Solvent Dependence of the Rheological Properties in Hydrogel Magnetorheological Plastomer
Abstract
1. Introduction
2. Results and Discussions
2.1. Materials Characterisation
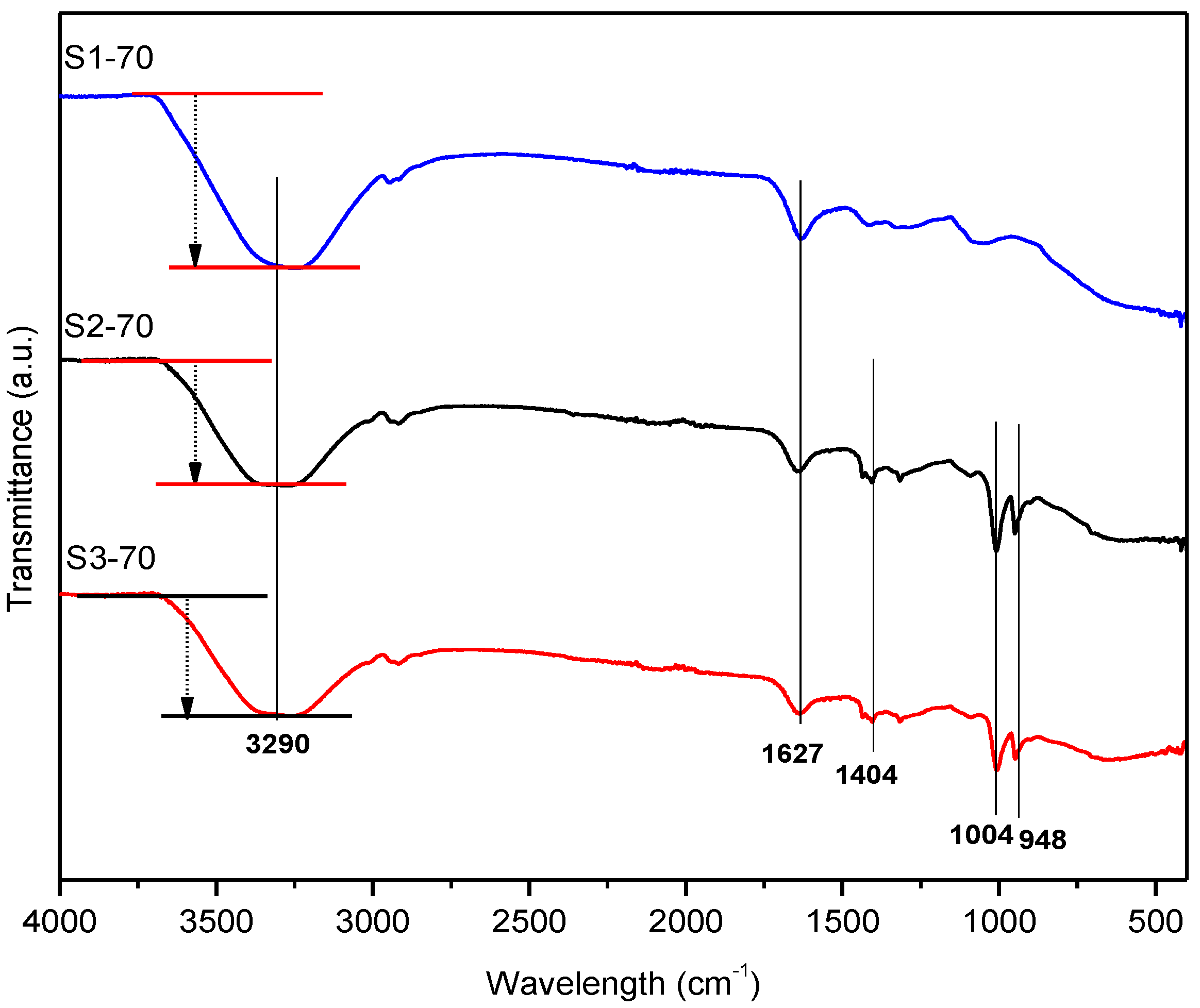
2.1.1. FTIR Measurement
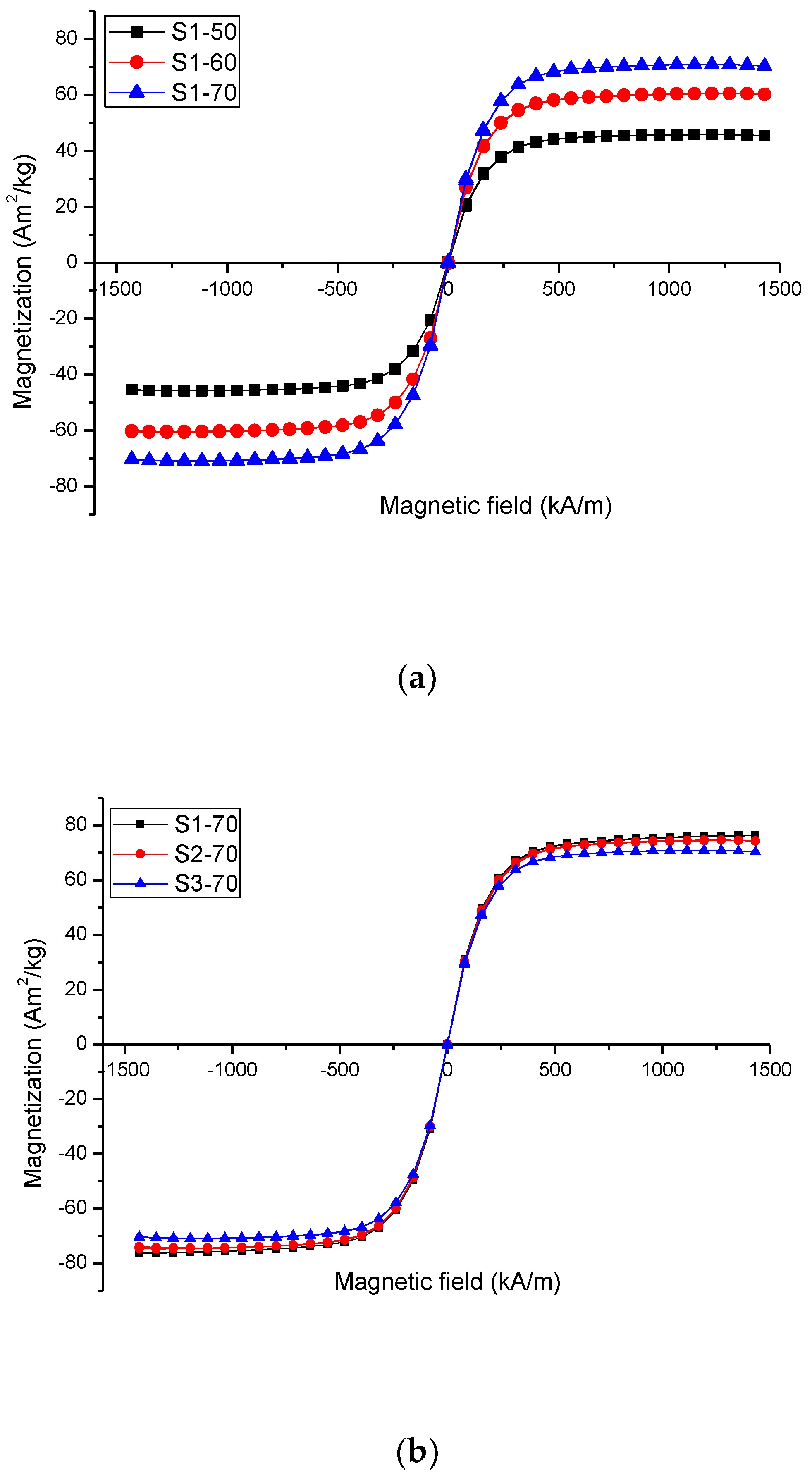
2.1.2. Vibrating Sample Magnetometer
2.2. Rheological Properties
2.2.1. Relative MR effect
2.2.2. Strain Amplitude Sweep
2.2.3. Damping Properties
2.3. Desiccation and Corrosion Observation
3. Materials and Methods
3.1. Material
3.2. Preparation of PVA MRP
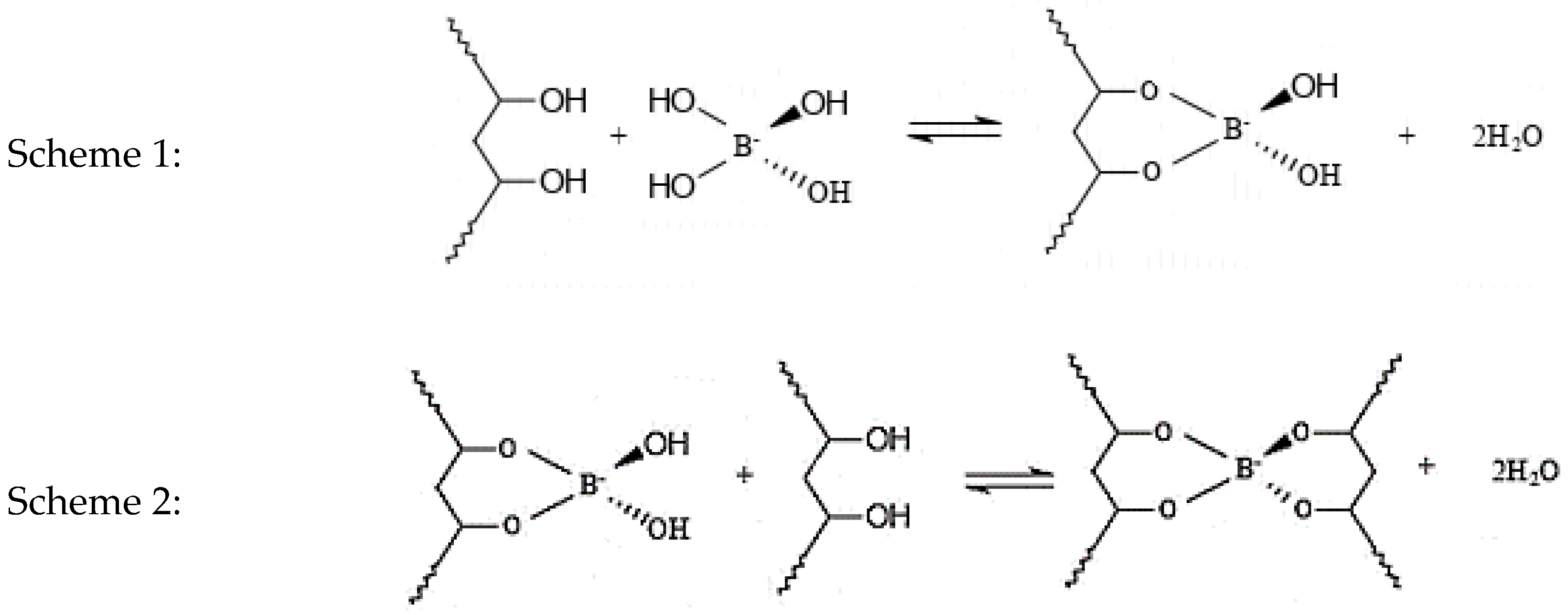
3.3. Mechanism of Chemically Crosslinking
3.4. Characterization and Rheological Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garg, D.P.; Anderson, G.L. Research in active composite materials and structures: An overview. Smart Struct. Mater. Act. Mater. Behav. Mech. 2000, 3992, 2–12. [Google Scholar]
- Ahmadian, M.; Poynor, J.C. An evaluation of magneto rheological dampers for controlling gun recoil dynamics. Shock Vib. 2001, 8, 147. [Google Scholar] [CrossRef]
- Ladipo, I.L.; Fadly, J.D.; Faris, W.F. Characterization of Magnetorheological Elastomer (MRE) Engine Mounts. Mater. Today Proc. 2016, 3, 411–418. [Google Scholar] [CrossRef]
- Bazinenkov, A.M.; Mikhailov, V.P. Active and semi active vibration isolation systems based on magnetorheological materials. Procedia Eng. 2015, 106, 170–174. [Google Scholar] [CrossRef]
- Ngatu, G.T.; Wereley, N.M.; Karli, J.O.; Bell, R.C. Dimorphic magnetorheological fluids: Exploiting partial substitution of microspheres by nanowires. Smart Mater. Struct. 2008, 17, 045022. [Google Scholar] [CrossRef]
- Chen, L.; Gong, X.-L.; Jiang, W.-Q.; Yao, J.-J.; Deng, H.-X.; Li, W.-H. Investigation on magnetorheological elastomers based on natural rubber. J. Mater. Sci. 2007, 42, 5483–5489. [Google Scholar] [CrossRef]
- Xuan, S.; Xu, Y.; Liu, T.; Gong, X. Recent progress on the magnetorheological plastomers. Int. J. Smart Nano Mater. 2015, 6, 135–148. [Google Scholar] [CrossRef]
- Sun, C.; Pang, H.; Xuan, S.; Gong, X. Glass microspheres strengthened magnetorheological plastomers for sound insulation. Mater. Lett. 2019, 256, 126611. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Y.; Gong, X.; Pang, H.; Xuan, S. Magneto-induced normal stress of magnetorheological plastomer. AIP Adv. 2013, 3, 082122. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Wan, Q.; Gong, X.; Xuan, S. The energy dissipation behaviors of magneto-sensitive polymer gel under cyclic shear loading. Mater. Lett. 2015, 158, 406–408. [Google Scholar] [CrossRef]
- Gong, X.; Xu, Y.; Xuan, S.; Guo, C.; Zong, L.; Jiang, W. The investigation on the nonlinearity of plasticine-like magnetorheological material under oscillatory shear rheometry. J. Rheol. 2012, 56, 1375–1391. [Google Scholar]
- Xu, Y.; Gong, X.; Liu, T.; Xuan, S. Squeeze flow behaviors of magnetorheological plastomers under constant volume. J. Rheol. 2014, 58, 659–679. [Google Scholar]
- Lee, S.J.; Kwak, J.W.; Kim, H.D.; Jeon, H.Y.; Kim, J.H.; Yoon, W.S.; Lyoo, W.S. Rheological properties of high molecular weight poly (vinyl alcohol)/dimethyl sulfoxide/water concentrated solutions. Polym. Polym. Compos. 2004, 12, 561–567. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Mitsumata, T.; Abe, N. Giant and reversible magnetorheology of carrageenan/iron oxide magnetic gels. Smart Mater. Struct. 2011, 20, 124003. [Google Scholar] [CrossRef]
- Mitsumata, T.; Sakai, K.; Takimoto, J.I. Giant reduction in dynamic modulus of κ-carrageenan magnetic gels. J. Phys. Chem. B 2006, 110, 20217–20223. [Google Scholar] [CrossRef] [PubMed]
- Negami, K.; Mitsumata, T. Magnetorheology of magnetic poly(vinyl alcohol) gels with high mechanical toughness. Chem. Lett. 2010, 39, 550–551. [Google Scholar] [CrossRef]
- Wu, J.; Gong, X.; Fan, Y.; Xia, H. Physically crosslinked poly(vinyl alcohol) hydrogels with magnetic field controlled modulus. Soft Matter 2011, 7, 6205–6212. [Google Scholar] [CrossRef]
- Lawrence, M.B.; Abbas, S.; Aswal, V.K. Structure of polyvinyl alcohol-borax ferrogels: A small angle neutron scattering study. J. Polym. Res. 2018, 25, 36. [Google Scholar] [CrossRef]
- Park, G.; McLaurin, E.J.; Faidley, L.E. Characterization of the actuator behavior of blended-system ferrogels. In Proceedings of the Behavior and Mechanics of Multifunctional and Composite Materials 2008, San Diego, CA, USA, 10–13 March 2008; Dapino, M.J., Ounaies, Z., Eds.; Volume 6929, p. 69292C. [Google Scholar]
- Akama, S.; Ikeda, J.; Kawai, M.; Mitsumata, T. A feature in magnetorheological effect for polysaccharide magnetic hydrogels. Chem. Lett. 2018, 47, 1240–1242. [Google Scholar] [CrossRef]
- Mitsumata, T.; Ohori, S.; Honda, A.; Kawai, M. Magnetism and viscoelasticity of magnetic elastomers with wide range modulation of dynamic modulus. Soft Matter 2013, 9, 904–912. [Google Scholar] [CrossRef]
- Kharine, A.; Manohar, S.; Seeton, R.; Kolkman, R.G.M.; Bolt, R.A.; Steenbergen, W.; de Mul, F.F.M. Poly(vinyl alcohol) gels for use as tissue phantoms in photoacoustic mammography. Phys. Med. Biol. 2003, 48, 357–370. [Google Scholar] [CrossRef]
- Noorjahan; Pathak, S.; Jain, K.; Pant, R.P. Improved magneto-viscoelasticity of cross-linked PVA hydrogels using magnetic nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 539, 273–279. [Google Scholar] [CrossRef]
- Shi, L.; Han, Q. Molecular dynamics study of the influence of solvents on the structure and mechanical properties of poly(vinyl alcohol) gels. J. Mol. Model. 2018, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Otsuka, E.; Suzuki, A. Swelling behavior of chemically crosslinked PVA gels in mixed solvents. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1978–1986. [Google Scholar] [CrossRef]
- Cha, W.; Hyon, S.; Ikada, Y. Transparent poly (viny1 alcohol) hydrogel with high water content and high strength. Macromol. Chem. Phys. 1992, 193, 1913–1925. [Google Scholar] [CrossRef]
- Gupta, D.; Jassal, M.; Agrawal, A.K. The electrospinning behavior of poly(vinyl alcohol) in DMSO-water binary solvent mixtures. RSC Adv. 2016, 6, 102947–102955. [Google Scholar] [CrossRef]
- Ju, B.; Tang, R.; Zhang, D.; Yang, B.; Yu, M.; Liao, C.; Yuan, X.; Zhang, L.; Liu, J. Dynamic mechanical properties of magnetorheological elastomers based on polyurethane matrix. Polym. Compos. 2016, 37, 1587–1595. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, J.; Choi, H. Magnetic Particle Filled Elastomeric Hybrid Composites and Their Magnetorheological Response. Materials (Basel) 2018, 11, 1040. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, W.W.W.; Jiang, W.W.W.; Ye, F.; Mao, Y.; Xuan, S.; Gong, X. Multifunctional polymer composite with excellent shear stiffening performance and magnetorheological effect. J. Mater. Chem. C 2014, 2, 7133–7140. [Google Scholar] [CrossRef]
- Krumova, M.; López, D.; Benavente, R.; Mijangos, C.; Pereña, J. Effect of crosslinking on the mechanical and thermal properties of poly(vinyl alcohol). Polymer (Guildf.) 2000, 41, 9265–9272. [Google Scholar] [CrossRef]
- Sorokin, V.V.; Ecker, E.; Stepanov, G.V.; Shamonin, M.; Monkman, G.J.; Kramarenko, Y.; Khokhlov, A.R.; Kramarenko, E.Y.; Khokhlov, A.R. Experimental study of the magnetic field enhanced Payne effect in magnetorheological elastomers. Soft Matter 2014, 10, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gong, X.; Xuan, S.; Zhang, W.; Fan, Y. A high-performance magnetorheological material: Preparation, characterization and magnetic-mechanic coupling properties. Soft Matter 2011, 7, 5246–5254. [Google Scholar] [CrossRef]
- Xu, Y.; Gong, X.; Xuan, S. Soft magnetorheological polymer gels with controllable rheological properties. Smart Mater. Struct. 2013, 22, 075029. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, H.; Wang, J.; Zheng, J.; Ouyang, Q. Dynamic rheological properties of polyurethane-based magnetorheological gels studied using oscillation shear tests. RSC Adv. 2019, 9, 10124–10134. [Google Scholar] [CrossRef]
- Mitsumata, T.; Ohori, S. Magnetic polyurethane elastomers with wide range modulation of elasticity. Polym. Chem. 2011, 2, 1063. [Google Scholar] [CrossRef]
- Jia, Y.; Bai, S.; Park, C.B.; Wang, Q. Effect of Boric Acid on the Foaming Properties and Cell Structure of Poly(vinyl alcohol) Foam Prepared by Supercritical-CO2 Thermoplastic Extrusion Foaming. Ind. Eng. Chem. Res. 2017, 56, 6655–6663. [Google Scholar] [CrossRef]
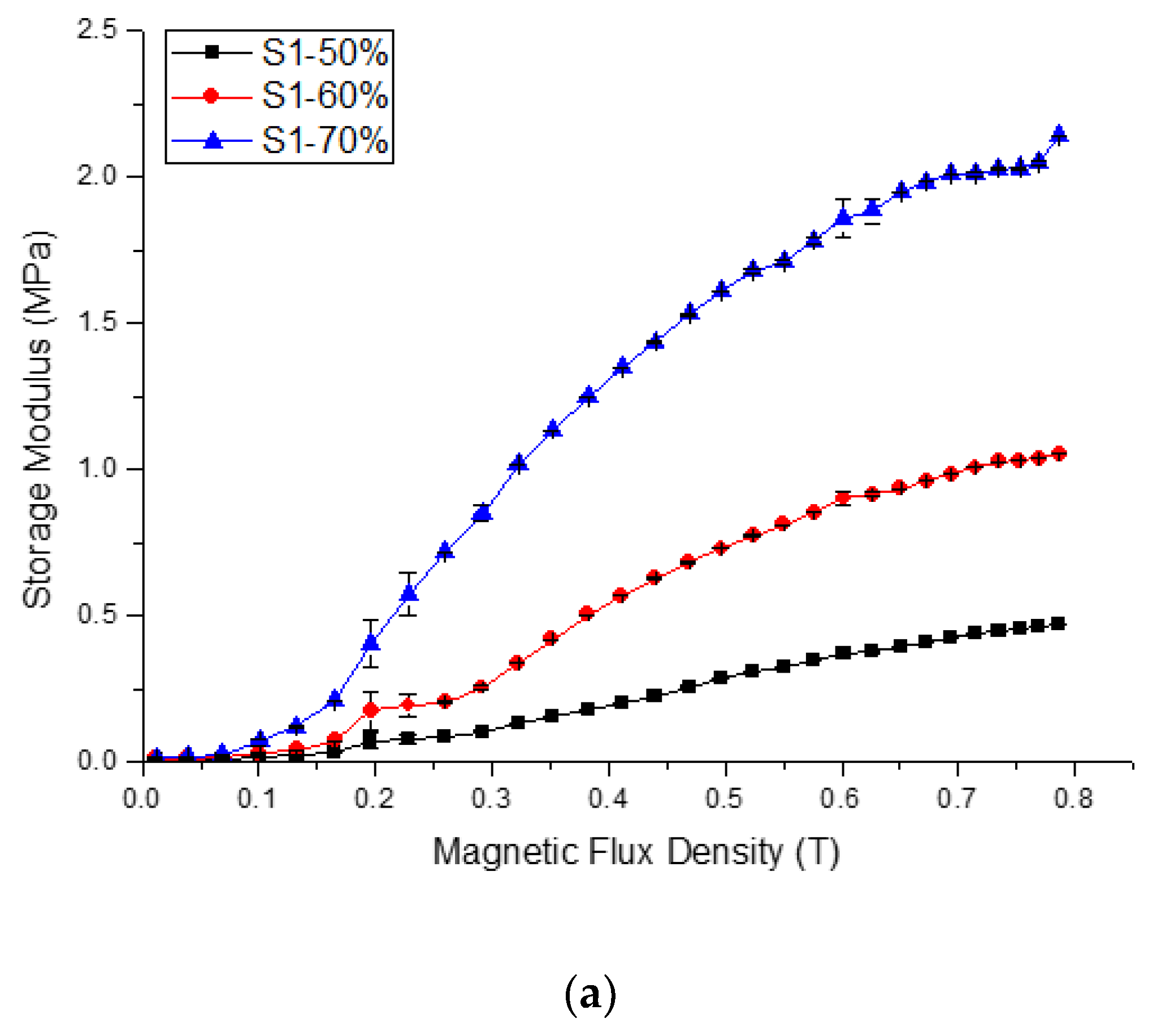
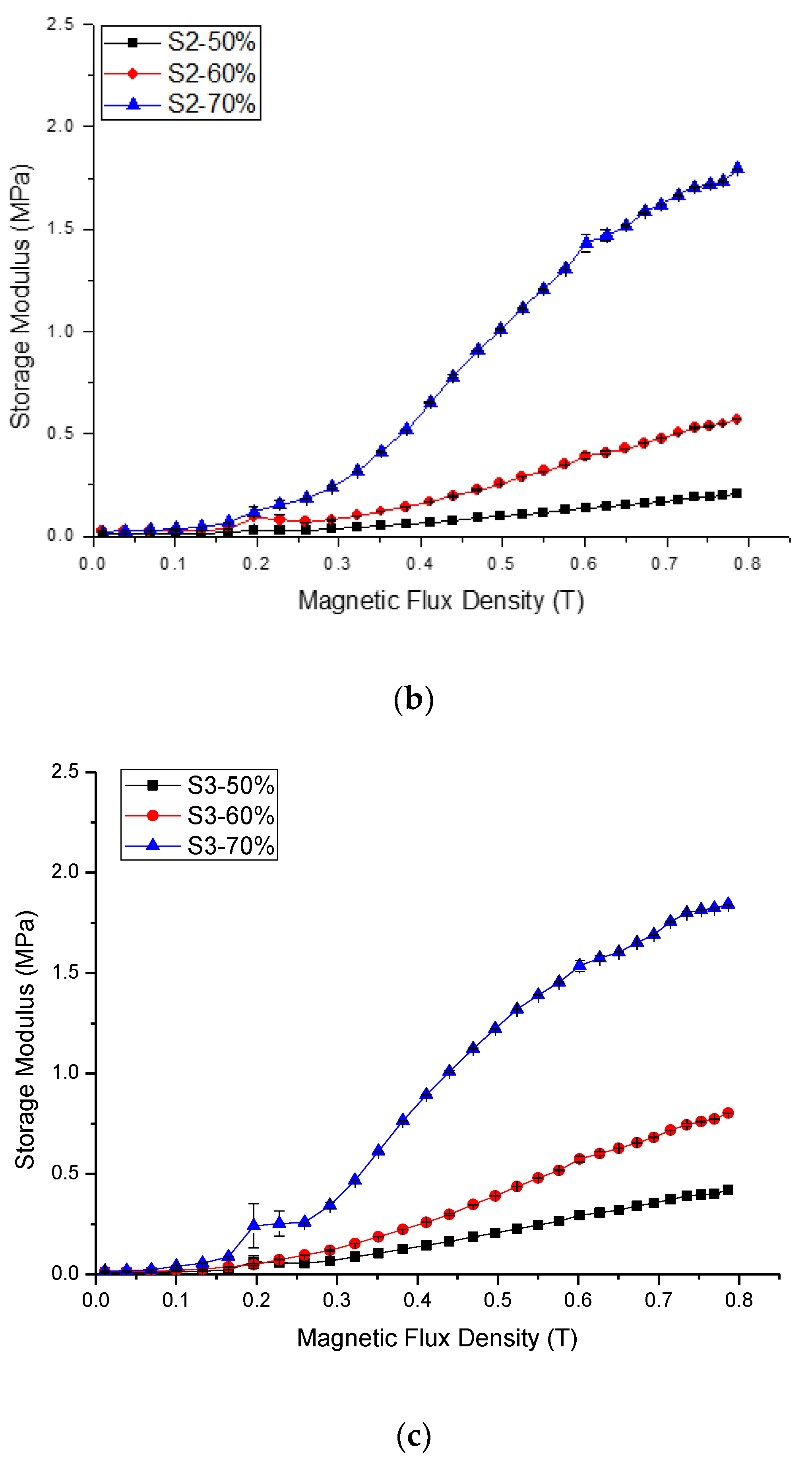
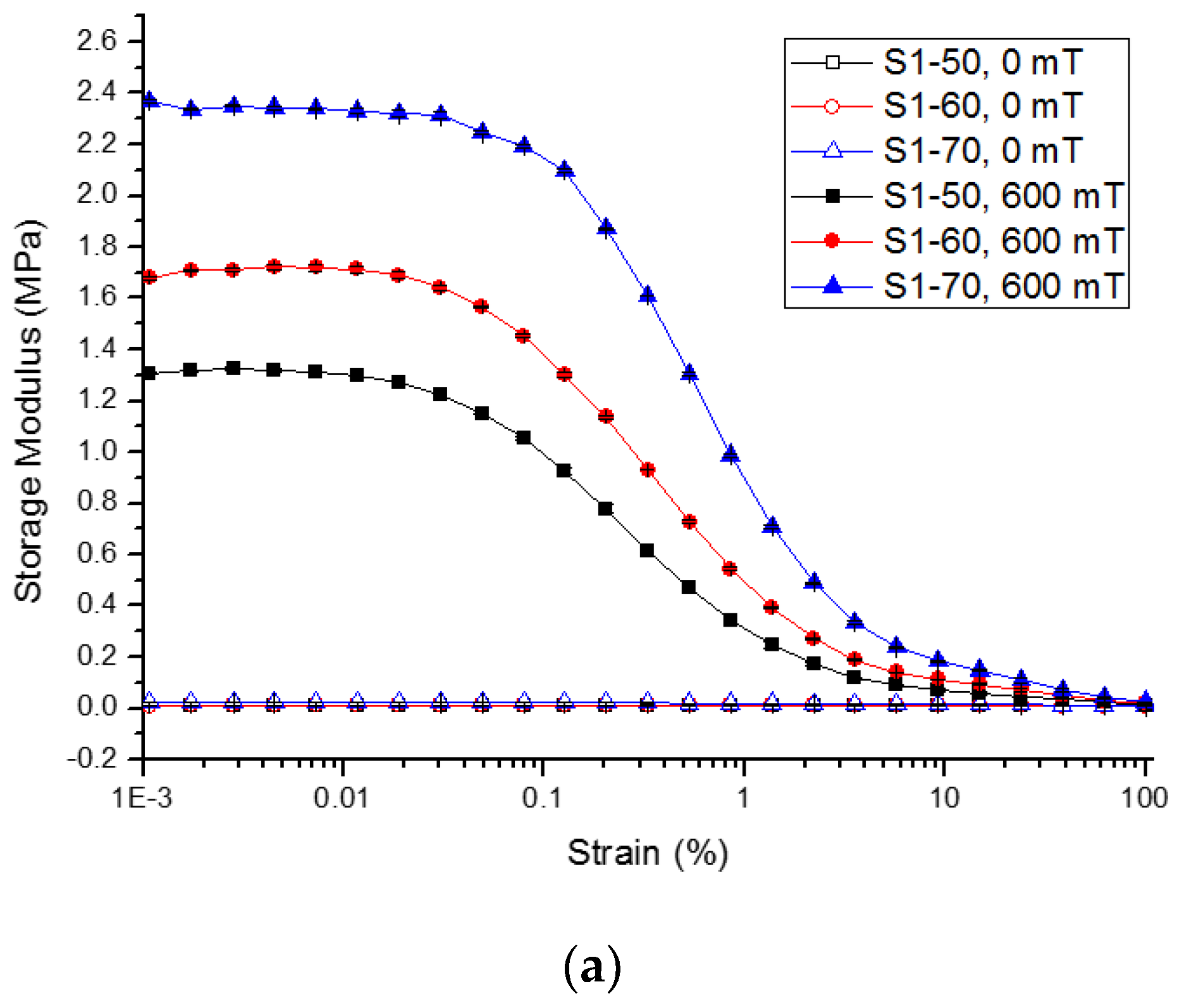
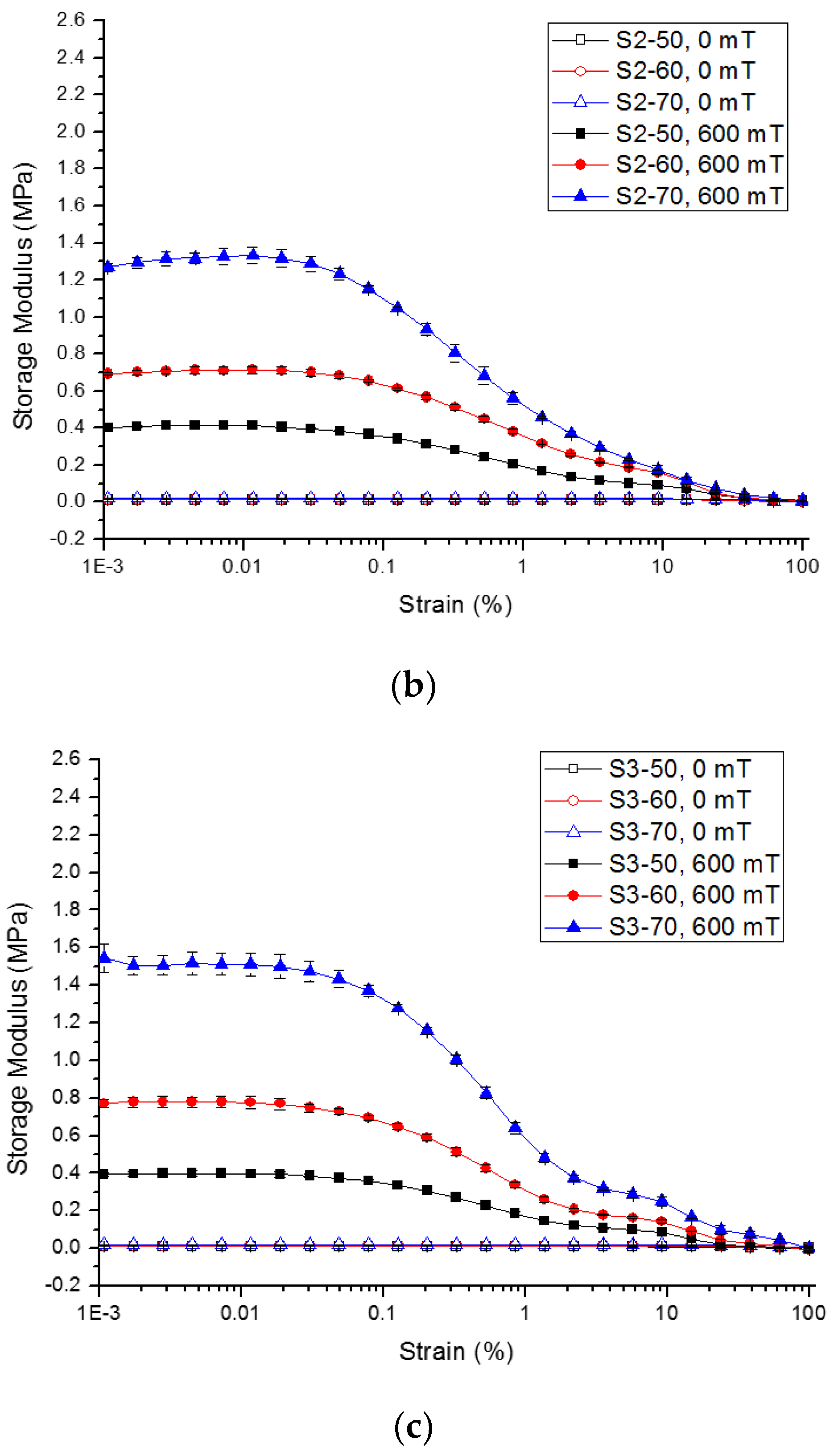
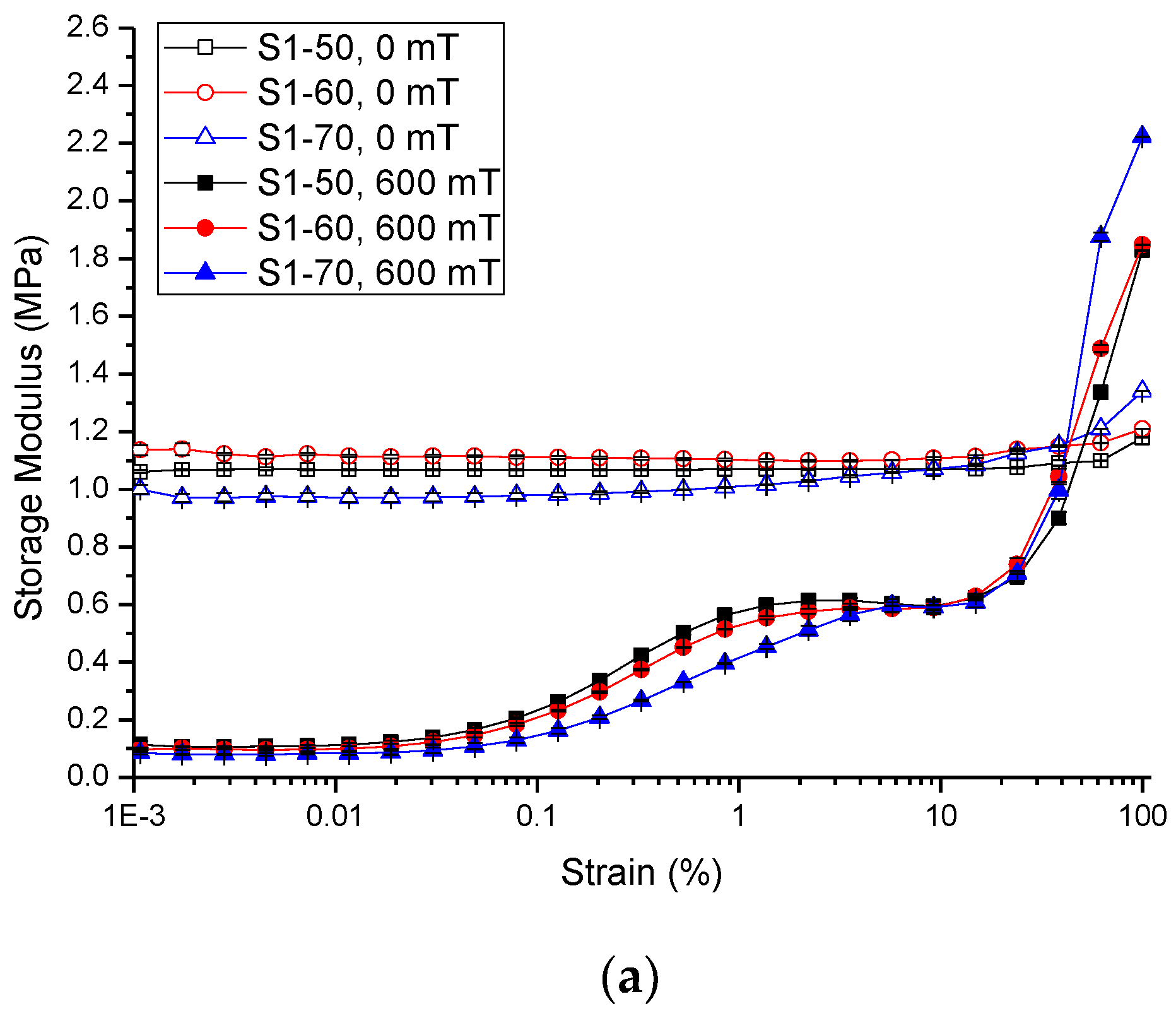
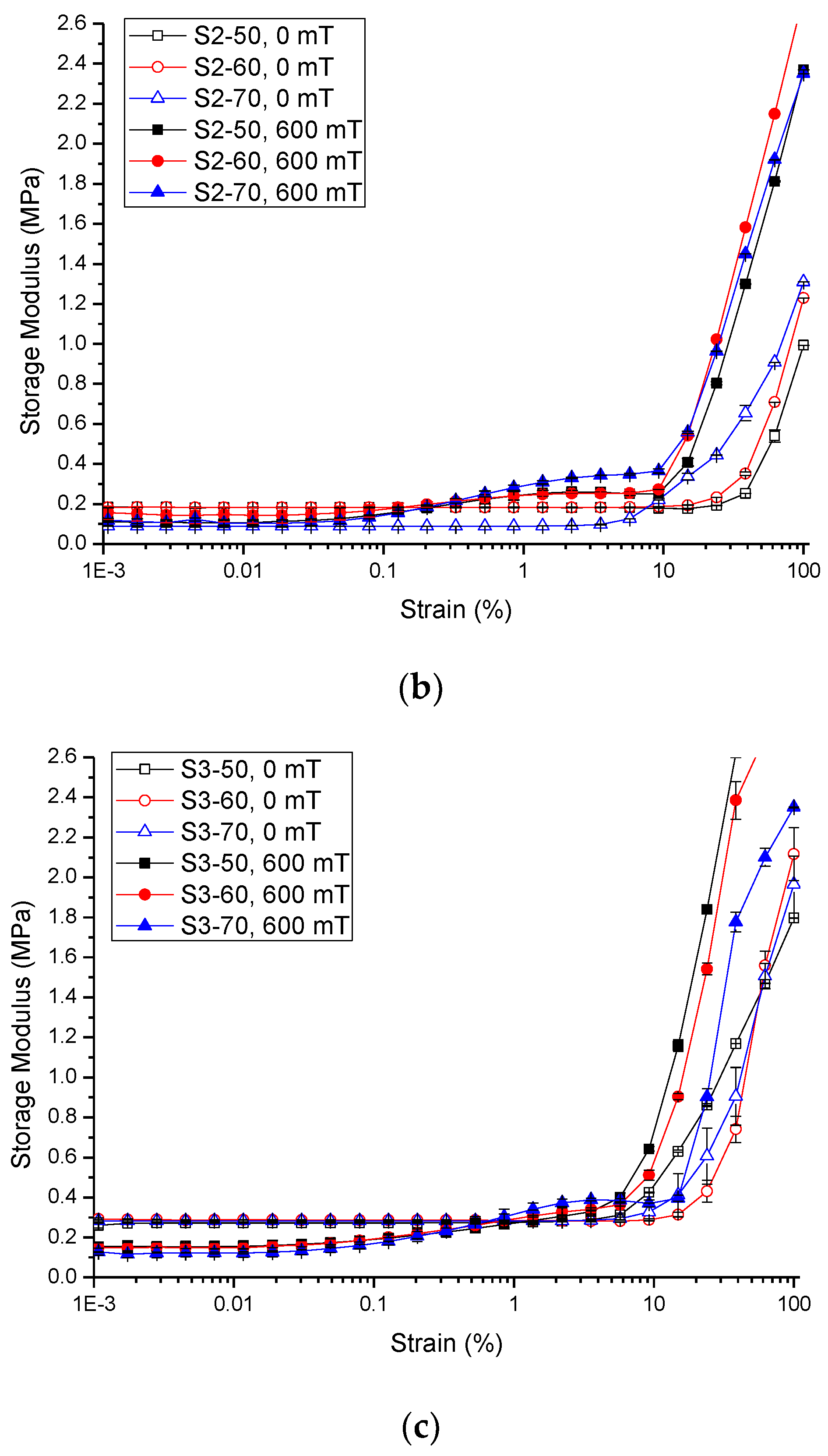

| Sample | Initial Storage Modulus, G′0 | Max. Storage Modulus, G′max | Avg. Absolute MR Effect | Avg. Relative MR Effect | |||||||||
| 1 | 2 | 3 | Mean | a SD | 1 | 2 | 3 | Mean | a SD | MPa | % | bp | |
| S1-50 | 0.008 | 0.008 | 0.009 | 0.008 | 0 | 0.472 | 0.473 | 0.473 | 0.473 | 0 | 56.307 | 5631 | 0.002 |
| S2-50 | 0.012 | 0.006 | 0.012 | 0.010 | 0.001 | 0.206 | 0.207 | 0.207 | 0.207 | 0 | 19.939 | 1994 | |
| S3-50 | 0.008 | 0.008 | 0.008 | 0.008 | 0.001 | 0.421 | 0.422 | 0.421 | 0.421 | 0 | 50.231 | 5023 | |
| S1-60 | 0.009 | 0.008 | 0.010 | 0.009 | 0.004 | 1.055 | 1.055 | 1.055 | 1.055 | 0 | 115.401 | 11,540 | < 0.001 |
| S2-60 | 0.025 | 0.021 | 0.024 | 0.023 | 0.002 | 0.570 | 0.572 | 0.570 | 0.571 | 0.001 | 23.442 | 2344 | |
| S3-60 | 0.013 | 0.011 | 0.013 | 0.012 | 0.002 | 0.802 | 0.803 | 0.802 | 0.802 | 0 | 63.426 | 6343 | |
| S1-70 | 0.013 | 0.015 | 0.013 | 0.014 | 0 | 2.140 | 2.140 | 2.140 | 2.140 | 0 | 155.448 | 15,544 | < 0.001 |
| S2-70 | 0.026 | 0.023 | 0.026 | 0.025 | 0.001 | 1.791 | 1.792 | 1.791 | 1.792 | 0.001 | 70.491 | 7049 | |
| S3-70 | 0.017 | 0.016 | 0.017 | 0.017 | 0.001 | 1.841 | 1.841 | 1.841 | 1.841 | 0 | 110.242 | 11,024 | |
| Sample | Initial Storage Modulus, G′0 | Max. Storage Modulus, G′max | |||||||||
| 1 | 2 | 3 | Mean | SD | 1 | 2 | 3 | Mean | SD | MPa | |
| S1-70 | 0.021 | 0.019 | 0.02 | 0.020 | 0.001 | 2.366 | 2.357 | 2.387 | 2.370 | 0.015 | 2.35 |
| S2-70 | 0.032 | 0.036 | 0.034 | 0.034 | 0.002 | 1.236 | 1.236 | 1.246 | 1.239 | 0.006 | 1.21 |
| S3-70 | 0.02 | 0.019 | 0.018 | 0.019 | 0.001 | 1.665 | 1.562 | 1.501 | 1.576 | 0.083 | 1.56 |
| Sample | CIPs Concentration (After a Month) | ||
|---|---|---|---|
| 50% | 60% | 70% | |
| 1 |  |  |  |
| 2 |  |  |  |
| 3 |  |  |  |
| Sample | CIPs Content, wt.% | PVA Content, wt.% | |
|---|---|---|---|
| Sample 1 (PVA/water) | S1-50 | 50 | 50 |
| S1-60 | 60 | 40 | |
| S1-70 | 70 | 30 | |
| Sample 2 (PVA/DMSO) | S2-50 | 50 | 50 |
| S2-60 | 60 | 40 | |
| S2-70 | 70 | 30 | |
| Sample 3 (PVA/DMSO:water) | S3-50 | 50 | 50 |
| S3-60 | 60 | 40 | |
| S3-70 | 70 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hapipi, N.M.; Mazlan, S.A.; Ubaidillah, U.; Abdul Aziz, S.A.; Ahmad Khairi, M.H.; Nordin, N.A.; Nazmi, N. Solvent Dependence of the Rheological Properties in Hydrogel Magnetorheological Plastomer. Int. J. Mol. Sci. 2020, 21, 1793. https://doi.org/10.3390/ijms21051793
Hapipi NM, Mazlan SA, Ubaidillah U, Abdul Aziz SA, Ahmad Khairi MH, Nordin NA, Nazmi N. Solvent Dependence of the Rheological Properties in Hydrogel Magnetorheological Plastomer. International Journal of Molecular Sciences. 2020; 21(5):1793. https://doi.org/10.3390/ijms21051793
Chicago/Turabian StyleHapipi, Norhiwani Mohd, Saiful Amri Mazlan, U. Ubaidillah, Siti Aishah Abdul Aziz, Muntaz Hana Ahmad Khairi, Nur Azmah Nordin, and Nurhazimah Nazmi. 2020. "Solvent Dependence of the Rheological Properties in Hydrogel Magnetorheological Plastomer" International Journal of Molecular Sciences 21, no. 5: 1793. https://doi.org/10.3390/ijms21051793
APA StyleHapipi, N. M., Mazlan, S. A., Ubaidillah, U., Abdul Aziz, S. A., Ahmad Khairi, M. H., Nordin, N. A., & Nazmi, N. (2020). Solvent Dependence of the Rheological Properties in Hydrogel Magnetorheological Plastomer. International Journal of Molecular Sciences, 21(5), 1793. https://doi.org/10.3390/ijms21051793







