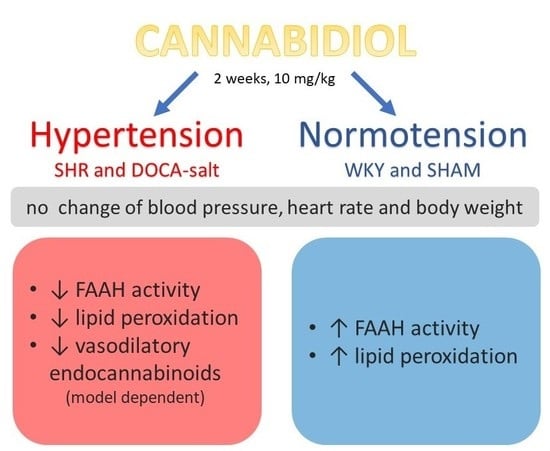
Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism
Abstract
1. Introduction
2. Results
2.1. General
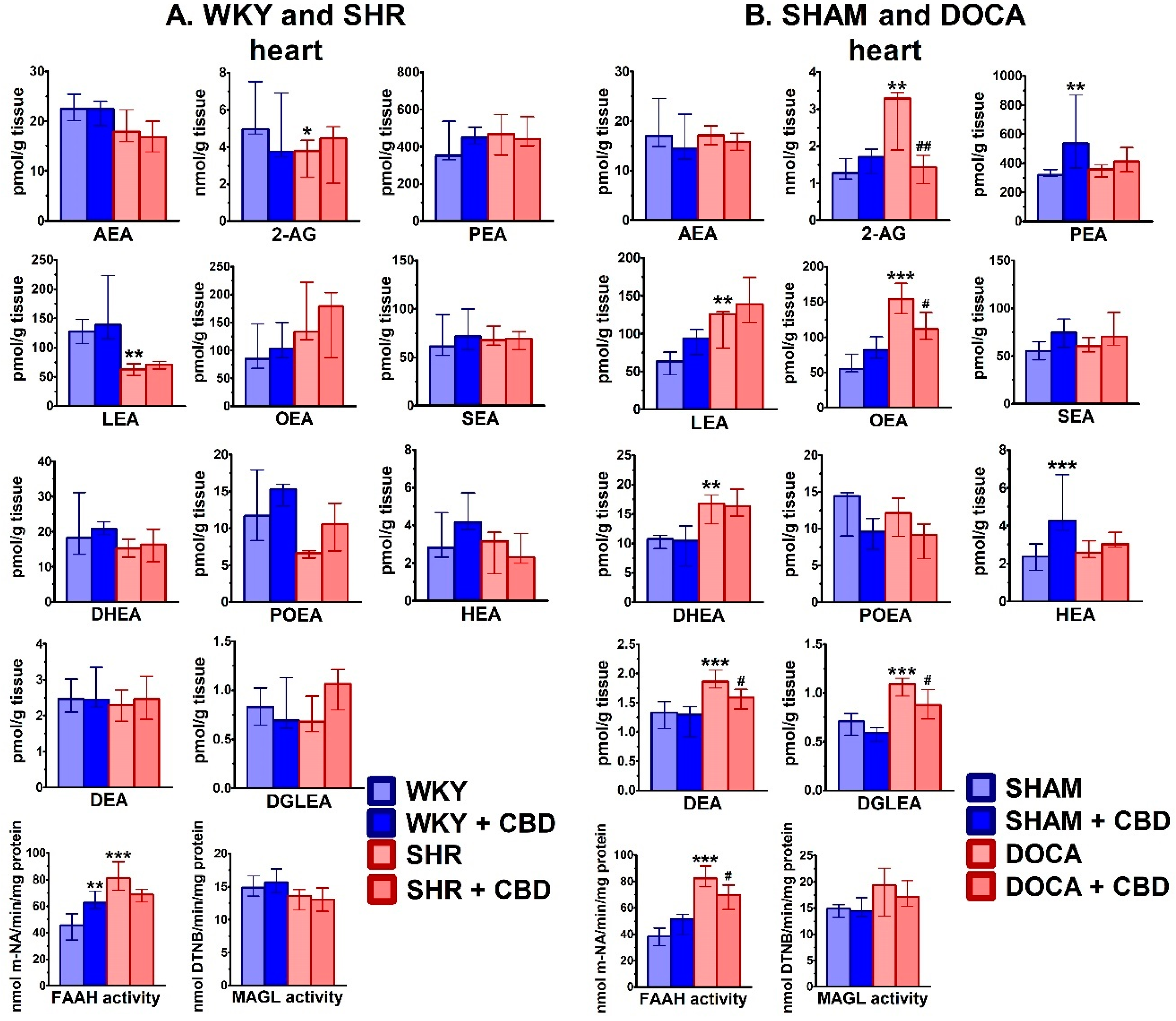
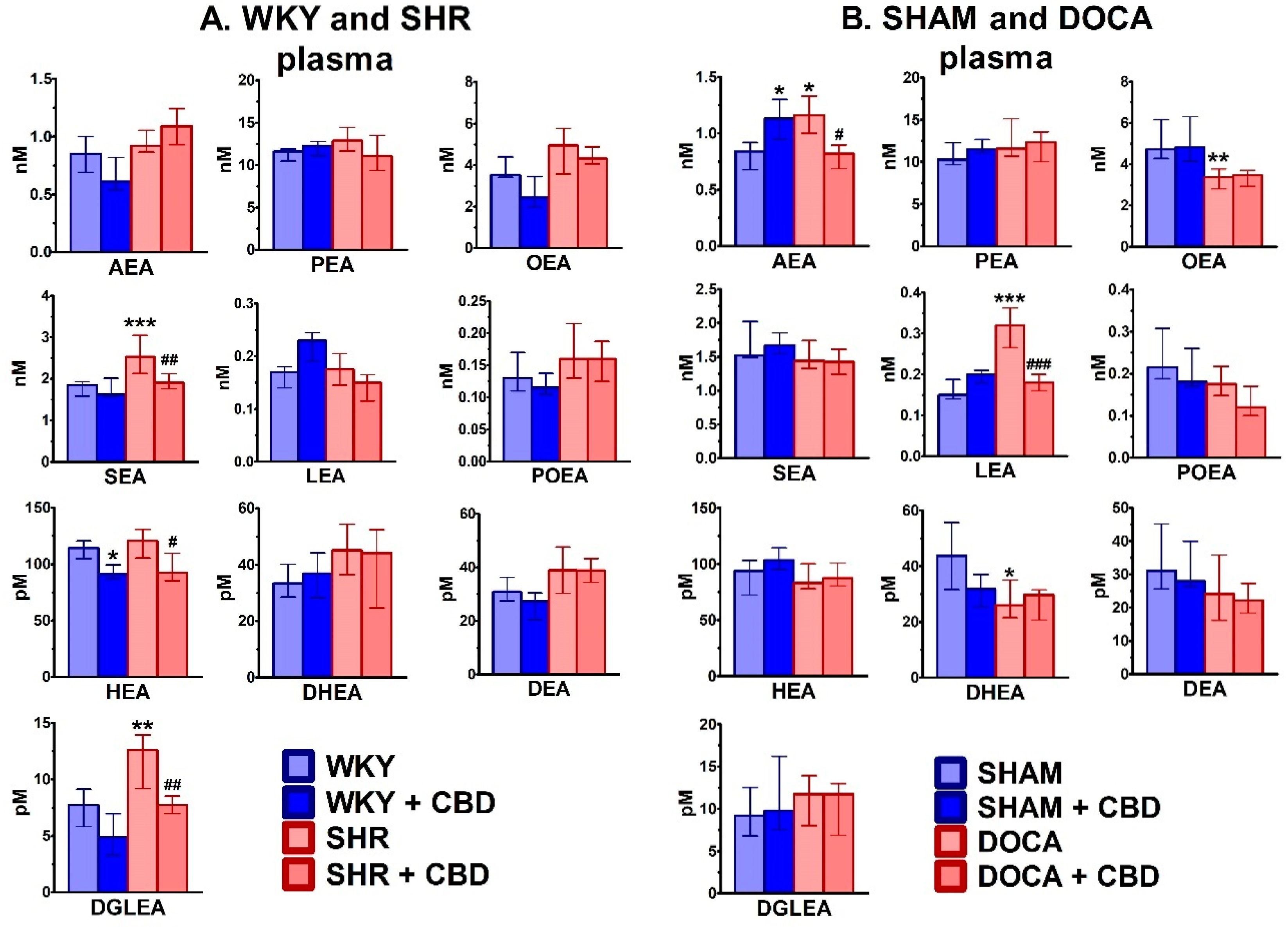
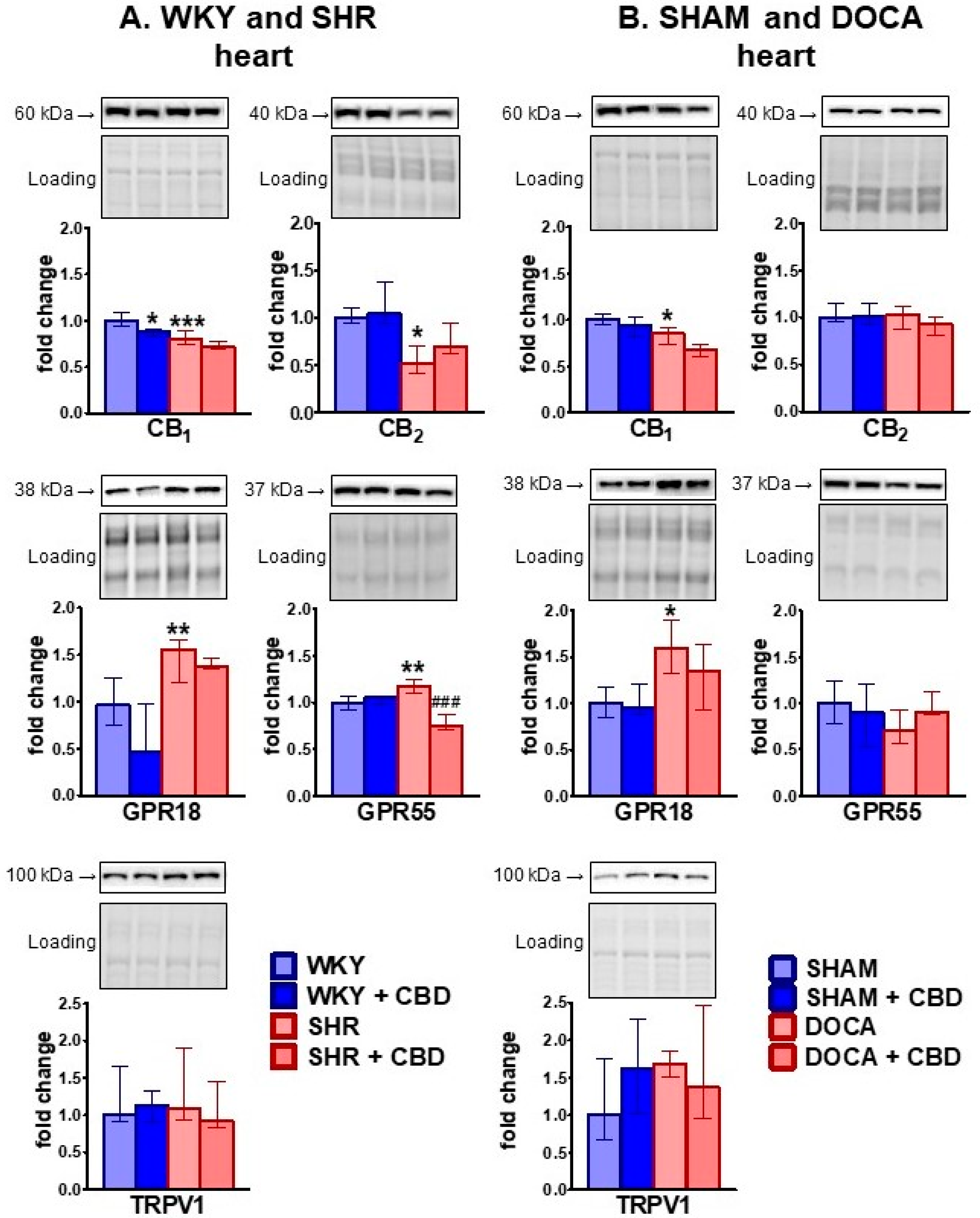
2.2. Influence of Hypertension and CBD on the Endocannabinoid System
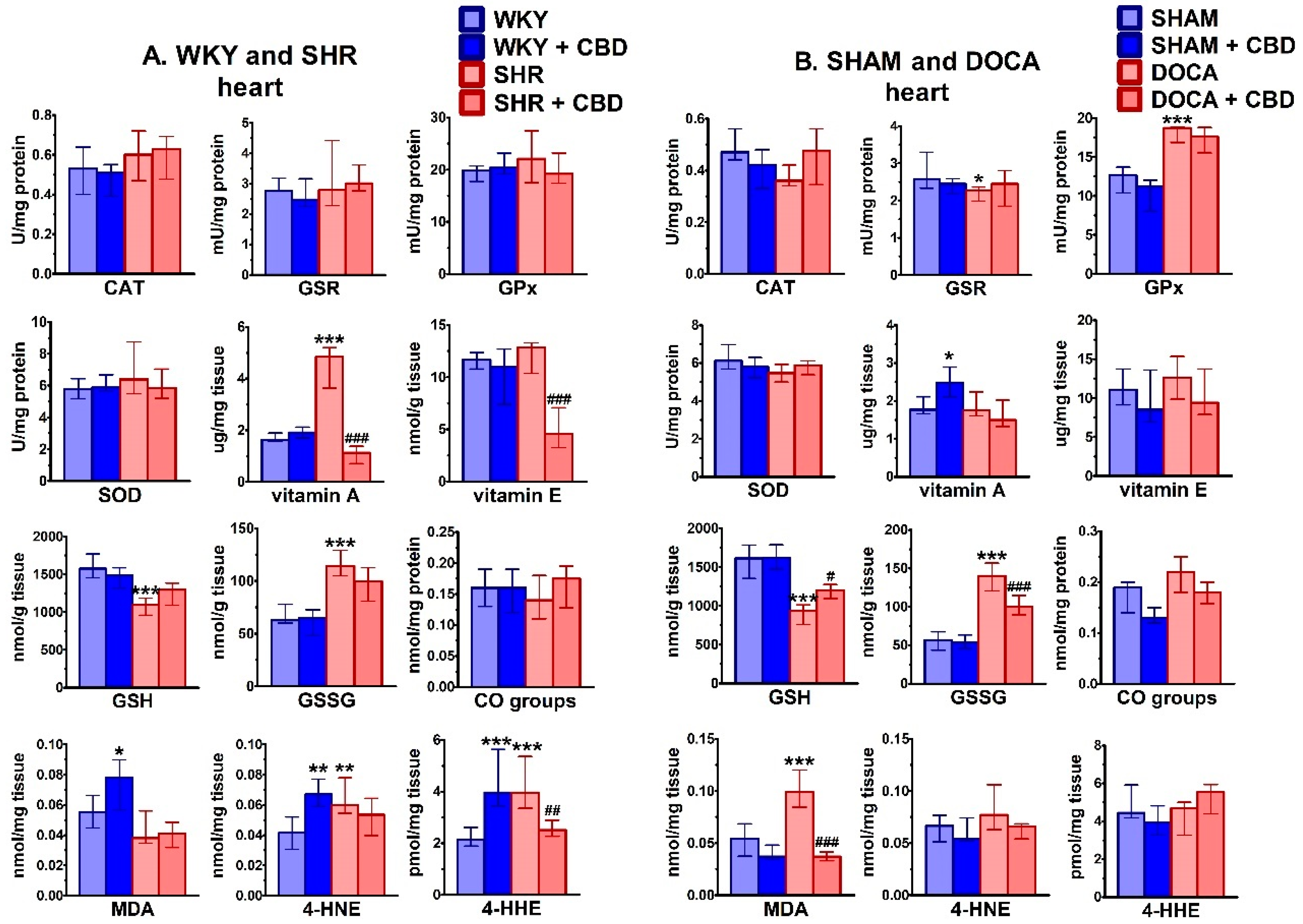
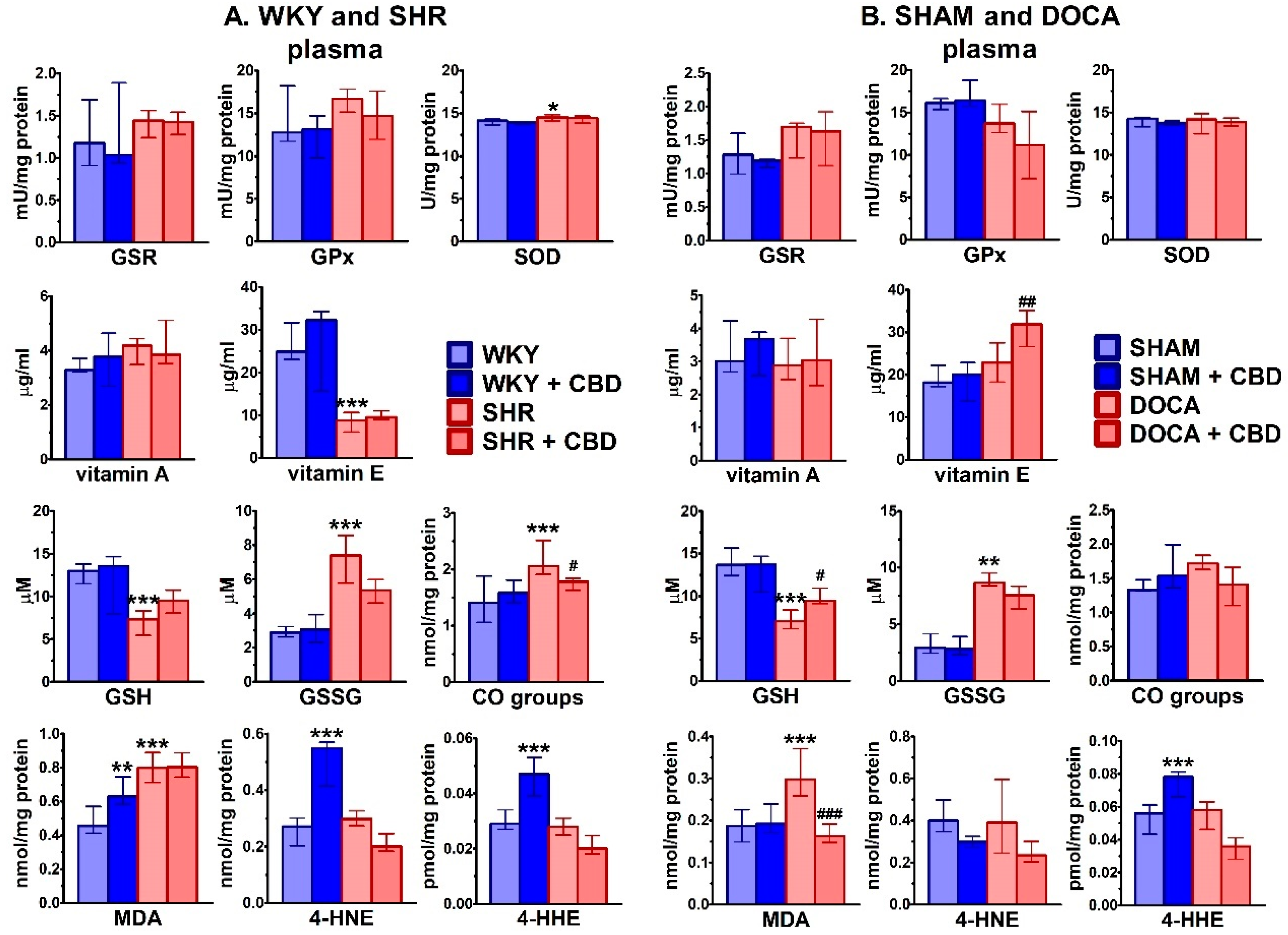
2.3. Influence of Hypertension and CBD on Oxidative Stress
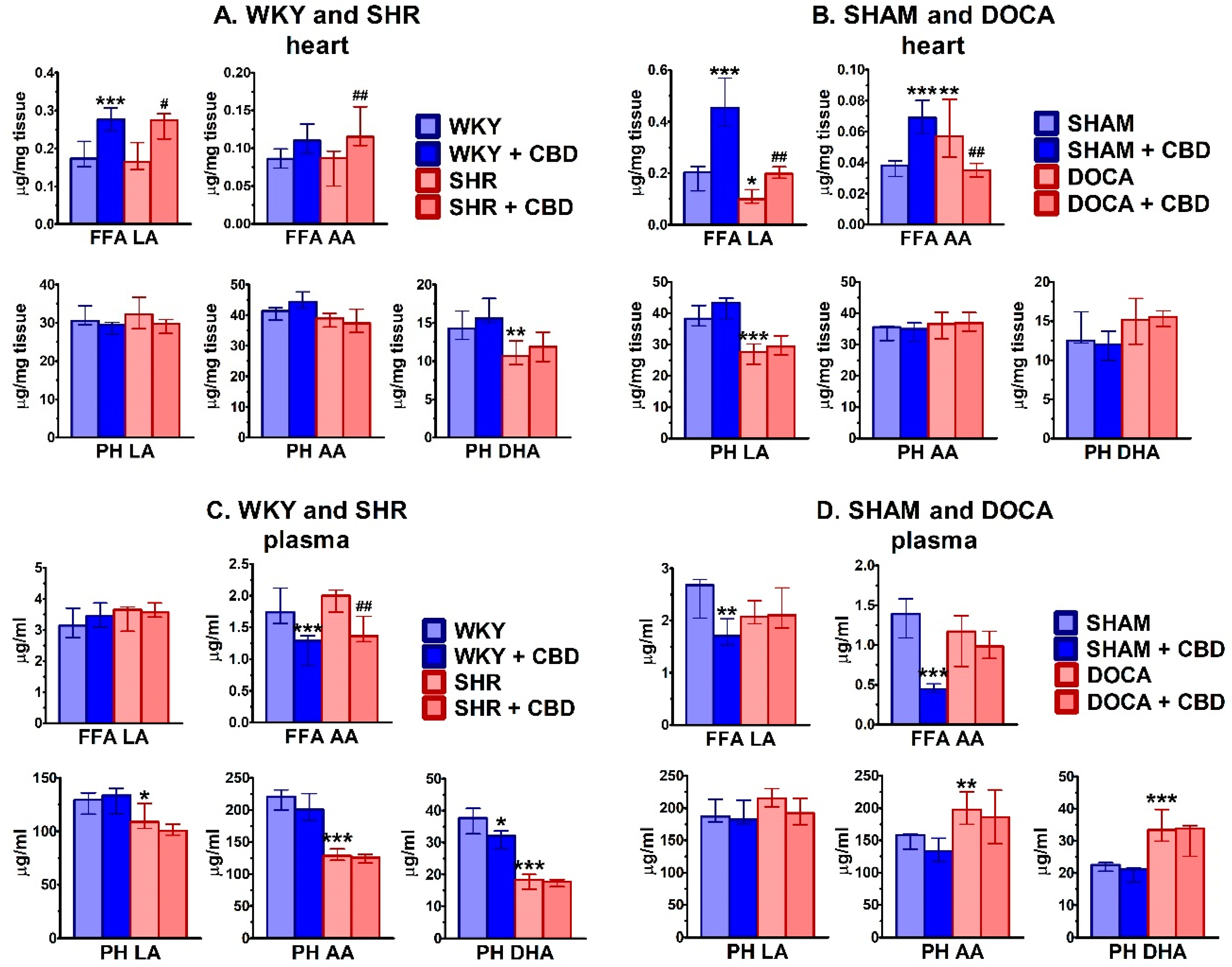
2.4. Influence of Hypertension and CBD on Lipids
3. Discussion
3.1. General
3.2. Effect of Hypertension
3.3. Effect of Chronic CBD in Hypertensive Animals
3.4. Effect of Chronic CBD in Normotensive Animals
3.5. Limitations of the Study
4. Materials and Methods
4.1. Animals
4.2. Experimental Groups and Protocol
4.3. Chronic CBD Administration
4.4. Determination of Cardiovascular Parameters in Conscious Rats
4.5. Tissue Preparation for Biochemical Examinations
4.6. Biochemical Studies
4.6.1. Determination of Endocannabinoids
4.6.2. Determination of FAAH and MAGL Activity
4.6.3. Western Blot Analysis
4.6.4. Determination of Antioxidant Enzyme Activity
4.6.5. Determination of Non-Enzymatic Antioxidant Level
4.6.6. Determination of Protein Modifications
4.6.7. Determination of Lipid Modifications
4.6.8. Determination of Fatty Acids
4.7. Statistical Analysis
4.8. Drugs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| 4-HHE | 4-hydroxyhexenal |
| 4-HNE | 4-hydroxynonenal |
| AA | arachidonic acid |
| ACN | acetonitrile |
| AEA | anandamide |
| A-1-TG | arachidonoyl-1-thio-glycerol |
| ANOVA | analysis of variance |
| BCA | bicinchoninic acid |
| BP | blood pressure |
| BSA | bovine serum albumin |
| CAT | catalase |
| CBD | cannabidiol |
| CE | capillary electrophoresis |
| CO | carbonyl groups |
| DBP | diastolic blood pressure |
| DEA | docosatetraenoyl ethanolamide |
| DGLEA | dihomo-γ-linolenoyl ethanolamide |
| DHA | docosahexaenoic acid |
| DHEA | docosahexaenoyl ethanolamide |
| DMF | dimethylformamide |
| DOCA | deoxycorticosterone acetate or DOCA-salt hypertensive rats |
| DOCA-salt | DOCA-based method to generate hypertension in rats or DOCA-salt hypertensive rats |
| DTNB | 5,5′-dithiobis-2-dinitrobenzoic acid |
| ECG | electrocardiography |
| ECS | endocannabinoid system |
| EDTA | ethylenediaminetetraacetic acid |
| ESI | electrospray ionization source |
| FAAH | fatty acid amide hydrolase |
| FAME | fatty acid methyl esters |
| FFA | free fatty acids |
| FID | flame ionization detector |
| GC | gas chromatography |
| GPR | G-protein coupled receptor |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GSR | glutathione-disulfide reductase |
| GSSG | glutathione disulfide |
| HEA | homo-γ-linolenyl ethanolamide |
| HPLC | high-performance liquid chromatography |
| HR | heart rate |
| ID | internal diameter |
| i.p. | intraperitoneal injection |
| IS | internal standard |
| LA | linoleic acid |
| LEA | linolenoyl ethanolamide |
| MAGL | monoacylglycerol lipase |
| MDA | malondialdehyde |
| MRM | multiple reaction monitoring |
| MS/MS | tandem mass spectrometry |
| m-NA | m-nitroaniline |
| NADA | N-arachidonoyl dopamine |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| OEA | oleoyl ethanolamide |
| O-PFBoxime | O-(2,3,4,5,6-pentafluorobenzyl) oxime |
| PEA | palmitoyl ethanolamide |
| PH | phospholipids |
| PIPES | piperazine-N,N′-bis(2-ethanesulfonic acid) |
| POEA | palmitoleoyl ethanolamide |
| PPR | peroxisome proliferator-activated receptor |
| PUFA | polyunsaturated fatty acids |
| PVDF | polyvinylidene difluoride |
| RAAS | renin-angiotensin-aldosterone system |
| RIPA | radioimmunoprecipitation assay |
| SBP | systolic blood pressure |
| SEA | stearoyl ethanolamide |
| SEM | standard error of the mean |
| SHAM | sham-operated rats |
| SHR | spontaneously hypertensive rats |
| SIM | selected ion monitoring mode |
| SOD | superoxide dismutase |
| s.c. | subcutaneous injection |
| THC | Δ9-tetrahydrocannabinol |
| TLC | thin-layer chromatography |
| TNB | 5-thio-2-nitrobenzoic acid |
| TRPV | transient receptor potential vanilloid |
| UPLC | ultrahigh performance liquid chromatography |
| WKY | Wistar-Kyoto rats |
References
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Booz, G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A systematic review of cannabidiol dosing in clinical populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Gruden, G.; Barutta, F.; Kunos, G.; Pacher, P. Role of the endocannabinoid system in diabetes and diabetic complications. Br. J. Pharmacol. 2016, 173, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Paloczi, J.; Varga, Z.V.; Haskó, G.; Pacher, P. Neuroprotection in oxidative stress-related neurodegenerative diseases: Role of endocannabinoid system modulation. Antioxid. Redox Signal. 2018, 29, 75–108. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Franco, V.; Perucca, E. Pharmacological and therapeutic properties of cannabidiol for epilepsy. Drugs 2019, 79, 1435–1454. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the cardiovascular system a therapeutic target for cannabidiol? Br. J. Clin. Pharmacol. 2013, 75, 313–322. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef]
- Sultan, S.R.; Millar, S.A.; England, T.J.; O’Sullivan, S.E. A systematic review and meta-analysis of the haemodynamic effects of cannabidiol. Front. Pharmacol. 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.R.; England, T.J.; O’Sullivan, S.E. Acute and chronic effects of cannabidiol on haemodynamics in healthy males. In Proceedings of the 29th Annual Symposium on the Cannabinoids, Research Triangle Park, NC, USA, 29 June–4 July 2019; p. 6. [Google Scholar]
- Resstel, L.B.; Tavares, R.F.; Lisboa, S.F.; Joca, S.R.; Corrêa, F.M.; Guimarães, F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Granjeiro, E.M.; Gomes, F.V.; Guimarães, F.S.; Corrêa, F.M.; Resstel, L.B. Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol. Biochem. Behav. 2011, 99, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Kossakowski, R.; Schlicker, E.; Toczek, M.; Weresa, J.; Malinowska, B. Cannabidiol affects the Bezold-Jarisch reflex via TRPV1 and 5-HT3 receptors and has peripheral sympathomimetic effects in spontaneously hypertensive and normotensive rats. Front. Pharmacol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Knock, G.A. NADPH oxidase in the vasculature: Expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic. Biol. Med. 2019, 145, 385–427. [Google Scholar] [CrossRef]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal models of hypertension: A scientific statement from the American Heart Association. Hypertension 2019, 73, e87–e120. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Sadowska, O.; Kozłowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: Modification by hypertension and the potential pharmacological opportunities. J. Hypertens. 2019, 37. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef]
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017, 11, 577–585. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Rozenfeld, R.; Wu, D.; Devi, L.A.; Zhang, Z.; Cederbaum, A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic. Biol. Med. 2014, 68, 260–267. [Google Scholar] [CrossRef]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 12064. [Google Scholar] [CrossRef]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdélyi, K.; Holovac, E.; Liaudet, L.; Lee, W.S.; Haskó, G.; Mechoulam, R.; Pacher, P. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef]
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef]
- Cassol, O.J., Jr.; Comim, C.M.; Silva, B.R.; Hermani, F.V.; Constantino, L.S.; Felisberto, F.; Petronilho, F.; Hallak, J.E.; De Martinis, B.S.; Zuardi, A.W.; et al. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010, 1348, 128–138. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol-from plant to human body: A promising bioactive molecule with multi-target effects in cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016, 6, 150276. [Google Scholar] [CrossRef]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; de Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the oxidative stress and lipid peroxidation by endocannabinoids and their lipid analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Mackie, K.; Pacher, P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 2010, 160, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Horváth, B.; Rajesh, M.; Matsumoto, S.; Saito, K.; Bátkai, S.; Patel, V.; Tanchian, G.; Gao, R.Y.; Cravatt, B.F.; et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic. Biol. Med. 2011, 50, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Bátkai, S.; Kechrid, M.; Mukhopadhyay, P.; Lee, W.S.; Horváth, B.; Holovac, E.; Cinar, R.; Liaudet, L.; Mackie, K.; et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 2012, 61, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Lenglet, S.; Braunersreuther, V.; Burger, F.; Pelli, G.; Bertolotto, M.; Mach, F.; Steffens, S. CB2 cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J. Mol. Cell. Cardiol. 2009, 46, 612–620. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Pan, H.; Patel, V.; Mukhopadhyay, B.; Batkai, S.; Gao, B.; Haskó, G.; Pacher, P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic. Biol. Med. 2010, 48, 457–467. [Google Scholar] [CrossRef]
- Sun, H.J.; Lu, Y.; Wang, H.W.; Zhang, H.; Wang, S.R.; Xu, W.Y.; Fu, H.L.; Yao, X.Y.; Yang, F.; Yuan, H.B. Activation of endocannabinoid receptor 2 as a mechanism of propofol pretreatment-induced cardioprotection against ischemia-reperfusion injury in rats. Oxid. Med. Cell. Longev. 2017, 2017, 2186383. [Google Scholar] [CrossRef]
- Duerr, G.D.; Heinemann, J.C.; Kley, J.; Eichhorn, L.; Frede, S.; Weisheit, C.; Wehner, S.; Bindila, L.; Lutz, B.; Zimmer, A.; et al. Myocardial maladaptation to pressure overload in CB2 receptor-deficient mice. J. Mol. Cell. Cardiol. 2019, 133, 86–98. [Google Scholar] [CrossRef]
- Matyas, C.; Erdelyi, K.; Trojnar, E.; Zhao, S.; Varga, Z.V.; Paloczi, J.; Mukhopadhyay, P.; Nemeth, B.T.; Haskó, G.; Cinar, R.; et al. Interplay of liver-heart inflammatory axis and cannabinoid 2 receptor signalling in an experimental model of hepatic cardiomyopathy. Hepatology 2019. [Google Scholar] [CrossRef]
- Rajaraman, G.; Simcocks, A.; Hryciw, D.H.; Hutchinson, D.S.; McAinch, A.J. G protein coupled receptor 18: A potential role for endocannabinoid signaling in metabolic dysfunction. Mol. Nutr. Food Res. 2016, 60, 92–102. [Google Scholar] [CrossRef]
- Matouk, A.I.; Taye, A.; El-Moselhy, M.A.; Heeba, G.H.; Abdel-Rahman, A.A. The effect of chronic activation of the novel endocannabinoid receptor GPR18 on myocardial function and blood pressure in conscious rats. J. Cardiovasc. Pharmacol. 2017, 69, 23–33. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Leishman, E.; Manchanda, M.; Thelen, R.; Miller, S.; Mackie, K.; Bradshaw, H.B. Cannabidiol’s upregulation of N-acyl ethanolamines in the central nervous system requires N-acyl phosphatidyl ethanolamine-specific phospholipase D. Cannabis Cannabinoid Res. 2018, 3, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef]
- Schloss, M.J.; Horckmans, M.; Guillamat-Prats, R.; Hering, D.; Lauer, E.; Lenglet, S.; Weber, C.; Thomas, A.; Steffens, S. 2-Arachidonoylglycerol mobilizes myeloid cells and worsens heart function after acute myocardial infarction. Cardiovasc. Res. 2019, 115, 602–613. [Google Scholar] [CrossRef]
- Biernacki, M.; Łuczaj, W.; Jarocka-Karpowicz, I.; Ambrożewicz, E.; Toczek, M.; Skrzydlewska, E. The effect of long-term administration of fatty acid amide hydrolase inhibitor URB597 on oxidative metabolism in the heart of rats with primary and secondary hypertension. Molecules 2018, 23, 2350. [Google Scholar] [CrossRef]
- Biernacki, M.; Malinowska, B.; Timoszuk, M.; Toczek, M.; Jastrząb, A.; Remiszewski, P.; Skrzydlewska, E. Hypertension and chronic inhibition of endocannabinoid degradation modify the endocannabinoid system and redox balance in rat heart and plasma. Prostaglandins Other Lipid Mediat. 2018, 138, 54–63. [Google Scholar] [CrossRef]
- Wheal, A.J.; Jadoon, K.; Randall, M.D.; O’Sullivan, S.E. In vivo cannabidiol treatment improves endothelium-dependent vasorelaxation in mesenteric arteries of Zucker diabetic fatty rats. Front. Pharmacol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Lee, W.S.; Erdelyi, K.; Matyas, C.; Mukhopadhyay, P.; Varga, Z.V.; Liaudet, L.; Haskó, G.; Čiháková, D.; Mechoulam, R.; Pacher, P. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: Implications to autoimmune disorders and organ transplantation. Mol. Med. 2016, 22, 136–146. [Google Scholar] [CrossRef]
- Pastor, A.; Farré, M.; Fitó, M.; Fernandez-Aranda, F.; de la Torre, R. Analysis of ECs and related compounds in plasma: Artifactual isomerization and ex vivo enzymatic generation of 2-MGs. J. Lipid Res. 2014, 55, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Bottemanne, P.; Makriyannis, A.; Muccioli, G.G. N-acylethanolamine-hydrolyzing acid amidase and fatty acid amide hydrolase inhibition differentially affect N-acylethanolamine levels and macrophage activation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Wortley, M.A.; Adcock, J.J.; Dubuis, E.D.; Maher, S.A.; Bonvini, S.J.; Delescluse, I.; Kinloch, R.; McMurray, G.; Perros-Huguet, C.; Papakosta, M.; et al. Targeting fatty acid amide hydrolase as a therapeutic strategy for antitussive therapy. Eur. Respir. J. 2017, 50, 1700782. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.A.; Coker, S.J.; Carswell, H.V.O. Detrimental effects of 2-arachidonoylglycerol on whole blood platelet aggregation and on cerebral blood flow after a focal ischemic insult in rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H967–H977. [Google Scholar] [CrossRef]
- Jehle, J.; Schöne, B.; Bagheri, S.; Avraamidou, E.; Danisch, M.; Frank, I.; Pfeifer, P.; Bindila, L.; Lutz, B.; Lütjohann, D.; et al. Elevated levels of 2-arachidonoylglycerol promote atherogenesis in ApoE-/- mice. PLoS ONE 2018, 13, e0197751. [Google Scholar] [CrossRef]
- Chanda, D.; Oligschlaeger, Y.; Geraets, I.; Liu, Y.; Zhu, X.; Li, J.; Nabben, M.; Coumans, W.; Luiken, J.; Glatz, J.F.C.; et al. 2-Arachidonoylglycerol ameliorates inflammatory stress-induced insulin resistance in cardiomyocytes. J. Biol. Chem. 2017, 292, 7105–7114. [Google Scholar] [CrossRef]
- Su, H.F.; Samsamshariat, A.; Fu, J.; Shan, Y.X.; Chen, Y.H.; Piomelli, D.; Wang, P.H. Oleylethanolamide activates Ras-Erk pathway and improves myocardial function in doxorubicin-induced heart failure. Endocrinology 2006, 147, 827–834. [Google Scholar] [CrossRef]
- Mattace Raso, G.; Simeoli, R.; Russo, R.; Santoro, A.; Pirozzi, C.; d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Paciello, O.; Pagano, T.B.; Orefice, N.S.; et al. N-Palmitoylethanolamide protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress. Pharmacol. Res. 2013, 76, 67–76. [Google Scholar] [CrossRef]
- Irvine, R.J.; White, J.; Chan, R. The influence of restraint on blood pressure in the rat. J. Pharmacol. Toxicol. Methods 1997, 38, 157–162. [Google Scholar] [CrossRef]
- Piomelli, D.; Tarzia, G.; Duranti, A.; Tontini, A.; Mor, M.; Compton, T.R.; Dasse, O.; Monaghan, E.P.; Parrott, J.A.; Putman, D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev. 2006, 12, 21–38. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.M.; Peng, J.; Zhang, Y.S.; Li, X.H.; Yuan, Q.; Hu, C.P.; Deng, H.W.; Li, Y.J. Role of anandamide transporter in regulating calcitonin gene-related peptide production and blood pressure in hypertension. J. Hypertens. 2009, 27, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, B.; Toczek, M.; Pędzińska-Betiuk, A.; Schlicker, E. Cannabinoids in arterial, pulmonary and portal hypertension—Mechanisms of action and potential therapeutic significance. Br. J. Pharmacol. 2019, 176, 1395–1411. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; Barrett, D.A.; Randall, M.D. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, B.; Baranowska-Kuczko, M.; Schlicker, E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br. J. Pharmacol. 2012, 165, 2073–2088. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An update on safety and side sffects of sannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Pędzińska-Betiuk, A.; Weresa, J.; Toczek, M.; Baranowska-Kuczko, M.; Kasacka, I.; Harasim-Symbor, E.; Malinowska, B. Chronic inhibition of fatty acid amide hydrolase by URB597 produces differential effects on cardiac performance in normotensive and hypertensive rats. Br. J. Pharmacol. 2017, 174, 2114–2129. [Google Scholar] [CrossRef]
- Kamon, T.; Kaneko, H.; Itoh, H.; Kiriyama, H.; Mizuno, Y.; Morita, H.; Yamamichi, N.; Komuro, I. Gender-specific association between the blood pressure category according to the updated ACC/AHA guidelines for hypertension and cardio-ankle vascular index: A community-based cohort study. J. Cardiol. 2019. [Google Scholar] [CrossRef]
- Mozos, I. Arrhythmia risk and obesity. J. Mol. Genet. Med. 2014, 1, 1747-0862. [Google Scholar] [CrossRef]
- Gasparova, I.; Kubatka, P.; Opatrilova, R.; Caprnda, M.; Filipova, S.; Rodrigo, L.; Malan, L.; Mozos, I.; Rabajdova, M.; Nosal, V.; et al. Perspectives and challenges of antioxidant therapy for atrial fibrillation. Naunyn Schmiedeberg Arch. Pharmacol. 2017, 390, 1–14. [Google Scholar] [CrossRef]
- Mozos, I. Laboratory markers of ventricular arrhythmia risk in renal failure. Biomed. Res. Int. 2014, 2014, 509204. [Google Scholar] [CrossRef]
- Lam, P.M.; Marczylo, T.H.; El-Talatini, M.; Finney, M.; Nallendran, V.; Taylor, A.H.; Konje, J.C. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Anal. Biochem. 2008, 380, 195–201. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; Calderón-Santiago, M.; Luque de Castro, M.D.; Priego-Capote, F. Study of sample preparation for determination of endocannabinoids and analogous compounds in human serum by LC-MS/MS in MRM mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef]
- Siegmund, S.V.; Seki, E.; Osawa, Y.; Uchinami, H.; Cravatt, B.F.; Schwabe, R.F. Fatty acid amide hydrolase determines anandamide-induced cell death in the liver. J. Biol. Chem. 2006, 281, 10431–10438. [Google Scholar] [CrossRef]
- Ulloa, N.M.; Deutsch, D.G. Assessment of a spectrophotometric assay for monoacylglycerol lipase activity. AAPS J. 2010, 12, 197–201. [Google Scholar] [CrossRef]
- Lessard, S.J.; MacDonald, T.L.; Pathak, P.; Han, M.S.; Coffey, V.G.; Edge, J.; Rivas, D.A.; Hirshman, M.F.; Davis, R.J.; Goodyear, L.J. JNK regulates muscle remodeling via myostatin/SMAD inhibition. Nat. Commun. 2018, 9, 3030. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Mize, C.E.; Langdon, R.G. Hepatic glutathione reductase. I. Purification and general kinetic properties. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Sykes, J.A.; McCormack, F.X., Jr.; O’Brien, T.J. A preliminary study of the superoxide dismutase content of some human tumors. Cancer Res. 1978, 38, 2759–2762. [Google Scholar]
- Vatassery, G.T.; Brin, M.F.; Fahn, S.; Kayden, H.J.; Traber, M.G. Effect of high doses of dietary vitamin E on the concentrations of vitamin E in several brain regions, plasma, liver, and adipose tissue of rats. J. Neurochem. 1988, 51, 621–623. [Google Scholar] [CrossRef]
- Maeso, N.; García-Martínez, D.; Rupérez, F.J.; Cifuentes, A.; Barbas, C. Capillary electrophoresis of glutathione to monitor oxidative stress and response to antioxidant treatments in an animal model. J. Chromatogr. B 2005, 822, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.P.; Yazdanpanah, M.; Bhooi, N.; Lehotay, D.C. Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatography-mass spectrometry. Anal. Biochem. 1995, 228, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In Advances in Lipid Methodology—Two; Christie, W.W., Ed.; Oily Press: Dundee, Scotland, 1993; pp. 69–111. [Google Scholar]

| Group | Treatment | n | Systolic Blood Pressure (mmHg) | Heart Rate (Beats/min) | Body Weight (g) | |||
|---|---|---|---|---|---|---|---|---|
| Before the First Dose of CBD or Its Solvent | 24 h After the Final Dose of CBD or Its Solvent | Before the First Dose of CBD or Its Solvent | 24 h After The Final Dose of CBD or Its Solvent | Before the First Dose of CBD or Its Solvent | 24 h After the Final Dose of CBD or Its Solvent | |||
| WKY | vehicle | 7 | 111 [95;122] | 98 [91;105] ! | 341 [321;366] | 314 [304;326] | 342 [329;361] | 380 [367;400] !!! |
| WKY | CBD | 7 | 101 [87;124] | 92 [87;107] | 334 [313;357] | 300 [298;321] ! | 325 [325;340] | 360 [353;390] ! |
| SHR | vehicle | 7 | 178 [161;199] *** | 174 [164;187] *** | 359 [351;369] | 367 [365;387] ** | 290 [282;292] *** | 300 [296;326] ***! |
| SHR | CBD | 7 | 184 [172;192] $$$ | 172 [170;176] $$$ | 355 [352;397] | 358 [344;380] $$ | 315 [300;325] | 328 [313;340] $!!! |
| SHAM | vehicle | 7 | 122 [111;132] | 120 [118;131] | 363 [351;371] | 336 [331;340] ! | 294 [270;304] | 320 [302;345] !!! |
| SHAM | CBD | 7 | 111 [100;129] | 121 [109;134] | 354 [333;374] | 349 [328;371] | 278 [256;294] | 322 [286;340] ! |
| DOCA | vehicle | 7 | 163 [111;175] | 175 [160;190] *** | 314 [303;360] | 324 [312;352] | 275 [233;276] | 280 [252;321] |
| DOCA | CBD | 6 | 150 [143;174] $ | 173 [150;189] $$$ | 327 [315;354] | 314 [301;348] | 280 [271;298] | 303 [297;312] ! |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. https://doi.org/10.3390/ijms21041295
Remiszewski P, Jarocka-Karpowicz I, Biernacki M, Jastrząb A, Schlicker E, Toczek M, Harasim-Symbor E, Pędzińska-Betiuk A, Malinowska B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. International Journal of Molecular Sciences. 2020; 21(4):1295. https://doi.org/10.3390/ijms21041295
Chicago/Turabian StyleRemiszewski, Patryk, Iwona Jarocka-Karpowicz, Michał Biernacki, Anna Jastrząb, Eberhard Schlicker, Marek Toczek, Ewa Harasim-Symbor, Anna Pędzińska-Betiuk, and Barbara Malinowska. 2020. "Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism" International Journal of Molecular Sciences 21, no. 4: 1295. https://doi.org/10.3390/ijms21041295
APA StyleRemiszewski, P., Jarocka-Karpowicz, I., Biernacki, M., Jastrząb, A., Schlicker, E., Toczek, M., Harasim-Symbor, E., Pędzińska-Betiuk, A., & Malinowska, B. (2020). Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. International Journal of Molecular Sciences, 21(4), 1295. https://doi.org/10.3390/ijms21041295