.B.; Ulloa-Aguirre, A.; Campo, S. Human FSH isoforms: Carbohydrate complexity as determinant of in-vitro bioactivity. Mol. Cell. Endocrinol. 2001, 174, 41–49. [Google Scholar] [CrossRef]
- Olijve, W.; de Boer, W.; Mulders, J.W.; van Wezenbeek, P.M. Molecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon). Mol. Hum. Reprod. 1996, 2, 371–382. [Google Scholar] [CrossRef]
- Olsson, H.; Sandström, R.; Grundemar, L. Different pharmacokinetic and pharmacodynamic properties of recombinant follicle-stimulating hormone (rFSH) derived from a human cell line compared with rFSH from a non-human cell line. J. Clin. Pharmacol. 2014, 54, 1299–1307. [Google Scholar] [CrossRef]
- Abd-Elaziz, K.; Duijkers, I.; Stöckl, L.; Dietrich, B.; Klipping, C.; Eckert, K.; Goletz, S. A new fully human recombinant FSH (follitropin epsilon): Two phase I randomized placebo and comparator-controlled pharmacokinetic and pharmacodynamic trials. Hum. Reprod. 2017, 32, 1639–1647. [Google Scholar] [CrossRef]
- Griesinger, G.; Dietrich, B.; Stöckl, L.; Eckert, K.; Goletz, S.; Tandler-Schneider, A. Fully human glyco-optimized recombinant FSH (follitropin epsilon)—Arandomized, comparator-controlled phase II clinical trial. Reprod. Biomed. Online 2020, 40, 331–341. [Google Scholar] [CrossRef]
- Riccetti, L.; Sperduti, S.; Lazzaretti, C.; Klett, D.; De Pascali, F.; Paradiso, E.; Limoncella, S.; Potì, F.; Tagliavini, S.; Trenti, T.; et al. Glycosylation Pattern and in vitro Bioactivity of Reference Follitropin alfa and Biosimilars. Front. Endocrinol. (Lausanne) 2019, 10, 503. [Google Scholar] [CrossRef]
- Koechling, W.; Plaksin, D.; Croston, G.E.; Jeppesen, J.V.; Macklon, K.T.; Andersen, C.Y. Comparative pharmacology of a new recombinant FSH expressed by a human cell line. Endocr. Connect. 2017, 6, 297–305. [Google Scholar] [CrossRef]
- Pouwer, A.W.; Farquhar, C.; Kremer, J.A.M. Long-acting FSH versus daily FSH for women undergoing assisted reproduction. Cochrane Database Syst. Rev. 2015, CD009577. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A. hCG, the wonder of today’s science. Reprod. Biol. Endocrinol. 2012, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of LH and hCG. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef] [PubMed]
- Verbost, P.; Sloot, W.N.; Rose, U.M.; de Leeuw, R.; Hanssen, R.G.J.M.; Verheijden, G.F.M. Pharmacologic profiling of corifollitropin alfa, the first developed sustained follicle stimulant. Eur. J. Pharmacol. 2011, 651, 227–233. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.; Alper, M.M.; Ledger, W.; Schoolcraft, W.B.; Zandvliet, A.; Mannaerts, B.M.J.L.; Engage Investigators. Pharmacokinetics and follicular dynamics of corifollitropin alfa versus recombinant FSH during ovarian stimulation for IVF. Reprod. Biomed. Online 2010, 21, 593–601. [Google Scholar] [CrossRef]
- Fares, F.A.; Suganuma, N.; Nishimori, K.; LaPolt, P.S.; Hsueh, A.J.; Boime, I. Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin beta subunit to the follitropin beta subunit. Proc. Natl. Acad. Sci. USA 1992, 89, 4304–4308. [Google Scholar] [CrossRef]
- De Lartigue, J. Corifollitropin alfa: A new option to treat female infertility. Drugs Today 2011, 47, 583–590. [Google Scholar]
- Sacchi, S.; Tenedini, E.; Tondelli, D.; Parenti, S.; Tagliasacchi, D.; Xella, S.; Marsella, T.; Tagliafico, E.; La Marca, A. Gene expression profiles of human granulosa cells treated with bioequivalent doses of corifollitropin alfa (CFA) or recombinant human follicle-stimulating hormone (recFSH). Gynecol. Endocrinol. 2019, 35, 623–627. [Google Scholar] [CrossRef]
- Fensore, S.; Di Marzio, M.; Tiboni, G.M. Corifollitropin alfa compared to daily FSH in controlled ovarian stimulation for in vitro fertilization: A meta-analysis. J. Ovarian Res. 2015, 8, 33. [Google Scholar] [CrossRef]
- Jonas, K.C.; Chen, S.; Virta, M.; Mora, J.; Franks, S.; Huhtaniemi, I.; Hanyaloglu, A.C. Temporal reprogramming of calcium signalling via crosstalk of gonadotrophin receptors that associate as functionally asymmetric heteromers. Sci. Rep. 2018, 8, 2239. [Google Scholar] [CrossRef]
- Rivero-Müller, A.; Chou, Y.-Y.; Ji, I.; Lajic, S.; Hanyaloglu, A.C.; Jonas, K.; Rahman, N.; Ji, T.H.; Huhtaniemi, I. Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc. Natl. Acad. Sci. USA 2010, 107, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, M.; Guan, R.; Segaloff, D.L. Heterodimerization Between the Lutropin and Follitropin Receptors is Associated With an Attenuation of Hormone-Dependent Signaling. Endocrinology 2013, 154, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.-F.; Liu, Z.X.; Nie, M.; Wang, X.; Xu, H.L.; Huang, B.K.; Zheng, J.J.; Min, L.; Kaiser, U.; Wu, X.Y. Pulsatile gonadotropin-releasing hormone therapy is associated with earlier spermatogenesis compared to combined gonadotropin therapy in patients with congenital hypogonadotropic hypogonadism. Asian J. Androl. 2017, 19, 680. [Google Scholar] [PubMed]
- Salenave, S.; Trabado, S.; Maione, L.; Brailly-Tabard, S.; Young, J. Male acquired hypogonadotropic hypogonadism: Diagnosis and treatment. Ann. Endocrinol. 2012, 73, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.D.C.; Kaiser, U.B. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat. Rev. Endocrinol. 2009, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C. Male infertility: Pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 271–285. [Google Scholar] [CrossRef]
- Zheng, J.; Mao, J.; Cui, M.; Liu, Z.; Wang, X.; Xiong, S.; Nie, M.; Wu, X. Novel FSHβ mutation in a male patient with isolated FSH deficiency and infertility. Eur. J. Med. Genet. 2017, 60, 335–339. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Hoang, X.-H.; Avbelj, M.; Hayes, F.J.; Thambundit, A.; Dwyer, A.; Au, M.; Plummer, L.; Crowley, W.F.; Pitteloud, N. Congenital idiopathic hypogonadotropic hypogonadism: Evidence of defects in the hypothalamus, pituitary, and testes. J. Clin. Endocrinol. Metab. 2010, 95, 3019–3027. [Google Scholar] [CrossRef]
- Pitteloud, N.; Hayes, F.J.; Dwyer, A.; Boepple, P.A.; Lee, H.; Crowley, W.F. Predictors of Outcome of Long-Term GnRH Therapy in Men with Idiopathic Hypogonadotropic Hypogonadism. J. Clin. Endocrinol. Metab. 2002, 87, 4128–4136. [Google Scholar] [CrossRef]
- Delemarre-Van de Waal, H.A.; Odink, R.J. Pulsatile GnRH treatment in boys and girls with idiopathic hypogonadotrophic hypogonadism. Hum. Reprod. 1993, 8, 180–183. [Google Scholar] [CrossRef]
- Rastrelli, G.; Corona, G.; Mannucci, E.; Maggi, M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: A meta-analytic study. Andrology 2014, 2, 794–808. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Herbison, A.E. The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology 2018, 159, 3723–3736. [Google Scholar] [CrossRef] [PubMed]
- Riccetti, L.; Yvinec, R.; Klett, D.; Gallay, N.; Combarnous, Y.; Reiter, E.; Simoni, M.; Casarini, L.; Ayoub, M.A. Human Luteinizing Hormone and Chorionic Gonadotropin Display Biased Agonism at the LH and LH/CG Receptors. Sci. Rep. 2017, 7, 940. [Google Scholar] [CrossRef] [PubMed]
- Riccetti, L.; De Pascali, F.; Gilioli, L.; Potì, F.; Giva, L.B.; Marino, M.; Tagliavini, S.; Trenti, T.; Fanelli, F.; Mezzullo, M.; et al. Human LH and hCG stimulate differently the early signalling pathways but result in equal testosterone synthesis in mouse Leydig cells in vitro. Reprod. Biol. Endocrinol. 2017, 15, 2. [Google Scholar] [CrossRef]
- Simoni, M.; Santi, D.; Negri, L.; Hoffmann, I.; Muratori, M.; Baldi, E.; Cambi, M.; Marcou, M.; Greither, T.; Baraldi, E.; et al. Treatment with human, recombinant FSH improves sperm DNA fragmentation in idiopathic infertile men depending on the FSH receptor polymorphism p.N680S: A pharmacogenetic study. Hum. Reprod. 2016, 31, 1960–1969. [Google Scholar] [CrossRef]
- Colacurci, N.; De Leo, V.; Ruvolo, G.; Piomboni, P.; Caprio, F.; Pivonello, R.; Papaleo, E.; La Verde, E.; Depalo, R.; Lispi, M.; et al. Recombinant FSH Improves Sperm DNA Damage in Male Infertility: A Phase II Clinical Trial. Front. Endocrinol. (Lausanne) 2018, 9, 383. [Google Scholar] [CrossRef]
- Patel, D.P.; Chandrapal, J.C.; Hotaling, J.M. Hormone-Based Treatments in Subfertile Males. Curr. Urol. Rep. 2016, 17, 56. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; Barbagallo, F.; Calogero, A.E.; La Vignera, S. Effects of the selective estrogen receptor modulators for the treatment of male infertility: A systematic review and meta-analysis. Expert Opin. Pharmacother. 2019, 20, 1517–1525. [Google Scholar] [CrossRef]
- Lunan, C.B.; Klopper, A. Antioestrogens: A review. Clin. Endocrinol. (Oxf.) 1975, 4, 551–572. [Google Scholar] [CrossRef]
- Chua, M.E.; Escusa, K.G.; Luna, S.; Tapia, L.C.; Dofitas, B.; Morales, M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: A meta-analysis. Andrology 2013, 1, 749–757. [Google Scholar] [CrossRef]
- Barbonetti, A.; Calogero, A.E.; Balercia, G.; Garolla, A.; Krausz, C.; La Vignera, S.; Lombardo, F.; Jannini, E.A.; Maggi, M.; Lenzi, A.; et al. The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2018, 41, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Colpi, G.M.; Francavilla, S.; Haidl, G.; Link, K.; Behre, H.M.; Goulis, D.G.; Krausz, C.; Giwercman, A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology 2018, 6, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Chehab, M.; Madala, A.; Trussell, J.C. On-label and off-label drugs used in the treatment of male infertility. Fertil. Steril. 2015, 103, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Santi, D. FSH treatment of male idiopathic infertility: Time for a paradigm change. Andrology 2020, 12746. [Google Scholar] [CrossRef]
- Santi, D.; Granata, A.R.M.; Simoni, M. FSH treatment of male idiopathic infertility improves pregnancy rate: A meta-analysis. Endocr. Connect. 2015, 4, R46–R58. [Google Scholar] [CrossRef]
- Attia, A.M.; Abou-Setta, A.M.; Al-Inany, H.G. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst. Rev. 2013, CD005071. [Google Scholar] [CrossRef]
- Foresta, C.; Selice, R.; Ferlin, A.; Garolla, A. Recombinant FSH in the treatment of oligozoospermia. Expert Opin. Biol. Ther. 2009, 9, 659–666. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, X.; Li, J.P.; Chen, S.; Zhang, R.; Tan, W.; Shi, X.J. Treatment of idiopathic oligozoospermia with recombinant human follicle-stimulating hormone: A prospective, randomized, double-blind, placebo-controlled clinical study in Chinese population. Clin. Endocrinol. (Oxf). 2015, 83, 866–871. [Google Scholar] [CrossRef]
- Foresta, C.; Bettella, A.; Merico, M.; Garolla, A.; Ferlin, A.; Rossato, M. Use of recombinant human follicle-stimulating hormone in the treatment of male factor infertility. Fertil. Steril. 2002, 77, 238–244. [Google Scholar] [CrossRef]
- Grigorova, M.; Punab, M.; Poolamets, O.; Sõber, S.; Vihljajev, V.; Žilaitienė, B.; Erenpreiss, J.; Matulevičius, V.; Tsarev, I.; Laan, M. Study in 1790 Baltic men: FSHR Asn680Ser polymorphism affects total testes volume. Andrology 2013, 1, 293–300. [Google Scholar] [CrossRef]
- Casarini, L.; Moriondo, V.; Marino, M.; Adversi, F.; Capodanno, F.; Grisolia, C.; La Marca, A.; La Sala, G.B.; Simoni, M. FSHR polymorphism p.N680S mediates different responses to FSH in vitro. Mol. Cell. Endocrinol. 2014, 393, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Selice, R.; Garolla, A.; Pengo, M.; Caretta, N.; Ferlin, A.; Foresta, C. The response to FSH treatment in oligozoospermic men depends on FSH receptor gene polymorphisms. Int. J. Androl. 2011, 34, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, A.; Ahda, Y.; Banaz-Yaşar, F.; Sonntag, B.; Nieschlag, E.; Simoni, M.; Gromoll, J. Single-nucleotide polymorphisms in the promoter region influence the expression of the human follicle-stimulating hormone receptor. Fertil. Steril. 2005, 84, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, L.; La Vignera, S.; Tomaselli, V.; Condorelli, R.A.; Cannarella, R.; Mongioì, L.M.; Calogero, A.E. The −29G/A FSH receptor gene polymorphism is associated with higher FSH and LH levels in normozoospermic men. J. Assist. Reprod. Genet. 2017, 34, 1289–1294. [Google Scholar] [CrossRef]
- Grigorova, M.; Punab, M.; Punab, A.M.; Poolamets, O.; Vihljajev, V.; Žilaitienė, B.; Erenpreiss, J.; Matulevičius, V.; Laan, M. Reproductive Physiology in Young Men Is Cumulatively Affected by FSH-Action Modulating Genetic Variants: FSHR -29G/A and c.2039 A/G, FSHB -211G/T. PLoS ONE 2014, 9, e94244. [Google Scholar] [CrossRef]
- Benson, C.A.; Kurz, T.L.; Thackray, V.G. A Human FSHB Promoter SNP Associated With Low FSH Levels in Men Impairs LHX3 Binding and Basal FSHB Transcription. Endocrinology 2013, 154, 3016–3021. [Google Scholar] [CrossRef][Green Version]
- Busch, A.S.; Tüttelmann, F.; Cremers, J.-F.; Schubert, M.; Nordhoff, V.; Schüring, A.N.; Zitzmann, M.; Gromoll, J.; Kliesch, S. FSHB −211 G>T Polymorphism as Predictor for TESE Success in Patients With Unexplained Azoospermia. J. Clin. Endocrinol. Metab. 2019, 104, 2315–2324. [Google Scholar] [CrossRef]
- Ferlin, A.; Vinanzi, C.; Selice, R.; Garolla, A.; Frigo, A.C.; Foresta, C. Toward a pharmacogenetic approach to male infertility: Polymorphism of follicle-stimulating hormone beta-subunit promoter. Fertil. Steril. 2011, 96, 1344–1349.e2. [Google Scholar] [CrossRef]
- Grigorova, M.; Punab, M.; Ausmees, K.; Laan, M. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum. Reprod. 2008, 23, 2160–2166. [Google Scholar] [CrossRef]
- Simoni, M.; Casarini, L. Mechanisms in endocrinology: Genetics of FSH action: A 2014-and-beyond view. Eur. J. Endocrinol. 2014, 170, R91–R107. [Google Scholar] [CrossRef]
- Tüttelmann, F.; Laan, M.; Grigorova, M.; Punab, M.; Sõber, S.; Gromoll, J. Combined Effects of the Variants FSHB −211G>T and FSHR 2039A>G on Male Reproductive Parameters. J. Clin. Endocrinol. Metab. 2012, 97, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.; Wang, C.; Katritch, V.; Han, G.W.; Huang, X.P.; Vardy, E.; McCorvy, J.D.; Jiang, Y.; Chu, M.; Siu, F.Y.; et al. Structural features for functional selectivity at serotonin receptors. Science 2013, 340, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, R.; Zou, Y.; Dror, R.O.; Mildorf, T.J.; Arlow, D.H.; Manglik, A.; Pan, A.C.; Liu, C.W.; Fung, J.J.; Bokoch, M.P.; et al. The dynamic process of β(2)-adrenergic receptor activation. Cell 2013, 152, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Kobilka, B.K. Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol. Sci. 2011, 32, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Chen, G.; Irving, P.; Way, J.; Chen, W.J.; Kenakin, T. The use of stimulus-biased assay systems to detect agonist-specific receptor active states: Implications for the trafficking of receptor stimulus by agonists. Mol. Pharmacol. 2000, 58, 1230–1238. [Google Scholar] [CrossRef]
- Galandrin, S.; Oligny-Longpré, G.; Bouvier, M. The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol. Sci. 2007, 28, 423–430. [Google Scholar] [CrossRef]
- Violin, J.D.; Lefkowitz, R.J. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007, 28, 416–422. [Google Scholar] [CrossRef]
- Reiter, E.; Ahn, S.; Shukla, A.K.; Lefkowitz, R.J. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 179–197. [Google Scholar] [CrossRef]
- Kenakin, T. Ligand-selective receptor conformations revisited: The promise and the problem. Trends Pharmacol. Sci. 2003, 24, 346–354. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef]
- Changeux, J.-P.; Christopoulos, A. Allosteric Modulation as a Unifying Mechanism for Receptor Function and Regulation. Cell 2016, 166, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Whalen, E.J.; Rajagopal, S.; Lefkowitz, R.J. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol. Med. 2011, 17, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.A.; Lucero Garcia-Rojas, E.Y.; Hegde, A.; Walker, J.K.L. Therapeutic Potential of Targeting ß-Arrestin. Front. Pharmacol. 2019, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, S.D.; Shen, E.S.; Holden, F.; Whitehorn, E.; Aguilar, B.; Tate, E.; Holmes, C.P.; Scheuerman, R.; MacLean, D.; Wu, M.M.; et al. Allosteric activation of the follicle-stimulating hormone (FSH) receptor by selective, nonpeptide agonists. J. Biol. Chem. 2006, 281, 13226–13233. [Google Scholar] [CrossRef]
- Arey, B.J.; Yanofsky, S.D.; Claudia Pérez, M.; Holmes, C.P.; Wrobel, J.; Gopalsamy, A.; Stevis, P.E.; López, F.J.; Winneker, R.C. Differing pharmacological activities of thiazolidinone analogs at the FSH receptor. Biochem. Biophys. Res. Commun. 2008, 368, 723–728. [Google Scholar] [CrossRef]
- Sriraman, V.; Denis, D.; de Matos, D.; Yu, H.; Palmer, S.; Nataraja, S. Investigation of a thiazolidinone derivative as an allosteric modulator of follicle stimulating hormone receptor: Evidence for its ability to support follicular development and ovulation. Biochem. Pharmacol. 2014, 89, 266–275. [Google Scholar] [CrossRef]
- Yu, H.N.; Richardson, T.E.; Nataraja, S.; Fischer, D.J.; Sriraman, V.; Jiang, X.; Bharathi, P.; Foglesong, R.J.; Haxell, T.F.N.; Heasley, B.H.; et al. Discovery of substituted benzamides as follicle stimulating hormone receptor allosteric modulators. Bioorg. Med. Chem. Lett. 2014, 24, 2168–2172. [Google Scholar] [CrossRef]
- Van Koppen, C.J.; Verbost, P.M.; van de Lagemaat, R.; Karstens, W.J.F.; Loozen, H.J.J.; van Achterberg, T.A.E.; van Amstel, M.G.A.; Brands, J.H.G.M.; van Doornmalen, E.J.P.; Wat, J.; et al. Signaling of an allosteric, nanomolar potent, low molecular weight agonist for the follicle-stimulating hormone receptor. Biochem. Pharmacol. 2013, 85, 1162–1170. [Google Scholar] [CrossRef]
- Van Straten, N.C.R.; van Berkel, T.H.J.; Demont, D.R.; Karstens, W.J.F.; Merkx, R.; Oosterom, J.; Schulz, J.; van Someren, R.G.; Timmers, C.M.; van Zandvoort, P.M. Identification of substituted 6-amino-4-phenyltetrahydroquinoline derivatives: Potent antagonists for the follicle-stimulating hormone receptor. J. Med. Chem. 2005, 48, 1697–1700. [Google Scholar] [CrossRef]
- Dias, J.A.; Bonnet, B.; Weaver, B.A.; Watts, J.; Kluetzman, K.; Thomas, R.M.; Poli, S.; Mutel, V.; Campo, B. A negative allosteric modulator demonstrates biased antagonism of the follicle stimulating hormone receptor. Mol. Cell. Endocrinol. 2011, 333, 143–150. [Google Scholar] [CrossRef][Green Version]
- Dias, J.A.; Campo, B.; Weaver, B.A.; Watts, J.; Kluetzman, K.; Thomas, R.M.; Bonnet, B.; Mutel, V.; Poli, S.M. Inhibition of follicle-stimulating hormone-induced preovulatory follicles in rats treated with a nonsteroidal negative allosteric modulator of follicle-stimulating hormone receptor. Biol. Reprod. 2014, 90, 19. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.A.; Yvinec, R.; Jégot, G.; Dias, J.A.; Poli, S.M.; Poupon, A.; Crépieux, P.; Reiter, E. Profiling of FSHR negative allosteric modulators on LH/CGR reveals biased antagonism with implications in steroidogenesis. Mol. Cell. Endocrinol. 2016, 436, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Newton, C.L.; Anderson, R.A.; Millar, R.P. Gonadotropins and Their Analogs: Current and Potential Clinical Applications. Endocr. Rev. 2018, 39, 911–937. [Google Scholar] [CrossRef]
- Sugahara, T.; Pixley, M.R.; Minami, S.; Perlas, E.; Ben-Menahem, D.; Hsueh, A.J.; Boime, I. Biosynthesis of a biologically active single peptide chain containing the human common alpha and chorionic gonadotropin beta subunits in tandem. Proc. Natl. Acad. Sci. USA 1995, 92, 2041–2045. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Lobel, L.; Pollak, S.; Ferin, M.; Xiao, E.; Sauer, M.; Lustbader, J. Pharmacokinetics and pharmacodynamics of single-chain recombinant human follicle-stimulating hormone containing the human chorionic gonadotropin carboxyterminal peptide in the rhesus monkey. Fertil. Steril. 2002, 77, 1248–1255. [Google Scholar] [CrossRef]
- Sugahara, T.; Grootenhuis, P.D.; Sato, A.; Kudo, M.; Ben-Menahem, D.; Pixley, M.R.; Hsueh, A.J.; Boime, I. Expression of biologically active fusion genes encoding the common alpha subunit and either the CG beta or FSH beta subunits: Role of a linker sequence. Mol. Cell. Endocrinol. 1996, 125, 71–77. [Google Scholar] [CrossRef]
- Lemke, E.P.; Adams, B.M.; Jablonka-Shariff, A.; Boime, I.; Adams, T.E. Single-chain human gonadotropin analogs induce follicle development in sheep. J. Endocrinol. 2008, 196, 593–600. [Google Scholar] [CrossRef]
- Rutigliano, H.M.; Adams, B.M.; Jablonka-Shariff, A.; Boime, I.; Adams, T.E. Effect of single-chain ovine gonadotropins with dual activity on ovarian function in sheep. Reproduction 2014, 148, 129–136. [Google Scholar] [CrossRef][Green Version]
- Kanda, M.; Jablonka-Shariff, A.; Sato, A.; Pixley, M.R.; Bos, E.; Hiro’oka, T.; Ben-Menahem, D.; Boime, I. Genetic fusion of an alpha-subunit gene to the follicle-stimulating hormone and chorionic gonadotropin-beta subunit genes: Production of a bifunctional protein. Mol. Endocrinol. 1999, 13, 1873–1881. [Google Scholar]
- Hutchings, C.J.; Koglin, M.; Olson, W.C.; Marshall, F.H. Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat. Rev. Drug Discov. 2017, 16, 787–810. [Google Scholar] [CrossRef]
- Mujić-Delić, A.; de Wit, R.H.; Verkaar, F.; Smit, M.J. GPCR-targeting nanobodies: Attractive research tools, diagnostics, and therapeutics. Trends Pharmacol. Sci. 2014, 35, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Staus, D.P.; Strachan, R.T.; Manglik, A.; Pani, B.; Kahsai, A.W.; Kim, T.H.; Wingler, L.M.; Ahn, S.; Chatterjee, A.; Masoudi, A.; et al. Allosteric nanobodies reveal the dynamic range and diverse mechanisms of G-protein-coupled receptor activation. Nature 2016, 535, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Ferasin, L.; Gabai, G.; Beattie, J.; Bono, G.; Holder, A.T. Enhancement of FSH bioactivity in vivo using site-specific antisera. J. Endocrinol. 1997, 152, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Glencross, R.G.; Lovell, R.D.; Holder, A.T. Monoclonal antibody enhancement of FSH-induced uterine growth in snell dwarf mice. J. Endocrinol. 1993, 136, R5–R7. [Google Scholar] [CrossRef] [PubMed]
- Licht, P.; Gallo, A.B.; Aggarwal, B.B.; Farmer, S.W.; Castelino, J.B.; Papkoff, H. Biological and binding activities of equine pituitary gonadotrophins and pregnant mare serum gonadotrophin. J. Endocrinol. 1979, 83, 311–322. [Google Scholar] [CrossRef]
- Guillou, F.; Combarnous, Y. Purification of equine gonadotropins and comparative study of their acid-dissociation and receptor-binding specificity. Biochim. Biophys. Acta 1983, 755, 229–236. [Google Scholar] [CrossRef]
- Combarnous, Y.; Guillou, F.; Martinat, N. Comparison of in vitro follicle-stimulating hormone (FSH) activity of equine gonadotropins (luteinizing hormone, FSH, and chorionic gonadotropin) in male and female rats. Endocrinology 1984, 115, 1821–1827. [Google Scholar] [CrossRef]
- Hervé, V.; Roy, F.; Bertin, J.; Guillou, F.; Maurel, M.-C. Antiequine chorionic gonadotropin (eCG) antibodies generated in goats treated with eCG for the induction of ovulation modulate the luteinizing hormone and follicle-stimulating hormone bioactivities of eCG differently. Endocrinology 2004, 145, 294–303. [Google Scholar] [CrossRef]
- Wehbi, V.; Decourtye, J.; Piketty, V.; Durand, G.; Reiter, E.; Maurel, M.C. Selective modulation of follicle-stimulating hormone signaling pathways with enhancing equine chorionic gonadotropin/antibody immune complexes. Endocrinology 2010, 151, 2788–2799. [Google Scholar] [CrossRef]
- Agrawal, G.; Dighe, R.R. Critical involvement of the hinge region of the follicle-stimulating hormone receptor in the activation of the receptor. J. Biol. Chem. 2009, 284, 2636–2647. [Google Scholar] [CrossRef]
- Crepin, R.; Veggiani, G.; Djender, S.; Beugnet, A.; Planeix, F.; Pichon, C.; Moutel, S.; Amigorena, S.; Perez, F.; Ghinea, N.; et al. Whole-cell biopanning with a synthetic phage display library of nanobodies enabled the recovery of follicle-stimulating hormone receptor inhibitors. Biochem. Biophys. Res. Commun. 2017, 493, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Guo, K.P.; Ji, S.Y.; Liu, X.M.; Wang, P.; Wu, J.; Gao, L.; Jiang, T.Q.; Xu, T.; Fan, H.Y. Development and characterization of a novel long-acting recombinant follicle stimulating hormone agonist by fusing Fc to an FSH-β subunit. Hum. Reprod. 2016, 31, 169–182. [Google Scholar] [CrossRef]
- Carr, R.; Benovic, J.L. From biased signalling to polypharmacology: Unlocking unique intracellular signalling using pepducins. Biochem. Soc. Trans. 2016, 44, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kahsai, A.W.; Wisler, J.W.; Lee, J.; Ahn, S.; Cahill, T.J., III; Dennison, S.M.; Staus, D.P.; Thomsen, A.R.B.; Anasti, K.M.; Pani, B.; et al. Conformationally selective RNA aptamers allosterically modulate the β2-adrenoceptor. Nat. Chem. Biol. 2016, 12, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Carrau, T.; Gärtner, U.; Seipp, A.; Taubert, A.; Felmer, R.; Sanchez, R.; Hermosilla, C. Leukocytes coincubated with human sperm trigger classic neutrophil extracellular traps formation, reducing sperm motility. Fertil. Steril. 2016, 106, 1053–1060.e1. [Google Scholar] [CrossRef]
| Drug Name | Status | Originator |
|---|---|---|
| GONAL-f | Marketed | Merck |
| Cinnal-f | Marketed | CinnaGen |
| DA-3801 | Marketed | Dong-A Pharmaceutical |
| Bemfola | Marketed | Gedeon Richter |
| Ovaleap | Marketed | Theramex |
| Gonapure | Marketed | Minapharm Pharmaceuticals |
| Follitropin alpha biosimilar—Allergan/Itero Biopharmaceuticals | Phase III | Itero Biopharmaceuticals |
| LM-001 | Preclinical | Alphamab |
| Follitropin alpha biosimilar—Cadila Healthcare | Phase III | Cadila Healthcare |
| Folitime | No development reported | GEMA |
| Primapur | Phase III | iVFarma |
| ProLease | Discontinued | Merck Serono |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarini, L.; Crépieux, P.; Reiter, E.; Lazzaretti, C.; Paradiso, E.; Rochira, V.; Brigante, G.; Santi, D.; Simoni, M. FSH for the Treatment of Male Infertility. Int. J. Mol. Sci. 2020, 21, 2270. https://doi.org/10.3390/ijms21072270
Casarini L, Crépieux P, Reiter E, Lazzaretti C, Paradiso E, Rochira V, Brigante G, Santi D, Simoni M. FSH for the Treatment of Male Infertility. International Journal of Molecular Sciences. 2020; 21(7):2270. https://doi.org/10.3390/ijms21072270
Chicago/Turabian StyleCasarini, Livio, Pascale Crépieux, Eric Reiter, Clara Lazzaretti, Elia Paradiso, Vincenzo Rochira, Giulia Brigante, Daniele Santi, and Manuela Simoni. 2020. "FSH for the Treatment of Male Infertility" International Journal of Molecular Sciences 21, no. 7: 2270. https://doi.org/10.3390/ijms21072270
APA StyleCasarini, L., Crépieux, P., Reiter, E., Lazzaretti, C., Paradiso, E., Rochira, V., Brigante, G., Santi, D., & Simoni, M. (2020). FSH for the Treatment of Male Infertility. International Journal of Molecular Sciences, 21(7), 2270. https://doi.org/10.3390/ijms21072270








