A Facile One-Pot Synthesis of Versatile PEGylated Platinum Nanoflowers and Their Application in Radiation Therapy
Abstract
1. Introduction
2. Results and Discussions
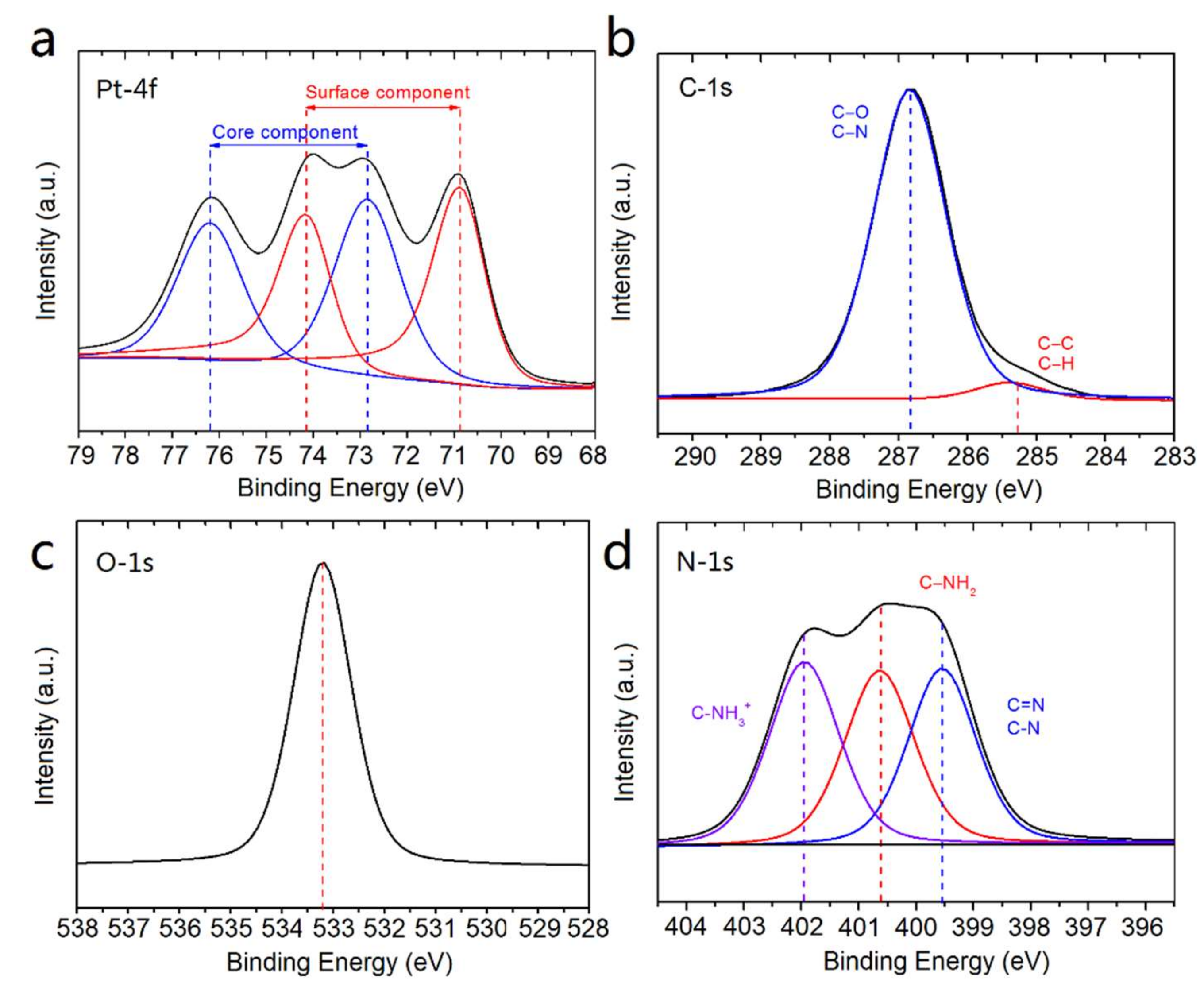
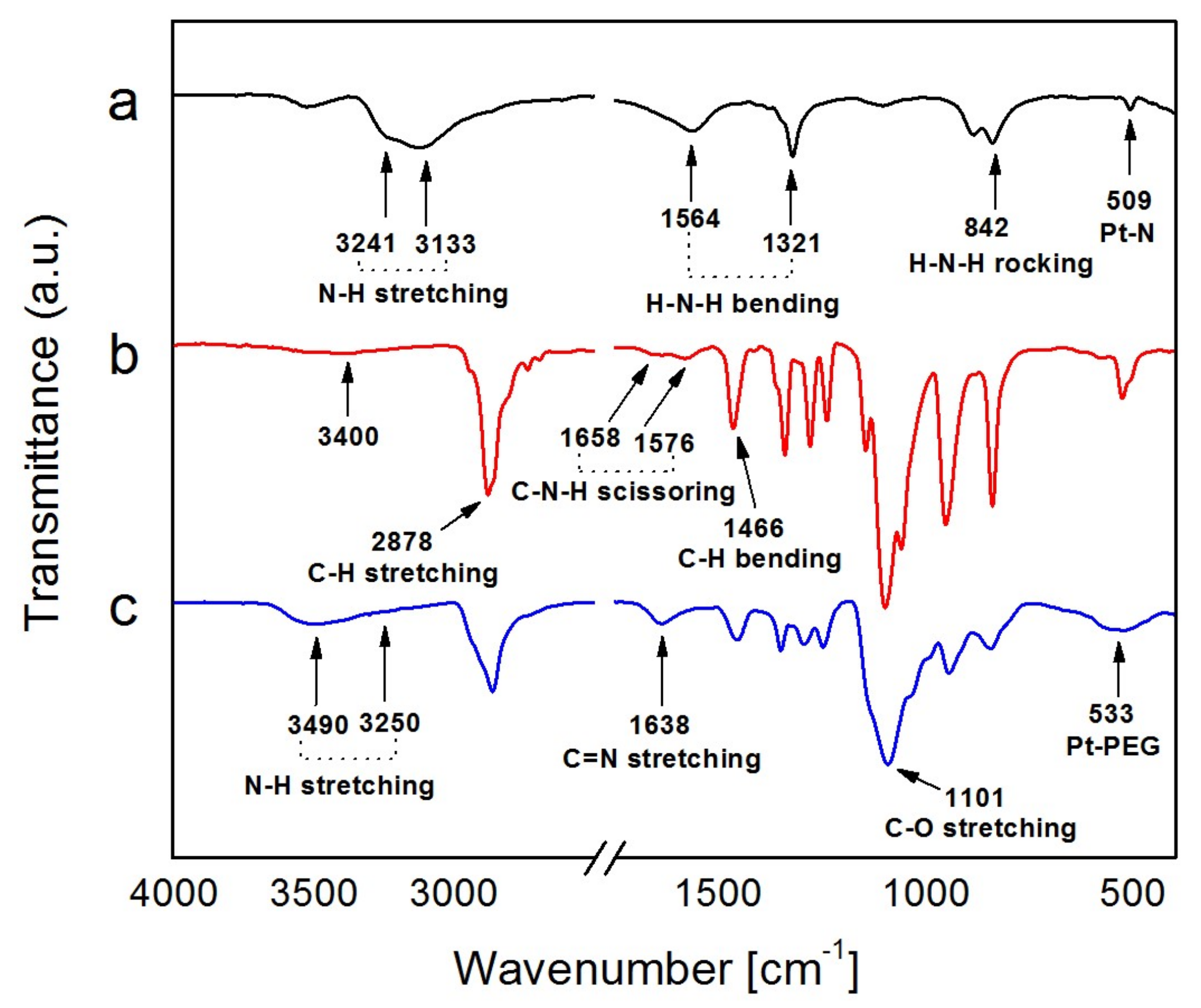
2.1. Synthesis and Characterization of Pt NFs
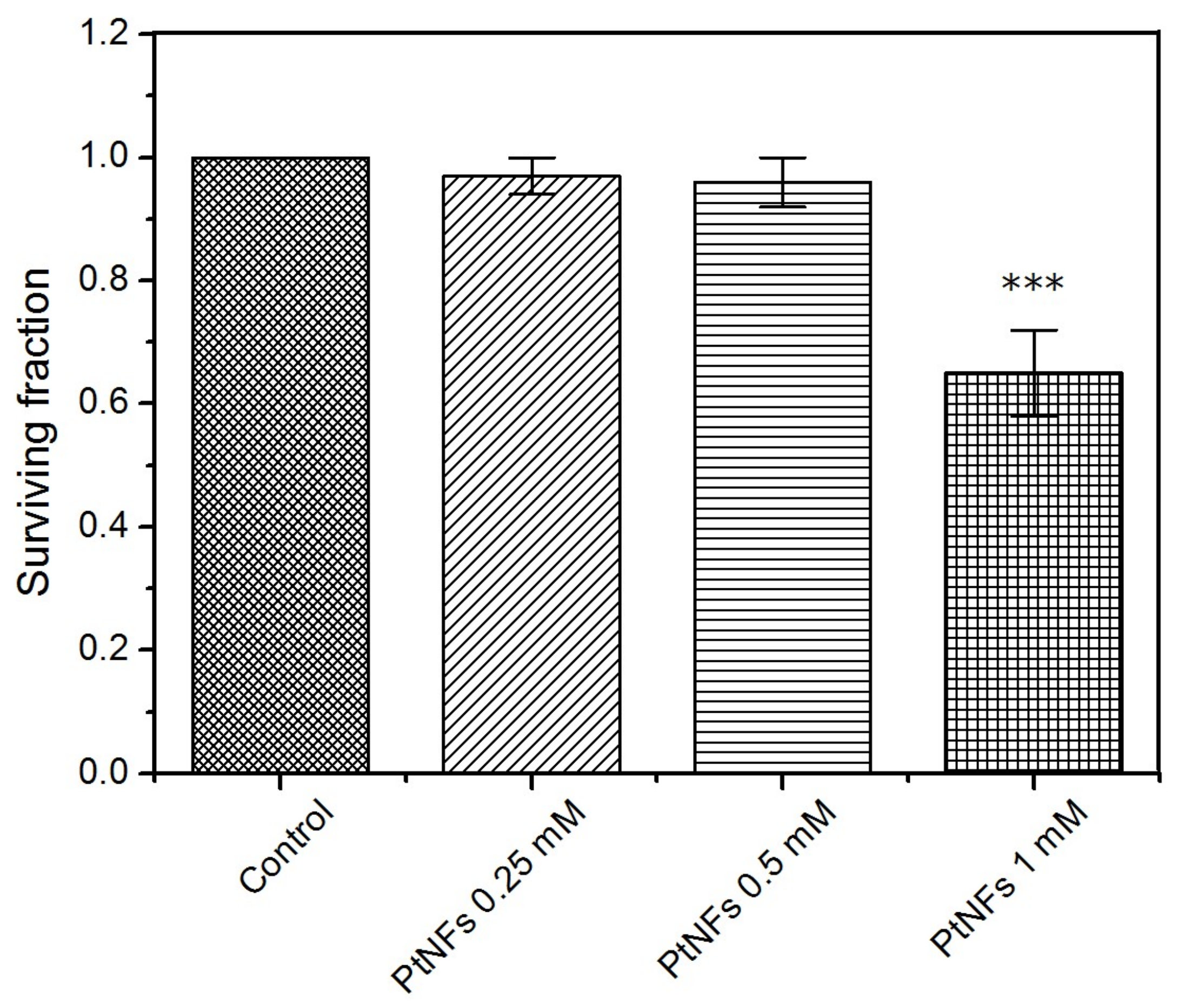
2.2. Toxicity of Pt NFs
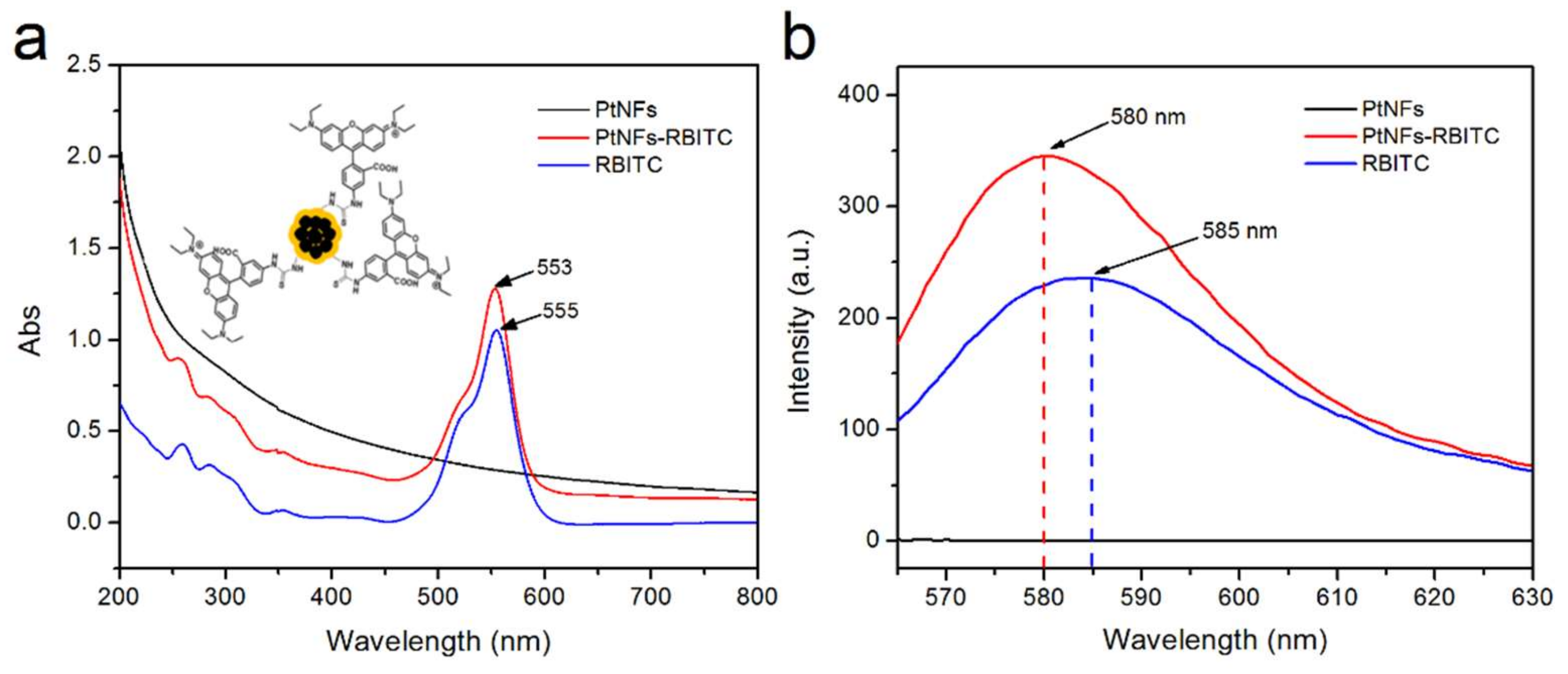
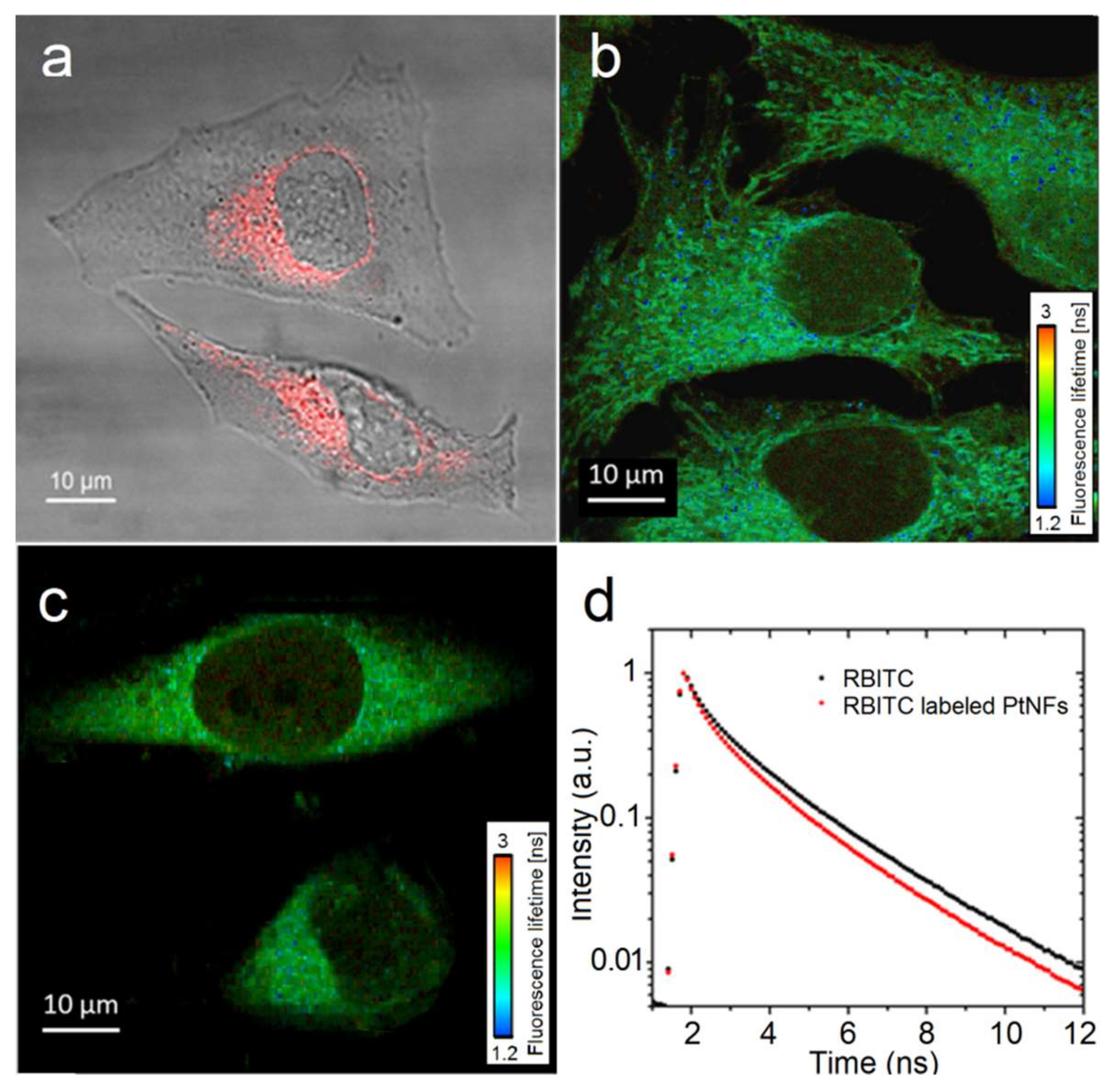
2.3. Fluorescent Labelling of Pt NFs
2.4. Pt NFs Localization and Quantification
2.5. Cell Irradiation
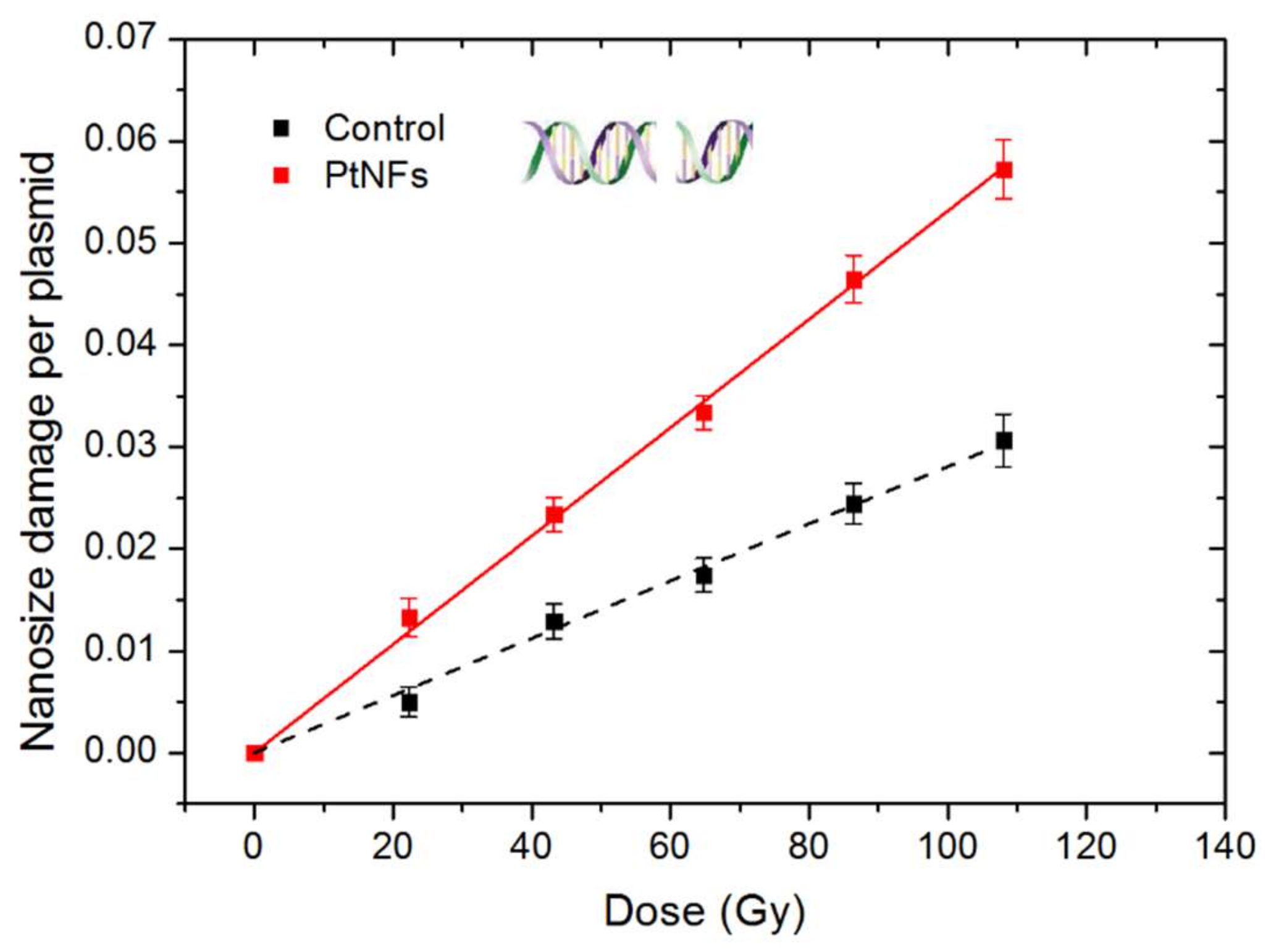
2.6. Nanosize Damage of Pt NFs
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Pt NFs
4.3. Characterization of Pt NFs
4.3.1. The Optical Absorption Measurements
4.3.2. Size, Shape and Charge Determination
4.3.3. XPS
4.3.4. FTIR
4.4. Cell Culture Conditions
4.5. Cytotoxicity Assessment
4.6. Fluorescent Labelling of Pt NFs
4.7. Pt NFs Localization and Quantification
4.8. Cell Irradiation with γ-Rays
4.9. Plasmid Damage Induced by Irradiation
4.10. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BEs | Binding Energies |
| DEF | Dose Enhancing Factor |
| DSBs | Doble Strand Breaks |
| EDTA | Ethylenediaminetetraacetic acid |
| EPR | Enhanced Permeability and Retention effect |
| FWHM | Full Width at Half Maximum |
| FTIR | Fourier Transform InfraRed |
| FLIM | Fluorescence Lifetime Imaging Microscopy |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| LMCT | Ligand-to-Metal Charge Transfer |
| LQM | Linear Quadratic Model |
| mAF | Molecular Amplification Factors |
| MEF | Metal Enhanced Fluorescence |
| NREs | Nano-Radio-Enhancers |
| PBS | Phosphate Buffer Saline |
| PEG | Poly (ethylene glycol) |
| PEG-diamine | Poly (ethylene glycol) diamine |
| Pt NPs | Platinum Nanoparticles |
| RBITC | Rhodamine Isothiocyanate |
| SER | Sensitizing Enhancement Ratio |
| SSBs | Single Strand Breaks |
| SF | Surviving Fraction |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Herold, D.M.; Stobbe, C.C.; Iyer, R.V.; Chapman, J.D. Gold microspheres: A selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000, 76, 1357–1364. [Google Scholar] [PubMed]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Štefančíková, L.; Porcel, E.; Eustache, P.; Li, S.; Salado, D.; Marco, S.; Guerquin-Kern, J.-L.; Réfrégiers, M.; Tillement, O.; Lux, F. Cell localisation of gadolinium-based nanoparticles and related radiosensitising efficacy in glioblastoma cells. Cancer Nanotechnol. 2014, 5, 6. [Google Scholar] [CrossRef]
- Miladi, I.; Aloy, M.-T.; Armandy, E.; Mowat, P.; Kryza, D.; Magné, N.; Tillement, O.; Lux, F.; Billotey, C.; Janier, M. Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 247–257. [Google Scholar] [CrossRef]
- Laurence, M.; Darmon, A.; Vivet, S.; Polrot, M.; Zhang, P.; Deutsch, E.; Bourhis, J.; Pottier, A.; Borghi, E.; Levy, L. NBTXR3 hafnium oxide nanoparticle activated by ionizing radiation demonstrates marked radio-enhancement and antitumor effect via high energy deposit in human soft tissue sarcoma. AACR Cancer Res. 2011, 71, 2665. [Google Scholar]
- Maggiorella, L.; Barouch, G.; Devaux, C.; Pottier, A.; Deutsch, E.; Bourhis, J.; Borghi, E.; Levy, L. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012, 8, 1167–1181. [Google Scholar] [CrossRef]
- Marill, J.; Anesary, N.M.; Zhang, P.; Vivet, S.; Borghi, E.; Levy, L.; Pottier, A. Hafnium oxide nanoparticles: Toward an in vitro predictive biological effect? Radiat. Oncol. 2014, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Sech, C.L.; Takakura, K.; Saint-Marc, C.; Frohlich, H.; Charlier, M.; Usami, N.; Kobayashi, K. Strand break induction by photoabsorption in DNA-bound molecules. Radiat. Res. 2000, 153, 454–458. [Google Scholar] [CrossRef]
- Kobayashi, K.; Frohlich, H.; Usami, N.; Takakura, K.; Le Sech, C. Enhancement of X-ray-induced breaks in DNA bound to molecules containing platinum: A possible application to hadrontherapy. Radiat. Res. 2002, 157, 32–37. [Google Scholar] [CrossRef]
- Porcel, E.; Kobayashi, K.; Usami, N.; Remita, H.; Le Sech, C.; Lacombe, S. Photosensitization of plasmid-DNA loaded with platinum nano-particles and irradiated by low energy X-rays. J. Phys. Conf. Ser. 2011, 261, 012004. [Google Scholar] [CrossRef]
- Porcel, E.; Li, S.; Usami, N.; Remita, H.; Furusawa, Y.; Kobayashi, K.; Le Sech, C.; Lacombe, S. Nano-Sensitization under gamma rays and fast ion radiation. J. Phys. Conf. Ser. 2012, 373. [Google Scholar] [CrossRef]
- Kim, J.-K.; Seo, S.-J.; Kim, H.-T.; Kim, K.-H.; Chung, M.-H.; Kim, K.-R.; Ye, S.-J. Enhanced proton treatment in mouse tumors through proton irradiated nanoradiator effects on metallic nanoparticles. Phys. Med. Biol. 2012, 57, 8309. [Google Scholar] [CrossRef]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1277–1283. [Google Scholar] [CrossRef]
- Schuemann, J.; Berbeco, R.; Chithrani, D.B.; Cho, S.H.; Kumar, R.; McMahon, S.J.; Sridhar, S.; Krishnan, S. Roadmap to clinical use of gold nanoparticles for radiation sensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, D.; Shen, X.; Liu, P.-X.; Fan, F.-Y.; Fan, S.-J. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials 2012, 33, 4628–4638. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Kumar, P.R.; Kale, B.B.; Sonawane, S.H. Synthesis of ultra-small platinum nanoparticles in a continuous flow microreactor. Colloid Interface Sci. Commun. 2016, 13, 6–9. [Google Scholar] [CrossRef]
- Le Gratiet, B.; Remita, H.; Picq, G.; Delcourt, M. CO-stabilized supported Pt catalysts for fuel cells: Radiolytic synthesis. J. Catal. 1996, 164, 36–43. [Google Scholar] [CrossRef]
- Kacenauskaite, L.; Quinson, J.; Schultz, H.; Kirkensgaard, J.J.K.; Kunz, S.; Vosch, T.; Arenz, M. UV-Induced Synthesis and Stabilization of Surfactant-Free Colloidal Pt Nanoparticles with Controlled Particle Size in Ethylene Glycol. ChemNanoMat 2017, 3, 89–93. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Aswitha, P.; Kiruthika, C.; Nagarajan, S.; Christy, A.N. Green synthesis of platinum nanoparticles using Azadirachta indica–An eco-friendly approach. Mater. Lett. 2016, 170, 175–178. [Google Scholar] [CrossRef]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.-L.; Delcourt, M.-O. Radiation-induced synthesis of mono-and multi-metallic clusters and nanocolloids. New J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Yang, W.; Ma, Y.; Tang, J.; Yang, X. “Green synthesis” of monodisperse Pt nanoparticles and their catalytic properties. Colloids Surf. A: Physicochem. Eng. Asp. 2007, 302, 628–633. [Google Scholar] [CrossRef]
- Remita, S.; Mostafavi, M.; Delcourt, M. Bimetallic Ag-Pt and Au-Pt aggregates synthesized by radiolysis. Radiat. Phys. Chem. 1996, 47, 275–279. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Nguyen, N.D.; Dang, V.P.; Phan, D.T.; Tran, T.H.; Nguyen, Q.H. Synthesis of Platinum Nanoparticles by Gamma Co-60 Ray Irradiation Method Using Chitosan as Stabilizer. Adv. Mater. Sci. Eng. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Shkilnyy, A.; Soucé, M.; Dubois, P.; Warmont, F.; Saboungi, M.-L.; Chourpa, I. Poly (ethylene glycol)-stabilized silver nanoparticles for bioanalytical applications of SERS spectroscopy. Analyst 2009, 134, 1868–1872. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Sarhid, I.; Abdellah, I.; Martini, C.; Huc, V.; Dragoe, D.; Beaunier, P.; Lampre, I.; Remita, H. Plasmonic catalysis for the Suzuki–Miyaura cross-coupling reaction using palladium nanoflowers. New J. Chem. 2019, 43, 4349–4355. [Google Scholar] [CrossRef]
- Shende, P.; Kasture, P.; Gaud, R. Nanoflowers: The future trend of nanotechnology for multi-applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Wang, N.; Quan, X.; Chen, Y. Controllable synthesis of ZnO nanoflowers and their morphology-dependent photocatalytic activities. Sep. Purif. Technol. 2008, 62, 727–732. [Google Scholar] [CrossRef]
- Grabowska, E.; Zaleska, A.; Sorgues, S.; Kunst, M.; Etcheberry, A.; Colbeau-Justin, C.; Remita, H. Modification of titanium (IV) dioxide with small silver nanoparticles: Application in photocatalysis. J. Phys. Chem. C 2013, 117, 1955–1962. [Google Scholar] [CrossRef]
- Ghosh, S.; Ramos, L.; Remita, H. Swollen hexagonal liquid crystals as smart nanoreactors: Implementation in materials chemistry for energy applications. Nanoscale 2018, 10, 5793–5819. [Google Scholar] [CrossRef]
- Gharibshahi, E.; Saion, E. Influence of dose on particle size and optical properties of colloidal platinum nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. [Google Scholar] [CrossRef]
- Inwati, G.K.; Rao, Y.; Singh, M. In situ free radical growth mechanism of platinum nanoparticles by microwave irradiation and electrocatalytic properties. Nanoscale Res. Lett. 2016, 11, 458. [Google Scholar] [CrossRef]
- Zhang, N.; Han, C.; Xu, Y.-J.; Foley IV, J.J.; Zhang, D.; Codrington, J.; Gray, S.K.; Sun, Y. Near-field dielectric scattering promotes optical absorption by platinum nanoparticles. Nat. Photonics 2016, 10, 473. [Google Scholar] [CrossRef]
- Choi, S.-H.; Zhang, Y.-P.; Gopalan, A.; Lee, K.-P.; Kang, H.-D. Preparation of catalytically efficient precious metallic colloids by γ-irradiation and characterization. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 165–170. [Google Scholar] [CrossRef]
- Cele, T.; Maaza, M.; Gibaud, A. Synthesis of platinum nanoparticles by gamma radiolysis. Mrs Adv. 2018, 3, 2537–2557. [Google Scholar] [CrossRef]
- Gharibshahi, E.; Saion, E.; Ashraf, A.; Gharibshahi, L. Size-controlled and optical properties of platinum nanoparticles by gamma radiolytic synthesis. Appl. Radiat. Isot. 2017, 130, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Privman, V. Diffusional nucleation of nanocrystals and their self-assembly into uniform colloids. arXiv 2008, arXiv:0806.464410, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, H.; Sánchez, E.H.; Brollo, M.E.; Asín, L.; Moerner, K.K.; Frandsen, C.; Lázaro, F.J.; Serna, C.J.; Veintemillas-Verdaguer, S.; Morales, M.P. Formation mechanism of maghemite nanoflowers synthesized by a polyol-mediated process. ACS Omega 2017, 2, 7172–7184. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Flores-Rojas, G.; López-Saucedo, F.; Bucio, E. Gamma-irradiation applied in the synthesis of metallic and organic nanoparticles: A short review. Radiat. Phys. Chem. 2018. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Radiation Synthesis and Modification of Polymers for Biomedical Applications; International Atomic Energy Agency: Vienna, Austria, 2002. [Google Scholar]
- Rosiak, J.; Ulański, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray photoelectron spectroscopy. Phys. Electron. 1995, 230–232. [Google Scholar]
- Puglia, C.; Nilsson, A.; Hernnäs, B.; Karis, O.; Bennich, P.; Mårtensson, N. Physisorbed, chemisorbed and dissociated O2 on Pt (111) studied by different core level spectroscopy methods. Surf. Sci. 1995, 342, 119–133. [Google Scholar] [CrossRef]
- Tu, W.; Takai, K.; Fukui, K.-i.; Miyazaki, A.; Enoki, T. Interface effect on the electronic structure of alkanethiol-coated platinum nanoparticles. J. Phys. Chem. B 2003, 107, 10134–10140. [Google Scholar] [CrossRef]
- Crispin, X.; Lazzaroni, R.; Crispin, A.; Geskin, V.; Bredas, J.; Salaneck, W.R. Understanding the initial stages of polymer grafting on metals: A photoelectron spectroscopy study of acrylonitrile adsorption on transition metal surfaces. J. Electron. Spectrosc. Relat. Phenom. 2001, 121, 57–74. [Google Scholar] [CrossRef]
- Galindo-Gonzalez, C.C.; Gantz, S.p.; Ourry, L.; Mammeri, F.; Ammar-Merah, S.; Ponton, A. Elaboration and rheological investigation of magnetic sensitive nanocomposite biopolymer networks. Macromolecules 2014, 47, 3136–3144. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, H.M.; Mandale, A.B.; Srivastava, R.; Adyanthaya, S.D.; Pasricha, R.; Sastry, M. Phase transfer of platinum nanoparticles from aqueous to organic solutions using fatty amine molecules. J. Chem. Sci. 2004, 116, 293–300. [Google Scholar] [CrossRef]
- Dandapat, A.; Mitra, A.; Gautam, P.K.; De, G. A facile synthesis of Pt nanoflowers composed of an ordered array of nanoparticles. Nanomater. Nanotechnol. 2013, 3, 11. [Google Scholar] [CrossRef][Green Version]
- Torres, M.G.; Cortez, J.C.; Pérez, M.G.; Talavera, R.R. Gamma radiation induced graft copolymerization of typical monomers onto poly (3-hydroxybutyrate). Int. J. Sci. Adv. Technol. 2012, 2, 106–119. [Google Scholar]
- Christensen, M.; Nielsen, O.F.; Jensen, P.; Schnell, U. Water structure in polyethylene glycols for preservation of wooden artefacts. A NIR-FT-Raman spectroscopic investigation. J. Mol. Struct. 2005, 735, 267–270. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Shi, H.; He, X.; Wang, K.; Yuan, Y.; Deng, K.; Chen, J.; Tan, W. Rhodamine B isothiocyanate doped silica-coated fluorescent nanoparticles (RBITC-DSFNPs)–based bioprobes conjugated to Annexin V for apoptosis detection and imaging. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 266–272. [Google Scholar] [CrossRef]
- Stobiecka, M.; Hepel, M. Multimodal coupling of optical transitions and plasmonic oscillations in rhodamine B modified gold nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 1131–1139. [Google Scholar] [CrossRef]
- Zhao, J.; Jensen, L.; Sung, J.; Zou, S.; Schatz, G.C.; Van Duyne, R.P. Interaction of plasmon and molecular resonances for rhodamine 6G adsorbed on silver nanoparticles. J. Am. Chem. Soc. 2007, 129, 7647–7656. [Google Scholar] [CrossRef] [PubMed]
- Diac, A.; Focsan, M.; Socaci, C.; Gabudean, A.-M.; Farcau, C.; Maniu, D.; Vasile, E.; Terec, A.; Veca, L.M.; Astilean, S. Covalent conjugation of carbon dots with Rhodamine B and assessment of their photophysical properties. Rsc. Adv. 2015, 5, 77662–77669. [Google Scholar] [CrossRef]
- Tira, D.S.; Focsan, M.; Ulinici, S.; Maniu, D.; Astilean, S. Rhodamine B-coated gold nanoparticles as effective “Turn-on” fluorescent sensors for detection of zinc II ions in water. Spectrosc. Lett. 2014, 47, 153–159. [Google Scholar] [CrossRef]
- Li, J.; Krasavin, A.V.; Webster, L.; Segovia, P.; Zayats, A.V.; Richards, D. Spectral variation of fluorescence lifetime near single metal nanoparticles. Sci. Rep. 2016, 6, 21349. [Google Scholar] [CrossRef]
- Kong, T.; Zeng, J.; Wang, X.; Yang, X.; Yang, J.; McQuarrie, S.; McEwan, A.; Roa, W.; Chen, J.; Xing, J.Z. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small 2008, 4, 1537–1543. [Google Scholar] [CrossRef]
- Li, S.; Porcel, E.; Remita, H.; Marco, S.; Refregiers, M.; Dutertre, M.; Confalonieri, F.; Lacombe, S. Platinum nanoparticles: An exquisite tool to overcome radioresistance. Cancer Nanotechnol. 2017, 8, 4. [Google Scholar] [CrossRef]
- Luchette, M.; Korideck, H.; Makrigiorgos, M.; Tillement, O.; Berbeco, R. Radiation dose enhancement of gadolinium-based AGuIX nanoparticles on HeLa cells. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1751–1755. [Google Scholar] [CrossRef]
- Deacon, J.; Peckham, M.; Steel, G. The radioresponsiveness of human tumours and the initial slope ofthe cell survival curve. Radiother. Oncol. 1984, 2, 317–323. [Google Scholar] [CrossRef]
- Subiel, A.; Ashmore, R.; Schettino, G. Standards and methodologies for characterizing radiobiological impact of high-Z nanoparticles. Theranostics 2016, 6, 1651. [Google Scholar] [CrossRef]
- Khoo, A.M.; Cho, S.H.; Reynoso, F.J.; Aliru, M.; Aziz, K.; Bodd, M.; Yang, X.; Ahmed, M.F.; Yasar, S.; Manohar, N. Radiosensitization of prostate cancers in vitro and in vivo to erbium-filtered orthovoltage x-rays using actively targeted gold nanoparticles. Sci. Rep. 2017, 7, 18044. [Google Scholar] [CrossRef]
- Lechtman, E.; Mashouf, S.; Chattopadhyay, N.; Keller, B.; Lai, P.; Cai, Z.; Reilly, R.; Pignol, J. A Monte Carlo-based model of gold nanoparticle radiosensitization accounting for increased radiobiological effectiveness. Phys. Med. Biol. 2013, 58, 3075. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Schlathölter, T.; Eustache, P.; Porcel, E.; Salado, D.; Stefancikova, L.; Tillement, O.; Lux, F.; Mowat, P.; Biegun, A.K.; Van Goethem, M.-J. Improving proton therapy by metal-containing nanoparticles: Nanoscale insights. Int. J. Nanomed. 2016, 11, 1549. [Google Scholar] [CrossRef] [PubMed]
- Porcel, E.; Tillement, O.; Lux, F.; Mowat, P.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Le Sech, C.; Li, S.; Lacombe, S. Gadolinium-based nanoparticles to improve the hadrontherapy performances. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Le Sech, C.; Lacombe, S. Platinum nanoparticles: A promising material for future cancer therapy? Nanotechnology 2010, 21, 085103. [Google Scholar] [CrossRef] [PubMed]



| Sample | α (Gy−1) | β (Gy−2) | R2 | SER | DEF |
|---|---|---|---|---|---|
| Control | 0.24 ± 0.01 | 0.049 ± 0.002 | 0.999 | − | − |
| Pt NFs | 0.37 ± 0.01 | 0.044 ± 0.003 | 0.999 | 23% | 1.20 |
| Sample | Nanosize Damage Per Plasmid and Per Gy (×10−5) | mAF (%) |
|---|---|---|
| Control | 28.1 ± 0.4 | − |
| Pt NFs | 53.1 ± 0.5 | 89.0 ± 4.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Salado-Leza, D.; Porcel, E.; González-Vargas, C.R.; Savina, F.; Dragoe, D.; Remita, H.; Lacombe, S. A Facile One-Pot Synthesis of Versatile PEGylated Platinum Nanoflowers and Their Application in Radiation Therapy. Int. J. Mol. Sci. 2020, 21, 1619. https://doi.org/10.3390/ijms21051619
Yang X, Salado-Leza D, Porcel E, González-Vargas CR, Savina F, Dragoe D, Remita H, Lacombe S. A Facile One-Pot Synthesis of Versatile PEGylated Platinum Nanoflowers and Their Application in Radiation Therapy. International Journal of Molecular Sciences. 2020; 21(5):1619. https://doi.org/10.3390/ijms21051619
Chicago/Turabian StyleYang, Xiaomin, Daniela Salado-Leza, Erika Porcel, César R. González-Vargas, Farah Savina, Diana Dragoe, Hynd Remita, and Sandrine Lacombe. 2020. "A Facile One-Pot Synthesis of Versatile PEGylated Platinum Nanoflowers and Their Application in Radiation Therapy" International Journal of Molecular Sciences 21, no. 5: 1619. https://doi.org/10.3390/ijms21051619
APA StyleYang, X., Salado-Leza, D., Porcel, E., González-Vargas, C. R., Savina, F., Dragoe, D., Remita, H., & Lacombe, S. (2020). A Facile One-Pot Synthesis of Versatile PEGylated Platinum Nanoflowers and Their Application in Radiation Therapy. International Journal of Molecular Sciences, 21(5), 1619. https://doi.org/10.3390/ijms21051619







