Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases †
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics
2.2. Biological Results
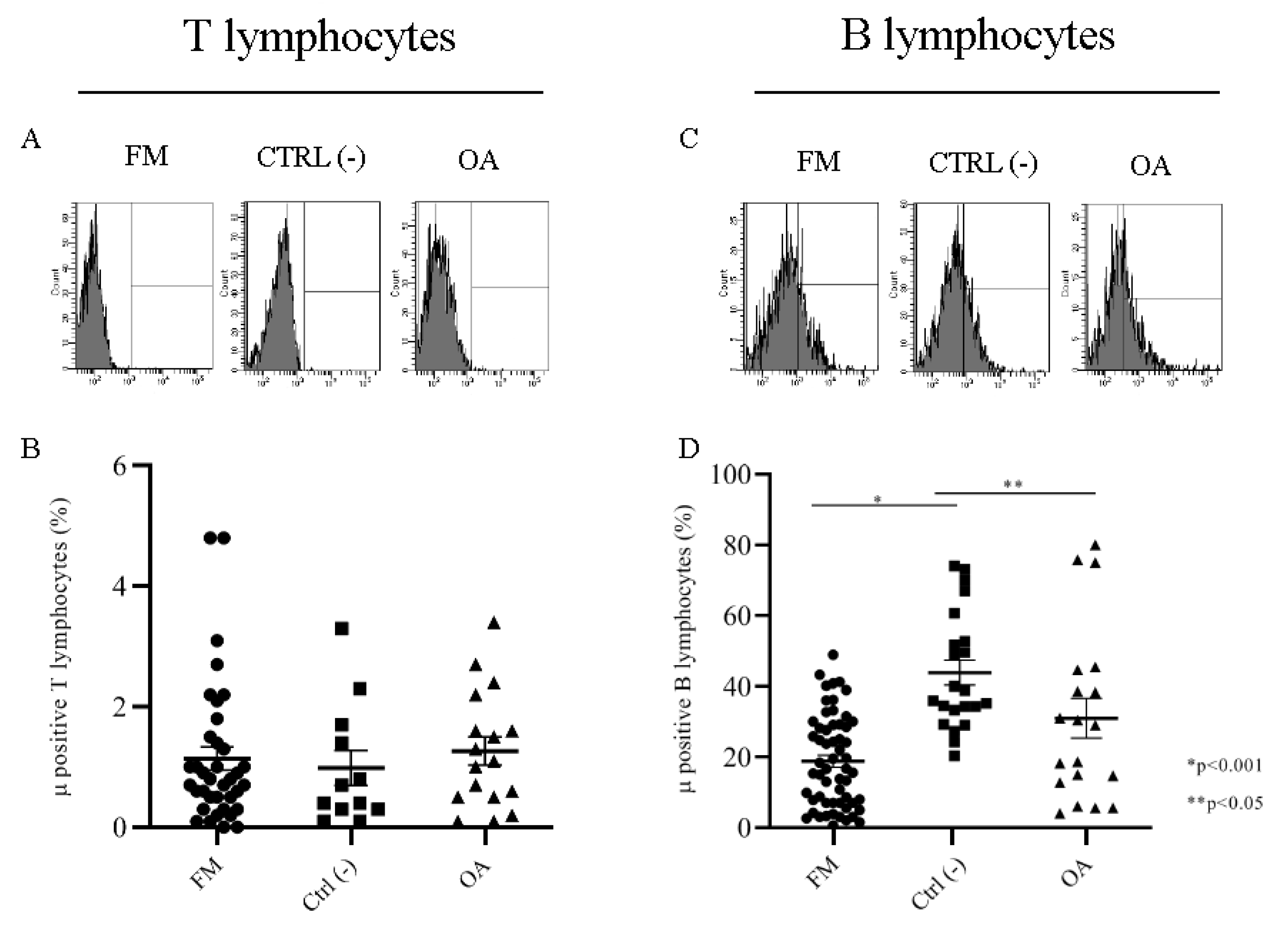
2.2.1. Mu Opioid Receptors on T and B Lymphocytes Membrane
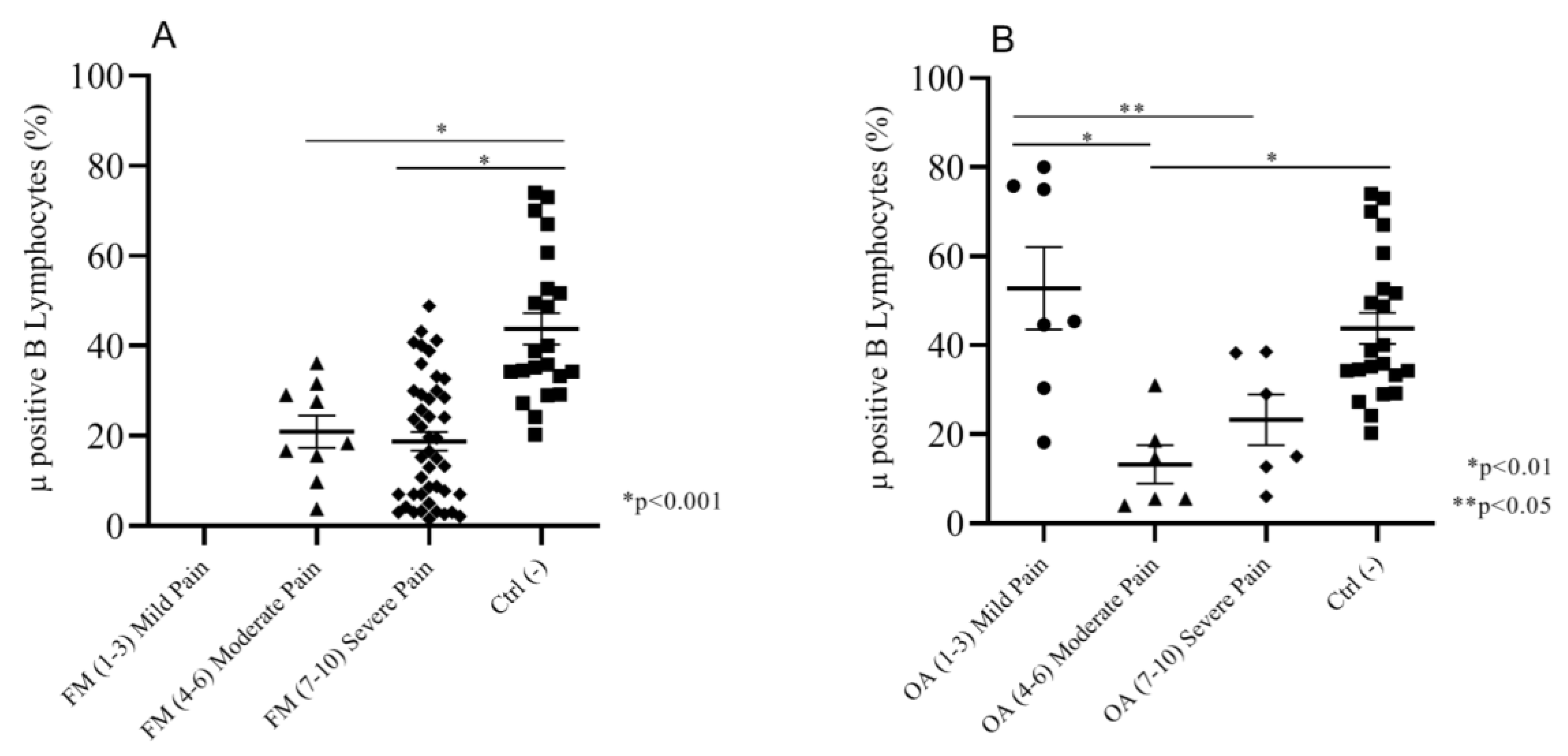
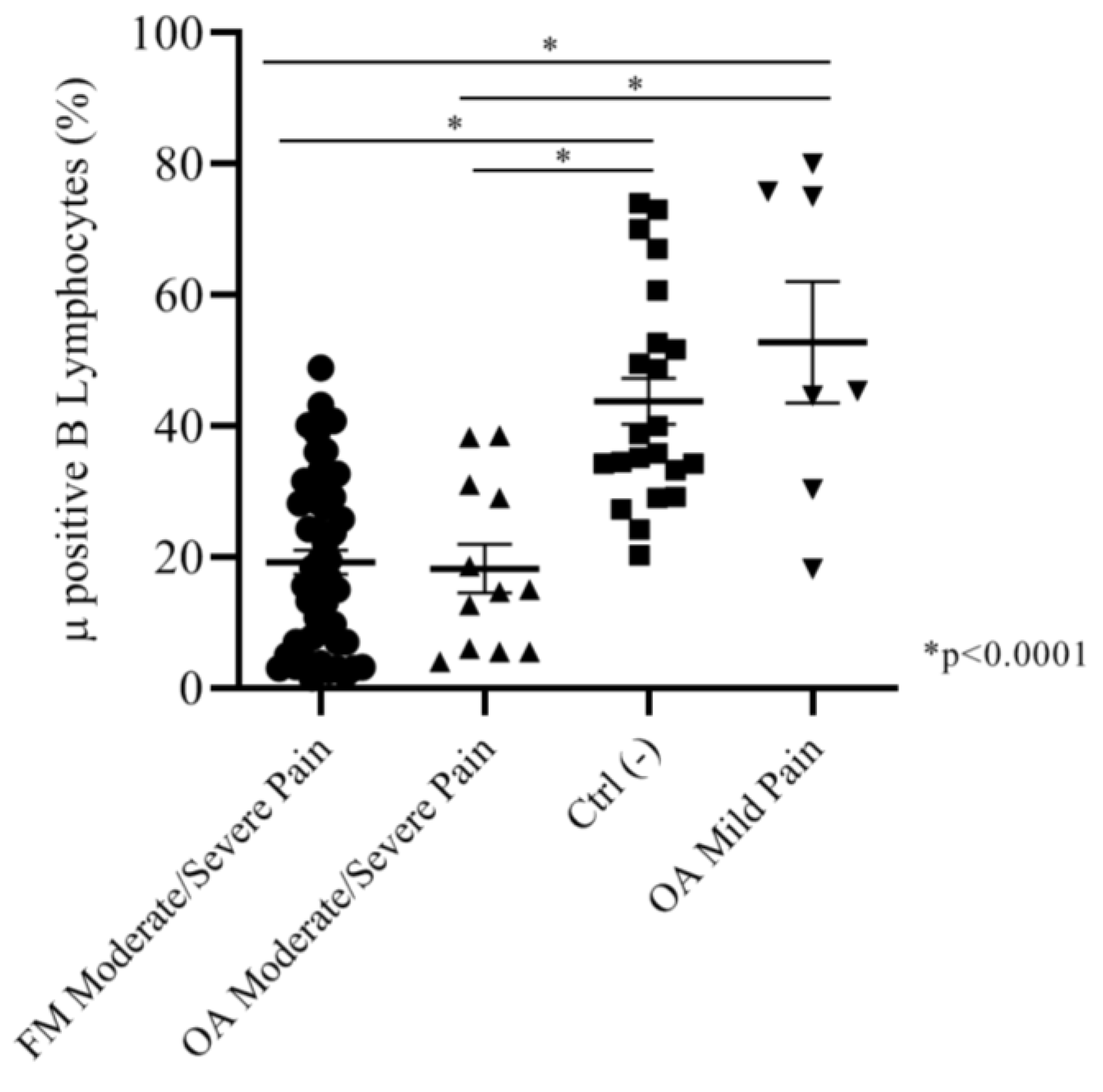
2.2.2. Correlation between Intensity of Pain (NRS Scale) and Mu-Positive B Cells
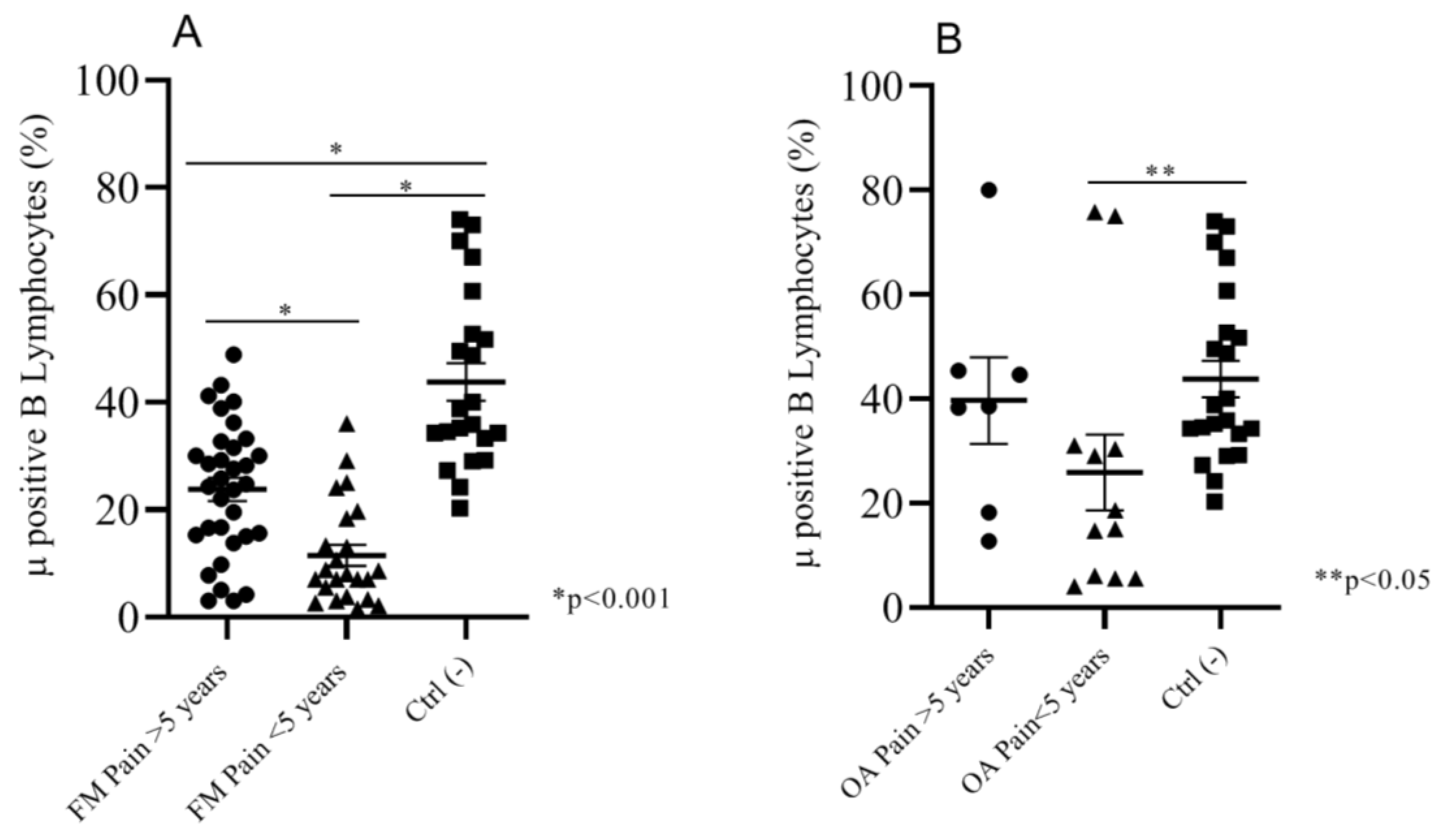
2.2.3. Correlation between Duration of Pain and Mu Positive B Cells
2.2.4. Correlation between Drugs Therapy before Enrollment and Mu-Positive BCells
2.3. Correlations between Mu-Positive B Cells and Psychological Variables
3. Discussion
4. Materials and Methods
4.1. Trial Design
4.2. Participants
4.3. Clinical and Psychological Measurements
4.4. Blood Assays
4.5. Biological Analysis
Immunophenotyping Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FM | Fibromyalgia |
| ACR | American College of Rheumatology |
| OA | Osteoarthritis |
| ICD-11 | International Classification of Diseases-11 |
| CWP | Chronic Widespread Pain |
| PBMC | Peripheral Blood Mononuclear Cell |
| uHPLC-PDA-MS | Ultra-High Performance Liquid Chromatography–Photo Diode Array–Mass-Spectrometry |
| HPLC | High Performance Liquid Chromatography |
| MOR | Mu Opioid Receptor |
| DOR | Delta Opioid Receptor |
| KOR | Kappa Opioid Receptor |
| IgM | Immunoglobulin M |
| IgG | Immunoglobulin G |
| CRF | Case Report Form |
| NRS | Numerical Rating Scale |
| NSAIDs | Non Steroidal Anti InflammatoryDrugs |
| ANOVA | Analysis of Variance |
| CTRL | Control |
| FIQ | Fibromyalgia Impact Questionnaire |
| OA-FIQ-R | Osteoarthritis- FIQ Revised |
| IPQ-R | Illness Perception Questionnaire – Revised |
| CSQ | Coping Strategies Questionnaire |
| CPAQ | Chronic Pain Acceptance Questionnaire |
| DASS-21 | Depression, Anxiety and Stress Scale-21 |
| IRCCS | Istituto di Ricovero e Cura a Carattere Scientifico |
| GDPR | General Data Protection Regulation |
References
- Hoffman, D.L.; Dukes, E.M. The health status burden of people with fibromyalgia: A review of studies that assessed health status with the SF-36 or the SF-12. Int. J. Clin. Pract. 2008, 62, 115–126. [Google Scholar] [CrossRef]
- Branco, J.C.; Bannwarth, B.; Failde, I.; Abello Carbonell, J.; Blotman, F.; Spaeth, M.; Saraiva, F.; Nacci, F.; Thomas, E.; Caubere, J.P.; et al. Prevalence of fibromyalgia: A survey in five European countries. Semin. Arthritis Rheum. 2010, 39, 448–453. [Google Scholar] [CrossRef]
- Queiroz, L.P. Worldwide epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef]
- Marques, A.P.; Santo, A.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of fibromyalgia: Literature review update. Rev. Bras. Reum. 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Ramiro Fde, S.; Lombardi Junior, I.; da Silva, R.C.; Montesano, F.T.; de Oliveira, N.R.; Diniz, R.E.; Alambert, P.A.; Padovani Rda, C. Investigation of stress, anxiety and depression in women with fibromyalgia: A comparative study. Rev. Bras. Reum. 2014, 54, 27–32. [Google Scholar] [CrossRef][Green Version]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Raffaeli, W.; Arnaudo, E. Pain as a disease: An overview. J. Pain Res. 2017, 10, 2003–2008. [Google Scholar] [CrossRef]
- Wolfe, F.; Hauser, W. Fibromyalgia diagnosis and diagnostic criteria. Ann. Med. 2011, 43, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Kumbhare, D.; Ahmed, S.; Watter, S. A narrative review on the difficulties associated with fibromyalgia diagnosis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 13–26. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Atzeni, F.; Masala, I.F.; Salaffi, F.; Chapman, J.; Choy, E. Are the ACR 2010 diagnostic criteria for fibromyalgia better than the 1990 criteria? Autoimmun. Rev. 2018, 17, 33–35. [Google Scholar] [CrossRef]
- Hauser, W.; Fitzcharles, M.A. Facts and myths pertaining to fibromyalgia. Dialogues Clin. Neurosci. 2018, 20, 53–62. [Google Scholar]
- Hackshaw, K.V.; Aykas, D.P.; Sigurdson, G.T.; Plans, M.; Madiai, F.; Yu, L.; Buffington, C.A.T.; Giusti, M.M.; Rodriguez-Saona, L. Metabolic fingerprinting for diagnosis of fibromyalgia and other rheumatologic disorders. J. Biol. Chem. 2019, 294, 2555–2568. [Google Scholar] [CrossRef]
- Choy, E.; Perrot, S.; Leon, T.; Kaplan, J.; Petersel, D.; Ginovker, A.; Kramer, E. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv. Res. 2010, 10, 102. [Google Scholar] [CrossRef]
- Di Franco, M.; Iannuccelli, C.; Bazzichi, L.; Atzeni, F.; Consensi, A.; Salaffi, F.; Pietropaolo, M.; Alessandri, C.; Basili, S.; Olivieri, M.; et al. Misdiagnosis in fibromyalgia: A multicentre study. Clin. Exp. Rheumatol. 2011, 29, S104–S108. [Google Scholar]
- Ghavidel-Parsa, B.; Bidari, A.; Amir Maafi, A.; Ghalebaghi, B. The Iceberg Nature of Fibromyalgia Burden: The Clinical and Economic Aspects. Korean J. Pain 2015, 28, 169–176. [Google Scholar] [CrossRef]
- Mengshoel, A.M.; Sim, J.; Ahlsen, B.; Madden, S. Diagnostic experience of patients with fibromyalgia—A meta-ethnography. Chronic Illn. 2018, 14, 194–211. [Google Scholar] [CrossRef]
- Hauser, W.; Sarzi-Puttini, P.; Fitzcharles, M.A. Fibromyalgia syndrome: Under-, over- and misdiagnosis. Clin. Exp. Rheumatol. 2019, 37, 90–97. [Google Scholar]
- Bazzichi, L.; Rossi, A.; Giacomelli, C.; Bombardieri, S. Exploring the abyss of fibromyalgia biomarkers. Clin. Exp. Rheumatol. 2010, 28, S125–S130. [Google Scholar] [PubMed]
- Giacomelli, C.; Talarico, R.; Baldini, C.; Bazzichi, L. Pain in Sjogren’s syndrome. Reumatismo 2014, 66, 39–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Behm, F.G.; Gavin, I.M.; Karpenko, O.; Lindgren, V.; Gaitonde, S.; Gashkoff, P.A.; Gillis, B.S. Unique immunologic patterns in fibromyalgia. BMC Clin. Pathol. 2012, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J.; Gavin, I.M.; Karpenko, O.; Barkhordar, F.; Gillis, B.S. Cytokine and chemokine profiles in fibromyalgia, rheumatoid arthritis and systemic lupus erythematosus: A potentially useful tool in differential diagnosis. Rheumatol. Int. 2015, 35, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.B.; Le-Niculescu, H.; Levey, D.F.; Roseberry, K.; Soe, K.C.; Rogers, J.; Khan, F.; Jones, T.; Judd, S.; McCormick, M.A.; et al. Towards precision medicine for pain: Diagnostic biomarkers and repurposed drugs. Mol. Psychiatry 2019, 24, 501–522. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Atzeni, F.; Mease, P.J. Chronic widespread pain: From peripheral to central evolution. Best Pract. Res. Clin. Rheumatol. 2011, 25, 133–139. [Google Scholar] [CrossRef]
- Stein, C. Opioids, sensory systems and chronic pain. Eur. J. Pharmacol. 2013, 716, 179–187. [Google Scholar] [CrossRef]
- Reilly, M.M.; Shy, M.E. Diagnosis and new treatments in genetic neuropathies. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1304–1314. [Google Scholar] [CrossRef]
- Liang, X.; Liu, R.; Chen, C.; Ji, F.; Li, T. Opioid System Modulates the Immune Function: A Review. Transl. Perioper. Pain Med. 2016, 1, 5–13. [Google Scholar]
- Brack, A.; Labuz, D.; Schiltz, A.; Rittner, H.L.; Machelska, H.; Schafer, M.; Reszka, R.; Stein, C. Tissue monocytes/macrophages in inflammation: Hyperalgesia versus opioid-mediated peripheral antinociception. Anesthesiology 2004, 101, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovac, J.; Cupic, B.; Zapletal, E.; Brozovic, A. IFN-gamma up-regulates kappa opioid receptors (KOR) on murine macrophage cell line J774. J. Neuroimmunol. 2012, 245, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Endres-Becker, J.; Heppenstall, P.A.; Mousa, S.A.; Labuz, D.; Oksche, A.; Schafer, M.; Stein, C.; Zollner, C. Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol. Pharmacol. 2007, 71, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Provinciali, M.; Di Stefano, G.; Raffaeli, W.; Pari, G.; Desiderio, F.; Fabris, N. Evaluation of NK and LAK cell activities in neoplastic patients during treatment with morphine. Int. J. Neurosci. 1991, 59, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Lauro, F.; Dagostino, C.; Ilari, S.; Giancotti, L.A.; Gliozzi, M.; Costa, N.; Carresi, C.; Musolino, V.; Casale, F.; et al. Olea Europea-derived phenolic products attenuate antinociceptive morphine tolerance: An innovative strategic approach to treat cancer pain. J. Biol. Regul. Homeost. Agents 2014, 28, 105–116. [Google Scholar] [PubMed]
- Lauro, F.; Giancotti, L.A.; Ilari, S.; Dagostino, C.; Gliozzi, M.; Morabito, C.; Malafoglia, V.; Raffaeli, W.; Muraca, M.; Goffredo, B.M.; et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE 2016, 11, e0156039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muscoli, C.; Doyle, T.; Dagostino, C.; Bryant, L.; Chen, Z.; Watkins, L.R.; Ryerse, J.; Bieberich, E.; Neumman, W.; Salvemini, D. Counter-regulation of opioid analgesia by glial-derived bioactive sphingolipids. J. Neurosci. 2010, 30, 15400–15408. [Google Scholar] [CrossRef]
- Muscoli, C.; Cuzzocrea, S.; Ndengele, M.M.; Mollace, V.; Porreca, F.; Fabrizi, F.; Esposito, E.; Masini, E.; Matuschak, G.M.; Salvemini, D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J. Clin. Invest. 2007, 117, 3530–3539. [Google Scholar] [CrossRef]
- Doyle, T.; Bryant, L.; Muscoli, C.; Cuzzocrea, S.; Esposito, E.; Chen, Z.; Salvemini, D. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci. Lett. 2010, 483, 85–89. [Google Scholar] [CrossRef]
- Provinciali, M.; Di Stefano, G.; Stronati, S.; Raffaeli, W.; Pari, G.; Fabris, N. Role of prolactin in the modulation of NK and LAK cell activity after short- or long-term morphine administration in neoplastic patients. Int. J. Immunopharmacol. 1996, 18, 577–586. [Google Scholar] [CrossRef]
- Raffaeli, W.; Samolsky Dekel, B.G.; Landuzzi, D.; Caminiti, A.; Righetti, D.; Balestri, M.; Montanari, F.; Romualdi, P.; Candeletti, S. Nociceptin levels in the cerebrospinal fluid of chronic pain patients with or without intrathecal administration of morphine. J. Pain Symptom Manag. 2006, 32, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Dersh, J.; Polatin, P.B.; Gatchel, R.J. Chronic pain and psychopathology: Research findings and theoretical considerations. Psychosom. Med. 2002, 64, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Fietta, P.; Fietta, P.; Manganelli, P. Fibromyalgia and psychiatric disorders. Acta Biomed. 2007, 78, 88–95. [Google Scholar] [PubMed]
- Loevinger, B.L.; Shirtcliff, E.A.; Muller, D.; Alonso, C.; Coe, C.L. Delineating psychological and biomedical profiles in a heterogeneous fibromyalgia population using cluster analysis. Clin. Rheumatol. 2012, 31, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hobro, N.; Weinman, J.; Hankins, M. Using the self-regulatory model to cluster chronic pain patients: The first step towards identifying relevant treatments? Pain 2004, 108, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Dysvik, E.; Natvig, G.K.; Eikeland, O.J.; Lindstrom, T.C. Coping with chronic pain. Int. J. Nurs. Studies 2005, 42, 297–305. [Google Scholar] [CrossRef]
- McCracken, L.M.; Vowles, K.E. Acceptance of chronic pain. Curr. Pain Headache Rep. 2006, 10, 90–94. [Google Scholar] [CrossRef]
- van Wilgen, C.P.; van Ittersum, M.W.; Kaptein, A.A.; van Wijhe, M. Illness perceptions in patients with fibromyalgia and their relationship to quality of life and catastrophizing. Arthritis Rheum. 2008, 58, 3618–3626. [Google Scholar] [CrossRef]
- Quartana, P.J.; Campbell, C.M.; Edwards, R.R. Pain catastrophizing: A critical review. Expert Rev. Neurother. 2009, 9, 745–758. [Google Scholar] [CrossRef]
- Jones, K.R.; Vojir, C.P.; Hutt, E.; Fink, R. Determining mild, moderate, and severe pain equivalency across pain-intensity tools in nursing home residents. J. Rehabil. Res. Dev. 2007, 44, 305–314. [Google Scholar] [CrossRef]
- Apkarian, A.V. Definitions of nociception, pain, and chronic pain with implications regarding science and society. Neurosci. Lett. 2019, 702, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.; Brahler, E.; Wolfe, F.; Henningsen, P. Patient Health Questionnaire 15 as a generic measure of severity in fibromyalgia syndrome: Surveys with patients of three different settings. J. Psychosom. Res. 2014, 76, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Thudium, C.S.; Lofvall, H.; Karsdal, M.A.; Bay-Jensen, A.C.; Bihlet, A.R. Protein biomarkers associated with pain mechanisms in osteoarthritis. J. Proteom. 2019, 190, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Malafoglia, V.; Celi, M.; Muscoli, C.; Ilari, S.; Lauro, F.; Giancotti, L.A.; Morabito, C.; Feola, M.; Tarantino, U.; Raffaeli, W. Lymphocyte opioid receptors as innovative biomarkers of osteoarthritic pain, for the assessment and risk management of opioid tailored therapy, before hip surgery, to prevent chronic pain and opioid tolerance/addiction development: OpMarkArt (Opioids-Markers-Arthroprosthesis) study protocol for a randomized controlled trial. Trials 2017, 18, 605. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A., Jr.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Campana, G.; Sarti, D.; Spampinato, S.; Raffaeli, W. Long-term intrathecal morphine and bupivacaine upregulate MOR gene expression in lymphocytes. Int. Immunopharmacol. 2010, 10, 1149–1152. [Google Scholar] [CrossRef]
- Luna, I.E.; Kehlet, H.; Petersen, M.A.; Aasvang, E.K. Clinical, nociceptive and psychological profiling to predict acute pain after total knee arthroplasty. Acta Anaesthesiol. Scand. 2017, 61, 676–687. [Google Scholar] [CrossRef]
- Perrot, S. Osteoarthritis pain. Best Pract. Res. Clin. Rheumatol. 2015, 29, 90–97. [Google Scholar] [CrossRef]
- Harris, R.E.; Clauw, D.J.; Scott, D.J.; McLean, S.A.; Gracely, R.H.; Zubieta, J.K. Decreased central mu-opioid receptor availability in fibromyalgia. J. Neurosci. 2007, 27, 10000–10006. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Atzeni, F.; Fiorini, T.; Panni, B.; Randisi, G.; Turiel, M.; Carrabba, M. Validation of an Italian version of the Fibromyalgia Impact Questionnaire (FIQ-I). Clin. Exp. Rheumatol. 2003, 21, 459–464. [Google Scholar]
- Giardini, A.; Majani, G.; Pierobon, A.; Gremigni, P.; Catapano, I. Contribution to the Italian validation of the IPQ-R. G. Ital. Med. Lav. Ergon. 2007, 29, A64–A74. [Google Scholar] [PubMed]
- Monticone, M.; Ferrante, S.; Giorgi, I.; Galandra, C.; Rocca, B.; Foti, C. The 27-item coping strategies questionnaire-revised: Confirmatory factor analysis, reliability and validity in Italian-speaking subjects with chronic pain. Pain Res. Manag. 2014, 19, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Bottesi, G.; Ghisi, M.; Altoe, G.; Conforti, E.; Melli, G.; Sica, C. The Italian version of the Depression Anxiety Stress Scales-21: Factor structure and psychometric properties on community and clinical samples. Compr. Psychiatry 2015, 60, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Bernini, O.; Pennato, T.; Cosci, F.; Berrocal, C. The psychometric properties of the chronic pain acceptance questionnaire in Italian patients with chronic pain. J. Health Psychol. 2010, 15, 1236–1245. [Google Scholar] [CrossRef]
| ClinicalCharacteristics | % Of Patients (n=102) | |
|---|---|---|
| Diagnosis | ||
| Fibromyalgia (FM) | 57.8 (n = 59) | |
| Musculoskeletaldisorders (OA) | 18.6 (n = 19) | |
| Healthypeople | 23.5 (n = 24) | |
| Intensity of Pain (NRS) | FM | OA |
| (1–3) MildPain | (n = 0) | 42.1 (n = 8) |
| (4–6) Moderate Pain | 15.3 (n = 9) | 26.4 (n = 5) |
| (7–10) Severe Pain | 84.7 (n = 50) | 31.5 (n = 6) |
| None | ||
| Duration of Pain | FM | OA |
| <5 years | 59.3 (n = 35) | 63.1 (n = 12) |
| >5 years | 40.7 (n = 24) | 36.9 (n = 7) |
| Mu+ B Cells % of Expression | |
|---|---|
| FIQ | −0.39 |
| IPQ-R—Timeline acute/chronic | −0.101 |
| IPQ-R—Timeline cyclical | −0.062 |
| IPQ-R—Consequences | −0.093 |
| IPQ-R—Personal control | 0.056 |
| IPQ-R—Treatment control | 0.089 |
| IPQ-R—Illnesscoherence | 0.046 |
| IPQ-R—Emotional representation | 0.090 |
| CSQ—Distraction | 0.135 |
| CSQ—Catastrophizing | −0.012 |
| CSQ—Ignoring pain sensations | 0.057 |
| CSQ—Distancing from pain | 0.107 |
| CSQ—Coping self-statements | 0.028 |
| CSQ—Praying | −0.032 |
| CPAQ | −0.021 |
| DASS—Depression | 0.064 |
| DASS—Anxiety | 0.053 |
| DASS—Stress | 0.105 |
| Mu+ B Cells % of Expression | |
|---|---|
| FIQ/OA-FIQ-R | −0.027 |
| IPQ-R—Timeline acute/chronic | −0.241 |
| IPQ-R—Timeline cyclical | −0.067 |
| IPQ-R—Consequences | −0.155 |
| IPQ-R—Personal control | 0.022 |
| IPQ-R—Treatment control | 0.122 |
| IPQ-R—Illness coherence | 0.006 |
| IPQ-R—Emotional representation | 0.133 |
| CSQ—Distraction | 0.055 |
| CSQ—Catastrophizing | −0.014 |
| CSQ—Ignoring pain sensations | −0.043 |
| CSQ—Distancing from pain | 0.036 |
| CSQ—Coping self-statements | 0.065 |
| CSQ—Praying | 0.024 |
| CPAQ | 0.031 |
| DASS—Depression | −0.015 |
| DASS—Anxiety | 0.074 |
| DASS—Stress | 0.105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffaeli, W.; Malafoglia, V.; Bonci, A.; Tenti, M.; Ilari, S.; Gremigni, P.; Iannuccelli, C.; Gioia, C.; Di Franco, M.; Mollace, V.; et al. Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases. Int. J. Mol. Sci. 2020, 21, 1499. https://doi.org/10.3390/ijms21041499
Raffaeli W, Malafoglia V, Bonci A, Tenti M, Ilari S, Gremigni P, Iannuccelli C, Gioia C, Di Franco M, Mollace V, et al. Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases. International Journal of Molecular Sciences. 2020; 21(4):1499. https://doi.org/10.3390/ijms21041499
Chicago/Turabian StyleRaffaeli, William, Valentina Malafoglia, Antonello Bonci, Michael Tenti, Sara Ilari, Paola Gremigni, Cristina Iannuccelli, Chiara Gioia, Manuela Di Franco, Vincenzo Mollace, and et al. 2020. "Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases" International Journal of Molecular Sciences 21, no. 4: 1499. https://doi.org/10.3390/ijms21041499
APA StyleRaffaeli, W., Malafoglia, V., Bonci, A., Tenti, M., Ilari, S., Gremigni, P., Iannuccelli, C., Gioia, C., Di Franco, M., Mollace, V., Vitiello, L., Tomino, C., & Muscoli, C. (2020). Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases. International Journal of Molecular Sciences, 21(4), 1499. https://doi.org/10.3390/ijms21041499







