IL-27Rα: A Novel Molecular Imaging Marker for Allograft Rejection
Abstract
1. Introduction
2. Results
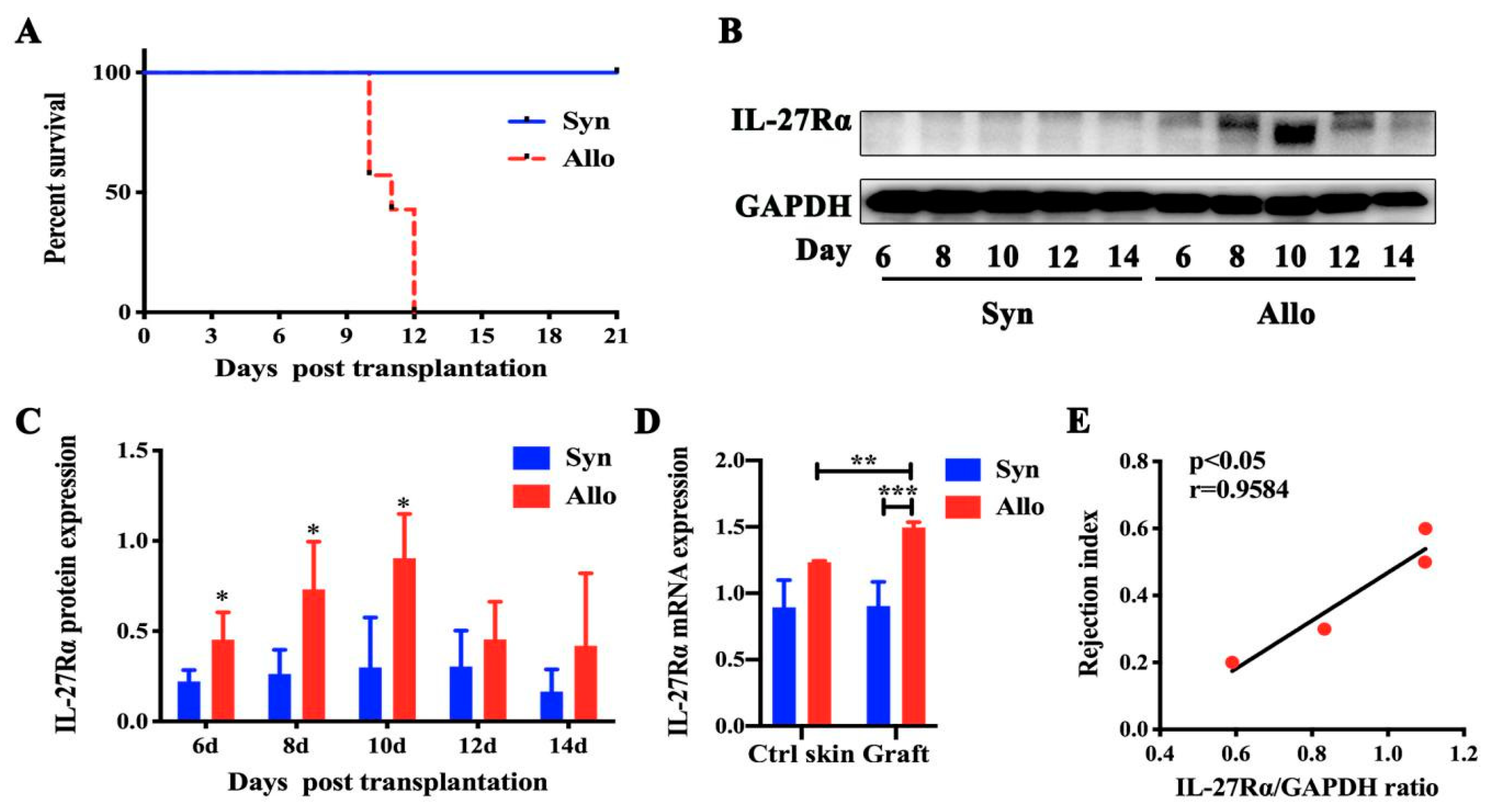
2.1. The Dynamic Expression of IL-27Rα in Grafts Post Transplantation
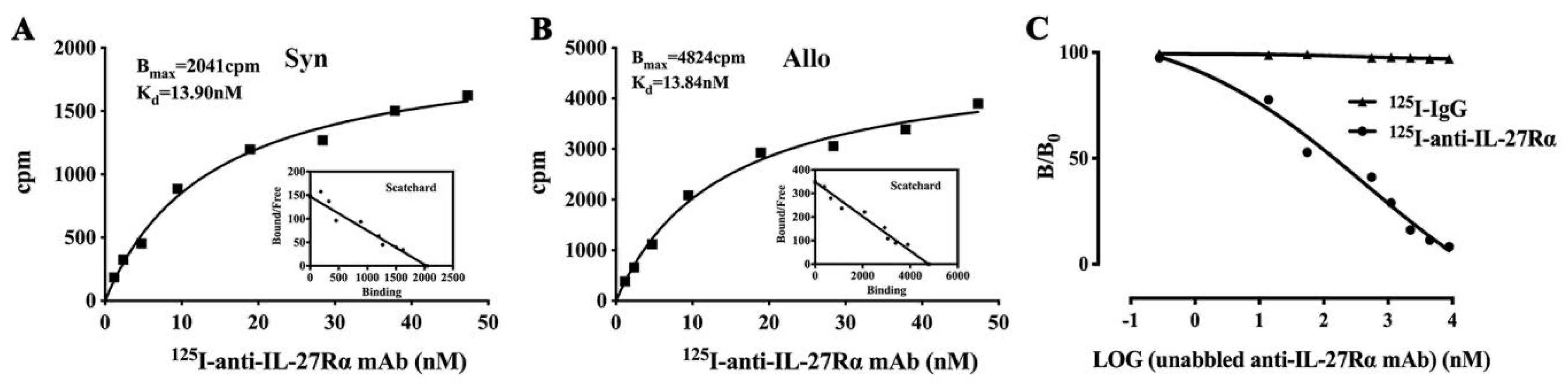
2.2. Preparation of 125I-anti-IL-27Rα mAb/125I-IgG
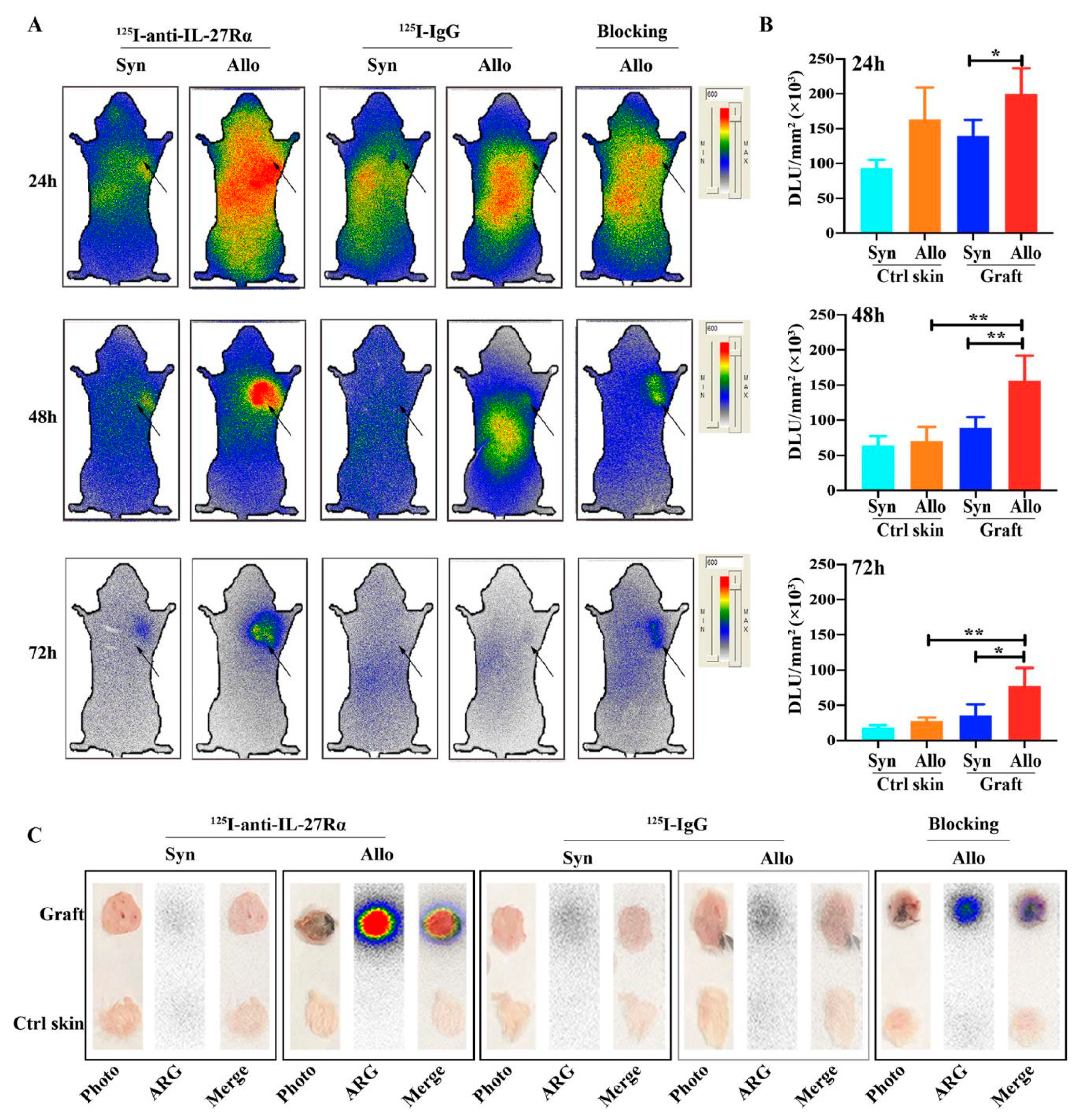
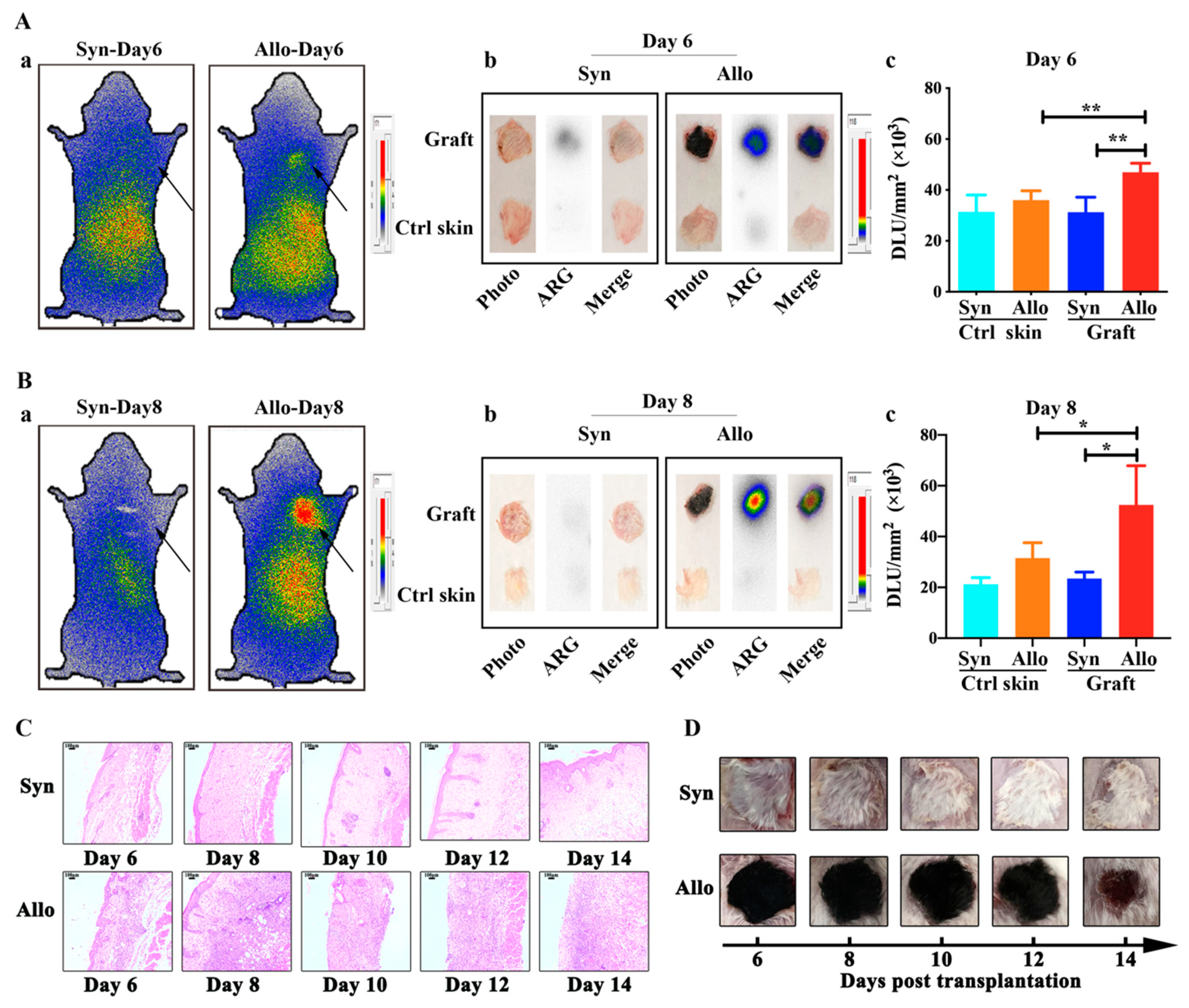
2.3. Whole-Body Phosphor-Autoradiography of Model Mice
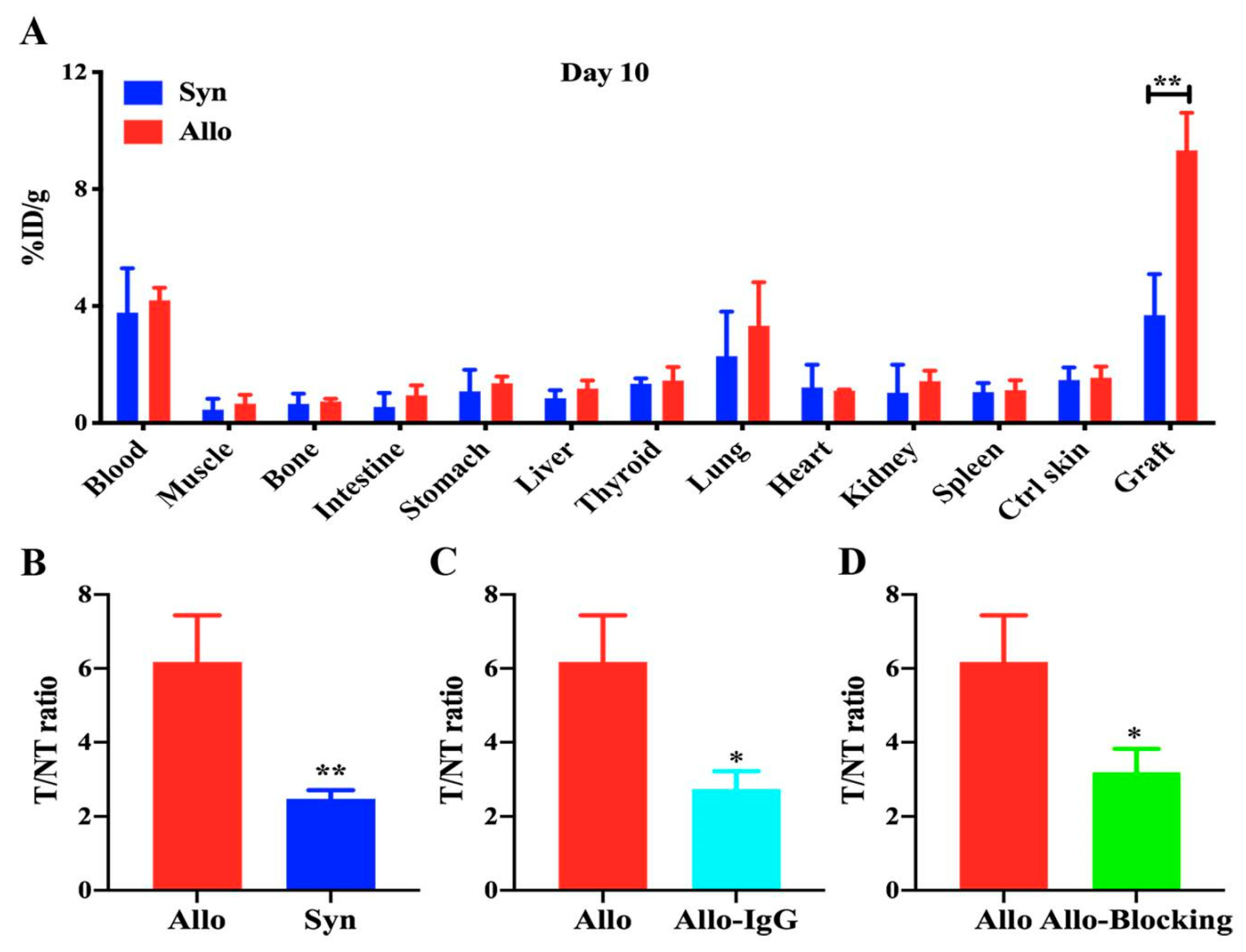
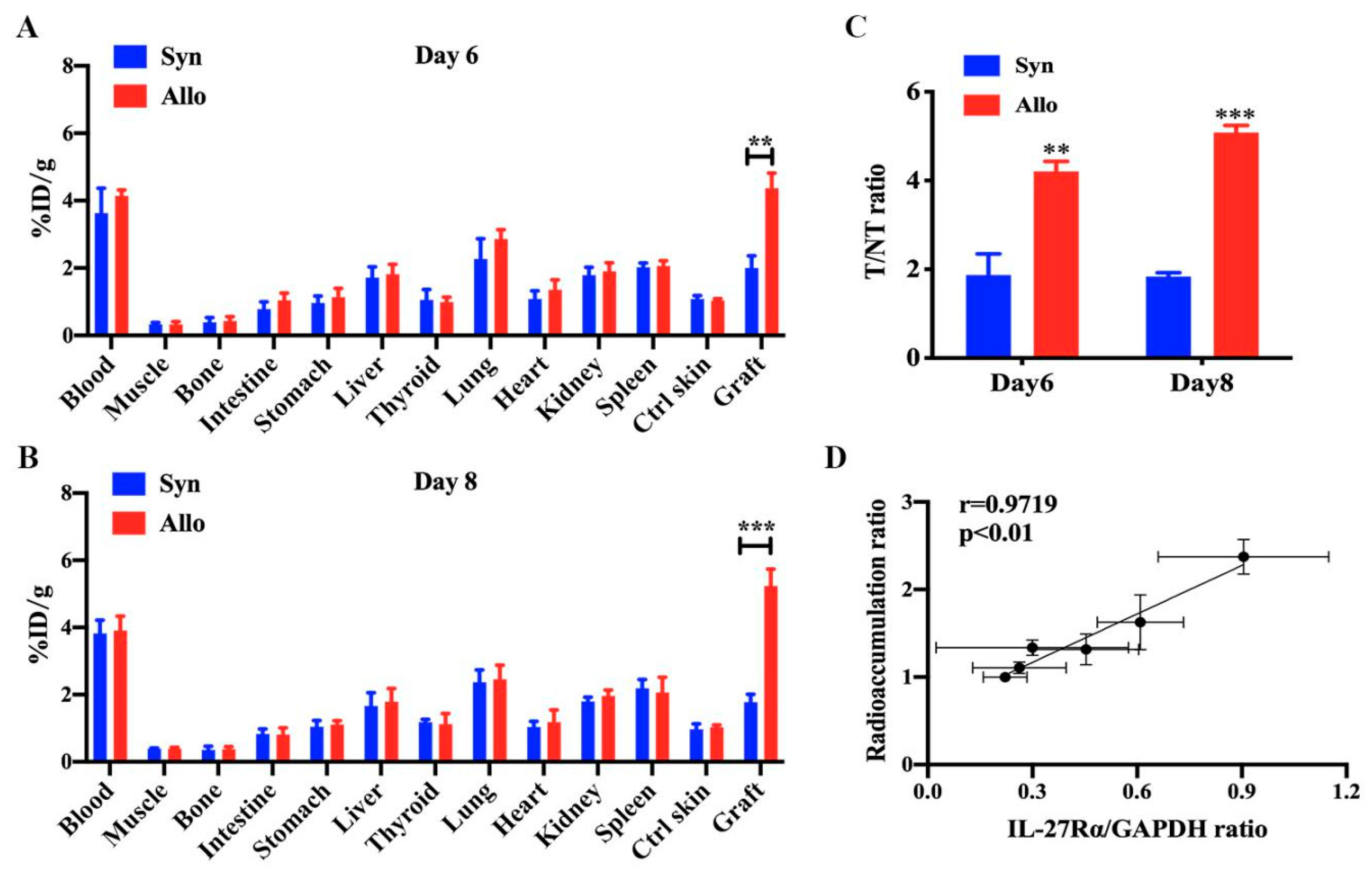
2.4. Ex Vivo Biodistribution of 125I-anti-IL-27Rα mAb/125I-IgG
2.5. 125I-Anti-IL-27Rα mAb Specifically Accumulated in Allogeneic Grafts in the Early Stage of Allorejection
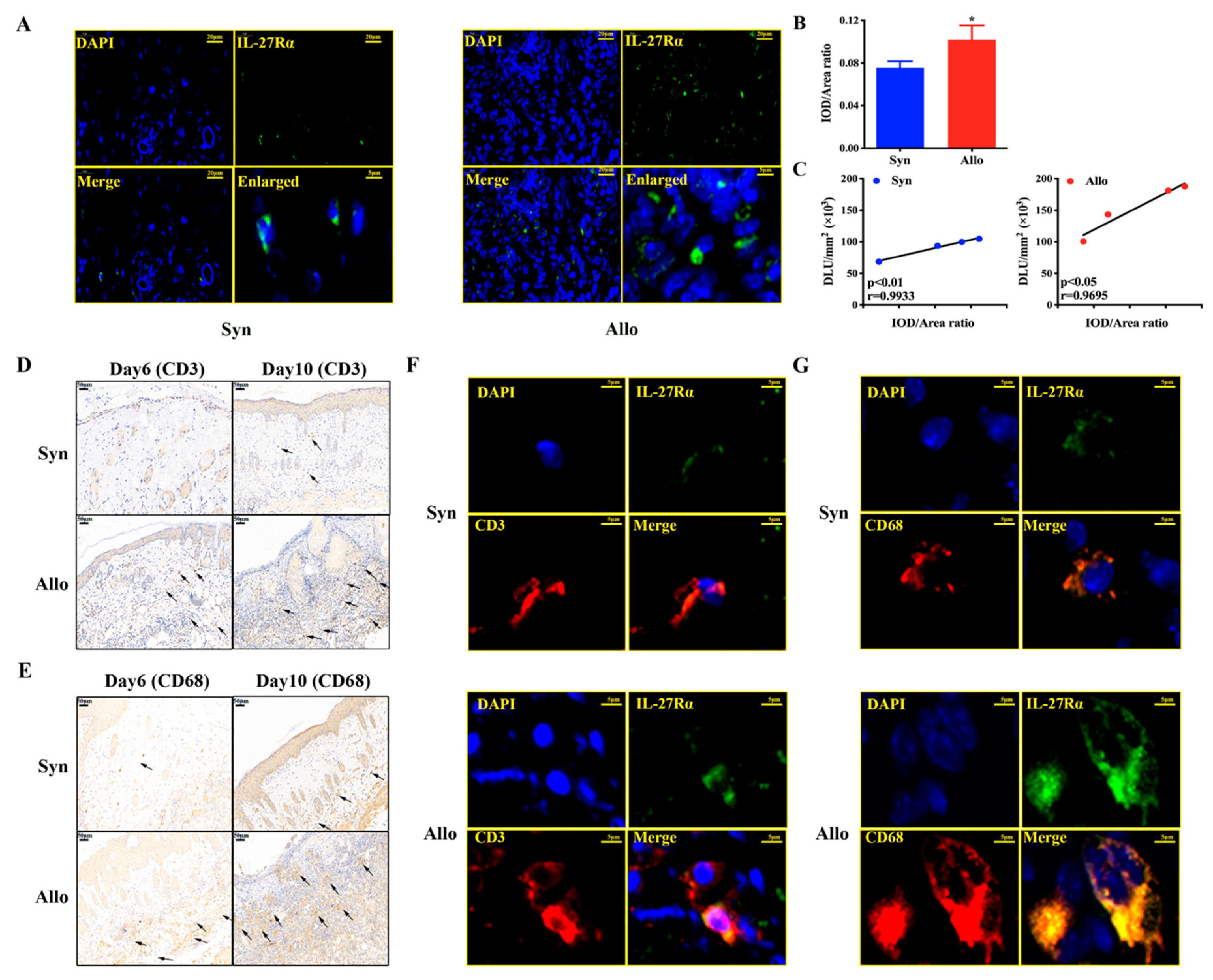
2.6. 125I-anti-IL-27Rα mAb Targeted on Graft-Infiltrated CD3+/CD68+ IL-27Rα Positive Cells Specifically
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Western Blot
4.3. RT-PCR
4.4. Preparation of 125I-anti-IL-27Rα mAb/ 125I-IgG
4.5. Binding Assay of 125I-anti-IL-27Rα mAb
4.6. Dynamic Phosphor-Autoradiography and Biodistribution Assay
4.7. Ex Vivo Biodistribution Study
4.8. H & E (Hematoxylin–Eosin) Staining
4.9. Immumohistochemical Staining and Immunofluorescence Staining
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | acute rejection |
| Treg cell | regulatory T cell |
| CT | computed Tomography |
| MRI | magnetic resonance imaging |
| 18F-FDG | 18F-labeled -fluorodeoxyglucose |
| GVHD | graft-versus-host disease |
| ARG | autoradiography |
| miRNA | microRNA |
| lncRNA | long non-coding RNA |
| dd-cfDNA | donor-derived cell-free DNA |
| ACR | acute renal allogeneic graft rejection |
| SERCA2a | sarcoplasmic reticulum Ca2+-ATPase |
| FEV1 | forced expiratory volume in one second |
| sCr | serum creatinine |
| ABMR HP SPF | antibody-mediated rejection Hyperpolarized Specific Pathogen Free |
| RIPA | Radio-Immuno-Precipitation-Assay |
| PMSF | Phenylmethanesulfonyl fluoride |
| Bmax | maximum binding ability |
| Kd | dissociation constant |
| DLU/mm2 | Digital Light Units per square millimeter |
| %ID/g T/NT H & E | the percentage injected dose per gram target/non target Hematoxylin–Eosin |
References
- Grenda, R.; Kaliciński, P. Combined and sequential liver-kidney transplantation in children. Pediatr. Nephrol. 2018, 33, 2227–2237. [Google Scholar] [CrossRef]
- Jalalzadeh, M.; Mousavinasab, N.; Peyrovi, S.; Ghadiani, M.H. The impact of acute rejection in kidney transplantation on long-term allograft and patient outcome. Nephrourol. Mon. 2015, 7, e24439. [Google Scholar] [CrossRef]
- Tsai, M.-K.; Wu, F.-L.L.; Lai, l.-R.U.E.; Lee, C.-Y.; Hu, R.-H.; Lee, P.-H. Decreased Acute Rejection and Improved Renal Allograft Survival Using Sirolimus and Low-Dose Calcineurin Inhibitors without Induction Therapy. Int. J. Artif. Organs 2009, 32, 371–380. [Google Scholar] [CrossRef]
- Halloran, P.F.; Famulski, K.; Reeve, J. The molecular phenotypes of rejection in kidney transplant biopsies. Curr. Opin. Organ Transplant. 2015, 20, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Roden, A.; Aisner, D.; Allen, T.; Aubry, M.; Barrios, R.; Beasley, M.; Cagle, P.; Capelozzi, V.; Dacic, S.; Ge, Y.; et al. Diagnosis of Acute Cellular Rejection and Antibody-Mediated Rejection on Lung Transplant Biopsies: A Perspective from Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2016, 141. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.A.; Turka, L.A. Tolerance signatures in transplant recipients. Curr. Opin. Organ Transplant. 2015, 20, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sadowski, E.A.; Artz, N.S.; Seo, S.; Djamali, A.; Grist, T.M.; Fain, S.B. Measurement and comparison of T1 relaxation times in native and transplanted kidney cortex and medulla. J. Magn. Reson. Imaging 2011, 33, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Köhnke, R.; Kentrup, D.; Schütte-Nütgen, K.; Schäfers, M.; Schnöckel, U.; Hoerr, V.; Reuter, S. Update on imaging-based diagnosis of acute renal allograft rejection. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 110–126. [Google Scholar] [PubMed]
- Benzimra, M.; Calligaro, G.L.; Glanville, A.R. Acute rejection. J. Thorac. Dis. 2017, 9, 5440–5457. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Sun, C.; Sun, L.; Feng, C.; Yang, F.; Xu, Y.; Zhao, Y. Primed macrophages directly and specifically reject allografts. Cell. Mol. Immunol. 2019. [Google Scholar] [CrossRef]
- Ayasoufi, K.; Zwick, D.B.; Fan, R.; Hasgur, S.; Nicosia, M.; Gorbacheva, V.; Keslar, K.S.; Min, B.; Fairchild, R.L.; Valujskikh, A. Interleukin-27 promotes CD8+ T cell reconstitution following antibody-mediated lymphoablation. JCI Insight 2019, 4, e125489. [Google Scholar] [CrossRef]
- Fink, A.F.; Ciliberti, G.; Popp, R.; Sirait-Fischer, E.; Frank, A.-C.; Fleming, I.; Sekar, D.; Weigert, A.; Brüne, B. IL27Rα Deficiency Alters Endothelial Cell Function and Subverts Tumor Angiogenesis in Mammary Carcinoma. Front. Oncol. 2019, 9, 1022. [Google Scholar] [CrossRef]
- Qiu, S.-L.; Duan, M.-C.; Liang, Y.; Tang, H.-J.; Liu, G.-N.; Zhang, L.-M.; Yang, C.-M. Cigarette Smoke Induction of Interleukin-27/WSX-1 Regulates the Differentiation of Th1 and Th17 Cells in a Smoking Mouse Model of Emphysema. Front. Immunol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Petes, C.; Mintsopoulos, V.; Finnen, R.L.; Banfield, B.W.; Gee, K. The effects of CD14 and IL-27 on induction of endotoxin tolerance in human monocytes and macrophages. J. Biol. Chem. 2018, 293, 17631–17645. [Google Scholar] [CrossRef]
- Belle, L.; Agle, K.; Zhou, V.; Yin-Yuan, C.; Komorowski, R.; Eastwood, D.; Logan, B.; Sun, J.; Ghilardi, N.; Cua, D.; et al. Blockade of interleukin-27 signaling reduces GVHD in mice by augmenting Treg reconstitution and stabilizing Foxp3 expression. Blood 2016, 128, 2068–2082. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Zhang, C.; Song, J.; Liang, T.; Jin, W.; Kim, Y.C.; Wang, S.M.; Hou, G. Alternatively Expressed Genes Identified in the CD4+ T Cells of Allograft Rejection Mice. Cell Transplant. 2011, 20, 333–350. [Google Scholar] [CrossRef]
- Christakoudi, S.; Runglall, M.; Mobillo, P.; Tsui, T.-L.; Duff, C.; Domingo-Vila, C.; Kamra, Y.; Delaney, F.; Montero, R.; Spiridou, A.; et al. Development of a multivariable gene-expression signature targeting T-cell-mediated rejection in peripheral blood of kidney transplant recipients validated in cross-sectional and longitudinal samples. EBioMedicine 2019, 41, 571–583. [Google Scholar] [CrossRef]
- Angeletti, A. Looking into the Graft without a Biopsy: Biomarkers of Acute Rejection in Renal Transplantation. Contrib. Nephrol. 2017, 190, 181–193. [Google Scholar] [CrossRef]
- Xu, J.; Hu, J.; Xu, H.; Zhou, H.; Liu, Z.; Zhou, Y.; Liu, R.; Zhang, W. Long Non-coding RNA Expression Profiling in Biopsy to Identify Renal Allograft at Risk of Chronic Damage and Future Graft Loss. Appl. Biochem. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Hamdorf, M.; Kawakita, S.; Everly, M. The Potential of MicroRNAs as Novel Biomarkers for Transplant Rejection. J. Immunol. Res. 2017, 2017, 4072364. [Google Scholar] [CrossRef]
- Lemerle, M.; Garnier, A.-S.; Planchais, M.; Brilland, B.; Subra, J.-F.; Blanchet, O.; Blanchard, S.; Croue, A.; Duveau, A.; Augusto, J.-F. CD45RC Expression of Circulating CD8(+) T Cells Predicts Acute Allograft Rejection: A Cohort Study of 128 Kidney Transplant Patients. J. Clin. Med. 2019, 8, 1147. [Google Scholar] [CrossRef]
- Xu, X.; Han, Y.; Huang, H.; Bi, L.; Kong, X.; Ma, X.; Shi, B.; Xiao, L. Circulating NK cell subsets and NKT-like cells in renal transplant recipients with acute T-cell-mediated renal allograft rejection. Mol. Med. Rep. 2019, 19, 4238–4248. [Google Scholar] [CrossRef] [PubMed]
- Tarazón, E.; Ortega, A.; Gil, C.; Sánchez-Lacuesta, E.; Marín, P.; Lago, F.; González-Juanatey, J.; Martinez-Dolz, L.; Portoles, M.; Rivera, M.; et al. SERCA2a: A Potential Noninvasive Biomarker of Cardiac Allograft Rejection. J. Heart Lung Transplant. 2017, 36. [Google Scholar] [CrossRef] [PubMed]
- Barbas, A.S.; Lin, L.; McRae, M.; MacDonald, A.L.; Truong, T.; Yang, Y.; Brennan, T.V. Heparan sulfate is a plasma biomarker of acute cellular allograft rejection. PLoS ONE 2018, 13, e0200877. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C. Recent advances in lung transplantation. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Shirakawa, H.; Imaizumi, Y.; Ogawa, H.; Yoshikawa, K.; Kono, M.; Saito, T.; Ishiwatari, A.; Sano, N.; Kawanishi, T.; et al. Preemptive Living Donor Kidney Transplantation and Kidney Function at the Initial Hospital Visit: A Single-Center Case-Control Study. Transplant. Proc. 2016, 48, 827–830. [Google Scholar] [CrossRef]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef]
- Schütz, E.; Fischer, A.; Beck, J.; Harden, M.; Koch, M.; Wuensch, T.; Stockmann, M.; Nashan, B.; Kollmar, O.; Matthaei, J.; et al. Graft-derived cell-free DNA, a noninvasive early rejection and graft damage marker in liver transplantation: A prospective, observational, multicenter cohort study. PLoS Med. 2017, 14. [Google Scholar] [CrossRef]
- Liao, T.; Zhang, Y.; Ren, J.; Zheng, H.; Zhang, H.; Li, X.; Liu, X.; Yin, T.; Sun, Q. Noninvasive quantification of intrarenal allograft C4d deposition with targeted ultrasound imaging. Am. J. Transplant. 2019, 19, 259–268. [Google Scholar] [CrossRef]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transplant. 2018, 18, 293–307. [Google Scholar] [CrossRef]
- Siddiqui, S.; Habertheuer, A.; Xin, Y.; Pourfathi, M.; Tao, J.Q.; Hamedani, H.; Kadlecek, S.; Duncan, I.; Vallabhajosyula, P.; Naji, A.; et al. Detection of lung transplant rejection in a rat model using hyperpolarized [1-13C] pyruvate-based metabolic imaging. NMR Biomed. 2019, 32, e4107. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Kreisel, D.; Fullerton, J.; Gilroy, D.; Goldstein, D. Inflammatory triggers of acute rejection of organ allografts. Immunol. Rev. 2014, 258, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Siu, J.H.Y.; Surendrakumar, V.; Richards, J.A.; Pettigrew, G.J. T cell Allorecognition Pathways in Solid Organ Transplantation. Front. Immunol. 2018, 9, 2548. [Google Scholar] [CrossRef] [PubMed]
- Panzer, S.E.; Wilson, N.A.; Verhoven, B.M.; Xiang, D.; Rubinstein, C.D.; Redfield, R.R.; Zhong, W.; Reese, S.R. Complete B Cell Deficiency Reduces Allograft Inflammation and Intragraft Macrophages in a Rat Kidney Transplant Model. Transplantation 2018, 102, 396–405. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Fujio, K.; Okamura, T.; Yamamoto, K. Interleukin-27 in T cell immunity. Int. J. Mol. Sci. 2015, 16, 2851–2863. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Roberts, L.L.; Robinson, C.M. The presence of interleukin-27 during monocyte-derived dendritic cell differentiation promotes improved antigen processing and stimulation of T cells. Immunology 2015, 144, 649–660. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Jang, E.; Le, H.T.; Kim, S.; Kim, D.; Dvorina, N.; Aronica, M.A.; Baldwin, W.M., 3rd; Asosingh, K.; Comhair, S.; et al. IL-27 targets Foxp3+ Tregs to mediate antiinflammatory functions during experimental allergic airway inflammation. JCI Insight 2019, 4, e123216. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Y.-Z.; Feng, X.-Y.; Fan, T.-T.; Jiang, L.; Guo, R.; Liu, Q. Interleukin-27 is elevated in sepsis-induced myocardial dysfunction and mediates inflammation. Cytokine 2016, 88, 1–11. [Google Scholar] [CrossRef]
- Peshkova, I.O.; Aghayev, T.; Fatkhullina, A.R.; Makhov, P.; Titerina, E.K.; Eguchi, S.; Tan, Y.F.; Kossenkov, A.V.; Khoreva, M.V.; Gankovskaya, L.V.; et al. IL-27 receptor-regulated stress myelopoiesis drives abdominal aortic aneurysm development. Nat Commun 2019, 10, 5046. [Google Scholar] [CrossRef]
- Salcido-Ochoa, F.; Hue, S.S.-S.; Peng, S.; Fan, Z.; Li, R.L.; Iqbal, J.; Allen, J.C., Jr.; Loh, A.H.L. Histopathological analysis of infiltrating T cell subsets in acute T cell-mediated rejection in the kidney transplant. World J. Transplant. 2017, 7, 222–234. [Google Scholar] [CrossRef]
- Dai, Y.; Cheng, X.; Yu, J.; Chen, X.; Xiao, Y.; Tang, F.; Li, Y.; Wan, S.; Su, W.; Liang, D. Hemin Promotes Corneal Allograft Survival Through the Suppression of Macrophage Recruitment and Activation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3952–3962. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Chen, S.; Zheng, H.; Huang, S.; Lu, F. Increased IL-27/IL-27R expression in association with the immunopathology of murine ocular toxoplasmosis. Parasitol. Res. 2018, 117, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.S.; Alegre, M.-L. The impact of infection and tissue damage in solid-organ transplantation. Nat. Rev. Immunol. 2012, 12, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhang, C.; Liang, T.; Song, J.; Hou, G. rFliC prolongs allograft survival in association with the activation of recipient Tregs in a TLR5-dependent manner. Cell. Mol. Immunol. 2014, 11, 206–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, D.; Liu, W.; Zhao, S.; Zhang, C.; Liang, T.; Hou, G. TLR5 is a new reporter for triple-negative breast cancer indicated by radioimmunoimaging and fluorescent staining. J. Cell. Mol. Med. 2019, 23, 8305–8313. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.; Zhang, Y.; Gu, Q.; Huang, F.; Zheng, W.; Li, Z. Gestational zinc deficiency impairs humoral and cellular immune responses to hepatitis B vaccination in offspring mice. PLoS ONE 2013, 8, e73461. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Shi, D.; Su, C.; Jiang, W.; Zhang, C.; Liang, T.; Hou, G. IL-27Rα: A Novel Molecular Imaging Marker for Allograft Rejection. Int. J. Mol. Sci. 2020, 21, 1315. https://doi.org/10.3390/ijms21041315
Zhao S, Shi D, Su C, Jiang W, Zhang C, Liang T, Hou G. IL-27Rα: A Novel Molecular Imaging Marker for Allograft Rejection. International Journal of Molecular Sciences. 2020; 21(4):1315. https://doi.org/10.3390/ijms21041315
Chicago/Turabian StyleZhao, Shanshan, Dai Shi, Chen Su, Wen Jiang, Chao Zhang, Ting Liang, and Guihua Hou. 2020. "IL-27Rα: A Novel Molecular Imaging Marker for Allograft Rejection" International Journal of Molecular Sciences 21, no. 4: 1315. https://doi.org/10.3390/ijms21041315
APA StyleZhao, S., Shi, D., Su, C., Jiang, W., Zhang, C., Liang, T., & Hou, G. (2020). IL-27Rα: A Novel Molecular Imaging Marker for Allograft Rejection. International Journal of Molecular Sciences, 21(4), 1315. https://doi.org/10.3390/ijms21041315



