Healthy Effects of Plant Polyphenols: Molecular Mechanisms
Abstract
1. Introduction
2. Polyphenols: Important Players of the Mediterranean/Asian Diets
3. The Beneficial Effects of Plant Polyphenols Are Supported by Population Studies and Clinical Trials
4. Hormesis: A New General Concept Supporting Polyphenol Benefits
5. Plant Polyphenols Rescue Altered Homeostatic Systems in Cells
5.1. Redox Homeostasis and the Inflammatory Response
5.2. Metabolic Homeostasis
5.3. Proteostasis
6. Epigenetic Implications
6.1. DNA Methylation
6.2. Histone Modifications
6.3. Noncoding RNAs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stefani, M.; Rigacci, S. Beneficial properties of natural phenols: Highlight on protection against pathological conditions associated with amyloid aggregation. BioFactors 2014, 40, 482–493. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Mediterranean Diet Foundation Expert Group. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Tangney, C.C.; Kwasny, M.J.; Li, H.; Wilson, R.S.; Evans, D.A.; Morris, M.C. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am. J. Clin. Nutr. 2011, 93, 601–607. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nutr. Rev. 1998, 56, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Debnath, S.; Banik, R. Naturally Occurring Iridoids and Secoiridoids. An Updated Review, Part 4. Chem. Pharm. Bull. (Tokyo) 2011, 59, 803–833. [Google Scholar] [CrossRef]
- Dinda, B.; Dubnath, S.; Harigaya, Y. Naturally Occurring Iridoids. A Review, Part 1. Chem. Pharm. Bull. (Tokyo) 2007, 55, 159–222. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, A.; Marchegiani, D.; Contento, S.; Girardi, F.; Nicolosi, M.P.; Brullo, M.D. Variations of the iridoid oleuropein in Italian olive varieties during growth and maturation. Eur. J. Lipid Sci. Technol. 2009, 111, 678–687. [Google Scholar] [CrossRef]
- Servili, M.; Montedoro, G.F. Contribution of phenolic compounds in virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Tsukahara, C.; Ikeda, N.; Sone, Y.; Ishikawa, T.; Ichi, I.; Koike, T.; Aoki, Y. Oleuropein improves insulin resistance in skeletal muscle by promoting the translocation of GLUT4. J. Clin. Biochem. Nutr. 2017, 61, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Velander, P.; Liu, D.; Xu, B. Olive Component Oleuropein Promotes β-Cell Insulin Secretion and Protects β-Cells from Amylin Amyloid-Induced Cytotoxicity. Biochemistry 2017, 56, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: In vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Ed Dami, T.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The polyphenol oleuropein aglycone protects TgCRNDmice against Aβ plaque pathology. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Casapullo, A. Modulation of tau protein fibrillization by oleocanthal. J. Nat. Prod. 2012, 75, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Parri, M.; Nediani, C.; Cerbai, E.; Stefani, M.; Berti, A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010, 8, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Daccache, A.; Lion, C.; Sibille, N.; Gerard, M.; Slomianny, C.; Lippens, G.; Cotelle, P. Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem. Int. 2011, 58, 700–707. [Google Scholar] [CrossRef]
- Qosa, H.; Mohamed, L.A.; Batarseh, Y.S.; Alqahtani, S.; Ibrahim, B.; LeVine, H., III; Keller, J.N.; Kaddoumi, A. Extra-virgin olive oil attenuates amyloid-β and tau pathologies in the brains of TgSwDI mice. J. Nutr. Biochem. 2015, 26, 1479–1490. [Google Scholar] [CrossRef]
- Leri, M.; Natalello, A.; Bruzzone, E.; Stefani, M.; Bucciantini, M. Oleuriopein aglycone and hydroxytyrosol interfere differently with toxic Aβ1-aggregation. Food Chem. Toxicol. 2019, 129, 1–12. [Google Scholar] [CrossRef]
- Yu, G.; Deng, A.; Tang, W.; Ma, J.; Yuan, C.; Ma, J. Hydroxytyrosol induces phase II detoxifying enzyme expression and effectively protects dopaminergic cells against dopamine- and 6-hydroxydopamine induced cytotoxicity. Neurochem. Int. 2016, 96, 113–120. [Google Scholar] [CrossRef]
- Nardiello, P.; Pantano, D.; Lapucci, A.; Stefani, M.; Casamenti, F. Diet supplementation with hydroxytyrosol ameliorates brain pathology and restores cognitive functions in a mouse model of amyloid-β deposition. J. Alzheimers Dis. 2018, 63, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurol. 2015, 16, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Bedse, G.; Di Domenico, F.; Serviddio, G.; Cassano, T. Aberrant insulin signaling in Alzheimer’s disease: Current knowledge. Front. Neurosci. 2015, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Ayissi, V.B.O.; Ebrahimi, A.; Schluesenner, H. Epigenetic effects of natural polyphenols: A focus on SIRT1-mediated mechanisms. Mol. Nutr. Food Res. 2014, 58, 22–32. [Google Scholar] [CrossRef]
- Bartolini, G.; Petruccelli, R. Classifications, Origins, Diffusion and History of the Olive; Rome Food and Agricolture Organisation in the United Nations: Roma, Italy, 2002. [Google Scholar]
- Flemmig, J.; Rusch, D.; Czerwinska, M.E.; Ruwald, H.W.; Arnhold, J. Components of a standardized olive leaf dry extract (Ph. Eur.) promote hypothiocyanate production by lactoperoxidase. Arch. Biochem. Biophys. 2014, 549, 17–25. [Google Scholar] [CrossRef]
- Flemming, J.; Kuchta, K.; Arnhold, J.; Rauwald, H.W. Olea europaea leaf (Ph. Eur.) extract as well as several of its isolated phenolics inhibit the gout-related enzyme xanthine oxidase. Phytomedicine 2011, 18, 561–566. [Google Scholar] [CrossRef]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar]
- Colomer, R.; Sarrats, A.; Lupu, R.; Puig, T. Natural Polyphenols and their Synthetic Analogs as Emerging Anticancer Agents. Curr. Drug Targets 2017, 18, 147–159. [Google Scholar] [CrossRef]
- Toric, J.; Markovic, A.K.; Brala, C.J.; Barbaric, M. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 2019, 69, 461–482. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceuticals and amyloid neurodegenerative diseases: A focus on natural polyphenols. Exp. Rev. Neurother. 2014, 15, 41–52. [Google Scholar] [CrossRef]
- Tomaselli, S.; La Vitola, P.; Pagano, K.; Brandi, E.; Santamaria, G.; Galante, D.; D’Arrigo, C.; Moni, L.; Lambruschini, C.; Banfi, L.; et al. Biophysical and in vivo studies identify a new natural-based polyphenol, counteracting Aβ oligomerization in vitro and Aβ oligomer-mediated memory impairment and neuroinflammation in an acute mouse model of Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 4452–4465. [Google Scholar] [CrossRef] [PubMed]
- Curti, V.; Zaccaria, V.; Tsetegho Sokeng, A.J.; Dacrema, M.; Masiello, I.; Mascaro, A.; D’Antona, G.; Daglia, M. Bioavailability and In Vivo Antioxidant Activity of a Standardized Polyphenol Mixture Extracted from Brown Propolis. Int. J. Mol. Sci. 2019, 20, 1250. [Google Scholar] [CrossRef] [PubMed]
- Gumireddy, A.; Christman, R.; Kumari, D.; Tiwari, A.; North, E.J.; Chauhan, H. Preparation, Characterization, and In vitro Evaluation of Curcumin- and Resveratrol-Loaded Solid Lipid Nanoparticles. AAPS Pharm. Sci. Tech. 2019, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Jaisamut, P.; Wiwattanawongsa, K.; Wiwattanapatapee, R. A Novel Self-Microemulsifying System for the Simultaneous Delivery and Enhanced Oral Absorption of Curcumin and Resveratrol. Planta Med. 2017, 83, 461–467. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Larrosa, M.; Possemiers, S.; Tomás-Barberán, F.A.; Espín, J.C. Bioavailability of phenolics from an oleuropein-rich olive (Olea europaea) leaf extract and its acute effect on plasma antioxidant status: Comparison between pre- and postmenopausal women. Eur. J. Nutr. 2014, 53, 1015–1027. [Google Scholar] [CrossRef]
- Luccarini, I.; Grossi, C.; Rigacci, S.; Coppi, E.; Pugliese, A.M.; Pantano, D.; la Marca, G.; ed Dami, T.; Berti, A.; Stefani, M.; et al. Oleuropein aglycone protects against pyroglutamylated-amyloid-β toxicity: Biochemical, epigenetic and functional correlates. Neurobiol. Aging 2015, 36, 648–663. [Google Scholar] [CrossRef]
- Lòpez de las Hazas, M.-C.; Godinho-Pereira, J.; Macià, A.; Filipa Almeida, A.; Ventura, M.R.; Motilva, M.-J.; Santos, C.N. Brain uptake of hydroxytyrosol and its main circulating metabolites: Protective potential in neuronal cells. J. Funct. Foods 2018, 46, 110–117. [Google Scholar] [CrossRef]
- De Pablos, R.M.; Espinosa-Oliva, A.M.; Hornado-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of ots effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharm. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Casamenti, F.; Stefani, M. Olive polyphenols: New promising agents to combat aging-associated neurodegeneration. Exp. Rev. Neurother. 2017, 17, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.; Baum, L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tgmice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Keys. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease; Harvard University Press: Cambridge, MA, USA, 1980. [Google Scholar]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Berr, C.; Portet, F.; Carriere, I.; Akbaraly, T.N.; Feart, C.; Gourlet, V.; Combe, N.; Barberger-Gateau, P.; Ritchie, K. Olive oil and cognition: Results from the three-city study. Dement. Geriatr. Cogn. Disord. 2009, 28, 357–364. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julián, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.Á. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Galbete, C.; Corella, D.; Toledo, E.; Buil-Cosiales, P.; Salas-Salvado, J.; Ros, E.; Martinez-Gonzalez, M.A. Genotype patterns at CLU, CR1, PICALM and APOE, cognition and Mediterranean diet: The PREDIMED-NAVARRA trial. Genes Nutr. 2014, 9, 393. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimers Dis. 2012, 29, 773–782. [Google Scholar] [CrossRef]
- Murie-Fernandez, M.; Irimia, P.; Toledo, E.; Martínez-Vila, E.; Buil-Cosiales, P.; Serrano-Martínez, M.; Ruiz-Gutiérrez, V.; Ros, E.; Estruch, R.; Martínez-González, M.Á. PREDIMED Investigators. Carotid intima-media thickness changes with Mediterranean diet: A randomized trial (PREDIMED-Navarra). Atherosclerosis 2011, 219, 158–162. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.A.; Medina-Remón, A.; Castañer, O.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.M. Effects of Polyphenol, Measured by a Biomarker of Total Polyphenols in Urine, on Cardiovascular Risk Factors After a Long-Term Follow-Up in the PREDIMED Study. Oxid. Med. Cell Longev. 2016, 2016, 2572606. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Int. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Estruch, R.; Salas-Salvadò, J.; Martínez-Gonzalez, M.A.; Arós, F.; Vila, J.; Corella, D.; Díaz, O.; Sáez, G.; de la Torre, R.; et al. Effect of the Mediterranean diet on heart failure biomarkers: A randomized sample from the PREDIMED trial. Eur. J. Heart Fail. 2014, 16, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Rojo-Martinez, G.; Goday, A.; Bosch-Comas, A.; Bordiu, E.; Caballero-Diaz, F.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; et al. Olive oil has a beneficial effect on impaired glucose regulation and other cardiometabolic risk factors. Di@bet.es study. Eur. J. Clin. Nutr. 2013, 67, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hruby, A.; Salas-Salvadò, J.; Martinez-Gonzàlez, M.A.; Sun, Q.; Willett, W.C.; Hu, F.B. Olive oil consumption and risk of type diabetes in US women. Am. J. Clin. Nutr. 2015, 102, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL-cholesterol in healthy subjects. Nutr. Diabetes 2015, 5, e172. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Int. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Hernáez, Á.; Remaley, A.T.; Farràs, M.; Fernández-Castillejo, S.; Subirana, I.; Schröder, H.; Fernández-Mampel, M.; Muñoz-Aguayo, D.; Sampson, M.; Solà, R.; et al. Olive Oil Polyphenols Decrease LDL Concentrations and LDL Atherogenicity in Men in a Randomized Controlled Trial. J. Nutr. 2015, 145, 1692–1697. [Google Scholar] [CrossRef]
- Hernáez, Á.; Fernàndez-Castillejo, S.; Farràs, M.; Catalán, Ú.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, Ó.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans. A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrera, A.; Delgado-Lista, J.; Torres-Sanchez, L.A.; Rangel-Zuñiga, O.A.; Camargo, A.; Moreno-Navarrete, J.M.; Garcia-Olid, B.; Quintana-Navarro, G.M.; Alcala-Diaz, J.F.; Muñoz-Lopez, C.; et al. The postprandial inflammatory response after ingestion of heated oils in obese persons is reduced by the presence of phenol compounds. Mol. Nutr. Food Res. 2012, 56, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Rainer, M.; Mucke, H.; Schlaefke, S. Ginkgo biloba extract EGb in the treatment of dementia: A pharmacoeconomic analysis of the Austrian setting. Wien Klin. Wochenschr. 2013, 125, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of mg. J. Psychiatr. Res. 2012, 46, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Bachinskaya, N.; Hoerr, R.; Ihl, R. Alleviating neuropsychiatric symptoms in dementia: The effects of Ginkgo biloba extract EGb 761. Findings from a randomized controlled trial. Neuropsychiatr. Dis. Treat. 2011, 7, 209–215. [Google Scholar]
- Oliveras-López, M.J.; Molina, J.J.; Mir, M.V.; Rey, E.F.; Martin, F.; de la Serrana, H.L. Extra virgin olive oil (EVOO) consumption and antioxidant status in healthy institutionalized elderly humans. Arch. Gerontol. Geriatr. 2013, 57, 234–242. [Google Scholar] [CrossRef]
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2772–2795. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Féart, C.; Samieri, C.; Allès, B.; Barberger-Gateau, P. Potential benefits of adherence to the Mediterranean diet on cognitive health. Proc. Nutr. Soc. 2013, 72, 140–152. [Google Scholar] [CrossRef]
- Knight, A.; Bryan, J.; Wilson, C.; Hodgson, J.; Murphy, K. A randomised controlled intervention trial evaluating the efficacy of a Mediterranean dietary pattern on cognitive function and psychological wellbeing in healthy older adults: The MedLey study. BMC Geriatr. 2015, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Int. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Yamada, M. Curcumin and Alzheimer’s Disease. CNS Neurosci. Ther. 2010, 16, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; da Rosa, J.C.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.L.; Breslow, J.; Holt, P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. J. Clin. Transl. Res. 2018, 4, 122–135. [Google Scholar]
- Najaf Najafi, M.; Salehi, M.; Ghazanfarpour, M.; Hoseini, Z.S.; Khadem-Rezaiyan, M. The association between green tea consumption and breast cancer risk: A systematic review and meta-analysis. Phytother. Res. 2018, 32, 1855–1864. [Google Scholar] [CrossRef]
- Kristen, A.V.; Lehrke, S.; Buss, S.; Mereles, D.; Steen, H.; Ehlermann, P.; Hardt, S.; Giannitsis, E.; Schreiner, R.; Haberkorn, U.; et al. Green tea halts progression of cardiac transthyretin amyloidosis: An observational report. Clin. Res. Cardiol. 2012, 101, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.J.; Perry, E. The role of phytochemicals in the treatment and prevention of dementia. Drugs Aging 2011, 28, 439–468. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.F.; Mao, X.Y.; Du, M. Prevention of breast cancer by dietary polyphenols-role of cancer stem cells. Crit. Rev. Food Sci. Nutr. 2019, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gardeazabal, I.; Romanos-Nanclares, A.; Martínez-González, M.A.; Sánchez-Bayona, R.; Vitelli-Storelli, F.; Gaforio, J.J.; Aramendía-Beitia, J.M.; Toledo, E. Total polyphenol intake and breast cancer risk in the SUN cohort. Br. J. Nutr. 2018, 27, 1–23. [Google Scholar]
- Afshari, K.; Haddadi, N.S.; Haj-Mirzaian, A.; Farzaei, M.H.; Rohani, M.M.; Akramian, F.; Naseri, R.; Sureda, A.; Ghanaatian, N.; Abdolghaffari, A.H. Natural flavonoids for the prevention of colon cancer: A comprehensive review of preclinical and clinical studies. J. Cell Physiol. 2019, 234, 21519–21546. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. Biomed. Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef]
- De Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Secoiridoids delivered as olive leaf extract onduce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomized, double-blind, placebo-controlled, crioss over trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabbà, C.; Palasciano, G.; Moschetta, A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Biophys. Acta 2016, 1861, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Carpi, S.; Massaro, M.; Pellegrino, M.; Polini, B.; Carluccio, M.A.; Wabitsch, M.; Verri, T.; Nieri, P.; De Caterina, R. Hydroxytyrosol modulates adipocyte gene and miRNA expression under inflammatory condition. Nutrients 2019, 11, 2493. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Gualtieri, P.; Gratteri, S.; Ali, W.; Sergi, D.; Muscoli, S.; Cammarano, A.; Bernardini, S.; Di Rienzo, L.; Romeo, E. Effects of postprandial hydroxytyrosol and derivates on oxidation of LDL, cardiometabolic state and gene expression: A nurtrigenomic approach for cardiovascular prevention. J. Cardiovasc. Med. 2019, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Botek, M.; McKune, A.J.; Sergi, D.; Georgousopoulou, E.; Mellor, D.D.; Naumovski, N. The effect of dietary polyphenols on circulating cardiovascular disease biomarkers and iron status: A systematic review. Nutr. Metab. Insights 2019, 12, 1178638819882739. [Google Scholar] [CrossRef]
- Carlos, S.; De La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Razquin, C.; Rico-Campà, A.; Martínez-González, M.; Ruiz-Canela, A. Mediterranean Diet and Health Outcomes in the SUN Cohort. Nutrients 2018, 10, E439. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis Defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis and Ginkgo biloba (GB): Numerous biological effects of GB are mediated via hormesis. Ageing Res. Rev. 2019. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, D.; Kapoor, R.; Mattson, M.P.; Rattan, S.I.S. Curcumin and hormesis with particular emphasis on neural cells. Food Chem. Toxicol. 2019, 129, 399–404. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. The hormesis database: The occurrence of hormetic dose responses in the toxicological literature. Reg. Toxicol. Pharmacol. 2011, 61, 73–81. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Abbasi, N.; Akhavan, M.M.; Rohbar-Roshandel, N.; Shafiei, M. The effects of low and high concentrations of luteolin on cultured human endothelial cells under normal and glucotoxic conditions: Involvement of integrin-linked kinase and cyclooxygenase-2. Phtyother. Res. 2014, 28, 1301–1307. [Google Scholar] [CrossRef]
- Abbasi, N.; Khosravi, A.; Aidy, A.; Shafiei, M. Biphasic response to luteolin in MG-osteobalst-like cells under high glucose-induced oxidative stress. IJMS 2016, 41, 118–125. [Google Scholar]
- Moghadam, F.H.; Mesbah-Artdakani, M.; Nasr-Esfahani, M.H. Ferulic acid exerts concentration-dependent anti-apoptotic and neuronal differentiation-inducing effects in PCand mouse neural stem cells. Eur. J. Pharmacol. 2018, 741, 104–112. [Google Scholar] [CrossRef]
- Woo, S.M.; Choi, W.R.; Jang, D.; Yi, C.S.; Kim, H.L.; Kim, K.H.; Kim, J.T.; Choi, W.H.; Hang, S.H.; Kim, M.J.; et al. Immune enhancement effect of an herb complex extract through the activation of natural killer cells and the regulation of cytokine levels in a cyclophosphamide-induced immunosuppression rat model. Asian Pacif. J. Trop. Med. 2018, 11, 653–658. [Google Scholar]
- Trovato Salinaro, A.; Cornelius, C.; Koverech, G.; Koverech, A.; Scuto, M.; Lodato, F.; Fronte, V.; Muccilli, V.; Reibaldi, M.; Longo, A.; et al. Cellular stress response, redox status, and vitagenes in glaucoma: A systemic oxidant disorderlinked to Alzheimer’s disease. Front. Pharmacol. 2014, 5, 129. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to post-natal lethality due to constitutive Nrfactivation. Nat. Genet. 2003, 35, 238–245. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. RedoxSignal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing 2018, 15, 8. [Google Scholar] [CrossRef]
- Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Trovato Salinaro, A.; Maiolino, L.; Calabrese, V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention inNeuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2019, 21, E284. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis and disease resistance: Activation of cellular stressresponse pathways. Hum. Exp. Toxicol. 2008, 27, 155–162. [Google Scholar] [CrossRef]
- Cornelius, C.; Trovato Salinaro, A.; Scuto, M.; Fronte, V.; Cambria, M.T.; Pennisi, M.; Bella, R.; Milone, P.; Graziano, A.; Crupi, R.; et al. Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: Role of vitagenes. Immun. Ageing 2013, 10, 41. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R.; Calabrese, V. The hemeoxygenase/biliverdin reductase system: A potential drug target in Alzheimers disease. J. Biol. Regul. Homeost. Agents 2013, 27 (Suppl. 2), 75–87. [Google Scholar]
- Naviaux, R.K. Metabolic features and regulation of the healing cycle-A new modelfor chronic disease pathogenesis and treatment. Mitochondrion 2019, 46, 278–297. [Google Scholar] [CrossRef]
- Scuto, M.C.; Mancuso, C.; Tomasello, B.; Laura Ontario, M.; Cavallaro, A.; Frasca, F.; Maiolino, L.; Trovato Salinaro, A.; Calabrese, E.J.; Calabrese, V. Curcumin, Hormesis and the Nervous System. Nutrients 2019, 11, 2417. [Google Scholar] [CrossRef]
- Frey, B.; Hehlgans, S.; Rodel, F.; Gaipl, U.S. Modulation of inflamma- tion by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef]
- Large, M.; Hehlgans, S.; Reichert, S.; Gaipl, U.S.; Fournier, C.; Rodel, C.; Weiss, C.; Rodel, F. Study of the anti- inflammatory effect of low-dose radiation. The contribution of biphasic regulation of the antioxidative system in endothelial cells. Strahlenther. Onkol. 2015, 191, 742–749. [Google Scholar] [CrossRef]
- Wunderlich, R.; Ernst, A.; Roedel, F.; Fietkau, R.; Ott, O.; Lauber, K.; Frey, B.; Gaipl, U.S. Low and moderate doses of ionizing radiation up to Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin. Exp. Immunol. 2015, 179, 50–61. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.T.; Maiolino, L.; et al. Redox modulation of cellular stress response and lipoxin Aexpression by Hericium Erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016, 13, 23. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Fronte, V.; Koverech, G.; Luca, M.; Serra, A.; Toscano, M.A.; Petralia, A.; et al. Redox modulation of cellular stress response and lipoxin Aexpression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology 2016, 53, 350–358. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hong, J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Tan, L. The NLRPinflammasome in Alzheimer’s disease. Mol. Neurobiol. 2013, 48, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Cedillos, R.; Choyke, S.; Lukic, Z.; McGuire, K.; Marvin, S.; Burrage, A.M.; Sudholt, S.; Rana, A.; O’Connor, C.; et al. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxy-gen species following endocytosis. PLoS ONE 2013, 8, e62143. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRPis activated in Alzheimer’s disease and contributes to pathology in APP/PSmice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Codolo, G.; Plotegher, N.; Pozzobon, T.; Brucale, M.; Tessari, I.; Bubacco, L.; de Bernard, M. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE 2013, 8, e55375. [Google Scholar] [CrossRef]
- Kostomoiri, M.; Fragkouli, A.; Sagnou, M.; Skaltsounis, L.A.; Pelecanou, M.; Tsilibary, E.C.; Tzinia, A.K. Oleuropein, an anti-oxidant polyphenol constituent of olivepromotes α-secretase cleavage of the amyloid precursor protein (AβPP). Cell. Mol. Neurobiol. 2013, 33, 147–154. [Google Scholar] [CrossRef]
- Ladiwala, A.R.; Mora-Pale, M.; Lin, J.C.; Bale, S.S.; Fishman, Z.S.; Dordick, J.S.; Tessier, P.M. Polyphenolic glycosides and aglycones utilize opposing pathways to selectively remodel and inactivate toxic oligomers of amyloid β. Chembiochem 2011, 12, 1749–1758. [Google Scholar] [CrossRef]
- Kim, M.H.; Min, J.S.; Lee, J.Y.; Chae, U.; Yang, E.J.; Song, K.S.; Lee, H.S.; Lee, H.J.; Lee, S.R.; Lee, D.S. Oleuropein isolated from Fraxinus rhynchophylla inhibits glutamate-induced neuronal cell death by attenuating mitochondrial dysfunction. Nutr. Neurosci. 2018, 21, 520–528. [Google Scholar] [CrossRef]
- Diomede, L.; Rigacci, S.; Romeo, M.; Stefani, M.; Salmona, M. Oleuropein Aglycone Protects Transgenic, C. elegans Strains Expressing Aβby Reducing Plaque Load and Motor Deficit. PLoS ONE 2013, 8, e58893. [Google Scholar] [CrossRef]
- Shibani, F.; Sahamsizadeh, A.; Fatemi, I.; Allahtavakoli, M.; Hasanshahi, J.; Rahmani, M.; Azin, M.; Hassanipour, M.; Mozafari, N.; Kaeidi, A. Effect of oleuropein on morphine-induced hippocampus neurotoxicity and memory impairments in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1383–1391. [Google Scholar] [CrossRef]
- De la Torre, R.; Covas, M.I.; Pujadas, M.A.; Fito, M.; Farre, M. Is dopamine behind the health benefits of red wine? Eur. J. Nutr. 2006, 45, 307–310. [Google Scholar] [CrossRef]
- Xu, C.L.; Sim, M.K. Reduction of dihydroxyphenylacetic acid by a novel enzyme in the rat brain. Biochem. Pharmacol. 1995, 50, 1333–1337. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Jinsmaa, Y.; Sullivan, P.; Holmes, C.; Kopin, I.J.; Sharabi, Y. 3,4-Dihydroxyphenylethanol (Hydroxytyrosol) Mitigates the Increase in Spontaneous Oxidation of Dopamine During Monoamine Oxidase Inhibition in PCCells. Neurochem. Res. 2016, 41, 2173–2178. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Isonaka, R.; Sharabi, Y.; Goldstein, D. 3,4-Dihydroxyphenylacetaldehyde is more efficient than dopamine in oligomerizing and quinonizing alpha-synuclein. J. Pharmacol. Exp. Ther. 2019. [Google Scholar] [CrossRef]
- Safouris, A.; Tsivgoulis, G.; Sergentanis, T.N.; Psaltopoulou, T. Mediterranean Diet and Risk of Dementia. Curr. Alzheimer Res. 2015, 12, 736–744. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Sergentanis, T.N. Mediterranean diet may reduce Alzheimer’s risk. Evid. Based. Med. 2015, 20, 202. [Google Scholar] [CrossRef]
- Peng, Y.; Hou, C.; Yang, Z.; Li, C.; Jia, L.; Liu, J.; Tang, Y.; Shi, L.; Li, Y.; Long, J.; et al. Hydroxytyrosol mildly improve cognitive function independent of APP processing inAPP/PSmice. Mol. Nutr. Food Res. 2016, 60, 2331–2342. [Google Scholar] [CrossRef]
- Palazzi, L.; Leri, M.; Cesaro, S.; Stefani, M.; Bucciantini, M.; Polverino de Laureto, P. Insight into the molecular mechanism underlying the inhibition of α-synuclein aggregation by hydroxytyrosol. Biochem. Pharmacol. 2019, in press. [Google Scholar] [CrossRef]
- Reutzel, M.; Grewal, R.; Silaidos, C.; Zotzel, J.; Marx, S.; Tretzel, J.; Eckert, G.P. Effects of Long-Term Treatment with a Blend of Highly Purified Olive Secoiridoids on Cognition and Brain ATP Levels in Aged NMRI Mice. Oxid. Med. Cell. Longev. 2018, 2018, 4070935. [Google Scholar] [CrossRef]
- Funakohi-Tago, M.; Sakata, T.; Fujiwara, S.; Sakakura, A.; Sugai, T.; Tago, K.; Tamura, H. Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-axis in SH-SY5Y cells. Eur. J. Pharmacol. 2018, 834, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, X.; Hou, C.; Yang, L.; Li, H.; Guo, J.; Huo, C.; Wang, M.; Miao, Y.; Liu, J.; et al. Oleuropein improves mitochondrial function to attenuate oxidative stress by activating the Nrfpathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Neuropharmacology 2017, 113, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Kusunoki, M.; Miyazaki, H. Role of Hydroxytyrosol-dependent Regulation of HO-Expression in Promoting Wound Healing of Vascular Endothelial Cells via NrfDe Novo Synthesis and Stabilization. Phytother. Res. 2015, 29, 1011–1018. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2018, 17, 1493–1498. [Google Scholar] [CrossRef]
- Montoya, T.; Aparicio-Soto, M.; Castejón, M.L.; Rosillo, M.Á.; Sánchez-Hidalgo, M.; Begines, P.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-induced inflammatory response in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HOand JAK/STAT signaling pathways. J. Nutr. Biochem. 2018, 57, 110–120. [Google Scholar] [CrossRef]
- Kumar, R.; Nigam, L.; Singh, A.P.; Singh, K.; Subbarao, N.; Dey, S. Design, synthesis of allosteric peptide activator for human SIRTand its biological evaluation in cellular model of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 909–916. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. SIRTin neurodevelopment and brain senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.Y.; Mao, Y.W.; Gräff, J.; Guan, J.S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.H. A novel pathway regulates memory and plasticity via SIRTand miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef]
- Zhi, L.Q.; Yao, S.X.; Liu, H.L.; Li, M.; Duan, N.; Ma, J.B. Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via SIRT6-mediated autophagy. Mol. Med. Rep. 2018, 17, 4035–4042. [Google Scholar] [CrossRef]
- Sun, T.; Chen, Q.; Zhu, S.Y.; Wu, Q.; Liao, C.R.; Wang, Z.; Wu, X.H.; Wu, H.T.; Chen, J.T. Hydroxytyrosol promotes autophagy by regulating SIRTagainst advanced oxidation protein product-induced NADPH oxidase and inflammatory response. Int. J. Mol. Med. 2019, 44, 1531–1540. [Google Scholar] [PubMed]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin, protocatechuic acid and hydroxytyrosol effects on vitagenes system against alpha-synuclein toxicity. Food Chem. Toxicol. 2019, 134, 110817. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jing, T.; Yang, X.; He, Y.; Wang, B.; Xiao, Y.; Shang, C.; Zhang, J.; Lin, R. Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1-mediated signaling pathway. Can. J. Physiol. Pharmacol. 2018, 96, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jing, T.; Li, Y.; He, Y.; Zhang, W.; Wang, B.; Xiao, Y.; Wang, W.; Zhang, J.; Wei, J.; et al. Hydroxytyrosol Attenuates LPS-Induced Acute Lung Injury in Mice by Regulating Autophagy and Sirtuin Expression. Curr. Mol. Med. 2017, 17, 149–159. [Google Scholar] [CrossRef]
- Calabrese, V.; Scapagnini, G.; Davinelli, S.; Koverech, G.; Koverech, A.; De Pasquale, C.; Salinaro, A.T.; Scuto, M.; Calabrese, E.J.; Genazzani, A.R. Sex hormonal regulation and hormesis in aging and longevity: Role of vitagenes. J. Cell. Commun. Signal. 2014, 8, 369–384. [Google Scholar] [CrossRef]
- Amara, I.; Timoumi, R.; Annabi, E.; Di Rosa, G.; Scuto, M.; Najjar, M.F.; Calabrese, V.; Abid-Essefi, S. Di (2-ethylhexyl) phthalate targets the thioredoxin system and the oxidative branch of the pentose phosphate pathway in liver of Balb/c mice. Environ Toxicol. 2020, 35, 78–86. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, B.; Yao, J.; Duan, D.; Fang, J. Dual protection of hydroxytyrosol, an olive oil polyphenol, against oxidative damage in PCcells. Food Funct. 2015, 6, 2091–2100. [Google Scholar] [CrossRef]
- Zhang, Y.; Ahn, Y.H.; Benjamin, I.J.; Honda, T.; Hicks, R.J.; Calabrese, V.; Cole, P.A.; Dinkova-Kostova, A.T. HSF1-dependent upregulation of Hspby sulfhydryl-reactive inducers of the KEAP1/NRF2/ARE pathway. Chem. Biol. 2011, 18, 1355–1361. [Google Scholar] [CrossRef]
- An, Y.W.; Jhang, K.A.; Woo, S.Y.; Kang, J.L.; Chong, Y.H. Sulforaphane exerts its anti-inflammatory effect against amyloid-β peptide via STAT-dephosphorylation and activation of Nrf2/HO-cascade in human THP-macrophages. Neurobiol. Aging 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.R.; Kim, J.; La Ferla, F.M.; Park, J.H.Y.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears Amyloid-β and Tau in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2018, 62, e1800240. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Li, W.F.; Li, F.; Zhang, Z.; Dai, Y.D.; Xu, A.L.; Qi, C.; Gao, J.M.; Gao, J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem. Biophys. Res. Commun. 2013, 435, 597–602. [Google Scholar] [CrossRef]
- Laudati, G.; Mascolo, L.; Guida, N.; Sirabella, R.; Pizzorusso, V.; Bruzzaniti, S.; Serani, A.; Di Renzo, G.; Canzoniero, L.M.T.; Formisano, L. Resveratrol treatment reduces the vulnerability of SH-SY5Y cells and cortical neurons overexpressing SOD1-G93A to Thimerosal toxicity through SIRT1/DREAM/PDYN pathway. Neurotoxicology 2019, 71, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, L.; Liu, X.; Lu, T.; Xie, C.; Jia, J. Resveratrol Attenuates Microglial Activation via SIRT1-SOCSPathway. Evid. Based. Complement Alternat. Med. 2017, 2017, 8791832. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Feng, J.; Zhang, R.; Chen, J.; Han, D.; Li, X.; Yang, B.; Li, X.; Fan, M.; Li, C.; et al. SIRTActivation by Resveratrol Alleviates Cardiac Dysfunction via Mitochondrial Regulation in Diabetic Cardiomyopathy Mice. Oxid. Med. Cell. Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Chengyong, T.; Cheng, L.; Haixia, H.; Yuanda, Z.; Weihua, Y. Resveratrol attenuates the cytotoxicity induced by amyloid-β 1–in PCcells by upregulating heme oxygenase-via the PI3K/Akt/Nrfpathway. Neurochem. Res. 2018, 43, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, Y.; Yu, H.; Shan, A.; Shen, J.; Zhou, C.; Zhao, Y.; Fang, H.; Wang, X.; Wang, J.; et al. Resveratrol inhibits aflatoxin B1-induced oxidative stress and apoptosis in bovine mammary epithelial cells and is involved the Nrfsignaling pathway. Toxicon 2019, 164, 10–15. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L. Resveratrol Suppresses Aβ-Induced Microglial Activation Through the TXNIP/TRX/NLRPSignaling Pathway. DNA Cell Biol. 2019, 38, 874–879. [Google Scholar] [CrossRef]
- Mhillaj, E.; Cuomo, V.; Trabace, L.; Mancuso, C. The HemeOxygenase/Biliverdin Reductase System as Effector of the Neuroprotective Outcomes of Herb-BasedNutritionalSupplements. Front. Pharmacol. 2019, 10, 1298. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Weng, Q.; Xiao, L.; Li, Q. Curcumin analogues attenuate Aβ 25—Induced oxidative stress in PCcells via Keap1/Nrf2/HO-signaling pathways. Chem. Biol. Interact. 2019, 305, 171–179. [Google Scholar] [CrossRef]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRPInflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef] [PubMed]
- Maggi, S.; Noale, M.; Gallina, P.; Bianchi, D.; Marzari, C.; Limongi, F.; Crepaldi, G. ILSA Working Group. Metabolic syndrome, diabetes, and cardiovascular disease in an elderly Caucasian cohort: The Italian Longitudinal Study on Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Schnack, L.L.; Romani, A.M.P. The Metabolic Syndrome and the Relevance of Nutrients for its Onset. Recent Pat. Biotechnol. 2017, 11, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Naska, A.; DAFNE III Group. European food availability databank based on household budget surveys: The Data Food Networking initiative. Eur. J. Public Health 2003, 13 (Suppl. 3), 24–28. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Peltonen, M.; Eriksson, J.G.; Louheranta, A.; Fogelholm, M.; Uusitupa, M.; Tuomilehto, J. High-fibre, low-fat diet predicts long-term weight loss and decreased type diabetes risk: The Finnish Diabetes Prevention Study. Diabetologia 2006, 49, 912–920. [Google Scholar] [CrossRef]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Valdivielso, P.; Puerta, S.; Rioja, J.; Alonso, I.; Ariza, M.J.; Sánchez-Chaparro, M.A.; Palacios, R.; González-Santos, P. Postprandial apolipoprotein Bis associated with asymptomatic peripheral arterial disease: A study in patients with type diabetes and controls. Clin. Chim. Acta 2010, 411, 433–437. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S.H. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef]
- Meigs, J.B.; Nathan, D.M.; D’Agostino, R.B., Sr.; Wilson, P.W.; Framingham Offspring Study. Fasting and postchallenge glycemia and cardiovascular disease risk: The Framingham offspring study. Diabetes Care 2002, 25, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Harte, A.L.; Varma, M.C.; Tripathi, G.; McGee, K.C.; Al-Daghri, N.M.; Al-Attas, O.S.; Sabico, S.; O’Hare, J.P.; Ceriello, A.; Saravanan, P.; et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type diabetic subjects. Diabetes Care 2012, 35, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Nocella, C.; Carnevale, R.; Bartimoccia, S.; Novo, M.; Cangemi, R.; Pastori, D.; Calvieri, C.; Pignatelli, P.; Violi, F. Lipopolysaccharide as trigger of platelet aggregation via eicosanoid over-production. Thromb. Haemost. 2017, 117, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- El-Amin, M.; Virk, P.; Elobeid, M.A.; Almarhoon, Z.M.; Hassan, Z.K.; Omer, S.A.; Merghani, N.M.; Daghestani, M.H.; Al-Olayan, E.M. Anti-diabetic effect of Murraya koenigii (L) and Olea europaea (L) leaf extracts on streptozotocin induced diabetic rats. Pak. J. Pharm. Sci. 2013, 26, 359–365. [Google Scholar]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra virgin olive oil blunt postprandial oxidative stress via NOXdown-regulation. Atherosclerosis 2014, 35, 649–658. [Google Scholar] [CrossRef]
- Carnevale, R.; Pastori, D.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Novo, M.; Del Ben, M.; Farcomeni, A.; Angelico, F.; Violi, F. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Noxactivation in patients with impaired fasting glucose tolerance: Effect of extra-virgin olive oil. Eur. J. Nutr. 2019, 58, 843–851. [Google Scholar] [CrossRef]
- Wilson, T.; Singh, A.P.; Vorsa, N.; Goettl, C.D.; Kittleson, K.M.; Roe, C.M.; Kastello, G.M.; Ragsdale, F.R. Human glycemic response and phenolic content of unsweetened cranberry juice. J. Med. Food 2008, 11, 46–54. [Google Scholar] [CrossRef]
- Calahorra, J.; Shenk, J.; Wielanga, V.H.; Verweij, V.; Geenen, B.; Dederen, P.J.; Peinado, M.A.; Siles, E.; Wiesmann, M.; Kiliaan, A.J. Hydroxytyroaol, the major phenolic compound of olive oil, as as acute therapeutic strategy after ischemic stroke. Nutrients 2019, 11, 2430. [Google Scholar] [CrossRef]
- Thilagam, E.; Parimaladevi, B.; Kumarappan, C.; Mandal, S.C. α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. J. Acupunct. Meridian Stud. 2013, 6, 24–30. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Fujiwara, M.; Yao, Z. Postprandial hyperglycemia and postprandial hypertriglyceridemia in type diabetes. J. Biomed. Res. 2019, 33, 1–16. [Google Scholar]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. alpha-Glucosidase inhibitory profile of catechins and theaflavins. J. Agric. Food Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Park, C.E.; Kim, M.J.; Lee, J.H.; Min, B.I.; Bae, H.; Choe, W.; Kim, S.S.; Ha, J. Resveratrol stimulates glucose transport in C2Cmyotubes by activating AMP-activated protein kinase. Exp. Mol. Med. 2007, 39, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid inhibition of sodium-dependent vitamin C transporter (SVCT1) and glucose transporter isoform (GLUT2), intestinal transporters for vitamin C and Glucose. J. Biol. Chem. 2002, 277, 15252–15260. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Santoro, A.; Trovato Salinaro, A.; Modafferi, S.; Scuto, M.; Albouchi, F.; Monti, D.; Giordano, J.; Zappia, M.; Franceschi, C.; et al. Hormetic approaches to the treatment of Parkinson’s disease: Perspectives and possibilities. J. Neurosci. Res. 2018, 96, 1641–1662. [Google Scholar] [CrossRef] [PubMed]
- Montagut, G.; Onnockx, S.; Vaqué, M.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pirson, I. Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J. Nutr. Biochem. 2010, 21, 476–481. [Google Scholar] [CrossRef]
- Cummings, E.; Hundal, H.S.; Wackerhage, H.; Hope, M.; Belle, M.; Adeghate, E.; Singh, J. Momordica charantia fruit juice stimulates glucose and amino acid uptakes in Lmyotubes. Mol. Cell. Biochem. 2004, 261, 99–104. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Shibayama, Y.; Inoue, S.; Soga, M.; Takikawa, M.; Ito, C.; Nanba, F.; Yoshida, T.; Yamashita, Y.; Ashida, H.; et al. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. J. Agric. Food Chem. 2013, 61, 5558–5564. [Google Scholar] [CrossRef]
- Babu, P.V.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Li, Q.; Liang, J.; Dai, X.Q.; Ding, Y.; Wang, J.B.; Li, Y. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat lmuscle cells exposed to dexamethasone condition. Phytomedicine 2010, 17, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Mahmoudi, A.; Bouallagui, Z.; Feki, I.; Isoda, H.; Feve, B.; Sayadi, S. Evaluation of hypocholesterolemic effect of oleuropein in cholesterol-fed rats. Chem. Biol. Interact. 2016, 252, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Lin, J.K. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRSand AMPK signaling in rat pancreatic beta cells. J. Agric. Food Chem. 2009, 57, 9817–9827. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; da Silva, X.G.; Leclerc, I. Roles of -AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem. J. 2003, 375, 1–16. [Google Scholar] [CrossRef]
- Kim, E.K.; Kwon, K.B.; Song, M.Y.; Han, M.J.; Lee, J.H.; Lee, Y.R.; Lee, J.H.; Ryu, D.G.; Park, B.H.; Park, J.W. Flavonoids protect against cytokine-induced pancreatic beta-cell damage through suppression of nuclear factor κB activation. Pancreas 2007, 35, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Drira, R.; Chen, S.; Sakamoto, K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in T3-Lcells. Life Sci. 2011, 89, 708–716. [Google Scholar] [CrossRef]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-Ladipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated/SNFprotein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Sabatini, D.M. mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Koh, H.; Kim, M.; Kim, Y.; Lee, S.Y.; Karess, R.E.; Lee, S.H.; Shong, M.; Kim, J.M.; Kim, J.; et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 2007, 447, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging (Albany N. Y.) 2019, 11, 2217–2240. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget 2015, 6, 35344–35357. [Google Scholar] [CrossRef] [PubMed]
- Bronner, M.; Hertz, R.; Bar-Tana, J. Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor alpha by AMP-activated protein kinase. Biochem. J. 2004, 384, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Marsin, A.S.; Bouzin, C.; Bertrand, L.; Hue, L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 2002, 277, 30778–30783. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Fletcher, J.M.; Dunne, A. Plant-Derived Polyphenols Modulate Human Dendritic Cell Metabolism and Immune Function via AMPK-Dependent Induction of Heme Oxygenase-1. Front. Immunol. 2019, 10, 345. [Google Scholar] [CrossRef]
- Kundu, J.; Kim, D.H.; Kundu, J.K.; Chun, K.S. Thymoquinone induces heme oxygenase-expression in HaCaT cells via Nrf2/ARE activation: Akt and AMPKα as upstream targets. Food Chem. Toxicol. 2014, 65, 18–26. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Schroder, A.; Reyes-Reyes, E.M.; Franco, R. mTOR/AMPK signaling in the brain: Cell metabolism, proteostasis and survival. Curr. Opin. Toxicol. 2018, 8, 102–110. [Google Scholar] [CrossRef]
- Li, X.N.; Song, J.; Zhang, L.; LeMaire, S.A.; Hou, X.; Zhang, C.; Coselli, J.S.; Chen, L.; Wang, X.L.; Zhang, Y.; et al. Activation of the AMPK-FOXOpathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes 2009, 58, 2246–2257. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK(hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Collodet, C.; Foretz, M.; Deak, M.; Bultot, L.; Metairon, S.; Viollet, B.; Lefebvre, G.; Raymondm, F.; Parisi, A.; Civiletto, G.; et al. AMPK promotes induction of the tumor suppressor FLCN through activation of TFEB independently of mTOR. FASEB J. 2019, 33, 12374–12391. [Google Scholar] [CrossRef] [PubMed]
- Furtado, L.M.; Somwar, R.; Sweeney, G.; Niu, W.; Klip, A. Activation of the glucose transporter GLUTby insulin. Biochem. Cell Biol. 2002, 80, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Kanzaki, M.; Pessin, J.E. Regulated membrane trafficking of the insulin-responsive glucose transporter in adipocytes. Endocr. Rev. 2004, 25, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.L. TSCmediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRTin cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, A.; Feng, X.; Hou, T.; Liu, K.; Liu, B.; Zhang, N. Metformin and resveratrol ameliorate muscle insulin resistance through preventing lipolysis and inflammation in hypoxic adipose tissue. Cell Signal. 2016, 28, 1401–1411. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, H.F.; Gi, S.J.; Chi, T.H.; Cheng, C.K.; Hsu, C.F.; Ma, Y.S.; Wei, Y.H.; Liu, C.S.; Hsieh, M. Decreased heat shock protein expression and altered autophagy in human cells harboring A8344G mitochondrial DNA mutation. Mitochondrion 2011, 11, 739–749. [Google Scholar] [CrossRef]
- Carra, S.; Rusmini, P.; Crippa, V.; Giorgetti, E.; Boncoraglio, A.; Cristofani, R.; Naujock, M.; Meister, M.; Minoia, M.; Kampinga, H.H.; et al. Different anti-aggregation and pro-degradative functions of the members of the mammalian sHSP family in neurological disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20110409. [Google Scholar] [CrossRef]
- Vos, M.J.; Zijlstra, M.P.; Carra, S.; Sibon, O.C.; Kampinga, H.H. Small heat shock proteins, protein degradation and protein aggregation diseases. Autophagy 2011, 7, 101–103. [Google Scholar] [CrossRef]
- Korolchuk, V.I.; Mansilla, A.; Menzies, F.M.; Rubinsztein, D.C. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol. Cell 2009, 33, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Baixauli, F.; López-Otín, C.; Mittelbrunn, M. Exosomes and autophagy: Coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014, 5, 403. [Google Scholar] [CrossRef] [PubMed]
- Labbadia, J.; Morimoto, R.I. The Biology of Proteostasis in Aging and Disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Wang, T.; Araujo, T.L.S.; Sharma, S.; Brodsky, J.L.; Chiosis, G. Adapting to stress—Chaperome networks in cancer. Nat. Rev. Cancer 2018, 18, 562–575. [Google Scholar] [CrossRef]
- Han, Y.S.; Bastianetto, S.; Dumont, Y.; Quirion, R. Specific plasma membrane binding sites for polyphenols, including resveratrol, in the rat brain. J. Pharmacol. Exp. Ther. 2006, 318, 238–245. [Google Scholar] [CrossRef]
- Chung, J.H.; Manganiello, V.; Dyck, J.R. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012, 22, 546–554. [Google Scholar] [CrossRef]
- Lan, F.; Weikel, K.A.; Cacicedo, J.M.; Ido, Y. Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application. Nutrients 2017, 9, 751. [Google Scholar] [CrossRef]
- Miki, H.; Uehara, N.; Kimura, A.; Tsubura, A. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int. J. Oncol. 2012, 40, 1020–1028. [Google Scholar] [CrossRef]
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Singh Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIMand the mTOR pathway. Mol. Carcinog. 2016, 55, 818–831. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol Inhibits Breast Cancer Stem-Like Cells and Induces Autophagy via Suppressing Wnt/β-catenin Signaling Pathway. PLoS ONE 2014, 9, e102535. [Google Scholar] [CrossRef] [PubMed]
- Villa-Cuesta, E.; Boylan, J.N.M.; Tatar, M.; Gruppuso, P.A. Resveratrol inhibits protein translation in hepatic cells. PLoS ONE 2011, 6, e29513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.; Zhou, Y.; Lu, Y.; Wang, D. Regulation of eIF”α expression and renal interstitial fibrosis by resveratrol in rat renal tissue after unilateral uretral obstruction. Ren. Fail. 2016, 38, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef]
- Teiten, M.H.; Reuter, S.; Schmucker, S.; Dicato, M.; Diederich, M. Induction of heat shock response by curcumin in human leukemia cells. Cancer Lett. 2009, 279, 145–154. [Google Scholar] [CrossRef]
- Graikou, K.; Kapeta, S.; Aligiannis, N.; Sotiroudis, G.; Chondrogianni, N.; Gonos, E.; Chinou, I. Chemical analysis of Greek pollen—Antioxidant, antimicrobial and proteasome activation properties. Chem. Cent. J. 2011, 5, 33. [Google Scholar] [CrossRef]
- Kuzuhara, T.; Suganuma, M.; Fujiki, H. Green tea catechin as a chemical chaperone in cancer prevention. Cancer Lett. 2008, 261, 12–20. [Google Scholar] [CrossRef]
- De Rijk, M.C.; Breteler, M.M.; den Breeijen, J.H.; Launer, L.J.; Grobbee, D.E.; van der Meche, F.G.; Hofman, A. Dietary antioxidants and Parkinson disease. The Rotterdam Study. Arch. Neurol. 1997, 54, 762–765. [Google Scholar] [CrossRef]
- Miceli, C.; Santin, Y.; Manzella, N.; Coppini, R.; Berti, A.; Stefani, M.; Parini, A.; Mialet-Perez, J.; Nediani, C. Oleuropein aglycone protects against MAO-A-induced autophagy impairment and cardiomyocyte death through activation of TFEB. Oxid. Med. Cell. Long. 2018, 2018, 8067592. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, D.M.; Lee, S.H. NrfExpression and Apoptosis in Quercetin-treated Malignant Mesothelioma Cells. Mol. Cells 2015, 38, 416–425. [Google Scholar] [CrossRef]
- Kwak, M.K.; Wakabayashi, N.; Greenlaw, J.L.; Yamamoto, M.; Kensler, T.W. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrfsignaling pathway. Mol. Cell. Biol. 2003, 23, 8786–8794. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Rizzello, C.; Gilardini Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Korzus, E. Manipulating the brain with epigenetics. Nat. Neurosci. 2010, 13, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ushijima, T. Diagnostic and therapeutic applications of epigenetics. Jap. J. Clin. Oncol. 2005, 35, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Peedicayil, J. Epigenetic therapy: A new development in pharmacology. Indian J. Med. Res. 2006, 123, 17–24. [Google Scholar]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. J. Nutrigenet. Nutrigenom. 2010, 3, 220–230. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Ghantous, A.; Bajbouj, K.; Saikali, M.; Darwiche, N. Epigenetic mechanisms of plant-derived anticancer drugs. Front. Biosci. 2012, 17, 129–173. [Google Scholar] [CrossRef]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Hellebrekers, D.M.; Griffioen, A.W.; van Engeland, M. Dual targeting of epigenetic therapy in cancer. Biochim. Biophys. Acta 2007, 1775, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Lim, J.; Lee, S.; Jeong, J.; Kang, H.; Kim, Y.; Kang, J.W.; Yu, H.Y.; Jeong, E.M.; Kim, K.; et al. SirtRegulates DNA Methylation and Differentiation Potential of Embryonic Stem Cells by Antagonizing Dnmt3l. Cell Rep. 2017, 18, 1930–1945. [Google Scholar] [CrossRef] [PubMed]
- Fulco, M.; Sartorelli, V. Comparing and contrasting the roles of AMPK and SIRTin metabolic tissues. Cell Cycle 2008, 7, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Wang, X.N.; Huang, C.C.; Hu, L.; Xiao, Y.F.; Guan, X.H.; Qian, Y.S.; Deng, K.Y.; Xin, H.B. Inhibition of NAMPT aggravates high fat diet-induced hepatic steatosis in mice through regulating Sirt1/AMPKα/SREBPsignaling pathway. Lipids Health Dis. 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Bai, Q.; Zou, L.Y.; Zhang, Q.Y.; Zhou, Y.; Chang, H.; Yi, L.; Zhu, J.D.; Mi, M.T. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Geneschromosomes Cancer 2014, 53, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRTand DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef]
- Heger, V.; Tyni, J.; Hunyadi, A.; Horáková, L.; Lahtela-Kakkonen, M.; Rahnasto-Rilla, M. Quercetin based derivatives as sirtuin inhibitors. Biomed. Pharmacother. 2019, 111, 1326–1333. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729–741. [Google Scholar] [CrossRef]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (–)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Utley, R.T.; Ikeda, K.; Grant, P.A.; Côté, J.; Steger, D.J.; Eberharter, A.; John, S.; Workman, J.L. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 1998, 394, 498. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310. [Google Scholar] [CrossRef]
- Okonkwo, A.; Mitra, J.; Johnson, G.S.; Li, L.; Dashwood, W.M.; Hegde, M.L.; Yue, C.; Dashwood, R.H.; Rajendran, P. Heterocyclic analogs of sulforaphane trigger DNA damage and impede DNA repair in colon cancer cells: Interplay of HATs and HDACs. Mol. Nutr. Food Res. 2018, 62, 1800228. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Jung, M.G.; Lee, Y.H.; Yoon, J.C.; Kwon, S.H.; Kang, H.B.; Kim, M.J.; Cha, J.H.; Kim, Y.J.; Jun, W.J.; et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009, 69, 583–592. [Google Scholar] [CrossRef]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin suppresses cyclooxygenase-expression and angiogenesis through inactivation of Psignaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef]
- Lee, W.J.; Chen, Y.R.; Tseng, T.H. Quercetin induces FasL-related apoptosis, in part, through promotion of histone Hacetylation in human leukemia HL-cells. Oncol. Rep. 2011, 25, 583–591. [Google Scholar]
- Ling, D.; Marshall, G.M.; Liu, P.Y.; Xu, N.; Nelson, C.A.; Iismaa, S.E.; Liu, T. Enhancing the anticancer effect of the histone deacetylase inhibitor by activating transglutaminase. Eur. J. Cancer 2012, 48, 3278–3287. [Google Scholar] [CrossRef]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol. Cancer 2013, 4, 12–19. [Google Scholar] [CrossRef]
- Majid, S.; Dar, A.A.; Shahryari, V.; Hirata, H.; Ahmad, A.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Dahiya, R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene in prostate cancer. Cancer 2010, 116, 66–76. [Google Scholar] [CrossRef]
- Majid, S.; Dar, A.A.; Ahmad, A.E.; Hirata, H.; Kawakami, K.; Shahryari, V.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Khatri, G.; et al. BTGtumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer. Carcinogenesis 2009, 30, 662–670. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, Y.; Wen, Y.F.; Chen, Y.F.; Na, L.X.; Li, S.T.; Sun, C.H. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-induced by palmitate in 3T3-Ladipocytes through NF-kappa B and JNK pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef]
- Gräff, J.; Rei, D.; Guan, J.S.; Wang, W.Y.; Seo, J.; Hennig, K.M.; Nieland, T.J.; Fass, D.M.; Kao, P.F.; Kahn, M.; et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 2012, 483, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Lithner, C.U.; Lacor, P.N.; Zhao, W.Q.; Mustafiz, T.; Klein, W.L.; Sweatt, J.D.; Hernandez, C.M. Disruption of neocortical histone Hhomeostasis by soluble Aβ: Implications for Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.L.; Le, K.X.; Cynis, H.; Ekpo, E.; Kleinschmidt, M.; Palmour, R.M.; Ervin, F.R.; Snigdha, S.; Cotman, C.W.; Saido, T.C.; et al. Pyroglutamate-amyloid-β deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am. J. Pathol. 2013, 183, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Zeitschel, U.; Hoffmann, T.; Heiser, U.; Francke, M.; Kehlen, A.; Holzer, M.; Hutter-Paier, B.; Prokesch, M.; Windisch, M.; et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer’s disease-like pathology. Nat. Med. 2008, 14, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Kikuno, N.; Shiina, H.; Urakami, S.; Kawamoto, K.; Hirata, H.; Tanaka, Y.; Majid, S.; Igawa, M.; Dahiya, R. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int. J. Cancer 2008, 123, 552–560. [Google Scholar] [CrossRef]
- Juli, G.; Oliverio, M.; Bellizzi, D.; Gallo Cantafio, M.E.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma. Cancers 2019, 11, 990. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834. [Google Scholar] [CrossRef]
- Lopez-Serra, P.; Esteller, M. DNA methylation-associated silencing of tumor-suppressor microRNAs in cancer. Oncogene 2012, 31, 1609. [Google Scholar] [CrossRef]
- Calin, G.A.; Ferracin, M.; Cimmino, A.; Di Leva, G.; Shimizu, M.; Wojcik, S.E.; Iorio, M.V.; Visone, R.; Sever, N.I.; Fabbri, M. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 353, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. Transactivation of miR-34a by pbroadly influences gene expression and promotes apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell 2007, 26, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Kwok, T.T. Epigallocatechin gallate up-regulation of miR-and induction of apoptosis in human cancer cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Khandkar, M.; Banik, N.L.; Ray, S.K. Alterations in expression of specific microRNAs by combination of 4-HPR and EGCG inhibited growth of human malignant neuroblastoma cells. Brain Res. 2012, 1454, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Deval, C.; Gouranton, E.; Landrier, J.F.; Scalbert, A.; Morand, C.; Mazur, A. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: A new mechanism of the action of polyphenols. PLoS ONE 2012, 7, e29837. [Google Scholar] [CrossRef]
- Sun, M.; Estrov, Z.; Ji, Y.; Coombes, K.R.; Harris, D.H.; Kurzrock, R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 464–473. [Google Scholar] [CrossRef]
- Pilipenko, V.; Narbute, K.; Amara, I.; Trovato, A.; Scuto, M.; Pupure, J.; Janson, B.; Poikans, J.; Bisenieks, E.; Klusa, V.; et al. GABA-containing compound gammapyrone protects against brain impairments in Alzheimer’s disease model male rats and prevents mitochondrial dysfunction in cell culture. J. Neurosci. Res. 2019, 97, 708–726. [Google Scholar] [CrossRef]
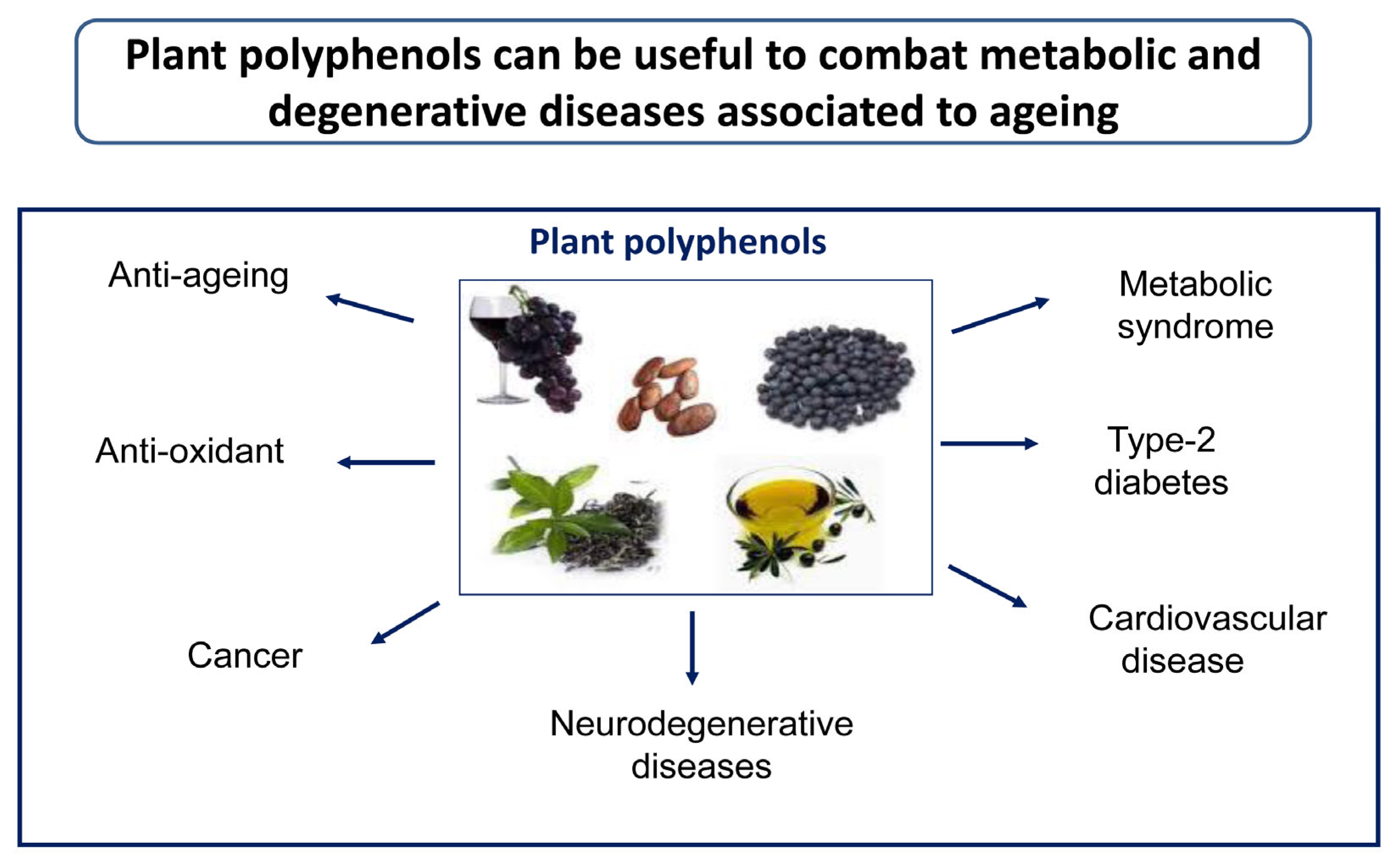
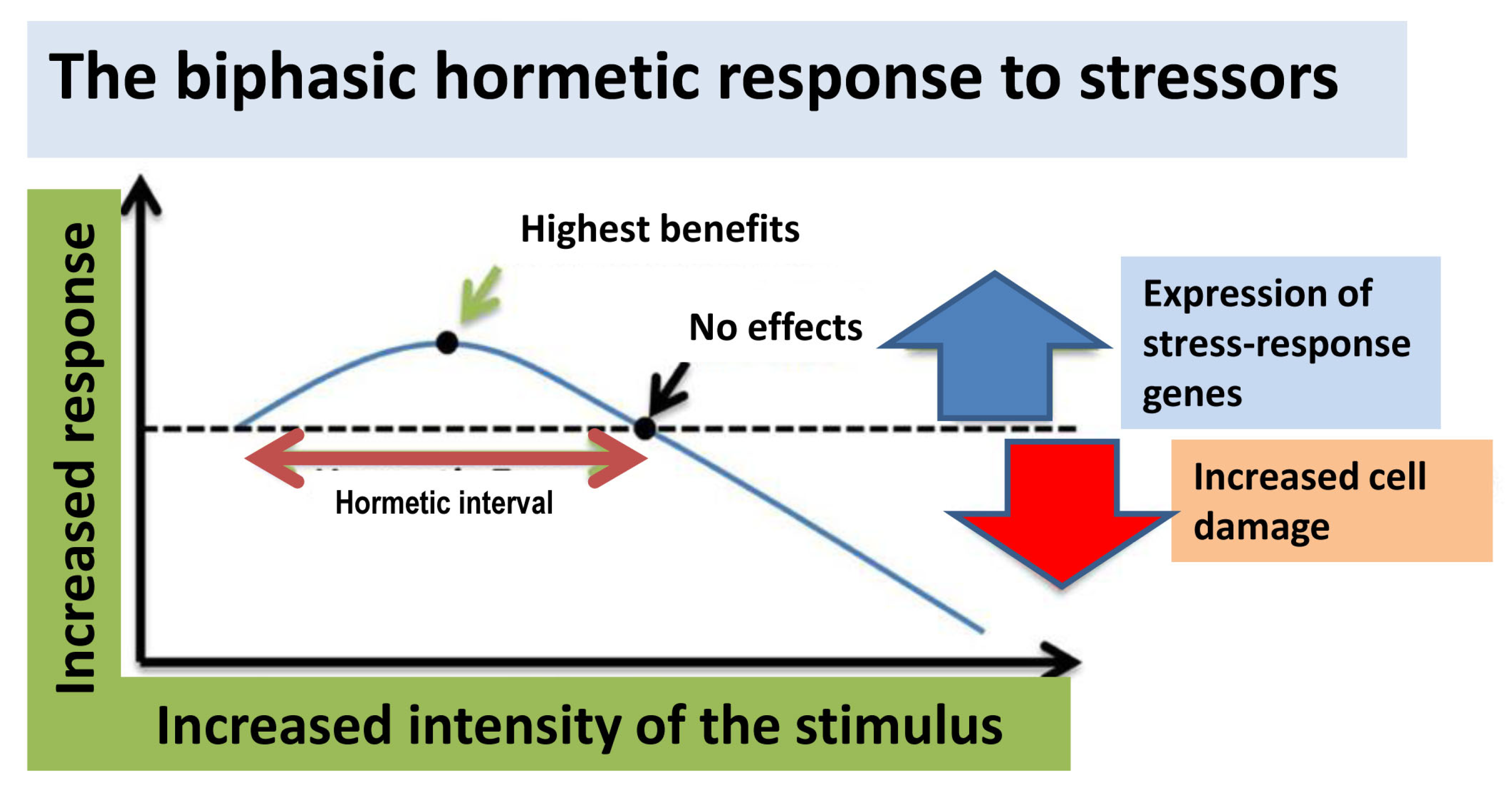
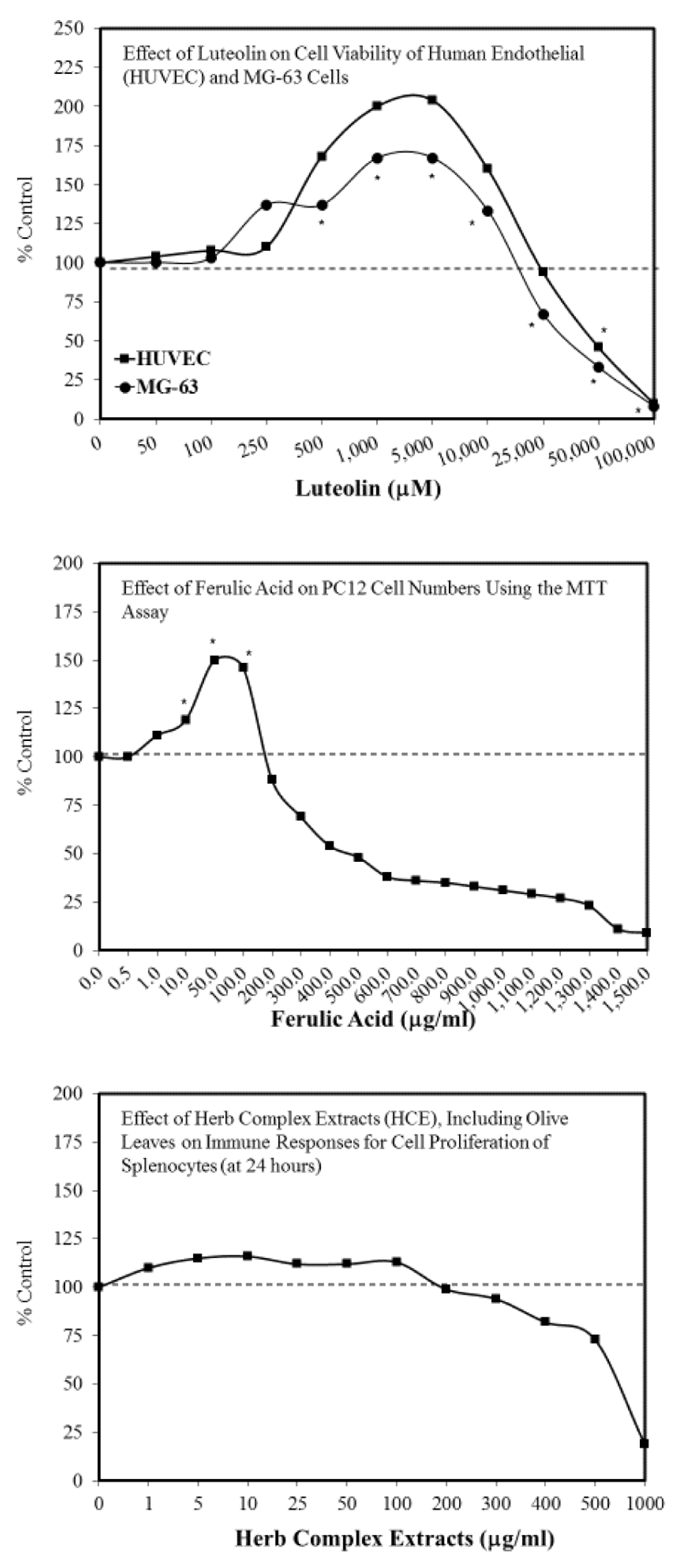
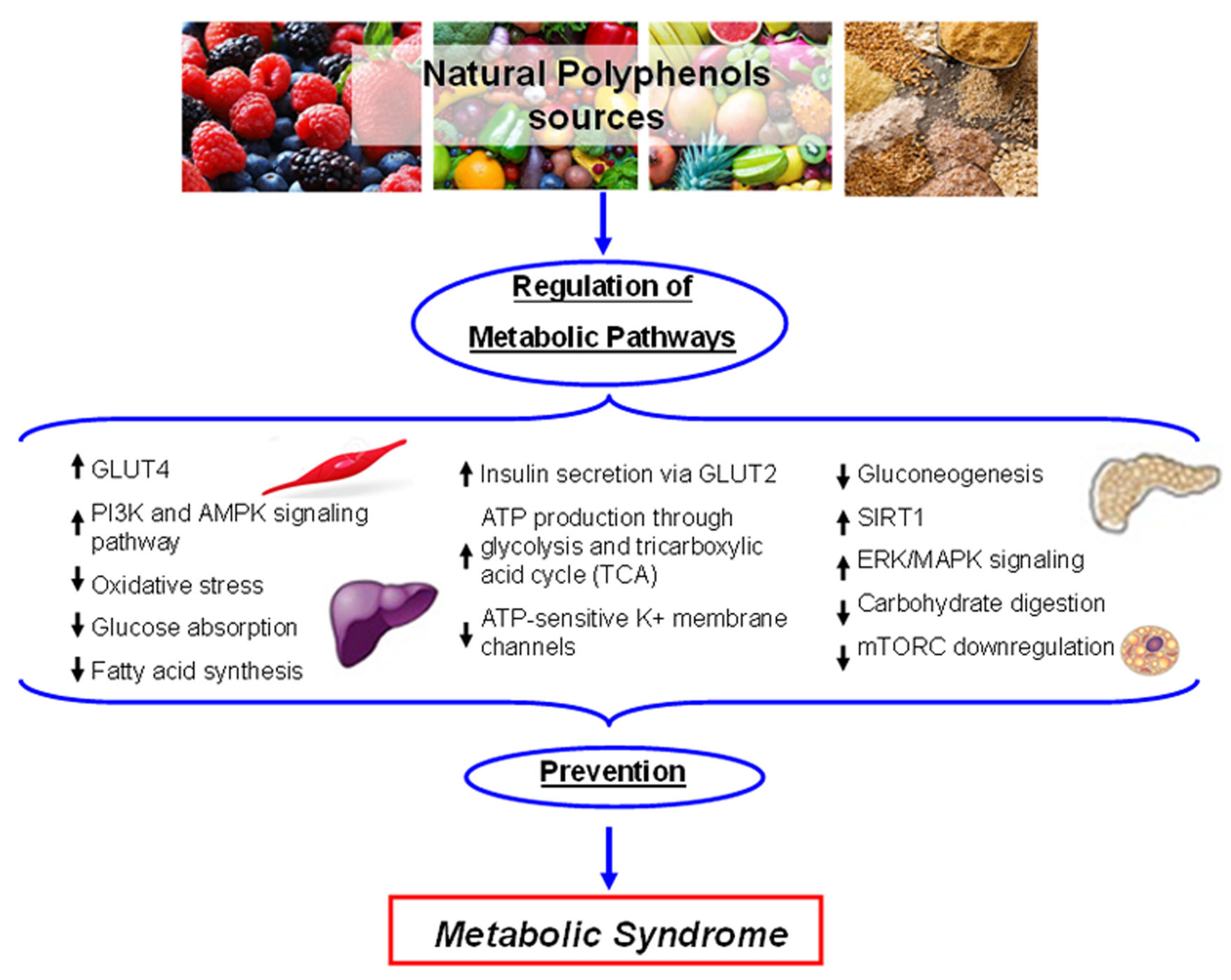
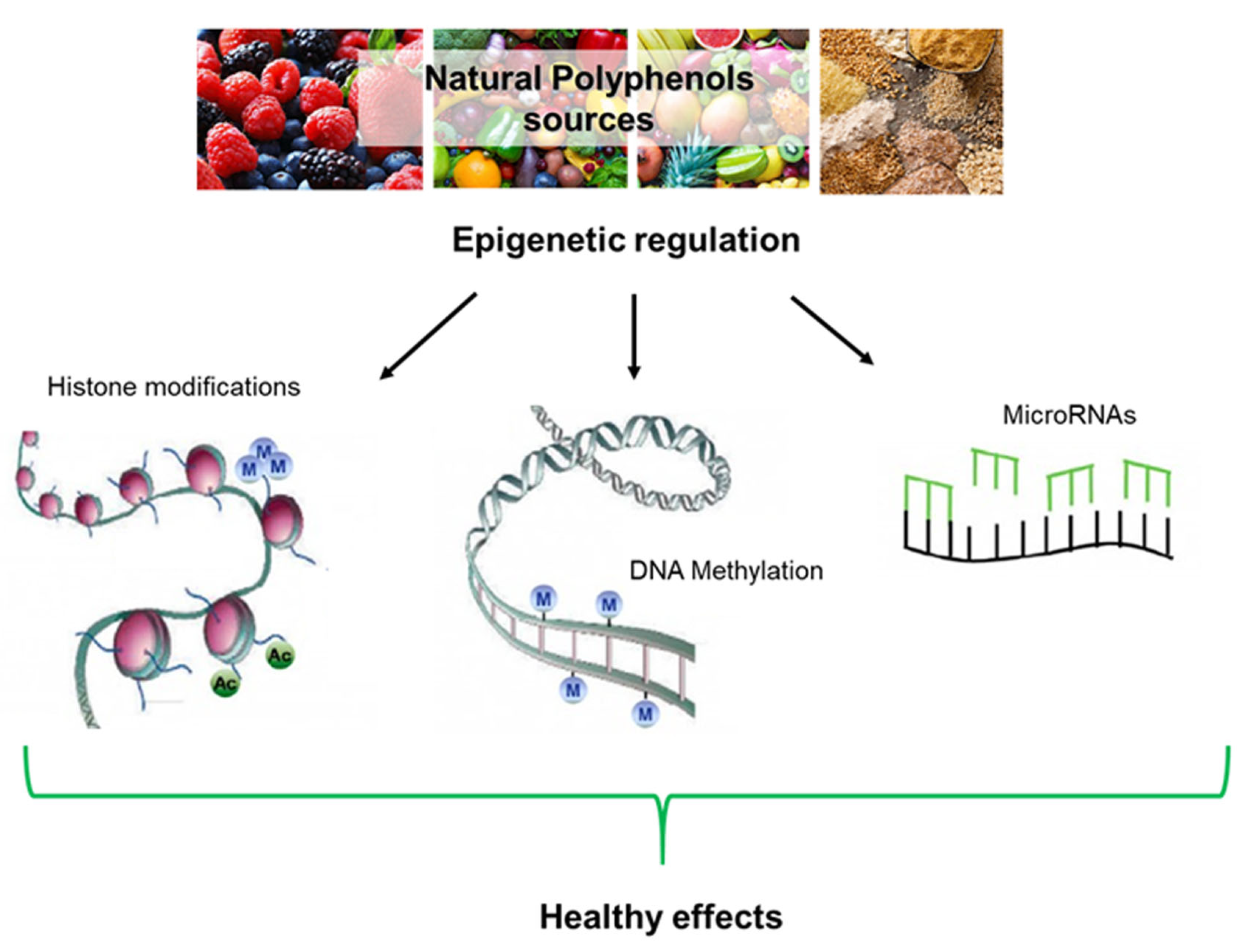
| Name/Number | N° People | Age | Type of Study | Treatment | Pathology | Ref |
|---|---|---|---|---|---|---|
| Seven Countries Study | 12,763 | 40–59 | Population, longitudinal | Traditional eating | CVD | [46] |
| Lyon Diet Heart Study | 605 | Recur. | Random. sec. prevent. trial | MD | Myo infarct. | [47] |
| Three-City Study | 9077 | >65 | Multi-center cohort study | Olive oil | Cognition | [48] |
| PREDIMEDNAVARRA | 1877/522 | 74 (mean) | Observational study | MD/oil or nuts | CVD risk/Cognition | [49,50,51,52] |
| PREDIMED | 447 | 55–80 | Dietary-intervention trial | Polyphenol intake | CVD profile/cognition | [51] |
| Spanish people | 4572 | >18 | Cross-sect. popul. Study | Olive/sunflower oil | CVD risk factors | [57] |
| Nurses’ Health Study | 59,930 | 35–65 | Longitud. Associate. study | Olive oil | Risk of T2DM | [58] |
| NHS II | 85,157 | 26–45 | Longitud. Associate. study | Olive oil | Risk of T2DM | [58] |
| Not specified | 25 | unknown | Cross-over study | MD with EVOO | Gluc./LDL chol | [59] |
| EUROLIVE | 200 | various | Randomized crossover trial | EVOO | Ox.stress mark./Chol. | [60,61,62] |
| PREDIMED Study | 7447 | 55–80 | Multicenter trial | MD/EVOO or nuts | CVD risk | [63] |
| Not specified | 20 | various | Double-blinded rand. Cross. | EVOO | Gene express. | [64] |
| Not specified | 20 | various | Randomized crossover | Olive polyphenols | Postprandial infl. | [65] |
| Not specified | 62 | 65–96 | Observational study | EVOO | Antioxidant status | [69] |
| Not specified | 410 | elderly | Random. double-blind trial | Ginkgo extract | Dementia | [68] |
| MedLey | 166 | >65 | Random. control. Int. trial | MD | Cognition decay | [72] |
| ISRCTN35739639 | 447 | 66.9 (mean) | Randomized clinical trial | MD/EVOO or nuts | Cognition | [74] |
| Not specified | 20 | >50 | Random. double-blind clin. trial | 4 g Curcumin | Cognition | [76] |
| Not specified | 36 | aged | Random. double-blind clin. trial | 2/4 g Curcumin | AD | [77] |
| Trials with resveratrol | - | various | 244 clinical trials+ 27 ongoing | Resveratrol | various | [79,80] |
| Rockefeller Univ. Hosp. | 28 | various | Placebo contr. Clinical trial | 1g Resveratrol | MetS, ins. Res. | [81] |
| Not specified | 19 | various | Observational study | Green tea extracts | ATTR cardiomyop. | [83] |
| Not specified | - | various | Various | Ginkgo biloba extracts | Various | [84] |
| SUN Project | 22,786 | various | Prospective Med. cohort study | Polyphenols | Breast cancer | [86] |
| SUN Project | 22,786 | various | Prospective Med. cohort study | Polyphenols | CVD, T2DM, MetS | [97] |
| Not specified | 79 | various | Controlled clinical trial | 500 mg Olive leaf extract | T2DM | [89] |
| Not specified | 46 | 46.4 (mean) | Randomized crossover trial | Olive leaf extract | T2DM | [90] |
| Not specified | 60 | 45 (mean) | Randomized contr. Clin. trial | Olive leaf extract | CVD risk | [91] |
| NCT01479699 | 18 | various | Random., double-blind cross-over | OLE + HT | CVD markers | [92] |
| Not specified | 24 | various | Gene/miRNAs expression | High/low polyph. EVOO | MetS | [93] |
| Not specified | 22 | various | Random. Control. trial | Polyphenol-rich EVOO | Redox/Met. Stat | [94] |
| Polyphenols | Anti-Oxidant | Anti-Inflammatory | Anti-Aggregation |
|---|---|---|---|
| Oleuropein Aglycone | ↑Sirt1 ↑Nrf2 | ↑Sirt1, Sirt2, Sirt6 | Aβ, Tau, α-Syn |
| Hydroxytyrosol | ↑Sirt1 ↑Nrf-2/HO-1 ↑Nrf-2-ARE ↑Keap1-Nrf2-TRXR1 | ↑Sirt1, Sirt2, Sirt6 ↓NLRP3 inflammasome ↓IL-1β, IL-18 | α-Syn Aβ |
| Sulforaphane | ↑Hsp70-CHIP | ↓NLRP3 inflammasome ↓STAT1 | |
| Resveratrol | ↑Nrf-2/HO-1 ↑TRX ↓Nf-κB | ↑Sirt1 | Aβ, Tau |
| Curcumin | ↑HO-1 ↑Hsp70 ↑Hsp90 ↑Hsc70 | ↓IL-1β ↓NLRP3 | Aβ, Tau |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. https://doi.org/10.3390/ijms21041250
Leri M, Scuto M, Ontario ML, Calabrese V, Calabrese EJ, Bucciantini M, Stefani M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. International Journal of Molecular Sciences. 2020; 21(4):1250. https://doi.org/10.3390/ijms21041250
Chicago/Turabian StyleLeri, Manuela, Maria Scuto, Maria Laura Ontario, Vittorio Calabrese, Edward J. Calabrese, Monica Bucciantini, and Massimo Stefani. 2020. "Healthy Effects of Plant Polyphenols: Molecular Mechanisms" International Journal of Molecular Sciences 21, no. 4: 1250. https://doi.org/10.3390/ijms21041250
APA StyleLeri, M., Scuto, M., Ontario, M. L., Calabrese, V., Calabrese, E. J., Bucciantini, M., & Stefani, M. (2020). Healthy Effects of Plant Polyphenols: Molecular Mechanisms. International Journal of Molecular Sciences, 21(4), 1250. https://doi.org/10.3390/ijms21041250










