Abstract
Alpinia oxyphylla Miq. (i.e., A. oxyphylla), a traditional Chinese medicine, can exert neuroprotective effects in ameliorating mild cognitive impairment and improving the pathological hallmarks of Alzheimer’s disease (AD). Here, 50 active compounds and 164 putative targets were collected and identified with 251 clinically tested AD-associated target proteins using network pharmacology approaches. Based on the Gene Ontology/Kyoto Encyclopedia of Genes and Genomes pathway enrichments, the compound-target-pathway-disease/protein–protein interaction network constructions, and the network topological analysis, we concluded that A. oxyphylla may have neuroprotective effects by regulating neurotransmitter function, as well as brain plasticity in neuronal networks. Moreover, closely-related AD proteins, including the amyloid-beta precursor protein, the estrogen receptor 1, acetylcholinesterase, and nitric oxide synthase 2, were selected as the bottleneck nodes of network for further verification by molecular docking. Our analytical results demonstrated that terpene, as the main compound of A. oxyphylla extract, exerts neuroprotective effects, providing new insights into the development of a natural therapy for the prevention and treatment of AD.
1. Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder, characterized by brain atrophy, progressive cognitive dysfunction, and behavioral disturbances [1]. The cardinal neuropathological features of AD include extracellular accumulations of amyloid beta (Aβ) peptide [2], neurofibrillary tangles (NFTs) of hyper-phosphorylated tau proteins [3], neuropil threads, dystrophic neuritis [4,5], astrogliosis, microglial activation [6], and overall neurodegeneration of the brain [6,7,8]. Several hypotheses about cholinergic system dysfunction [9], Aβ deposits, oxidative stress [10], inflammatory pathways [11], calcium signaling dysfunction [12], hormone imbalance [13], and genetic components [14] have been considered to play important roles in the occurrence and development of AD, although the etiology of this disease is still not precisely known [15]. In addition, although several therapies have been used as compensation for the cholinergic neuronal loss and reduction or prevention of amyloid/tau aggregation and toxicity, such as gene therapy, vaccines, anti-inflammatory agents [16], cholesterol-lowering agents, anti-oxidants [17], and hormone therapy [18] for AD, these single targeted therapies have often been unsuccessful [19,20]. Traditional Chinese Medicine (TCM), with thousands of years of history in China, has received widespread attention, particularly given the few side effects it carries, multi-target treatments, and natural origins [21,22]. These medicines have been successfully utilized for the treatment of complex neurological disorders, such as AD [23,24].
Alpinia oxyphylla (A. oxyphylla), the dried ripe fruit of Alpinia oxyphylla Miq., has been long been thought to be “multivalent” for commonly treating diarrhea [25], enuresis (i.e., involuntary urination), dementia [26,27,28], inflammation [29], cancer [29], and other disorders [30,31]. Notably, terpene, the main component of A. oxyphylla, is regarded as a representative constituent with putative pharmacological activities by phytochemical studies. Recently, several pharmacology studies have indicated that A. oxyphylla has definite beneficial effects in ameliorating cognitive disorders and alleviating pathological brain injuries characteristic of neurodegenerative disorders [26,27,32,33,34]. A. oxyphylla extracts were shown to have neuroprotective effects against glutamate-induced apoptosis in cortical neurons [35], as well as biological activity towards regulating redox homeostasis, increasing the anti-oxidative properties of enzymes, improving the cholinergic system, and reducing Aβ peptide levels. Moreover, extracts also ameliorated the learning and memory impairment of AD mouse models after being administered by intracerebroventricular injection, which reduced β-amyloid levels significantly [26,36]. However, despite the known therapeutic effects of A. oxyphylla, its pharmacological and molecular mechanism of action have not been fully elucidated. Nonetheless, these aforementioned studies have given us a foundation to further study the molecular mechanisms of A. oxyphylla using systems pharmacology methodologies.
It is worth mentioning that our previous experiments have verified the neuroprotective effects of A. oxyphylla by extending the nonparalytic rate and reducing the mean paralysis rate on amyloid beta (1-42) transgenic Caenorhabditis elegans (C. elegans) (data not shown). In this paper, pharmacokinetic evaluation, target prediction, network, and pathway analysis using multiple available public databases and bioinformatics resources, have revealed potential pharmacological mechanisms and beneficial effects of A. oxyphylla on AD.
2. Results
2.1. Screening of Candidate Compounds and Predicting Putative Target Proteins
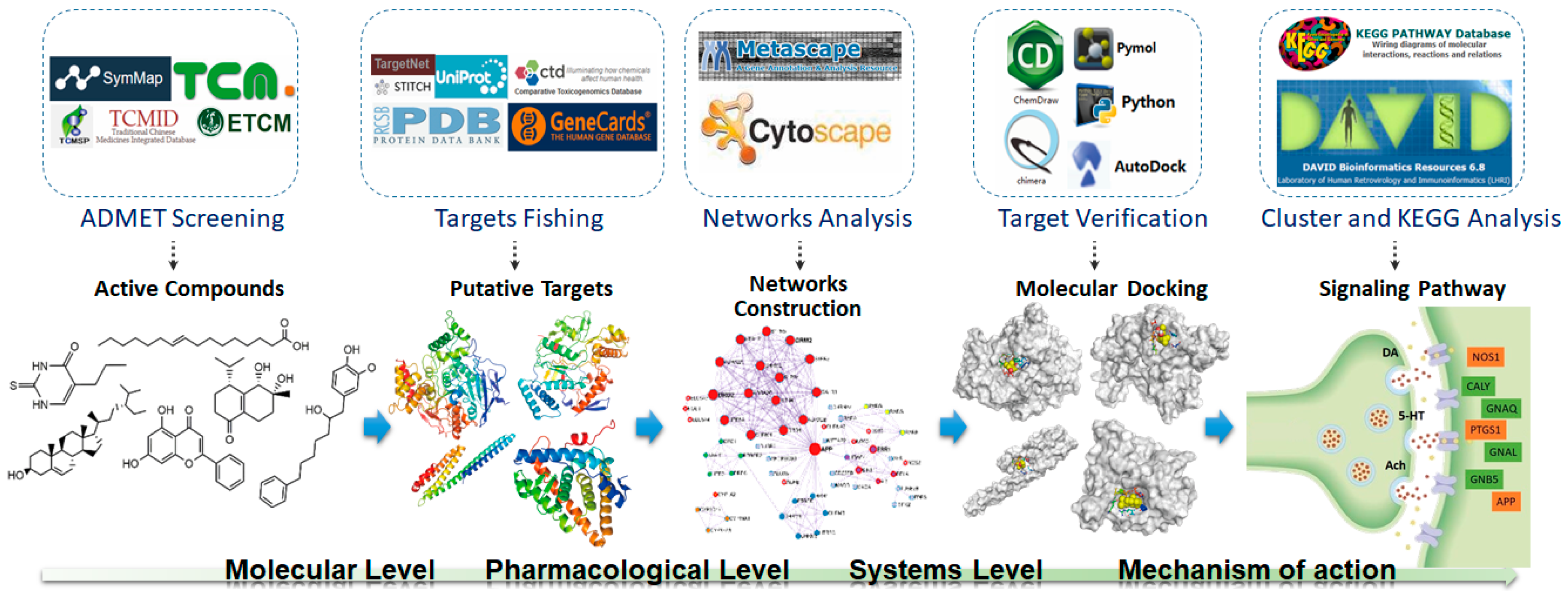
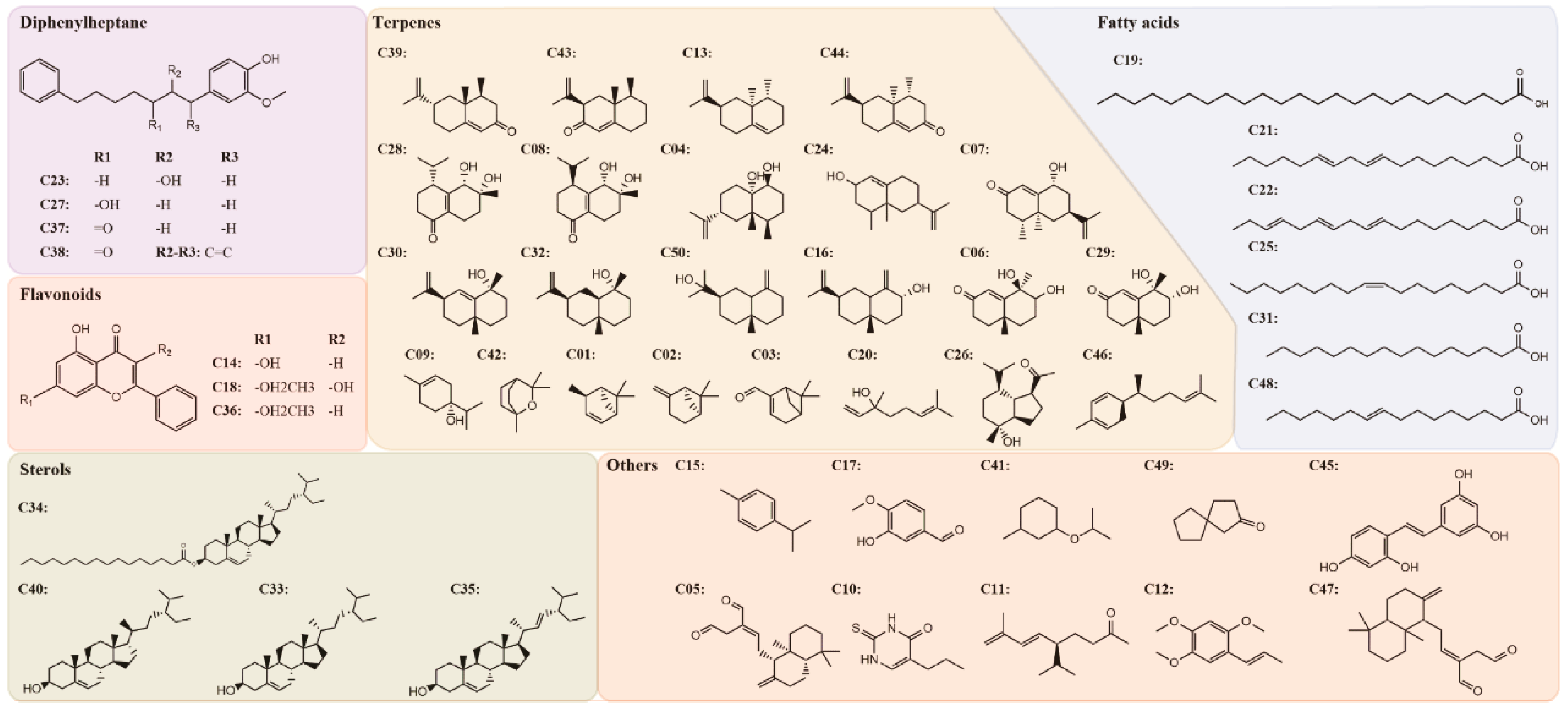
The potential pharmacological mechanisms and the details behind each step in defining the role of A. oxyphylla on AD using system pharmacology are shown in Figure 1. Fifty compounds were selected as candidates by screening and excluding duplicates from the native compounds of A. oxyphylla based on the Symmap, TCMSP, TCM-MESH, and TCMID database analyses. All 50 compounds were divided into 6 categories: 23 terpenes (e.g., oxyphyllol A, oxyphyllol B, zingiberol, among others), 6 fatty acids (e.g., lignoceric acid, oleic acid), 4 diphenylpeptanes (e.g., neonootkatol, oxyphyllacinol), 4 sterols (e.g., sitoterpene, sitoterpene palmitate), 3 flavonoids (chrysin, izalpinin, and tectochrysin), and 10 others (e.g., solanone, isovanilin, azaron, among others), as shown in Figure 2. Furthermore, 12 compounds presented a good score in the ADMET criterion, and 18 compounds presented moderate levels after evaluating them in the ETCM database (Supplementary Materials Table S1).
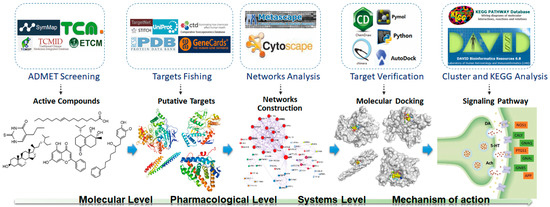
Figure 1.
Network pharmacology for deciphering pharmacological mechanisms of A. oxyphylla acting on Alzheimer’s disease.
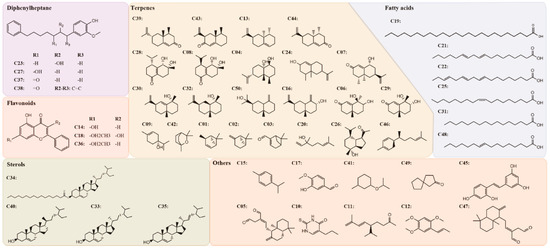
Figure 2.
The 2-dimensional (2D) molecular structures and the classification of 50 candidate compounds in A. oxyphylla. There are 6 classifications including diphenylheptane (4), flavonoids (3), sterols (4), terpenes (23), fatty acids (6) and others (10).
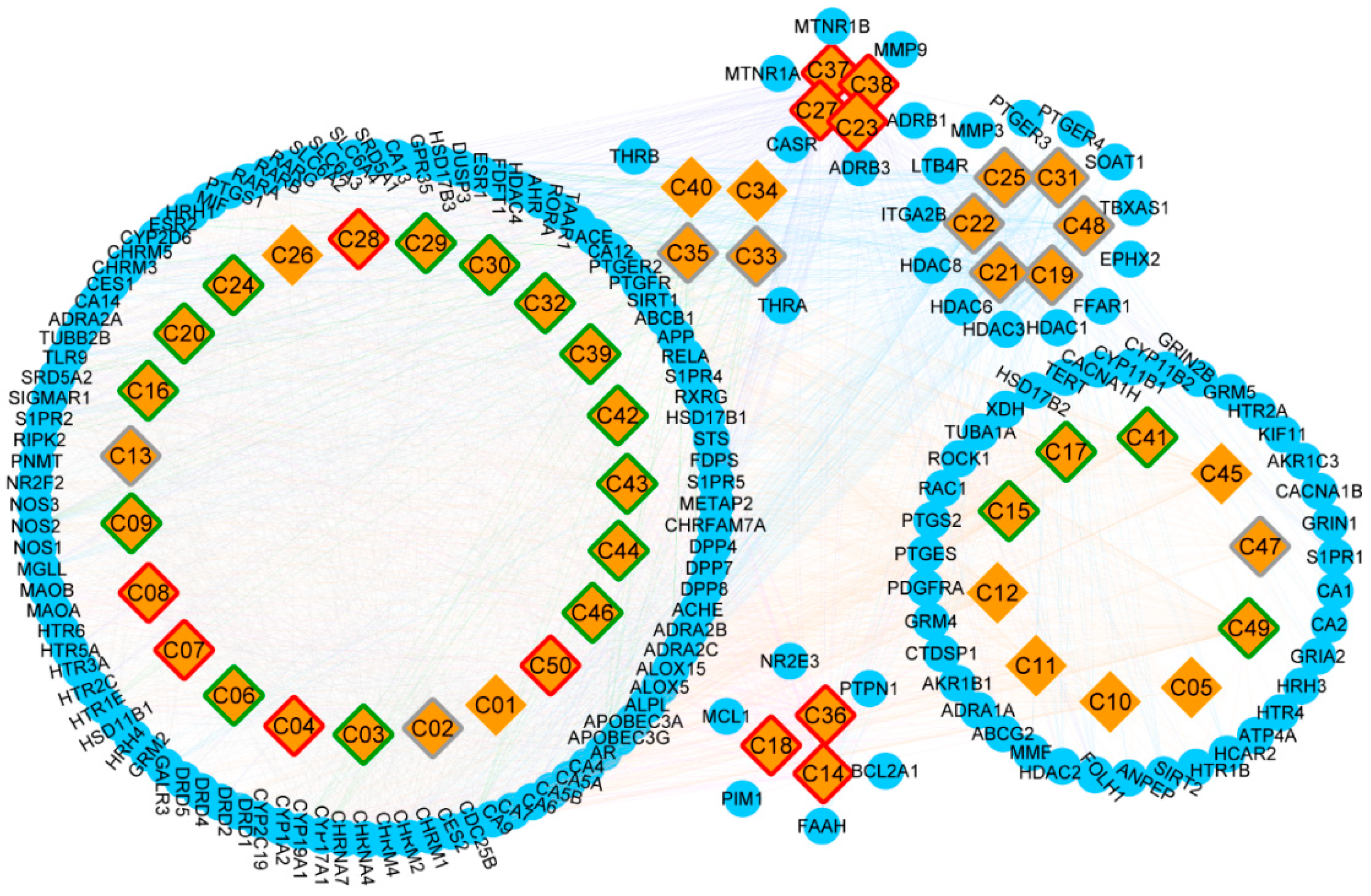
A total of 31150 target proteins were fished along with the 50 candidate compounds using the Lipinski Rule of Five in QSAR and overlapping them with STITCH results. A higher docking probability and combined score of putative target proteins indicated a closely integration with the compounds. Among these target proteins, 164 targets were screened as putative target proteins, 99 of which were putative target proteins of terpenes (Table S2). Furthermore, the CT network was constructed and illustrated based on the various categories of compounds and putative proteins aligned (Figure 3). Compound nodes with red, green, gray, and white borders represented good, moderate, weak, and N/A level of ADMET evaluation respectively. Among these, five terpenes (oxyphyllol B/C, oxyphyllenodiol A/B, and zingiberol), four diphenylpeptanes (neonootkatol, oxyphyllacinol, and yakuchinone A/B), and three flavonoids (chrysin, izalpinin, and tectochrysin) reflected good pharmacokinetic properties by the ADMET criterion. Moreover, our Venn diagram clearly showed the overlapping of terpene proteins, which represent more than half of the protein targets, than any of the other five groups (Figure S1). Thus, we speculated that terpenes may be the predominant and unique primary bioactive substances mediating the neuroprotective effects of A. oxyphylla.
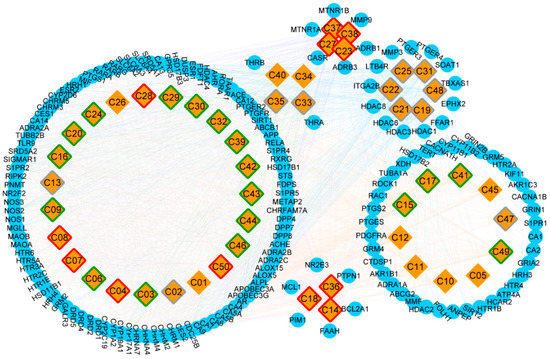
Figure 3.
The construction of Compound-Target network, and the classification of putative target proteins of A. oxyphylla. The candidate compounds (diamond mesh node with yellow) are divided into groups by structural category, the pharmacokinetic properties of which are divided into good (red border), moderate (green border), week (gray border), and N/A (white border). Similarly, the putative target proteins (the circular mesh node with blue) are grouped and surrounded with the corresponding compounds.
2.2. Exploration of A. oxyphylla Molecular Mechanism of Action
The 99 putative target proteins of terpenes were selected based on their integration score and GO and KEGG pathway enrichment analyses were initiated. After filtering by a parameter p-value cutoff of ≤ 0.01, 579 GO terms and 28 KEGG pathway terms for terpenes were identified, as shown in Tables S3 and S4 (Figure S2D). A total of 579 GO terms were included: 459 for biological processes, 76 for molecular function, and 44 for cellular components (Table S3). In order to show the results of the GO enrichment in an intuitive and explicit way, a bubble diagram was utilized. As shown in Figure S2A–C, p-values are given the highest priority in descending order. More importantly, the muscarinic acetylcholine receptor family M1-M5 (CHRM1, 2, 3, 4 and CHRM5), the neuronal acetylcholine receptor subunit alpha-4 and 7 (CHRNA4 and CHRNA7), the 5-hydroxytryptamine receptor 3A and 5A (HTR3A and HTR5A), the sodium-dependent serotonin transporter (SLC6A4), and the dopamine D1/D2 receptor (DRD1 and DRD2) were the most frequently occurring protein targets, mainly enriched in nervous (has04725, 04726, and 04728) and sensory systems (has04742), signaling molecules and interaction (hsa04080), signal transduction (hsa04020, 04022, 04024, 04066, and 04371), as well as substance dependence related signaling pathways, especially in neurotransmission (GO: 0030594, 0099536, and 0099537), neurons and synapse formation (GO: 0030425, 0045211, 0097447, 0097060, and 0099699), serotonin receptor (GO: 0099589), and G protein-coupled receptor (GPCR) signaling pathways (GO: 0004993, 0007187, 0007188, 0008227, and 0099528). Thus, we further identify that terpenes played a key regulating role in nervous system diseases, such as AD.
Furthermore, gene entries related to AD were collected from the CTD and GeneCards databases. As a result, 22150 and 6634 protein coding gene entries were collected from each database, respectively. A total of 251 gene entries with prioritized inference scores and relevance scores were screened and integrated from two databases, which served as the key putative target proteins of AD (Tables S5 and S6). Among the putative target proteins of A. oxyphylla, 21 target proteins associated with AD were found, including the amyloid-beta precursor protein (APP), the ATP-dependent translocase ABCB1 (ABCB1) protein, the neuronal acetylcholine receptor subunit alpha-7 (CHRNA7), the angiotensin-converting enzyme (ACE), the glutamate receptor ionotropic N-methyl-D-aspartate receptor (NMDA) 2B (GRIN2B), the cytochrome P450 1A2 (CYP1A2), acetylcholinesterase (ACHE), the 5-hydroxytryptamine receptor 2A (HTR2A), the sodium-dependent dopamine transporter (SLC6A3 and SLC6A4), nitric oxide synthase (brain: NOS1, inducible: NOS2, and endothelial: NOS3), neprilysin (MME), stromelysin-1 (MMP3), the matrix metalloproteinase-9 (MMP9), the NAD-dependent protein deacetylase sirtuin-1 (SIRT1), prostaglandin G/H synthase 1, 2 (PTGS1 and PTGS2), transcription factor p65 (RELA), and glutamate receptor ionotropic, NMDA 1 (GRIN1), as listed in Table S7. The overlap proteins from terpenes and the clinically tested AD-associated target proteins reflect the potential functions of terpenes in treating AD.
2.3. Construction and Analysis of the Integrated Network Model
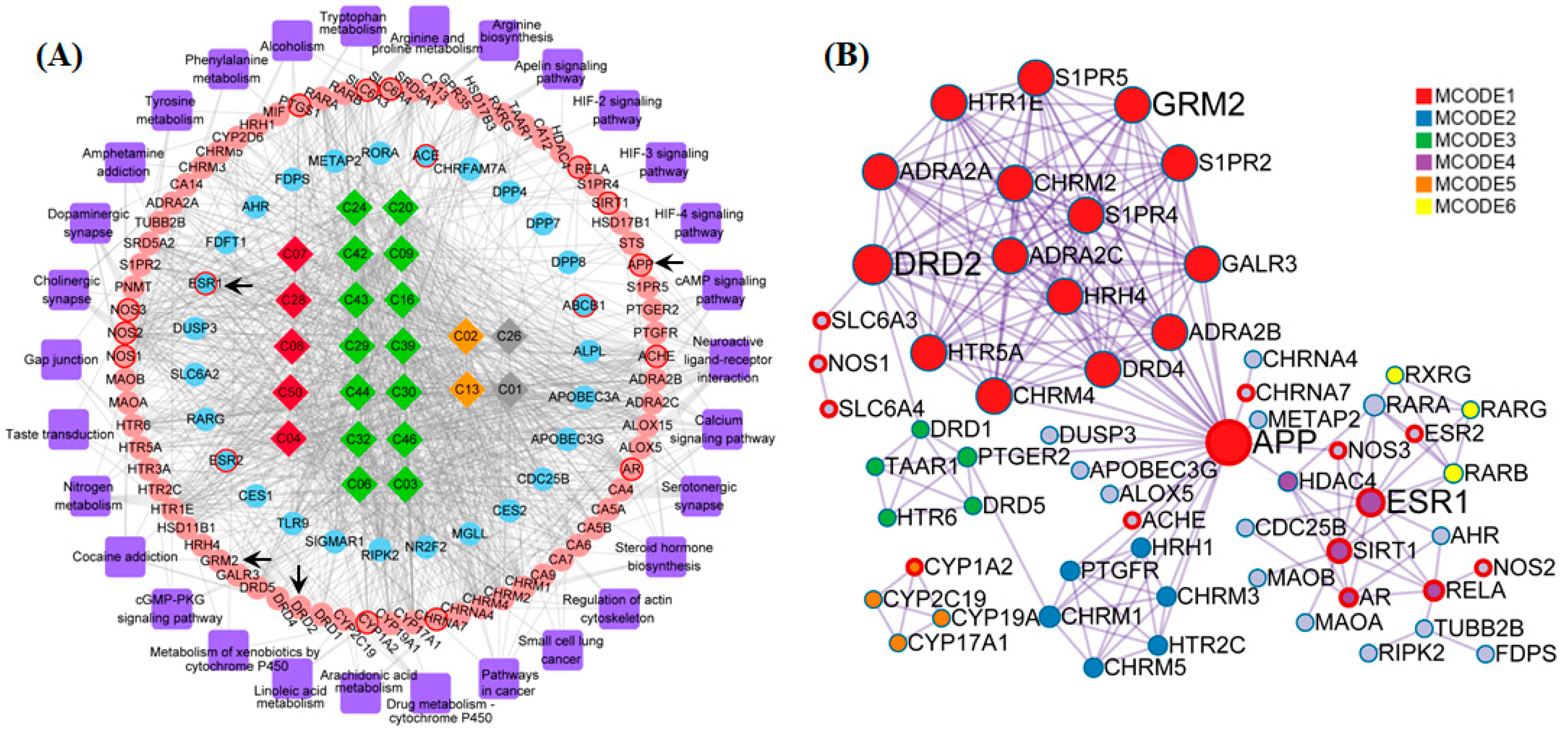
The compound-target-pathway-disease (CTPD) network of A. oxyphylla contained 234 nodes and 2594 edges, with the AD-related putative target proteins marked with a red border, and the other putative target proteins marked in blue, as shown in Figure S3. In order to further inquire into the underlying molecular mechanisms mediating the neuroprotective mechanisms of A. oxyphylla, the compound-target-pathway (CTP) and protein–protein interaction (PPI) network of terpene main ingredients were carried out in this research. Specifically, the CTP network model of terpenes contained 152 nodes and 995 edges, with 72 differentially expressed genes (p < 0.01) that were enriched in 28 pathways, based on the GO/KEGG pathway enrichment analysis (Figure 4A). The ellipse nodes with red border represent the 13 AD-associated proteins and 4 key proteins (from 13 hubba proteins, Table S8), which were filtered by topological analysis of 164 putative proteins from the HINT hqb as background network (Figure S4). Importantly, the PPI network is the premise and basis to obtain nodes with more substantial contributions. Overlap nodes were screened by valuable topological indices including degree, betweenness centrality, closeness centrality, and bottleneck nodes of the top 20 nodes, which have significant impact on the performance of the whole network. In the PPI network, the estrogen receptor (ESR1), APP, D2 dopamine receptor (DRD2), and metabotropic glutamate receptor 2 (GRM2) were filtered as bottleneck node proteins consistent with their molecular functions. Clustering and topology approaches were utilized to identify individual variations and similarities among various protein targets (Figure 4B).
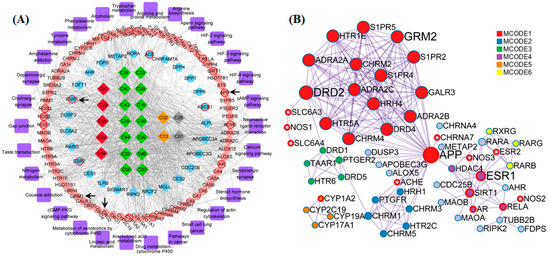
Figure 4.
The constructions and analysis of compound-target-pathway (CTP) network and the Protein-Protein Interaction (PPI) network of terpenes. (A) The candidate compounds with different pathological properties (good: Red diamond, moderate: Green diamond, week: Yellow diamond and N/A: Gray diamond), the ellipse nodes respect the KEGG enrichment terms with p < 0.01 (pink ellipse) and nodes not enriched in KEGG with p < 0.01 (blue ellipse), and AD-associated proteins (ellipse with red border) and the pathways (purple round rectangle) of Terpenes are constructed. (B) The protein-protein-interaction (PPI) network of Terpenes are illustrated with color by clusters and size by degree. Among these nodes, APP, ESR1, DRD2 and GRM2 are served as core targets with red border.
Furthermore, it is of great significance for compounds with better water solubility, blood–brain barrier permeability, and pharmacokinetic properties in thinking of drug design and development. For better understanding of the mechanism of action and structure-activity relationship of terpenes and its putative targets, terpene compounds with good pharmacokinetic properties by the ADMET screening criteria and its common targets (i.e., ACHE, AR, HTR1E, NOS2, SRD5A2, CHRM4, and CYP17A1) were selected. Among these target proteins, ACHE and NOS2 were found to be the overlapping nodes with AD- associated proteins, which is valuable for future studies.
2.4. Molecular Docking
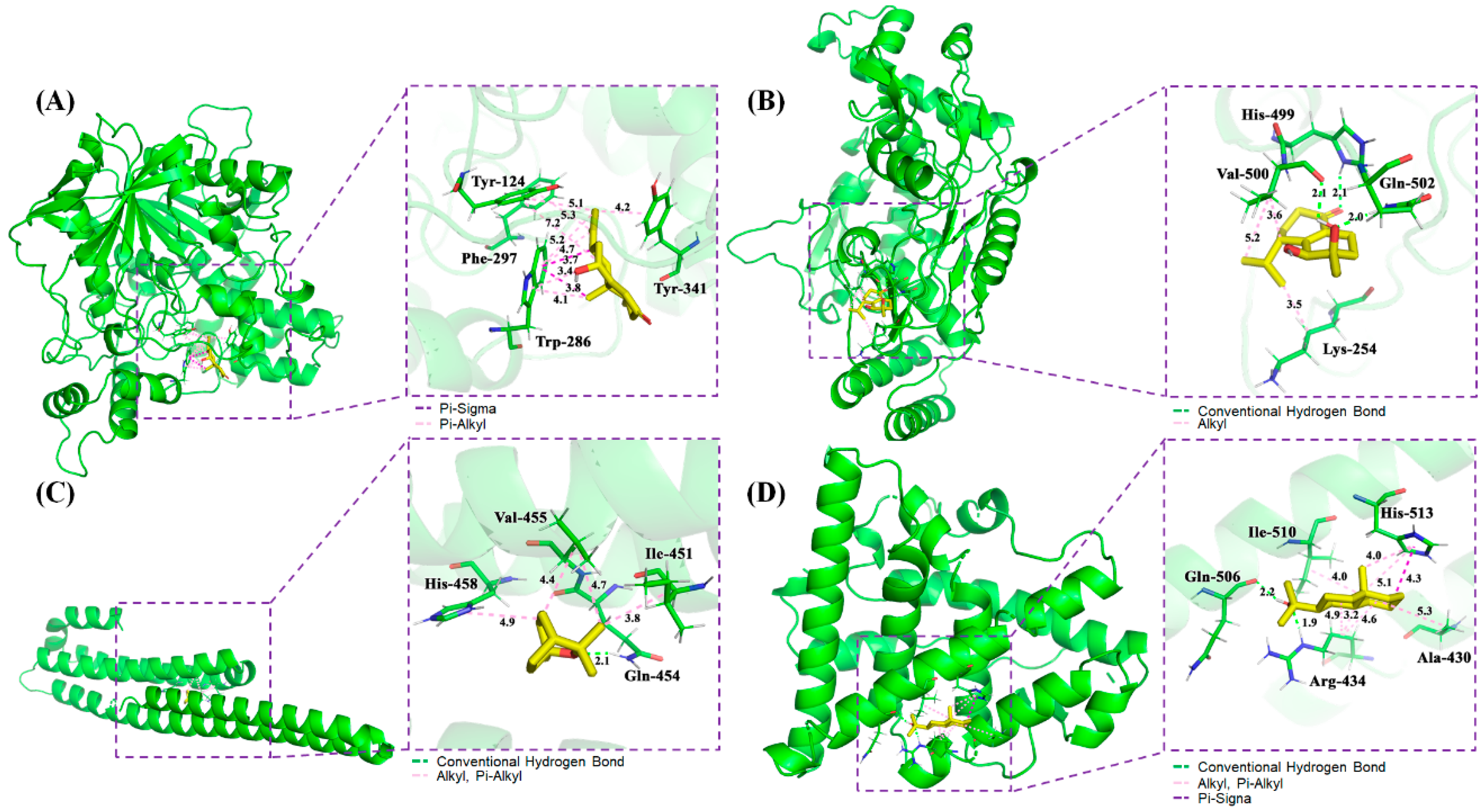
In order to further verify the functional effects of terpenes in AD, the interaction between the active compounds of terpenes with good pharmacokinetic properties and key putative proteins were tested and verified randomly. With the application of AutoDockTools-1.5.6, Discovery Studio 4.5 Client, and Pymol software, the interactions among the potential active compounds (i.e., oxyphyllol B, oxyphyllenodiol A, eucalyptol, and zingiberol), the AD-associated proteins (i.e., ACHE and NOS2), as well as the bottleneck node proteins (i.e., APP and ESR1) were elucidated. Notably, the lower of binding energy meant the binding between the compounds and the targets were stronger. The Van der Waals forces, hydrogen bonding, and aromatic stacking (Pi-Sigma, Pi-alkyl, and alkyl interactions) were found to be involved between the active site residues and the potential compounds.
The 3D mode and 2D dimensional representation of oxyphyllol B (C07) in the active site of ACHE (6o4w) is represented in Figure 5A and Figure S5A. Interestingly, the free binding energy of C07 with ACHE was found to be ‒4.8 kcal/mol. In addition, C07 showed two Pi-Sigma bond interactions with TRP 286, and eight Pi-alkyl bonds with TYR 124, PHE 297, TRP 286, and TYR 341. The interaction types and distance of binding complexes are also depicted in the enlarged drawing in Figure 5A. The following atoms, TYR 72, PHE 295, VAL 294, ARG 296, SER 293, and LEU 76 were served as the pocket atoms held together around the C07 compound by Van der Waals forces. Similarly, the binding interaction of oxyphyllenodiol A (C08) and NOS2 (4nos) is shown in Figure 5B and Figure S5B. The free binding energy of C08 with NOS2 was found to be -4.68 kcal/mol. In particular, C08 showed 3H-bond interactions with HIS 499, GLN 502, and VAL 500, respectively. VAL 500 and LYS 254 also presented alkyl interactions with C08. Additionally, THR498, GLN 310, GLY313, and ASP131 were able to bind with C08 by Van der Waals forces. The interaction types and distance of these binding complexes are also depicted in the Ray tracing diagram in Figure 5B. Moreover, the binding sites, interaction types and distances, as well as the active atoms of APP (5buo) with eucalyptol (C42) were analyzed and shown in Figure 5C and Figure S5C. The free binding energy of eucalyptol (C42) with APP (5buo) was found to be ‒3.9 kcal/mol. C42 showed 1H-bond interactions with GLN 454 and three alkyl, as well as Pi-alkyl interactions with ILE 451, VAL 455, and HIS 458, respectively. Meanwhile, C42 presented four Van der Waals forces among MET 383, HIS 436, THR 433, and GLU 387. The 2D/3D mode and the interaction types and distance of zingiberol (C50) in the active site of ESR1 (3os8 revised mutation site) is represented in Figure 5D and Figure S5D. The free binding energy of C07 with ACHE was found to be ‒5.28 kcal/mol. C50 showed 2H-bond interactions with ARG 434 and GLN 506, as well as one Pi-Sigma bond with HIS 513. Moreover, C50 also had seven Alkyl and Pi-Alkyl interactions with HIS 513, ALA 430, ILE 510, and ARG 434, respectively. There were two Van der Waals forces of THR 431 and LEU 509 with C50.
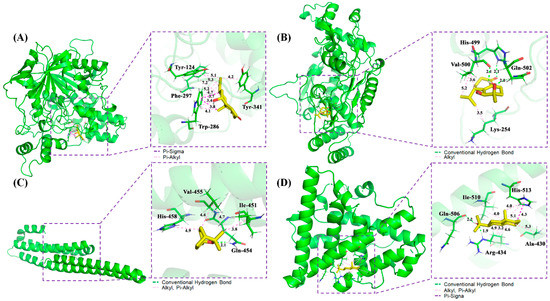
Figure 5.
Schematic 3D representation that the molecular docking model, active sites and binding distances, and the ray tracing of compound (A) oxyphyllol B (C07) in the protein ACHE (PDB ID: 6o4w), (B) oxyphyllenodiol A (C08) with NOS2 (4nos), (C) eucalyptol (C42) with APP (5buo), and (D) zingiberol (C50) with ESR1 (3os8 revised mutation site), respectively.
3. Discussion
TCM has been shown to be an effective treatment for the relieve of complicated diseases in a multi-target/multi-component manner, which makes it unique among all traditional medicines [37]. Importantly, TCM has been used in the treatment of neurological diseases for over thousands of years. The A. oxyphylla plant belongs to the Zingiber of Zingiberaceae genus, which is rich in terpenes, flavonoids, and diphenylpeptanes, among other compounds, which have been shown to have clinical activity in patients with diarrhea and enuresis [38,39,40,41]. Recently, a growing number of clinical trials and studies have shown that A. oxyphylla has a definite positive effect in ameliorating cognitive impairment and improving the characteristic pathological brain injuries in AD [26,37], although the underlying mechanism of action remains elusive. In addition, our previous results have shown that A. oxyphylla extracts have significant neuroprotective effects on reducing the paralysis ratio in AD transgenic C. elegans. (data not shown). This prompted us to apply an integrative and computational system pharmacology approach, as well as classical molecular dynamics and molecular docking models to explore the effective substances, putative targets, and potential pharmacological mechanisms of A. oxyphylla with its bioactive compounds for treating AD.
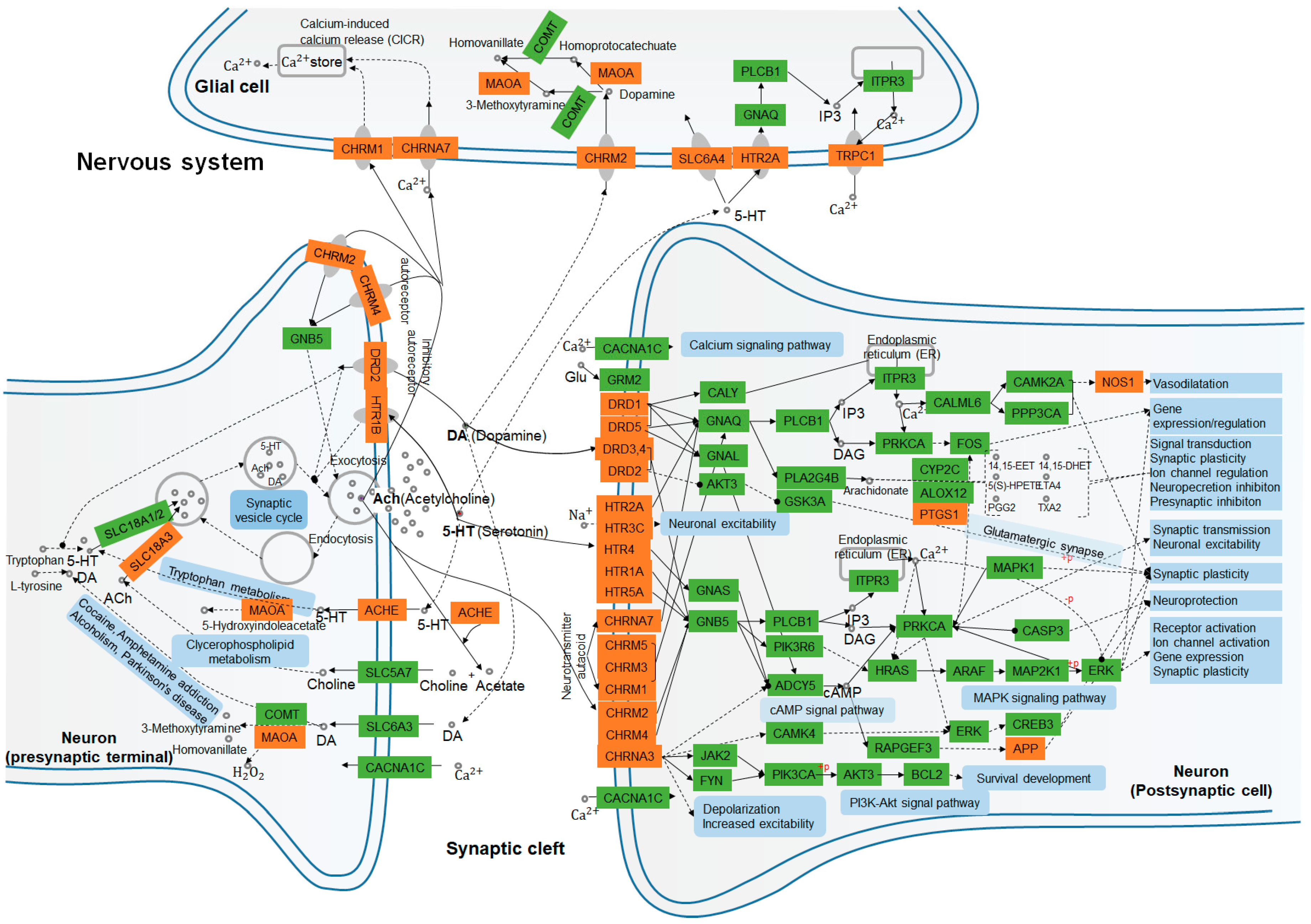
By incorporating SymMap, TCMSP, TCMID, TCM-ID, and ETCM databases, 50 candidate compounds were screened using the ADMET criteria, and 164 putative targets of A. oxyphylla (including 99 targets of terpenes) were selected using the QSAR-TargetNet screening criteria. The classification of compounds and the analysis of the compound-target network indicated that terpenes, flavonoids, and diphenylpeptanes with good pharmacokinetic properties served as the main compounds in A. oxyphylla, which is consistent with the existing experimental data. Subsequently, 579 GO and 28 KEGG pathway terms, along with 6 protein-associated diseases of A. oxyphylla were identified and classified. The functions of these putative target proteins included, G protein-coupled amines, neurotransmitter and serotonin receptor function proteins, dendrite and synaptic membrane components, and proteins involved in GPCR signaling pathways. The GO/KEGG enrichment pathway therefore underscores the potential relevance of the putative proteins of A. oxyphylla and AD. We then examined the link between the putative proteins and AD-associated proteins from CTD and GeneCards by CTPD network construction of A. oxyphylla, as well as CTP and PPI network construction of terpenes. Among the putative target proteins of A. oxyphylla, 21 target proteins associated with AD and 8 putative target proteins (HDAC1, ESR1, EGFR, RELA, ESR2, AR, RAC1, TP53) had the characteristics of bottleneck nodes by topology analysis. Furthermore, the putative target proteins of terpenes, which occupied a great part of the putative targets of A. oxyphylla, were annotated with KEGG pathways, and constructed as a protein–protein interaction network for the protein function prediction by clustering algorithm. Based on the manual annotation map, terpenes demonstrated a function on regulating the synthesis and release of neurotransmitters, as well as signal transmission, dendritic growth, and spine formation, including synaptic plasticity in the nervous system (Figure 6).
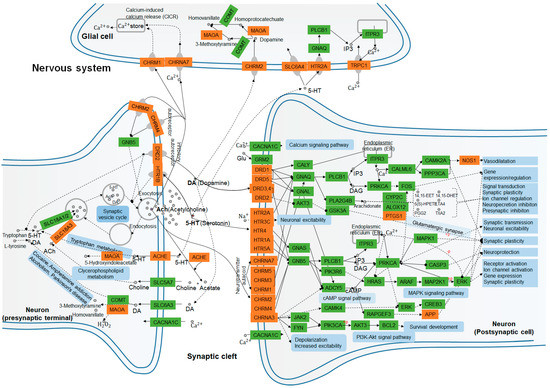
Figure 6.
Nervous system interaction effects of the KEGG enrichment pathway terms and putative target proteins identified in A. oxyphylla. and terpenes. Pathways and biological functions (blue rectangles) form the compressed nerve synapses signal transmission network. Orange and green rectangles represent the putative target proteins which are identified in this study, and the relative functional proteins in network, respectively. Arrows indicate activation; round-arrows indicate inhibition.
The left panel of Figure 6 shows that SLC18A1, 2, and 3 may have a role in synaptic vesicle cycling, acetylcholinesterase (ACHE) [42], and amine oxidase [flavin-containing] A (MAOA) [43,44] signaling, which have been shown to be involved in tryptophan metabolism, clycerophospholipid metabolism, and also related to cocaine and amphetamine addiction, as well as alcoholism, and Parkinson’s disease in the presynaptic nerve terminal. Neurotransmitters, such as dopamine (DA, hsa04728), serotonin (5-HT, hsa04726), and acetylcholine (ACh, has04725) work on muscarinic acetylcholine receptor M2 (CHRM2) [45] and MAOA for DA metabolism in glial cells, as well as on CHRNA7 [46] and CHRM1 [47] for calcium (Ca2+) storage by Ca2+-induced Ca2+ release (CICR) [48], and on HTR2A-SLC6A4-IP3-TRPC1 [49,50] pathway for Ca2+ transport, respectively. On the postsynaptic cell membrane, DA, is the prototypical slow neurotransmitter of the mammalian brain, which interacts with D1-like receptors DRD1 and DRD5 [51,52], both positively coupled to adenylyl cyclase (AC) and cAMP production, which are activated and regulated downstream of PTGS1 and NOS1 expression. While the activation of D2-like receptors DRD2, DRD3, and DRD4 have exactly the reverse effect on regulating the production of AC and cAMP in dopaminergic synapse pathway (hsa04728) [51] activity. More evidence has suggested that DA influences neuronal activity, synaptic plasticity, and behavior by diverse cAMP- and Ca2+-dependent and independent mechanisms (has 04020). Similarly, ACh binds and activates muscarinic acetylcholine receptors CHRM1, 2, 3, 4, and 5 [53], and directly alters cellular homeostasis of phospholipase C, inositol trisphosphate, cAMP, free Ca2+, and the activation of neuronal ACh receptors CHRNA4 and 7 [54]. It also regulates the rapid influx of sodium (Na+) and Ca2+, leading to the subsequent cellular depolarization by cAMP (hsa04024), MAPK (hsa04010), and phosphoinositide 3-kinase (PI3K)/AKT (hsa04151) signaling pathways. For monoamine neurotransmitters, serotonin (5-hydroxytryptamine, 5-HT) binds to ionotropic 5-HT3 receptors, GPCR 5-HT receptors, 5-HT1 (Gi/Go -coupled), 5-HT2 (Gq-coupled), 5-HT4/6/7 (Gs-coupled), and 5-HT5 receptors, which can be directly or indirectly involved in the regulation of synaptic transmission, neuronal excitability, synaptic plasticity, and neuroprotection [55,56]. Therefore, we hypothesize that A. oxyphylla or terpenoids are natural compounds that may play an important role in mediating anti-AD effects by functional annotation and enrichment analysis of neurotransmitter receptors.
Based on our topology analysis, hubba nodes screened, and protein–protein interaction network constructions of terpenes, two bottleneck node proteins, APP [57] and ESR1 [58], and two AD-associated targets, ACHE [59] and NOS2 [60], were chosen as the basis for the compound-ligand interaction analysis by AutoDock for preliminary studies aimed at investigating the anti-AD effects of terpenes. The good molecular docking scores and results reflected that terpenes possess suitable anti-AD activity. Other putative protein targets are also highly matched with several representative compounds in A. oxyphylla, however the specific actions and properties need to be further verified and probed in future studies.
4. Materials and Methods
4.1. Compound Database Building
In the present study, the chemical compound data of A. oxyphylla were collected from SymMap (http://symmap/org/) [61], which is an integrative database covering information from the Traditional Chinese Medicine System Pharmacology (TCMSP) database and analysis platform, http://ibts.hkbu.edu.hk/LSP/tcmsp.php) [62], the Traditional Chinese Medicine Integrated Database (TCMID) [63] the Traditional Chinese Medicine Information Database (TCM-ID) [64], and the Encyclopedia of Traditional Chinese Medicine (ETCM; http://www.nrc.ac.cn:9090/ETCM/) database [65]. The comprehensive drug-likeness grading and pharmacokinetic properties of A. oxyphylla were evaluated and filtered for the candidate compounds using the absorptions, distribution, metabolism, excretion, and toxicity (ADMET) criterion as oral bioavailability (OB ≥ 30%), drug-likeness (DL ≥ 0.18) evaluation, and blood–brain barrier (BBB ≥ −0.3), respectively. In addition, the two/three dimensional (2D/3D) structure, canonical smiles, and PubChem ID of the compounds were calibrated using the PubChem (https://pubchem.ncbi.nlm.nih.gov/) database.
4.2. AD Associated Proteins and Putative Target Protein Screening
AD-associated protein targets were identified using the comparative toxicogenomics database (CTD; http://ctdbase.org/) [66] and the GeneCards (http://www.genecards.org/) [67] database, with higher rank score, which respected the higher correlation with AD. Compounds were then scored using the Lipinski Rule of Five, and the putative target protein screening, which were fishing and filtered conditionally from the quantitative structure activity relationships-TargetNet (QSAR-TargetNet: http://targetnet.scbdd.com) [68]. The AD-associated proteins and putative target proteins of candidate compounds were verified by their unique UniProtKB ID and target names in the UniProt database (http://www.uniprot.org/) [69,70].
4.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment and Network Constructions
Metascape, a web-based portal for comprehensive gene annotation and analysis resources (http://metascape.org/) [71,72], combines a GO [73] and KEGG [74] pathway enrichment analysis search to leverage over 40 independent knowledge bases. The GO/KEGG pathway enrichment terms of the putative proteins with a p-value of ≤ 0.01 were regarded as significant and of interest.
In order to clarify the pathogenesis of AD and elucidate the mechanism of action of A. oxyphylla, the compound-target interaction (CT), compound-target-pathway (CTP), and compound-target-pathway-disease (CTPD) network were constructed and analyzed using topological parameters and the subnetwork of the bottleneck nodes were filtered and visualized using the Cytoscape 3.6.0 software (Institute for Systems Biology, Seattle, WA, USA; http://www.cytoscape.org/) [75]. Protein–protein interactions (PPI) were set up on Metascape and STRING (https://string-db.org/) [76] databases and also visualized using Cytoscape by cluster analysis.
4.4. Molecular Docking
The binding ability, sites, and interactions between compound and target proteins were achieved and analyzed by classical molecular dynamics using AutoDockTools-1.5.6, Pymol 2.3 and Discovery Studio 4.5 Client [77,78]. The 3D chemical structural formulas of candidate compounds were obtained from PubChem and energy minimizing employed to ChemBioDraw 3D. In addition, the crystal structures of putative targets were obtained from the Protein Data Bank (http: //www.pdb.org/) and decorated by removing the ligands and water motifs, revising it and optimizing the mutation sites, and adding hydrogen through the Pymol 2.3 and UCSF Chimera 1.14rc software.
5. Conclusions
In summary, A. oxyphylla has been shown to exert positive effects in the treatment of AD. In this study, we used network pharmacology and molecular docking approaches to investigate the mechanism of its anti-AD activity. Our findings show that A. oxyphylla as TCM, displays a holistic performance through multiple pathways with multi-targets. A. oxyphylla, especially terpenes, appear to possess neuroprotective effects on regulating the synthesis, release, and transmission of neurotransmitters, as well as in the formation and plasticity of dendritic spines and synapses in the nervous system, which contributes, as a theoretical basis, to new insights for the development of novel anti-AD drugs.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/6/2071/s1, Figure S1: Venn diagram illustrated that the six overlapping classifications of 164 putative target proteins which consistent with the compounds in A. oxyphylla. Figure S2: Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis for the top 20 putative target proteins of terpenes. Figure S3: The constructions and analysis of Compound-Target-Pathway-Disease (C-T-P-D) network of A. oxyphylla. Figure S4: The network topology analysis of A. oxyphylla. Figure S5: The molecular docking results of compounds (oxyphyllol B, oxyphyllenodiol A, eucalyptol and zingiberol) interacted with proteins (ACHE, NOS2, APP and ESR1), respectively. Table S1: Result of 50 candidate compounds of A. oxyphylla. Table S2: Result of 164 putative target proteins of A. oxyphylla. Table S3: Result of 597 GO terms enrichment of terpenes in A. oxyphylla. Table S4: Result of 28 KEGG terms enrichment of terpenes in A. oxyphylla. Table S5-1: AD-associated target proteins information collected from Comparative Toxicogenomics Database (CTD). Table S5-2: 164 AD-associated target proteins of Homo sapiens from CTD with an Inference Score of ≥50 were regarded as the more substantially contributing proteins. Table S6-1: AD-associated target proteins information collected from The Human Gene Database (GeneCards). Table S6-2: 118 AD-associated target proteins of Homo sapiens from Genecards with an Inference Score of ≥30 were regarded as the more substantially contributing proteins. Table S7: Result of the KEGG pathway enrichment analysis of A. oxyphylla. Table S8: 13 hubba node proteins of A. oxyphylla.
Author Contributions
J.X., and Q.W. conceived and designed the experiments; J.X., F.W., J.G., Q.W., performed the experiments and wrote the paper; C.X., Y.C., and Z.F., analyzed the data. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Nautral Science Foundation of China (U1503223), Key Research and Development Project of Ningbo (No. 2019B10034), and the initial Scientific Research Fund of Ningbo University (421911122).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.V.; Bonito-Oliva, A.; Sakmar, T.P. Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med. 2017, 68, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, M.; Fukumoto, H.; Newell, K.L.; Growdon, J.H.; Hedley-Whyte, E.T.; Frosch, M.P.; Albert, M.S.; Hyman, B.T.; Irizarry, M.C. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 2004, 62, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Fukuda, T.; Sahara, N.; Yamashita, S.; Murayama, M.; Mizoroki, T.; Yoshiike, Y.; Lee, B.; Sotiropoulos, I.; Maeda, S. Aggregation of Detergent-insoluble Tau Is Involved in Neuronal Loss but Not in Synaptic Loss. J. Biol. Chem. 2010, 285, 38692–38699. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Roychaudhuri, R.; Yang, M.; Hoshi, M.M.; Teplow, D.B. Amyloid beta-protein assembly and Alzheimer disease. J. Biol. Chem. 2009, 284, 4749–4753. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Bezprozvanny, I.; Mattson, M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008, 31, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.C.; Hrynchak, M.V.; Goncalves, I.; Quintela, T.; Santos, C.R. Sex Hormone Decline and Amyloid beta Synthesis, Transport and Clearance in the Brain. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Pivac, N. Genetic Markers of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1192, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76 Pt. A, 27–50. [Google Scholar] [CrossRef]
- Wang, J.; Tan, L.; Wang, H.F.; Tan, C.C.; Meng, X.F.; Wang, C.; Tang, S.W.; Yu, J.T. Anti-inflammatory drugs and risk of Alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimer’s Dis. 2015, 44, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zaulkffali, A.S.; Md Razip, N.N.; Syed Alwi, S.S.; Abd Jalil, A.; Abd Mutalib, M.S.; Gopalsamy, B.; Chang, S.K.; Zainal, Z.; Ibrahim, N.N.; Zakaria, Z.A.; et al. Vitamins D and E Stimulate the PI3K-AKT Signalling Pathway in Insulin-Resistant SK-N-SH Neuronal Cells. Nutrients 2019, 11, 2525. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Farlow, M.R.; Greig, N.H.; Sambamurti, K. Current drug targets for Alzheimer’s disease treatment. Drug Dev. Res. 2010, 56, 267–281. [Google Scholar] [CrossRef]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef]
- Eikelboom, W.S.; Singleton, E.; van den Berg, E.; Coesmans, M.; Mattace Raso, F.; van Bruchem, R.L.; Goudzwaard, J.A.; de Jong, F.J.; Koopmanschap, M.; den Heijer, T.; et al. Early recognition and treatment of neuropsychiatric symptoms to improve quality of life in early Alzheimer’s disease: Protocol of the BEAT-IT study. Alzheimer’s Res. Ther. 2019, 11, 48. [Google Scholar] [CrossRef]
- Tang, J.L.; Liu, B.Y.; Ma, K.W. Traditional Chinese Medicine. Lancet 2008, 372, 1938–1940. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, H.; Zhang, R.; Long, F.; Mao, D. Subchronic toxicity of herbal compound “Jiedu Huayu” granules in rats. Bmc Complementary Altern. Med. 2017, 17, 450. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Fogacci, F.; Banach, M. Botanicals and phytochemicals active on cognitive decline: The clinical evidence. Pharmacol. Res. 2018, 130, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cheng, X.; Jing, H.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Comparative pharmacokinetic study of the components in Alpinia oxyphylla Miq.-Schisandra chinensis (Turcz.) Baill. herb pair and its single herb between normal and Alzheimer’s disease rats by UPLC-MS/MS. J. Pharm Biomed. Anal. 2020, 177, 112874. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Zhang, J.; Huang, X.; Wang, Y.; Xu, X.; Zheng, B.; Zhou, X.; Tian, H.; Liu, L.; et al. Antidiarrheal effect of Alpinia oxyphylla Miq. (Zingiberaceae) in experimental mice and its possible mechanism of action. J. Ethnopharmacol. 2015, 168, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhao, X.; Li, H.; Liu, Z.; Liu, B.; Mao, X.; Guo, L.; Bi, K.; Jia, Y. 5-Hydroxymethylfurfural, an antioxidant agent from Alpinia oxyphylla Miq. improves cognitive impairment in Abeta 1-42 mouse model of Alzheimer’s disease. Int. Immunopharmacol. 2014, 23, 719–725. [Google Scholar] [CrossRef]
- Shi, S.H.; Zhao, X.; Liu, A.J.; Liu, B.; Li, H.; Wu, B.; Bi, K.S.; Jia, Y. Protective effect of n-butanol extract from Alpinia oxyphylla on learning and memory impairments. Physiol. Behav. 2015, 139, 13–20. [Google Scholar] [CrossRef]
- An, L.J.; Guan, S.; Shi, G.F.; Bao, Y.M.; Duan, Y.L.; Jiang, B. Protocatechuic acid from Alpinia oxyphylla against MPP+-induced neurotoxicity in PC12 cells. Food Chem. Toxicol. 2006, 44, 436–443. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, Y.; Hu, X.; Hu, X.; Lv, W.; Lv, D.; Chen, J.; Wu, M.; Song, Q.; Shentu, J. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miquel: A review. J. Ethnopharmacol. 2018, 224, 149–168. [Google Scholar] [CrossRef]
- Chang, Y.M.; Chang, H.H.; Tsai, C.C.; Lin, H.J.; Ho, T.J.; Ye, C.X.; Chiu, P.L.; Chen, Y.S.; Chen, R.J.; Huang, C.Y.; et al. Alpinia oxyphylla Miq. fruit extract activates IGFR-PI3K/Akt signaling to induce Schwann cell proliferation and sciatic nerve regeneration. Bmc Complementary Altern. Med. 2017, 17, 184. [Google Scholar] [CrossRef][Green Version]
- Duan, L.H.; Li, M.; Wang, C.B.; Wang, Q.M.; Liu, Q.Q.; Shang, W.F.; Shen, Y.J.; Lin, Z.H.; Sun, T.Y.; Wu, Z.Z.; et al. Protective effects of organic extracts of Alpinia oxyphylla against hydrogen peroxide-induced cytotoxicity in PC12 cells. Neural Regen. Res. 2020, 15, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Fan, K.; Li, T.; Yan, T.; Wu, B.; Bi, K.; Jia, Y. Protective effects of Alpinae Oxyphyllae Fructus extracts on lipopolysaccharide-induced animal model of Alzheimer’s disease. J. Ethnopharmacol 2018, 217, 98–106. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xu, F.; Yan, T.; Xiao, F.; Wu, B.; Wang, Y.; Bi, K.; Jia, Y. Tectochrysin from Alpinia Oxyphylla Miq. alleviates Abeta1-42 induced learning and memory impairments in mice. Eur J. Pharm. 2019, 842, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.S.; Lee, W.C.; Chang, Y.C.; Kim, C.H. Protective effects of alpinae oxyphyllae fructus (Alpinia oxyphylla MIQ) water-extracts on neurons from ischemic damage and neuronal cell toxicity. Phytother. Res.: Ptr 2004, 18, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; An, L.; Wang, Y.; Zhao, H.; Gao, C. Neuroprotective effect of Alpinia oxyphylla Miq. fruits against glutamate-induced apoptosis in cortical neurons. Toxicol. Lett. 2003, 144, 205–212. [Google Scholar] [CrossRef]
- Shi, S.H.; Zhao, X.; Liu, B.; Li, H.; Jia, Y. The Effects of Sesquiterpenes-Rich Extract of Alpinia oxyphylla Miq. on Amyloid-β-Induced Cognitive Impairment and Neuronal Abnormalities in the Cortex and Hippocampus of Mice. Oxidative Med. Cell. Longev. 2014, 2014, 451802. [Google Scholar] [CrossRef]
- Chao, J.; Dai, Y.; Verpoorte, R.; Lam, W.; Cheng, Y.C.; Pao, L.H.; Zhang, W.; Chen, S. Major achievements of evidence-based traditional Chinese medicine in treating major diseases. Biochem. Pharmacol. 2017, 139, 94–104. [Google Scholar] [CrossRef]
- Bian, Q.Y.; Wang, S.Y.; Xu, L.J.; Chan, C.O.; Mok, D.K.; Chen, S.B. Two new antioxidant diarylheptanoids from the fruits of Alpinia oxyphylla. J. Asian Nat. Prod. Res. 2013, 15, 1094–1099. [Google Scholar] [CrossRef]
- Ju, D.T.; Kuo, W.W.; Ho, T.J.; Paul, C.R.; Kuo, C.H.; Viswanadha, V.P.; Lin, C.C.; Chen, Y.S.; Chang, Y.M.; Huang, C.Y. Protocatechuic Acid from Alpinia oxyphylla Induces Schwann Cell Migration via ERK1/2, JNK and p38 Activation. Am. J. Chin. Med. 2015, 43, 653–665. [Google Scholar] [CrossRef]
- Chang, Y.M.; Velmurugan, B.K.; Kuo, W.-W.; Chen, Y.-S.; Ho, T.-J.; Tsai, C.-T.; Ye, C.-X.; Tsai, C.-H.; Tsai, F.-J.; Huang, C.-Y. Inhibitory effect of alpinate Oxyphyllae fructus extracts on Ang II-induced cardiac pathological remodeling-related pathways in H9c2 cardiomyoblast cells. Biomedicine 2013, 3, 148–152. [Google Scholar] [CrossRef]
- Li, Y.H.; Chen, F.; Wang, J.F.; Wang, Y.; Zhang, J.Q.; Guo, T. Analysis of nine compounds from Alpinia oxyphylla fruit at different harvest time using UFLC-MS/MS and an extraction method optimized by orthogonal design. Chem. Cent. J. 2013, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. Bmj (Clin. Res. Ed.) 2019, 367, l6217. [Google Scholar] [CrossRef]
- Mathew, B.; Parambi, D.; Mathew, G.E.; Uddin, M.S.; Inasu, S.T.; Kim, H.; Marathakam, A.; Unnikrishnan, M.K.; Carradori, S. Emerging therapeutic potentials of dual-acting MAO and AChE inhibitors in Alzheimer’s and Parkinson’s diseases. Arch. Der Pharm. 2019, 352, e1900177. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Georgieva, M.G.; Atanasov, A.G.; Tzvetkov, N.T. Monoamine Oxidases (MAOs) as Privileged Molecular Targets in Neuroscience: Research Literature Analysis. Front. Mol. Neurosci. 2019, 12, 143. [Google Scholar] [CrossRef]
- Chee, L.Y.; Cumming, A. Polymorphisms in the Cholinergic Receptors Muscarinic (CHRM2 and CHRM3) Genes and Alzheimer’s Disease. Avicenna J. Med. Biotechnol. 2018, 10, 196–199. [Google Scholar] [PubMed]
- Clarelli, F.; Mascia, E.; Santangelo, R.; Mazzeo, S.; Giacalone, G.; Galimberti, D.; Fusco, F.; Zuffi, M.; Fenoglio, C.; Franceschi, M.; et al. CHRNA7 Gene and Response to Cholinesterase Inhibitors in an Italian Cohort of Alzheimer’s Disease Patients. J. Alzheimer’s Dis. 2016, 52, 1203–1208. [Google Scholar] [CrossRef]
- Liu, H.C.; Hong, C.J.; Liu, T.Y.; Chi, C.W.; Tsai, S.J. Association Analysis for the Muscarinic M1 Receptor Genetic Polymorphisms and Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2005, 19, 42–45. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Sun, S.; Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling, excitability, and synaptic plasticity defects in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 45, 561–580. [Google Scholar] [CrossRef]
- Thome, J.; Retz, W.; Baader, M.; Pesold, B.; Hu, M.; Cowen, M.; Durany, N.; Adler, G.; Henn, F.A.; Rosler, M. Association analysis of HTR6 and HTR2A polymorphisms in sporadic Alzheimer’s disease. J. Neural Transm (Vienna) 2001, 108, 1175–1180. [Google Scholar] [CrossRef]
- Fehér, A.; Juhász, A.; László, A.; Pákáski, M.; Kálmán, J.; Janka, Z. Serotonin transporter and serotonin receptor 2A gene polymorphisms in Alzheimer’s disease. Neurosci. Lett. 2013, 534, 233–236. [Google Scholar] [CrossRef]
- Papenberg, G.; Becker, N.; Ferencz, B.; Naveh-Benjamin, M.; Laukka, E.J.; Backman, L.; Brehmer, Y. Dopamine Receptor Genes Modulate Associative Memory in Old Age. J. Cogn Neurosci. 2017, 29, 245–253. [Google Scholar] [CrossRef]
- Baetu, I.; Burns, N.R.; Urry, K.; Barbante, G.G.; Pitcher, J.B. Commonly-occurring polymorphisms in the COMT, DRD1 and DRD2 genes influence different aspects of motor sequence learning in humans. Neurobiol Learn. Mem 2015, 125, 176–188. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, A.; Tripathi, T.; Kumar, A. Muscarinic and nicotinic acetylcholine receptor agonists: Current scenario in Alzheimer’s disease therapy. J. Pharm. Pharmacol. 2018, 70, 985–993. [Google Scholar] [CrossRef]
- Nagele, R.G.; D’Andrea, M.R.; Anderson, W.J.; Wang, H.Y. Intracellular accumulation of β-amyloid 1–42 in neurons is facilitated by the α7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience. 2002, 110, 199–211. [Google Scholar] [CrossRef]
- Hashemi-Firouzi, N.; Komaki, A.; Asl, S.S.; Shahidi, S. The Effects of the 5-HT7 Receptor on Hippocampal Long-Term Potentiation and Apoptosis in a rat model of Alzheimer’s Disease. Brain Res. Bull. 2017, 135, 85–91. [Google Scholar] [CrossRef]
- Metaxas, A.; Vaitheeswaran, R.; Jensen, K.T.; Thygesen, C.; Finsen, B. Reduced Serotonin Transporter Levels and Inflammation in the Midbrain Raphe of 12 month old APPswe/PSEN1dE9 Mice. Curr. Alzheimer Res. 2017, 15, 420–428. [Google Scholar] [CrossRef]
- Wilson, C.A.; Doms, R.W.; Lee, V.M.-Y. Intracellular APP Processing and Aβ Production in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 1999, 58, 787–794. [Google Scholar] [CrossRef]
- Luckhaus, C.; Sand, P.G. Estrogen Receptor 1 gene (ESR1) variants in Alzheimer’s disease. Results of a meta-analysis. Aging Clin. Exp. Res. 2007, 19, 165–168. [Google Scholar] [CrossRef]
- Grimaldi, M.; Marino, S.D.; Florenzano, F.; Ciotta, M.T.; Nori, S.L.; Rodriquez, M.; Sorrentino, G.; D’Ursi, A.M.; Scrima, M. β-Amyloid-acetylcholine molecular interaction: New role of cholinergic mediators in anti-Alzheimer therapy? Future Med. Chem. 2016, 11, 1179–1189. [Google Scholar] [CrossRef]
- Colton, C.A.; Vitek, M.P.; Wink, D.A.; Xu, Q.; Cantillana, V.; Previti, M.L.; Van Nostrand, W.E.; Weinberg, J.B.; Dawson, H. NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 12867–12872. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, F.; Yang, K.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Hu, H.; Gao, K.; Wang, W.; et al. SymMap: An integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019, 47, D1110–D1117. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminformatics 2014, 6, 13. [Google Scholar] [CrossRef]
- Huang, L.; Xie, D.; Yu, Y.; Liu, H.; Shi, Y.; Shi, T.; Wen, C. TCMID 2.0: A comprehensive resource for TCM. Nucleic Acids Res. 2018, 46, D1117–D1120. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Liu, Y.B.; Wang, J.F.; Li, H.; Ung, C.Y.; Han, L.Y.; Cao, Z.W.; Chen, Y.Z. Database of traditional Chinese medicine and its application to studies of mechanism and to prescription validation. Br. J. Pharm. 2006, 149, 1092–1103. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, Y.Q.; Liu, Z.M.; Chen, T.; Lv, C.Y.; Tang, S.H.; Zhang, X.B.; Zhang, W.; Li, Z.Y.; Zhou, R.R.; et al. ETCM: An encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; McMorran, R.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. The Comparative Toxicogenomics Database: Update 2019. Nucleic Acids Res. 2019, 47, D948–D954. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54. [Google Scholar] [CrossRef]
- Yao, Z.J.; Dong, J.; Che, Y.J.; Zhu, M.F.; Wen, M.; Wang, N.N.; Wang, S.; Lu, A.P.; Cao, D.S. TargetNet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J. Comput.-Aided Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Pundir, S.; Martin, M.J.; O’Donovan, C.; UniProt Consortium. UniProt Tools. Curr. Protoc. Bioinform. 2016, 53. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Cheng, H.; Deaton, L.M.; Qiu, M.; Ha, S.; Pacoma, R.; Lao, J.; Tolley, V.; Moran, R.; Keeton, A.; Lamb, J.R.; et al. Tau overexpression exacerbates neuropathology after repeated mild head impacts in male mice. Neurobiol. Dis. 2020, 134, 104683. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2017, 45, D331–D338. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Cao, Q.; Shin, W.S.; Chan, H.; Vuong, C.K.; Dubois, B.; Li, B.; Murray, K.A.; Sawaya, M.R.; Feigon, J.; Black, D.L.; et al. Inhibiting amyloid-beta cytotoxicity through its interaction with the cell surface receptor LilrB2 by structure-based design. Nat. Chem. 2018, 10, 1213–1221. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, L.; Ju, Y.; Liu, C.; Meng, D. Chemical constituents and synergistic anti-gout studies on Eurycoma longifolia and potential mechanisms evaluation based on systemic analysis approach. Bioorg. Chem. 2019, 92, 103302. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).