Immunomonitoring of Tacrolimus in Healthy Volunteers: The First Step from PK- to PD-Based Therapeutic Drug Monitoring?
Abstract
1. Introduction
2. Results
2.1. Subject Characteristics
2.2. Whole Blood and Intracellular Pharmacokinetics
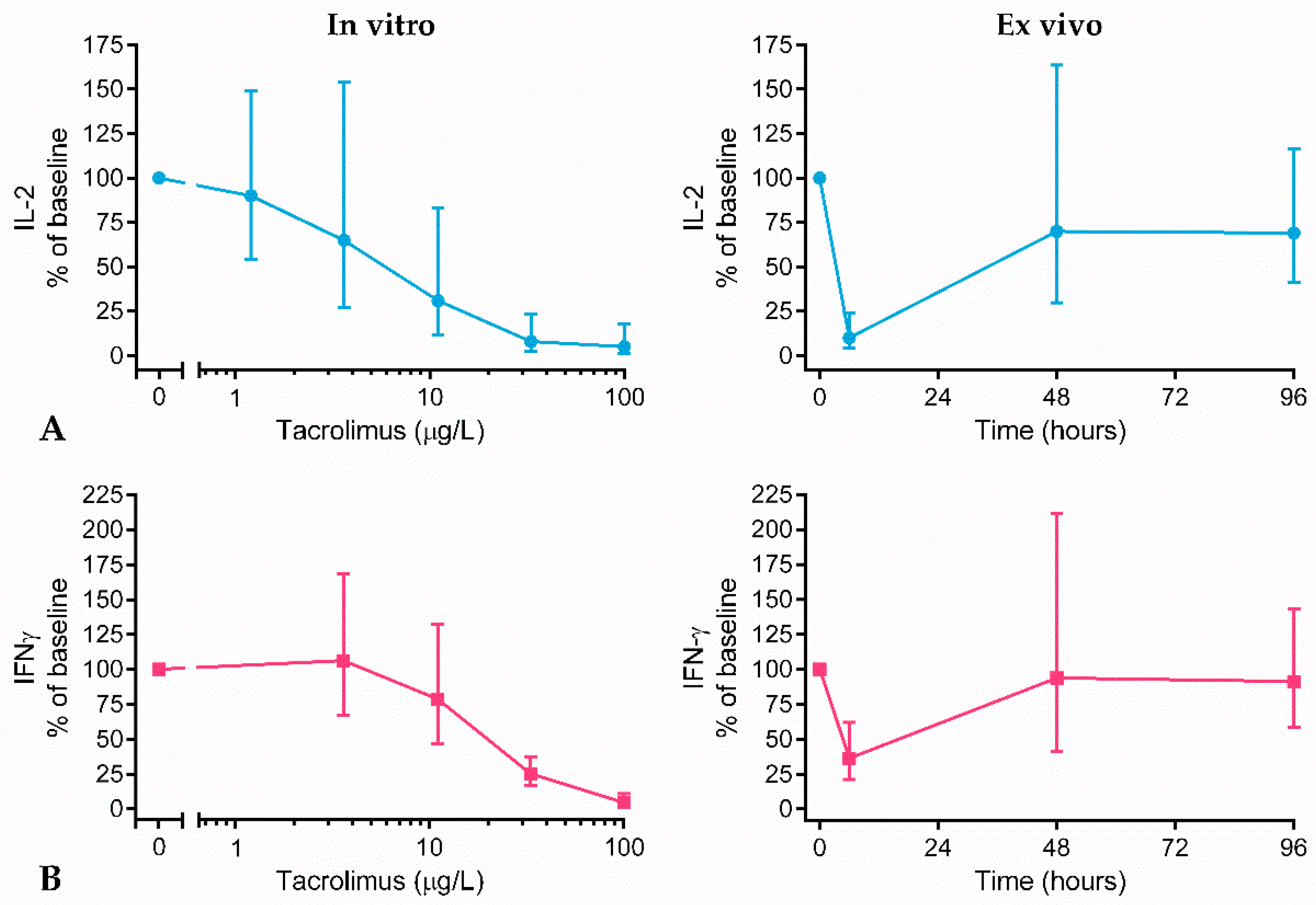
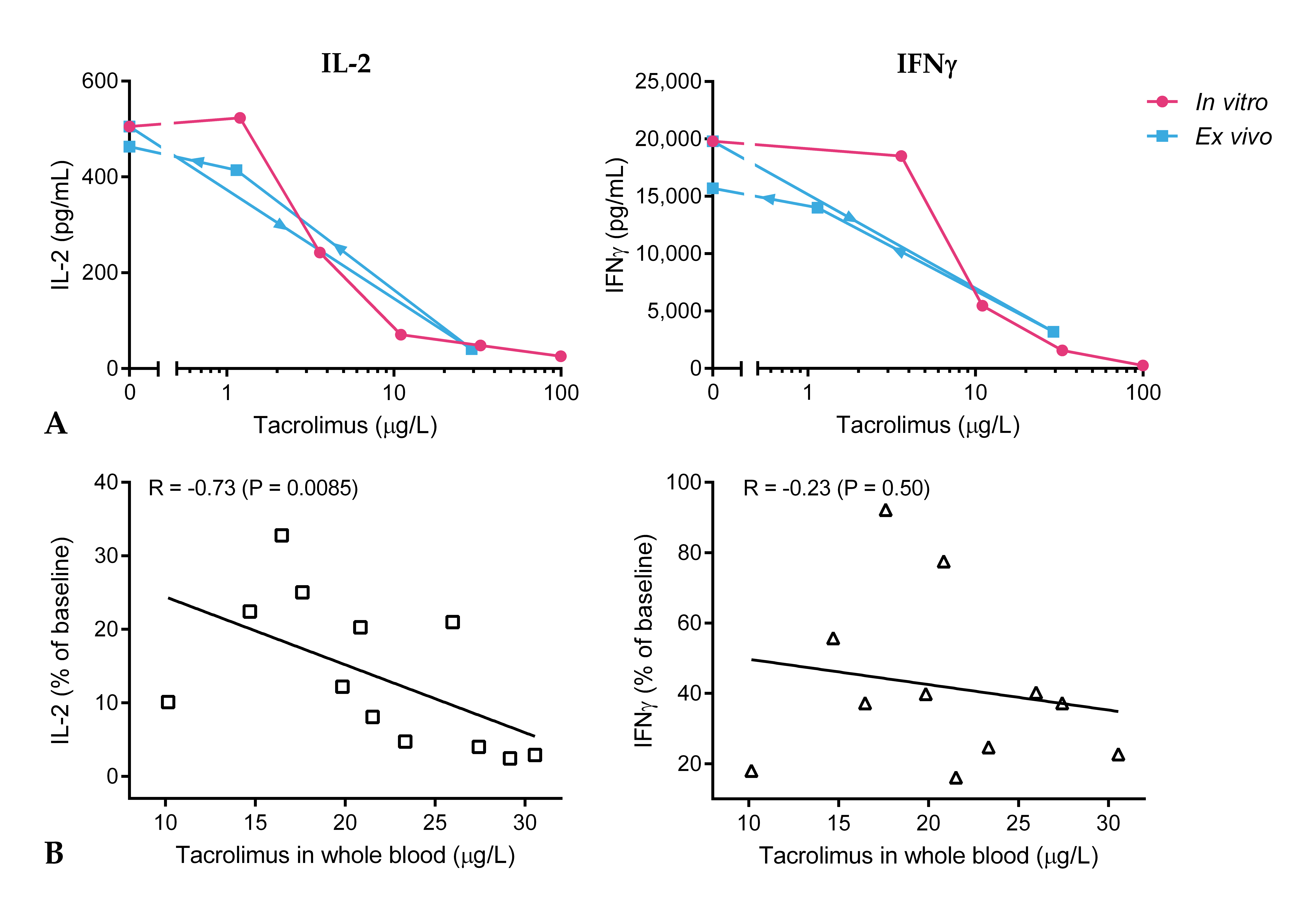
2.3. Cytokine Production
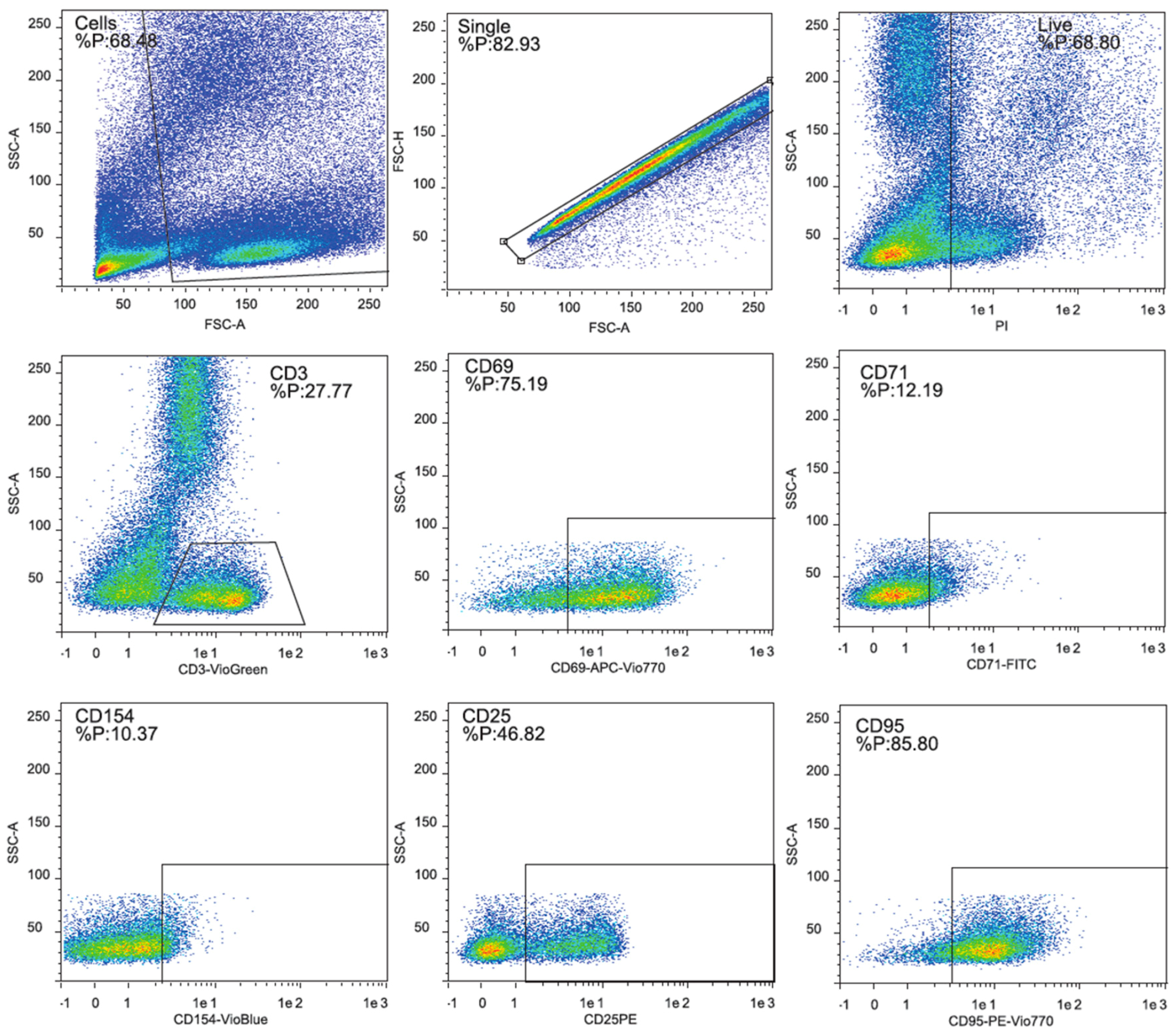
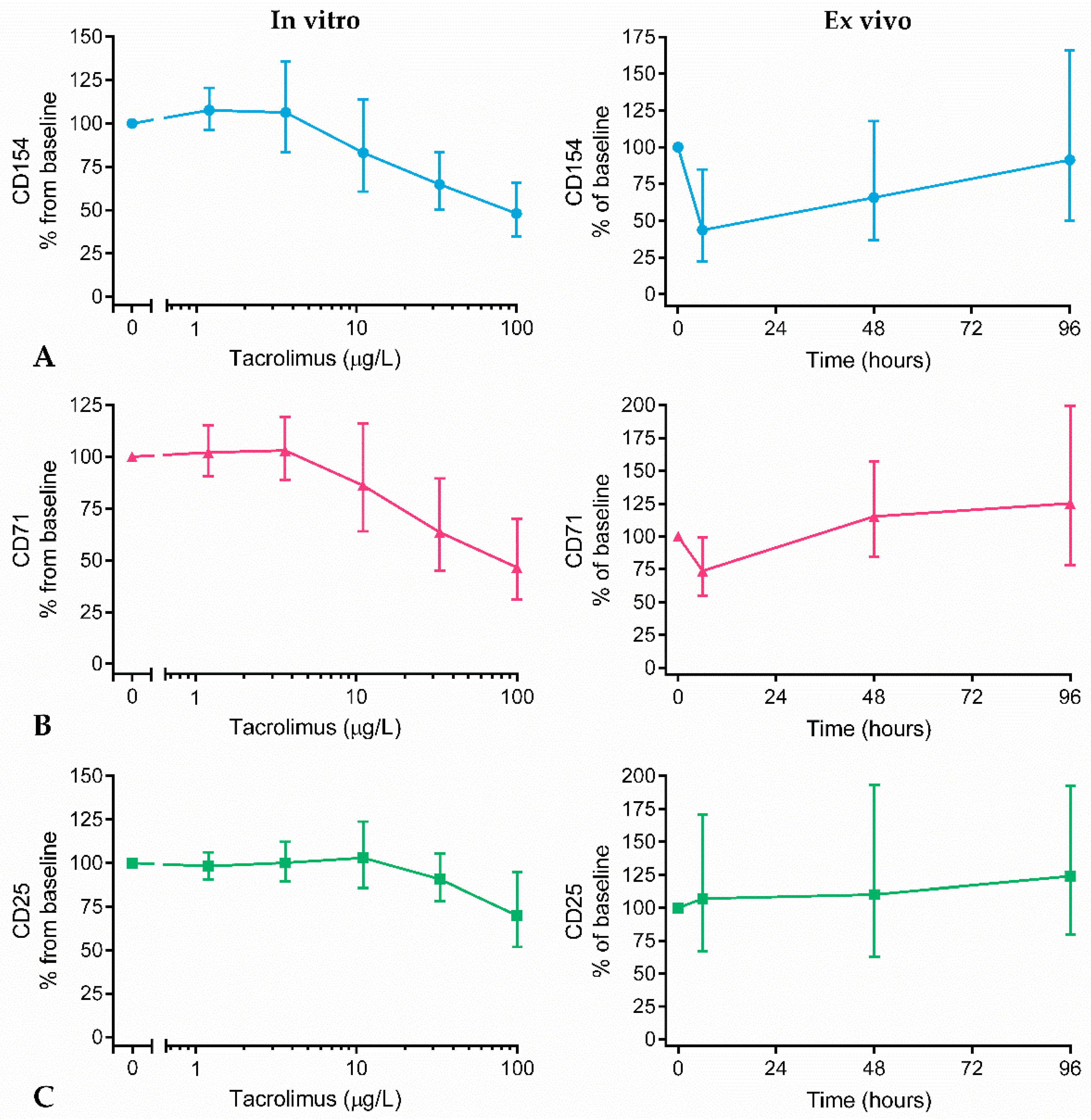
2.4. Surface Marker Expression
2.5. Calcineurin Activity
3. Discussion
4. Materials and Methods
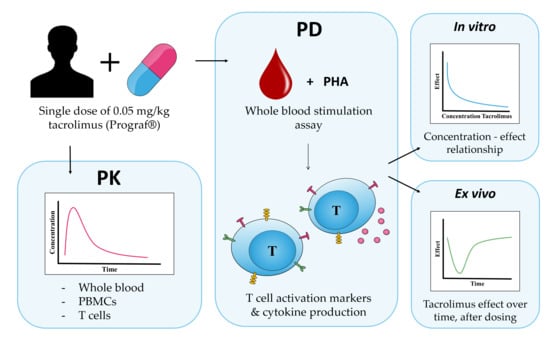
4.1. Study Design
4.2. Whole Blood and Intracellular PK
4.3. In Vitro and Ex Vivo Whole Blood Culture
4.4. Cytokine Production
4.5. Surface Marker Expression
4.6. Calcineurin Activity
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CNI | Calcineurin inhibitor |
| C0 | Pre-dose trough concentration |
| FK506 | Tacrolimus |
| FKBP | FK506 binding protein |
| IC50 | Half maximal inhibitory concentration |
| IFNγ | Interferon gamma |
| IL-2 | Interleukin 2 |
| MMF | Mycophenolate mofetil |
| NFAT | Nuclear factor of activated T cells |
| PBMCs | Peripheral blood mononuclear cells |
| PD | Pharmacodynamics |
| PHA | Phytohemagglutinin |
| PI | Propidium iodide |
| PK | Pharmacokinetics |
| SD | Standard deviation |
| TDM | Therapeutic drug monitoring |
| WB | Whole blood |
Appendix A
References
- Coemans, M.; Susal, C.; Dohler, B.; Anglicheau, D.; Giral, M.; Bestard, O.; Legendre, C.; Emonds, M.P.; Kuypers, D.; Molenberghs, G.; et al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int. 2018, 94, 964–973. [Google Scholar] [CrossRef]
- Gondos, A.; Dohler, B.; Brenner, H.; Opelz, G. Kidney graft survival in Europe and the United States: Strikingly different long-term outcomes. Transplantation 2013, 95, 267–274. [Google Scholar] [CrossRef]
- Shuker, N.; van Gelder, T.; Hesselink, D.A. Intra-patient variability in tacrolimus exposure: Causes, consequences for clinical management. Transplant. Rev. (Orlando) 2015, 29, 78–84. [Google Scholar] [CrossRef]
- Andrews, L.M.; Li, Y.; De Winter, B.C.M.; Shi, Y.Y.; Baan, C.C.; van Gelder, T.; Hesselink, D.A. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1225–1236. [Google Scholar] [CrossRef]
- Bouamar, R.; Shuker, N.; Hesselink, D.A.; Weimar, W.; Ekberg, H.; Kaplan, B.; Bernasconi, C.; van Gelder, T. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: A pooled analysis from three randomized-controlled clinical trials(dagger). Am. J. Transplant. 2013, 13, 1253–1261. [Google Scholar] [CrossRef]
- Klaasen, R.A.; Bergan, S.; Bremer, S.; Daleq, L.; Andersen, A.M.; Midtvedt, K.; Skauby, M.H.; Vethe, N.T. Longitudinal Study of Tacrolimus in Lymphocytes During the First Year After Kidney Transplantation. Ther. Drug Monit. 2018, 40, 558–566. [Google Scholar] [CrossRef]
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of action of tacrolimus (FK506): Molecular and cellular mechanisms. Ther. Drug Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef]
- Noceti, O.M.; Woillard, J.B.; Boumediene, A.; Esperon, P.; Taupin, J.L.; Gerona, S.; Valverde, M.; Tourino, C.; Marquet, P. Tacrolimus pharmacodynamics and pharmacogenetics along the calcineurin pathway in human lymphocytes. Clin. Chem. 2014, 60, 1336–1345. [Google Scholar] [CrossRef]
- Almawi, W.Y.; Assi, J.W.; Chudzik, D.M.; Jaoude, M.M.; Rieder, M.J. Inhibition of cytokine production and cytokine-stimulated T-cell activation by FK506 (tacrolimus)1. Cell Transplant. 2001, 10, 615–623. [Google Scholar] [CrossRef]
- Hartel, C.; Schumacher, N.; Fricke, L.; Ebel, B.; Kirchner, H.; Muller-Steinhardt, M. Sensitivity of whole-blood T lymphocytes in individual patients to tacrolimus (FK 506): Impact of interleukin-2 mRNA expression as surrogate measure of immunosuppressive effect. Clin. Chem. 2004, 50, 141–151. [Google Scholar] [CrossRef]
- Kannegieter, N.M.; Hesselink, D.A.; Dieterich, M.; de Graav, G.N.; Kraaijeveld, R.; Baan, C.C. Analysis of NFATc1 amplification in T cells for pharmacodynamic monitoring of tacrolimus in kidney transplant recipients. PLoS ONE 2018, 13, e0201113. [Google Scholar] [CrossRef]
- Keller, F.; Sommerer, C.; Giese, T.; Zeier, M.; Schroppel, B. Correlation between pharmacokinetics of tacrolimus and pharmacodynamics on NFAT-regulated gene expression in stable kidney transplant recipients. Clin. Nephrol. 2017, 87, 93–99. [Google Scholar] [CrossRef]
- Nowell, P.C. Phytohemagglutinin: An initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960, 20, 462–466. [Google Scholar]
- Sellar, K.J.; van Rossum, H.H.; Romijn, F.P.; Smit, N.P.; de Fijter, J.W.; van Pelt, J. Spectrophotometric assay for calcineurin activity in leukocytes isolated from human blood. Anal. Biochem. 2006, 358, 104–110. [Google Scholar] [CrossRef]
- Wallemacq, P.; Armstrong, V.W.; Brunet, M.; Haufroid, V.; Holt, D.W.; Johnston, A.; Kuypers, D.; Le Meur, Y.; Marquet, P.; Oellerich, M.; et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: Report of the European consensus conference. Ther. Drug Monit. 2009, 31, 139–152. [Google Scholar] [CrossRef]
- Hebert, M.F.; Park, J.M.; Chen, Y.L.; Akhtar, S.; Larson, A.M. Effects of St. John’s wort (Hypericum perforatum) on tacrolimus pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 2004, 44, 89–94. [Google Scholar]
- Autissier, P.; Soulas, C.; Burdo, T.H.; Williams, K.C. Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytometry A. 2010, 77, 410–419. [Google Scholar] [CrossRef]
- Laskin, B.L.; Jiao, J.; Baluarte, H.J.; Amaral, S.; Furth, S.L.; Akimova, T.; Hancock, W.W.; Levine, M.H.; Reese, P.P.; Beier, U.H. The Effects of Tacrolimus on T-Cell Proliferation Are Short-Lived: A Pilot Analysis of Immune Function Testing. Transplant. Direct 2017, 3, e199. [Google Scholar] [CrossRef]
- Bahmany, S.; de Wit, L.E.A.; Hesselink, D.A.; van Gelder, T.; Shuker, N.M.; Baan, C.; van der Nagel, B.C.H.; Koch, B.C.P.; de Winter, B.C.M. Highly sensitive and rapid determination of tacrolimus in peripheral blood mononuclear cells by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4416. [Google Scholar] [CrossRef]
- Han, S.S.; Yang, S.H.; Kim, M.C.; Cho, J.Y.; Min, S.I.; Lee, J.P.; Kim, D.K.; Ha, J.; Kim, Y.S. Monitoring the Intracellular Tacrolimus Concentration in Kidney Transplant Recipients with Stable Graft Function. PLoS ONE 2016, 11, e0153491. [Google Scholar] [CrossRef]
- Lemaitre, F.; Blanchet, B.; Latournerie, M.; Antignac, M.; Houssel-Debry, P.; Verdier, M.C.; Dermu, M.; Camus, C.; Le Priol, J.; Roussel, M.; et al. Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: Inside the white blood cells. Clin. Biochem. 2015, 48, 406–411. [Google Scholar] [CrossRef]
- Capron, A.; Lerut, J.; Latinne, D.; Rahier, J.; Haufroid, V.; Wallemacq, P. Correlation of tacrolimus levels in peripheral blood mononuclear cells with histological staging of rejection after liver transplantation: Preliminary results of a prospective study. Transplant. Int. 2012, 25, 41–47. [Google Scholar] [CrossRef]
- Wieland, E.; Shipkova, M. Lymphocyte surface molecules as immune activation biomarkers. Clin. Biochem. 2016, 49, 347–354. [Google Scholar] [CrossRef]
- Deng, M.C.; Erren, M.; Roeder, N.; Dreimann, V.; Gunther, F.; Kerber, S.; Baba, H.A.; Schmidt, C.; Breithardt, G.; Scheld, H.H. T-cell and monocyte subsets, inflammatory molecules, rejection, and hemodynamics early after cardiac transplantation. Transplantation 1998, 65, 1255–1261. [Google Scholar] [CrossRef]
- Lun, A.; Cho, M.Y.; Muller, C.; Staffa, G.; Bechstein, W.O.; Radke, C.; Neuhaus, P.; Renz, H. Diagnostic value of peripheral blood T-cell activation and soluble IL-2 receptor for acute rejection in liver transplantation. Clin. Chim. Acta 2002, 320, 69–78. [Google Scholar] [CrossRef]
- Ashokkumar, C.; Shapiro, R.; Tan, H.; Ningappa, M.; Elinoff, B.; Fedorek, S.; Sun, Q.; Higgs, B.W.; Randhawa, P.; Humar, A.; et al. Allospecific CD154+ T-cytotoxic memory cells identify recipients experiencing acute cellular rejection after renal transplantation. Transplantation 2011, 92, 433–438. [Google Scholar] [CrossRef]
- Antignac, M.; Hulot, J.S.; Boleslawski, E.; Hannoun, L.; Touitou, Y.; Farinotti, R.; Lechat, P.; Urien, S. Population pharmacokinetics of tacrolimus in full liver transplant patients: Modelling of the post-operative clearance. Eur. J. Clin. Pharmacol. 2005, 61, 409–416. [Google Scholar] [CrossRef]
- Bergmann, T.K.; Hennig, S.; Barraclough, K.A.; Isbel, N.M.; Staatz, C.E. Population pharmacokinetics of tacrolimus in adult kidney transplant patients: Impact of CYP3A5 genotype on starting dose. Ther. Drug Monit. 2014, 36, 62–70. [Google Scholar] [CrossRef]
- Andreu, F.; Colom, H.; Grinyo, J.M.; Torras, J.; Cruzado, J.M.; Lloberas, N. Development of a population PK model of tacrolimus for adaptive dosage control in stable kidney transplant patients. Ther. Drug Monit. 2015, 37, 246–255. [Google Scholar] [CrossRef]
- Staatz, C.E.; Willis, C.; Taylor, P.J.; Tett, S.E. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin. Pharmacol. Ther. 2002, 72, 660–669. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
in ‘t Veld, A.E.; Grievink, H.W.; Saghari, M.; Stuurman, F.E.; de Kam, M.L.; de Vries, A.P.J.; de Winter, B.C.M.; Burggraaf, J.; Cohen, A.F.; Moerland, M. Immunomonitoring of Tacrolimus in Healthy Volunteers: The First Step from PK- to PD-Based Therapeutic Drug Monitoring? Int. J. Mol. Sci. 2019, 20, 4710. https://doi.org/10.3390/ijms20194710
in ‘t Veld AE, Grievink HW, Saghari M, Stuurman FE, de Kam ML, de Vries APJ, de Winter BCM, Burggraaf J, Cohen AF, Moerland M. Immunomonitoring of Tacrolimus in Healthy Volunteers: The First Step from PK- to PD-Based Therapeutic Drug Monitoring? International Journal of Molecular Sciences. 2019; 20(19):4710. https://doi.org/10.3390/ijms20194710
Chicago/Turabian Stylein ‘t Veld, Aliede E., Hendrika W. Grievink, Mahdi Saghari, Frederik E. Stuurman, Marieke L. de Kam, Aiko P. J. de Vries, Brenda C. M. de Winter, Jacobus Burggraaf, Adam F. Cohen, and Matthijs Moerland. 2019. "Immunomonitoring of Tacrolimus in Healthy Volunteers: The First Step from PK- to PD-Based Therapeutic Drug Monitoring?" International Journal of Molecular Sciences 20, no. 19: 4710. https://doi.org/10.3390/ijms20194710
APA Stylein ‘t Veld, A. E., Grievink, H. W., Saghari, M., Stuurman, F. E., de Kam, M. L., de Vries, A. P. J., de Winter, B. C. M., Burggraaf, J., Cohen, A. F., & Moerland, M. (2019). Immunomonitoring of Tacrolimus in Healthy Volunteers: The First Step from PK- to PD-Based Therapeutic Drug Monitoring? International Journal of Molecular Sciences, 20(19), 4710. https://doi.org/10.3390/ijms20194710