Hydrogen Sulfide as Potential Regulatory Gasotransmitter in Arthritic Diseases
Abstract
1. Introduction
2. Oxidative Stress and Inflammation in Arthritis
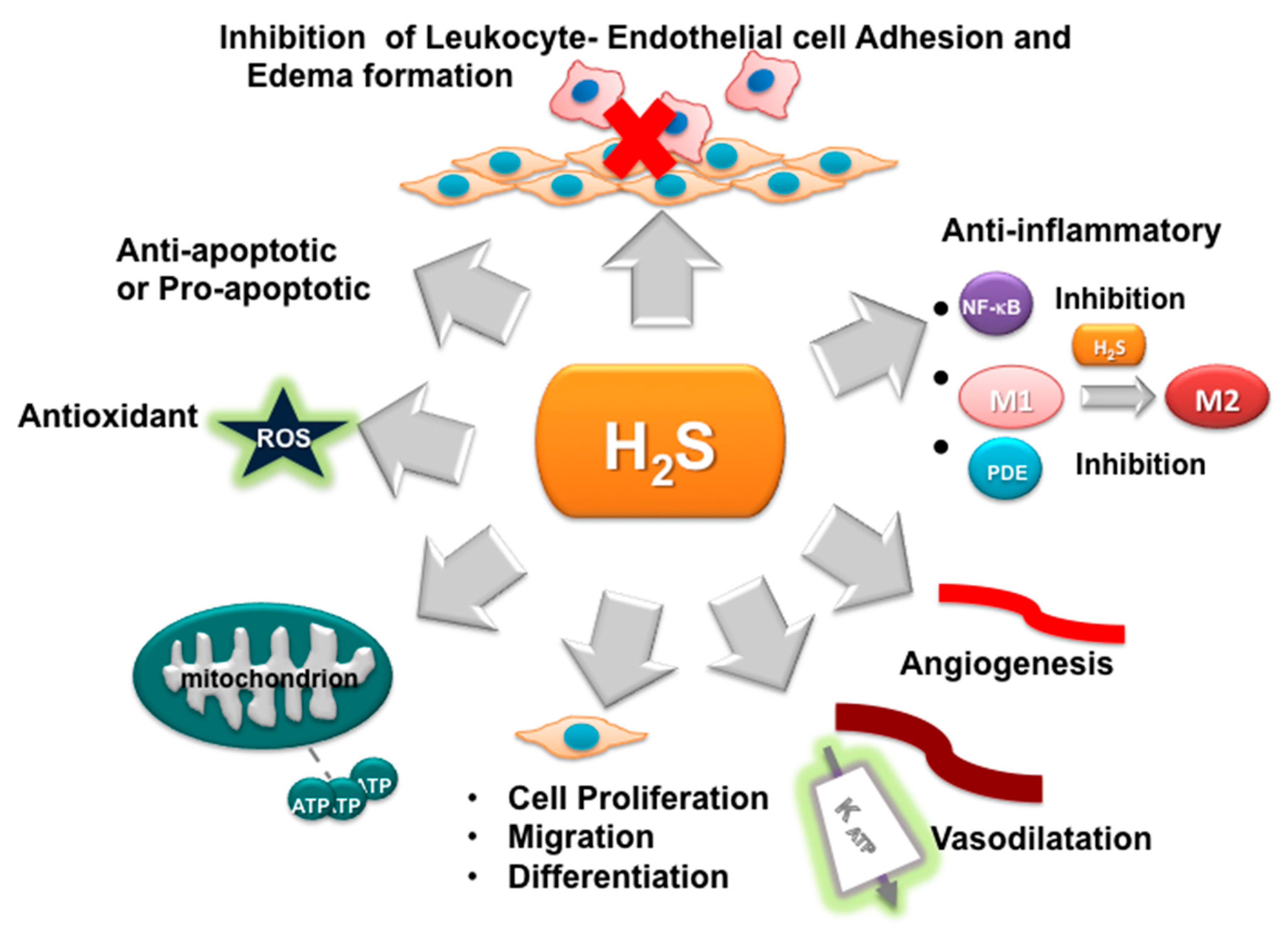
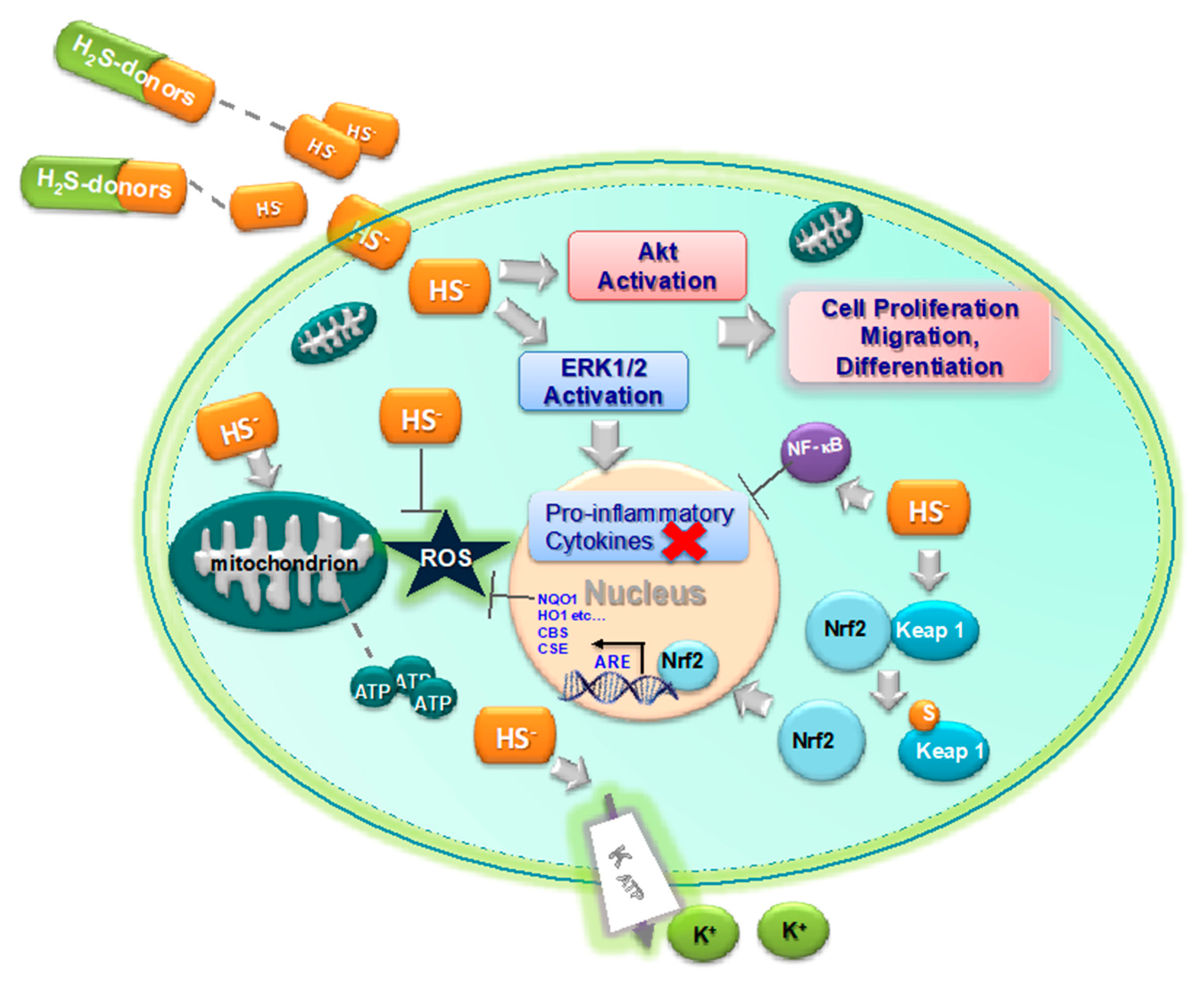
3. H2S as Inhibitor of Oxidative Stress and Inflammation
4. H2S and Arthritic Diseases
5. H2S-Donors as Potential Anti-Arthritis Drugs
6. Tissue Regeneration as a Therapeutic Approach in Arthritis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADSCs | adipose-derived stem cells |
| ADT-OH | 5-(4-hydroxyphenyl)-3H-1:2-dithiole-3-thione |
| ATB-429 | 4-(5-sulfanylidenedithiol-3-yl) phenyl 5-amino-2-hydroxybenzoate |
| ALG-CHO | partially oxidized alginate |
| anti CCP | anti-cyclic citrullinated peptide |
| ARE | antioxidant response element |
| APCs | antigen-presenting cells |
| bFGF | basic fibroblast growth factor |
| cAMP | cyclic adenosine monophosphate |
| CAT | cysteine aminotransferase |
| CAT | catalase |
| CD | cluster of differentiation |
| cGMP | cyclic guanosine monophosphate |
| COX 2 | cyclooxygenase 2 |
| CS | chondroitin sulfate |
| CBS | cystathionine beta-synthase |
| CSE | cystathionine gamma lyase |
| DAS | diallyl sulfide |
| DCs | dendritic cells |
| EC | endothelial cells |
| ECM | extracellular matrix |
| eIF2a | eukaryotic translation initiation factor 2 |
| ErK | extracellular signal-regulated kinase |
| FLS | fibroblast like synoviocytes |
| FOXP3 | forkhead box P3 |
| GaOS | garlic oil soluble extracts |
| GM-CSF | granulocyte macrophage colony stimulating factor |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | glutathione |
| GSSH | glutathione persulfide |
| GST | glutathione-S-transferase |
| HA | hyaluronic acid |
| hCPCs | human cardiac progenitor cells |
| HER 2 | human epidermal growth factor receptor 2 |
| HFFs | human foreskin fibroblasts |
| HO1 | heme oxygenase 1 |
| ICAM 1 | intercellular Adhesion Molecule 1 |
| IDO 1 | indoleamine-pyrrole 2:3- dioxygenase 1 |
| IGF 1 | insulin-like growth factor 1 |
| IκB/NF-κB | inhibitor of κB /Nuclear factor κ B |
| IKK | IκB kinase |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JK1 | H2S donors synthesized from phenylphosphonothioic dichloride |
| Keap 1 | Kelch-like ECH-associated protein 1 |
| LPS | lipopolysaccharides |
| M1 | macrophages M1 |
| M2 | macrophages M2 |
| MAPK | mitogen-activated protein kinase |
| MBs-PFHy | fibrinogen hydrogel incorporating albumin microbubbles |
| MHC-I | major histocompatibility complex |
| MMP | matrix metalloproteinase |
| MMP | pepmatrix metalloproteinase-sensitive peptides |
| MOCART | magnetic resonance observation of cartilage repair tissue |
| MSCs | mesenchymal stem cells |
| MST | 3-mercaptopyruvate sulfotransferase |
| NFκB | nuclear factor kappa beta |
| NOSH | nitric oxide and hydrogen sulfide |
| Nrf2 | nuclear erythroid factor 2-related factor 2 |
| NSHD 1 | N-benzoyl thio-benzamide |
| OA | osteoarthritis |
| OBs | osteoblast cell |
| OCs | osteoclasts cell |
| OSC | organosulfur compounds |
| PAG | propargylglycine |
| PCL | polycaprolactone |
| PDE | phosphodiesterases |
| PEG | polyethylene glycol |
| PERK | protein kinase RNA-like endoplasmic reticulum kinase |
| PFM | poly(lactic) acid fibrous |
| PKB | protein kinase B |
| PLA | polylactic acid |
| PLLA | poly (l-lactide) |
| PsA | psoriatic arthritis |
| PTPs | protein tyrosine phosphatases |
| QR | quinone reductase |
| RA | rheumatoid arthritis |
| RANTES | regulated upon activation, normal T Cell expressed and presumably secreted |
| RAW 264.7 | murine monocyte/macrophage-like lineage |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SATO | S-aroylthiooxime |
| SELENBP1 | selenium-binding protein 1 |
| SF | silk fibroin |
| SOD | superoxide dismutase |
| SpA | spondyloarthritis |
| STAT 3 | signal transducer and activator of transcription 3 |
| TBZ | 4-hydroxythiobenzamide |
| TGF-β1 | transforming growth factor beta 1 |
| Th | T helper cells |
| TNF α | tumor necrosis factor-α |
| TST | thiosulfate sulfurtransferase |
| VCAM 1 | vascular cell adhesion molecule 1 |
References
- Polhemus, D.J.; Lefer, D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014, 114, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004, 26, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Moore, P.K. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol. Sci. 2008, 29, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Nagahara, N.; Koike, S.; Nirasawa, T.; Kimura, H.; Ogasawara, Y. Alternative pathway of H2S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem. Biophys. Res. Commun. 2018, 496, 648–653. [Google Scholar] [CrossRef]
- Pol, A.; Renkema, G.H.; Tangerman, A.; Winkel, E.G.; Engelke, U.F.; de Brouwer, A.P.M.; Lloyd, K.C.; Araiza, R.S.; van den Heuvel, L.; Omran, H.; et al. Mutations in SELENBP1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat. Genet. 2018, 50, 120–129. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Collins, M.D. Interactive endogenous small molecule (gaseous) signaling: Implications for teratogenesis. Curr. Pharm. Des. 2007, 13, 2952–2978. [Google Scholar] [CrossRef]
- Cao, J.T.; Zhang, W.S.; Fu, X.L.; Wang, H.; Ma, S.H.; Liu, Y.M. Copper ion modified graphitic C3N4 nanosheets enhanced luminol-H2O2 chemiluminescence system: Toward highly selective and sensitive bioassay of H2S in human plasma. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118040. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef]
- Olson, K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Biophys. Acta 2009, 1787, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.F.K.; Kamaya, M. Determination of trace amounts of sulfide in human serum by high-performance liquid chromatography with fluorometric detection after derivatization with 1-amino-5-n, n-diethylaminotoluene and iron (III). J. Liq. Chrom. Relat. Technol. 1995, 18, 515. [Google Scholar] [CrossRef]
- Whiteman, M.; Haigh, R.; Tarr, J.M.; Gooding, K.M.; Shore, A.C.; Winyard, P.G. Detection of hydrogen sulfide in plasma and knee-joint synovial fluid from rheumatoid arthritis patients: Relation to clinical and laboratory measures of inflammation. Ann. N. Y. Acad. Sci. 2010, 1203, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Motl, N.; Banerjee, R. H2S and its role in redox signaling. Biochim. Biophys. Acta 2014, 1844, 1355–1366. [Google Scholar] [CrossRef]
- Searcy, D.G.; Lee, S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998, 282, 310–322. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H474–H480. [Google Scholar] [CrossRef]
- Jha, S.; Calvert, J.W.; Duranski, M.R.; Ramachandran, A.; Lefer, D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H801–H806. [Google Scholar] [CrossRef]
- Wallace, J.L. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid. Redox Signal. 2010, 12, 1125–1133. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Li, Y.; Song, J.N.; Pang, H.G. Role of hydrogen sulfide in secondary neuronal injury. Neurochem. Int. 2014, 64, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Ichinose, F. Hydrogen Sulfide and Neuroinflammation. Handb. Exp. Pharmacol. 2015, 230, 181–189. [Google Scholar] [PubMed]
- Wallace, J.L.; Ianaro, A.; Flannigan, K.L.; Cirino, G. Gaseous mediators in resolution of inflammation. Semin. Immunol. 2015, 27, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sen, N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017, 429, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M. H2S and Inflammation: An Overview. Handb. Exp. Pharmacol. 2015, 230, 165–180. [Google Scholar] [CrossRef]
- Coletta, C.; Szabo, C. Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Curr. Vasc. Pharmacol. 2013, 11, 208–221. [Google Scholar]
- Akter, F. The role of hydrogen sulfide in burns. Burns 2016, 42, 519–525. [Google Scholar] [CrossRef]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Ahmad, A.; Sattar, M.A.; Rathore, H.A.; Khan, S.A.; Lazhari, M.I.; Afzal, S.; Hashmi, F.; Abdullah, N.A.; Johns, E.J. A critical review of pharmacological significance of Hydrogen Sulfide in hypertension. Indian J. Pharmacol. 2015, 47, 243–247. [Google Scholar] [CrossRef]
- Wagner, F.; Asfar, P.; Calzia, E.; Radermacher, P.; Szabo, C. Bench-to-bedside review: Hydrogen sulfide-the third gaseous transmitter: Applications for critical care. Crit. Care 2009, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Szabo, C.; Ichinose, F.; Ahmed, A.; Whiteman, M.; Papapetropoulos, A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol. Sci. 2015, 36, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Coaccioli, S.; Panaccione, A.; Biondi, R.; Sabatini, C.; Landucci, P.; Del Giorno, R.; Fantera, M.; Monno Mondo, A.; Di Cato, L.; Paladini, A.; et al. Evaluation of oxidative stress in rheumatoid and psoriatic arthritis and psoriasis. Clin. Ter. 2009, 160, 467–472. [Google Scholar] [PubMed]
- Phillips, D.C.; Dias, H.K.; Kitas, G.D.; Griffiths, H.R. Aberrant reactive oxygen and nitrogen species generation in rheumatoid arthritis (RA): Causes and consequences for immune function, cell survival, and therapeutic intervention. Antioxid. Redox Signal. 2010, 12, 743–785. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Autoimmunity and oxidatively modified autoantigens. Autoimmun. Rev. 2008, 7, 567–573. [Google Scholar] [CrossRef]
- Fearon, U.; Reece, R.; Smith, J.; Emery, P.; Veale, D.J. Synovial cytokine and growth factor regulation of MMPs/TIMPs: Implications for erosions and angiogenesis in early rheumatoid and psoriatic arthritis patients. Ann. N. Y. Acad. Sci. 1999, 878, 619–621. [Google Scholar] [CrossRef]
- Ng, C.T.; Biniecka, M.; Kennedy, A.; McCormick, J.; Fitzgerald, O.; Bresnihan, B.; Buggy, D.; Taylor, C.T.; O’Sullivan, J.; Fearon, U.; et al. Synovial tissue hypoxia and inflammation in vivo. Ann. Rheum. Dis. 2010, 69, 1389–1395. [Google Scholar] [CrossRef]
- Biniecka, M.; Kennedy, A.; Fearon, U.; Ng, C.T.; Veale, D.J.; O’Sullivan, J.N. Oxidative damage in synovial tissue is associated with in vivo hypoxic status in the arthritic joint. Ann. Rheum. Dis. 2010, 69, 1172–1178. [Google Scholar] [CrossRef]
- Harty, L.C.; Biniecka, M.; O’Sullivan, J.; Fox, E.; Mulhall, K.; Veale, D.J.; Fearon, U. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann. Rheum. Dis. 2012, 71, 582–588. [Google Scholar] [CrossRef]
- Kruithof, E.; Baeten, D.; De Rycke, L.; Vandooren, B.; Foell, D.; Roth, J.; Canete, J.D.; Boots, A.M.; Veys, E.M.; De Keyser, F. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R569–R580. [Google Scholar] [CrossRef]
- Costello, P.J.; Winchester, R.J.; Curran, S.A.; Peterson, K.S.; Kane, D.J.; Bresnihan, B.; FitzGerald, O.M. Psoriatic arthritis joint fluids are characterized by CD8 and CD4 T cell clonal expansions appear antigen driven. J. Immunol. 2001, 166, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, I.; D’Angelo, S.; Palazzi, C.; Padula, A. Advances in the management of psoriatic arthritis. Nat. Rev. Rheumatol. 2014, 10, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Ospelt, C. Synovial fibroblasts in 2017. RMD Open 2017, 3, e000471. [Google Scholar] [CrossRef] [PubMed]
- Colucci, S.; Brunetti, G.; Cantatore, F.P.; Oranger, A.; Mori, G.; Quarta, L.; Cirulli, N.; Mancini, L.; Corrado, A.; Grassi, F.R.; et al. Lymphocytes and synovial fluid fibroblasts support osteoclastogenesis through RANKL, TNFalpha, and IL-7 in an in vitro model derived from human psoriatic arthritis. J. Pathol. 2007, 212, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 968549. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G.; Isenberg, D.A.; Mauri, C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Alivernini, S. Synovial tissue macrophages: Friend or foe? RMD Open 2017, 3, e000527. [Google Scholar] [CrossRef]
- Baeten, D.; Kruithof, E.; De Rycke, L.; Boots, A.M.; Mielants, H.; Veys, E.M.; De Keyser, F. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res. Ther. 2005, 7, R359–R369. [Google Scholar] [CrossRef]
- Canete, J.D.; Santiago, B.; Cantaert, T.; Sanmarti, R.; Palacin, A.; Celis, R.; Graell, E.; Gil-Torregrosa, B.; Baeten, D.; Pablos, J.L. Ectopic lymphoid neogenesis in psoriatic arthritis. Ann. Rheum. Dis. 2007, 66, 720–726. [Google Scholar] [CrossRef]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Jia-Ling, S.; Wong, B.S.; Cheung, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004, 90, 765–768. [Google Scholar] [CrossRef]
- Mitsuhashi, H.; Yamashita, S.; Ikeuchi, H.; Kuroiwa, T.; Kaneko, Y.; Hiromura, K.; Ueki, K.; Nojima, Y. Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock 2005, 24, 529–534. [Google Scholar] [CrossRef]
- Geng, B.; Chang, L.; Pan, C.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 2004, 318, 756–763. [Google Scholar] [CrossRef]
- Olas, B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta 2015, 439, 212–218. [Google Scholar] [CrossRef]
- Sun, W.H.; Liu, F.; Chen, Y.; Zhu, Y.C. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem. Biophys. Res. Commun. 2012, 421, 164–169. [Google Scholar] [CrossRef]
- De Beus, M.D.; Chung, J.; Colon, W. Modification of cysteine 111 in Cu/Zn superoxide dismutase results in altered spectroscopic and biophysical properties. Protein Sci. 2004, 13, 1347–1355. [Google Scholar] [CrossRef]
- Kalayarasan, S.; Prabhu, P.N.; Sriram, N.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur. J. Pharmacol. 2009, 606, 162–171. [Google Scholar] [CrossRef]
- Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.G.; Park, S.A.; Kundu, J.K.; Keum, Y.S.; Cha, Y.N.; Na, H.K.; Surh, Y.J. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS ONE 2014, 9, e85984. [Google Scholar] [CrossRef]
- Wu, W.J.; Jia, W.W.; Liu, X.H.; Pan, L.L.; Zhang, Q.Y.; Yang, D.; Shen, X.Y.; Liu, L.; Zhu, Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016, 10, 157–167. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid. Redox Signal. 2013, 19, 465–481. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Liu, Y.; Bian, J.S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid. Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef]
- Kimura, Y.; Dargusch, R.; Schubert, D.; Kimura, H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox Signal. 2006, 8, 661–670. [Google Scholar] [CrossRef]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Trevino-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Shakibaei, M.; Henrotin, Y. Antioxidants and Osteoarthritis. Syst. Biol. Free Radic. Antioxid. 2014, 2997–3026. [Google Scholar] [CrossRef]
- Whiteman, M.; Winyard, P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef]
- Dufton, N.; Natividad, J.; Verdu, E.F.; Wallace, J.L. Hydrogen sulfide and resolution of acute inflammation: A comparative study utilizing a novel fluorescent probe. Sci. Rep. 2012, 2, 499. [Google Scholar] [CrossRef]
- Wallace, J.L.; Ferraz, J.G.; Muscara, M.N. Hydrogen sulfide: An endogenous mediator of resolution of inflammation and injury. Antioxid. Redox Signal. 2012, 17, 58–67. [Google Scholar] [CrossRef]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 2009, 137, 569–578. [Google Scholar] [CrossRef]
- Miller, T.W.; Wang, E.A.; Gould, S.; Stein, E.V.; Kaur, S.; Lim, L.; Amarnath, S.; Fowler, D.H.; Roberts, D.D. Hydrogen sulfide is an endogenous potentiator of T cell activation. J. Biol. Chem. 2012, 287, 4211–4221. [Google Scholar] [CrossRef]
- Gemici, B.; Wallace, J.L. Anti-inflammatory and cytoprotective properties of hydrogen sulfide. Methods Enzymol. 2015, 555, 169–193. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Agbor, T.A.; Blackler, R.W.; Kim, J.J.; Khan, W.I.; Verdu, E.F.; Ferraz, J.G.; Wallace, J.L. Impaired hydrogen sulfide synthesis and IL-10 signaling underlie hyperhomocysteinemia-associated exacerbation of colitis. Proc. Natl. Acad. Sci. USA 2014, 111, 13559–13564. [Google Scholar] [CrossRef]
- Fiorucci, S.; Orlandi, S.; Mencarelli, A.; Caliendo, G.; Santagada, V.; Distrutti, E.; Santucci, L.; Cirino, G.; Wallace, J.L. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharmacol. 2007, 150, 996–1002. [Google Scholar] [CrossRef]
- Huang, C.W.; Feng, W.; Peh, M.T.; Peh, K.; Dymock, B.W.; Moore, P.K. A novel slow-releasing hydrogen sulfide donor, FW1256, exerts anti-inflammatory effects in mouse macrophages and in vivo. Pharmacol. Res. 2016, 113, 533–546. [Google Scholar] [CrossRef]
- Du, C.; Jin, M.; Hong, Y.; Li, Q.; Wang, X.H.; Xu, J.M.; Wang, F.; Zhang, Y.; Jia, J.; Liu, C.F.; et al. Downregulation of cystathionine beta-synthase/hydrogen sulfide contributes to rotenone-induced microglia polarization toward M1 type. Biochem. Biophys. Res. Commun. 2014, 451, 239–245. [Google Scholar] [CrossRef]
- Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, X.; Gao, L.; Chen, J.; Liu, Y.; Yu, C.; Zhang, N.; Zhang, X.; Zhao, J. Hydrogen sulfide suppresses high glucose-induced expression of intercellular adhesion molecule-1 in endothelial cells. J. Cardiovasc. Pharmacol. 2013, 62, 278–284. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Jin, H.; Wei, H.; Li, W.; Bu, D.; Tang, X.; Ren, Y.; Tang, C.; Du, J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 173–179. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Wu, D.; Zhu, Y.Z. Hydrogen sulfide attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS ONE 2011, 6, e19766. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Zheng, H.M.; Yang, H.B.; Gong, Q.H.; Zhu, Y.Z. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in endothelial cells. Int. J. Cardiol. 2012, 155, 327–332. [Google Scholar] [CrossRef]
- Mariggio, M.A.; Minunno, V.; Riccardi, S.; Santacroce, R.; De Rinaldis, P.; Fumarulo, R. Sulfide enhancement of PMN apoptosis. Immunopharmacol. Immunotoxicol. 1998, 20, 399–408. [Google Scholar] [CrossRef]
- Traves, P.G.; Pardo, V.; Pimentel-Santillana, M.; Gonzalez-Rodriguez, A.; Mojena, M.; Rico, D.; Montenegro, Y.; Cales, C.; Martin-Sanz, P.; Valverde, A.M.; et al. Pivotal role of protein tyrosine phosphatase 1B (PTP1B) in the macrophage response to pro-inflammatory and anti-inflammatory challenge. Cell Death Dis. 2014, 5, e1125. [Google Scholar] [CrossRef]
- Pao, L.I.; Badour, K.; Siminovitch, K.A.; Neel, B.G. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu. Rev. Immunol. 2007, 25, 473–523. [Google Scholar] [CrossRef]
- Camer, D.; Huang, X.F. The endothelin pathway: A protective or detrimental target of bardoxolone methyl on cardiac function in patients with advanced chronic kidney disease? Am. J. Nephrol. 2014, 40, 288–290. [Google Scholar] [CrossRef]
- Rivera Franco, M.M.; Leon Rodriguez, E.; Martinez Benitez, B.; Villanueva Rodriguez, L.G.; de la Luz Sevilla Gonzalez, M.; Armengol Alonso, A. Association of PTP1B with Outcomes of Breast Cancer Patients Who Underwent Neoadjuvant Chemotherapy. Breast Cancer (Auckl.) 2016, 10, 177–184. [Google Scholar]
- Berdnikovs, S.; Pavlov, V.I.; Abdala-Valencia, H.; McCary, C.A.; Klumpp, D.J.; Tremblay, M.L.; Cook-Mills, J.M. PTP1B deficiency exacerbates inflammation and accelerates leukocyte trafficking in vivo. J. Immunol. 2012, 188, 874–884. [Google Scholar] [CrossRef]
- Bleidorn, J.; Alamzad-Krabbe, H.; Gerhards, B.; Kraus, T.; Brand, P.; Krabbe, J.; Martin, C. The pro-inflammatory stimulus of zinc- and copper-containing welding fumes in whole blood assay via protein tyrosine phosphatase 1B inhibition. Sci. Rep. 2019, 9, 1315. [Google Scholar] [CrossRef]
- Song, G.J.; Jung, M.; Kim, J.H.; Park, H.; Rahman, M.H.; Zhang, S.; Zhang, Z.Y.; Park, D.H.; Kook, H.; Lee, I.K.; et al. A novel role for protein tyrosine phosphatase 1B as a positive regulator of neuroinflammation. J. Neuroinflamm. 2016, 13, 86. [Google Scholar] [CrossRef]
- Krishnan, N.; Fu, C.; Pappin, D.J.; Tonks, N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011, 4, ra86. [Google Scholar] [CrossRef] [PubMed]
- Andruski, B.; McCafferty, D.M.; Ignacy, T.; Millen, B.; McDougall, J.J. Leukocyte trafficking and pain behavioral responses to a hydrogen sulfide donor in acute monoarthritis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R814–R820. [Google Scholar] [CrossRef] [PubMed]
- Heneberg, P. Reactive nitrogen species and hydrogen sulfide as regulators of protein tyrosine phosphatase activity. Antioxid. Redox Signal. 2014, 20, 2191–2209. [Google Scholar] [CrossRef] [PubMed]
- Houslay, M.D.; Sullivan, M.; Bolger, G.B. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: Intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. Adv. Pharmacol. 1998, 44, 225–342. [Google Scholar] [PubMed]
- Haddad, J.J.; Land, S.C.; Tarnow-Mordi, W.O.; Zembala, M.; Kowalczyk, D.; Lauterbach, R. Immunopharmacological potential of selective phosphodiesterase inhibition. I. Differential regulation of lipopolysaccharide-mediated proinflammatory cytokine (interleukin-6 and tumor necrosis factor-alpha) biosynthesis in alveolar epithelial cells. J. Pharmacol. Exp. Ther. 2002, 300, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Modis, K.; Panopoulos, P.; Asimakopoulou, A.; Gero, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef]
- Panagiotis, P.; Yang, G.; Asimakopoulou, A.; Topouzis, T.; Wang, R.; Szabo, C.; Papapetropoulos, A. Selectivity of hydrogen sulfide toward cyclic nucleotide phosphodiesterases. Nitric Oxide 2015, 47, S39. [Google Scholar]
- Modis, K.; Panopoulos, P.; Coletta, C.; Papapetropoulos, A.; Szabo, C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem. Pharmacol. 2013, 86, 1311–1319. [Google Scholar] [CrossRef]
- Wells, A.F.; Edwards, C.J.; Kivitz, A.J.; Bird, P.; Nguyen, D.; Paris, M.; Teng, L.; Aelion, J.A. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: Results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology 2018, 57, 1253–1263. [Google Scholar] [CrossRef]
- Wallace, J.L.; Dicay, M.; McKnight, W.; Martin, G.R. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007, 21, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346). Br. J. Pharmacol. 2010, 159, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, H.R.; Mu, Q.; Rose, P.; Zhu, Y.Z. S-propargyl-cysteine protects both adult rat hearts and neonatal cardiomyocytes from ischemia/hypoxia injury: The contribution of the hydrogen sulfide-mediated pathway. J. Cardiovasc. Pharmacol. 2009, 54, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tamizhselvi, R.; Sun, J.; Koh, Y.H.; Bhatia, M. Effect of hydrogen sulfide on the phosphatidylinositol 3-kinase-protein kinase B pathway and on caerulein-induced cytokine production in isolated mouse pancreatic acinar cells. J. Pharmacol. Exp. Ther. 2009, 329, 1166–1177. [Google Scholar] [CrossRef]
- Oh, G.S.; Pae, H.O.; Lee, B.S.; Kim, B.N.; Kim, J.M.; Kim, H.R.; Jeon, S.B.; Jeon, W.K.; Chae, H.J.; Chung, H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006, 41, 106–119. [Google Scholar] [CrossRef]
- Li, L.; Salto-Tellez, M.; Tan, C.H.; Whiteman, M.; Moore, P.K. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic. Biol. Med. 2009, 47, 103–113. [Google Scholar] [CrossRef]
- Whiteman, M.; Li, L.; Rose, P.; Tan, C.H.; Parkinson, D.B.; Moore, P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010, 12, 1147–1154. [Google Scholar] [CrossRef]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef]
- Gong, Q.H.; Shi, X.R.; Hong, Z.Y.; Pan, L.L.; Liu, X.H.; Zhu, Y.Z. A new hope for neurodegeneration: Possible role of hydrogen sulfide. J. Alzheimer’s Dis. 2011, 24 (Suppl. S2), 173–182. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef]
- Shao, J.L.; Wan, X.H.; Chen, Y.; Bi, C.; Chen, H.M.; Zhong, Y.; Heng, X.H.; Qian, J.Q. H2S protects hippocampal neurons from anoxia-reoxygenation through cAMP-mediated PI3K/Akt/p70S6K cell-survival signaling pathways. J. Mol. Neurosci. 2011, 43, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Qu, C.; Zhou, Y.; Konkel, J.E.; Shi, S.; Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Chen, Y.; et al. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity 2015, 43, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Zhang, C.Y.; Wu, J.X.; Zhang, T. Changes in plasma hydrogen sulfide and nitric oxide levels and their clinical significance in children with Kawasaki disease. Chin. Med. J. 2011, 124, 3445–3449. [Google Scholar] [PubMed]
- Alshorafa, A.K.; Guo, Q.; Zeng, F.; Chen, M.; Tan, G.; Tang, Z.; Yin, R. Psoriasis is associated with low serum levels of hydrogen sulfide, a potential anti-inflammatory molecule. Tohoku J. Exp. Med. 2012, 228, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kloesch, B.; Liszt, M.; Broell, J. H2S transiently blocks IL-6 expression in rheumatoid arthritic fibroblast-like synoviocytes and deactivates p44/42 mitogen-activated protein kinase. Cell Biol. Int. 2010, 34, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kloesch, B.; Liszt, M.; Krehan, D.; Broell, J.; Kiener, H.; Steiner, G. High concentrations of hydrogen sulphide elevate the expression of a series of pro-inflammatory genes in fibroblast-like synoviocytes derived from rheumatoid and osteoarthritis patients. Immunol. Lett. 2012, 141, 197–203. [Google Scholar] [CrossRef]
- Brancaleone, V.; Esposito, I.; Gargiulo, A.; Vellecco, V.; Asimakopoulou, A.; Citi, V.; Calderone, V.; Gobbetti, T.; Perretti, M.; Papapetropoulos, A.; et al. D-Penicillamine modulates hydrogen sulfide (H2S) pathway through selective inhibition of cystathionine-gamma-lyase. Br. J. Pharmacol. 2016, 173, 1556–1565. [Google Scholar] [CrossRef]
- Tamizhselvi, R.; Koh, Y.H.; Sun, J.; Zhang, H.; Bhatia, M. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-kappaB and Src-family kinases pathway. Exp. Cell Res. 2010, 316, 1625–1636. [Google Scholar] [CrossRef]
- Fox, B.; Schantz, J.T.; Haigh, R.; Wood, M.E.; Moore, P.K.; Viner, N.; Spencer, J.P.; Winyard, P.G.; Whiteman, M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: Is H2S a novel cytoprotective mediator in the inflamed joint? J. Cell. Mol. Med. 2012, 16, 896–910. [Google Scholar] [CrossRef]
- Ha, C.; Tian, S.; Sun, K.; Wang, D.; Lv, J.; Wang, Y. Hydrogen sulfide attenuates IL-1beta-induced inflammatory signaling and dysfunction of osteoarthritic chondrocytes. Int. J. Mol. Med. 2015, 35, 1657–1666. [Google Scholar] [CrossRef]
- Li, L.; Fox, B.; Keeble, J.; Salto-Tellez, M.; Winyard, P.G.; Wood, M.E.; Moore, P.K.; Whiteman, M. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. J. Cell. Mol. Med. 2013, 17, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Vela-Anero, A.; Hermida-Gomez, T.; Gato-Calvo, L.; Vaamonde-Garcia, C.; Diaz-Prado, S.; Meijide-Failde, R.; Blanco, F.J.; Burguera, E.F. Long-term effects of hydrogen sulfide on the anabolic-catabolic balance of articular cartilage in vitro. Nitric Oxide 2017, 70, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.P.; Cao, Y.P.; Wen, L.C.; Chai, W.B.; Du, J.B.; Jin, H.F.; Liu, J.; Yang, X.; Meng, Z.C.; Liu, H.; et al. Hydrogen sulfide in cartilage and its inhibitory effect on matrix metalloproteinase 13 expression in chondrocytes induced by interlukin-1beta. Beijing Da Xue Xue Bao Yi Xue Ban 2016, 48, 194–202. [Google Scholar] [PubMed]
- Sieghart, D.; Liszt, M.; Wanivenhaus, A.; Broll, H.; Kiener, H.; Klosch, B.; Steiner, G. Hydrogen sulphide decreases IL-1beta-induced activation of fibroblast-like synoviocytes from patients with osteoarthritis. J. Cell. Mol. Med. 2015, 19, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, C.H.; Tsai, H.C.; Salter, D.M. Inhibition of cyclooxygenase 2 expression by diallyl sulfide on joint inflammation induced by urate crystal and IL-1beta. Osteoarthr. Cartil. 2009, 17, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Maniatis, D.T. Transcriptional Regulation of Endothelial Cell Adhesion Molecules: Nf-Kappa B And Cytokine-Inducible Enhancers. FASEB J. 1995, 9, 899–909. [Google Scholar]
- Perry, M.M.; Hui, C.K.; Whiteman, M.; Wood, M.E.; Adcock, I.; Kirkham, P.; Michaeloudes, C.; Chung, K.F. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 746–752. [Google Scholar] [CrossRef]
- Zhang, H.; Zhi, L.; Moochhala, S.; Moore, P.K.; Bhatia, M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L960–L971. [Google Scholar] [CrossRef]
- Braun, T.; Zwerina, J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, 235. [Google Scholar] [CrossRef]
- Frantzias, J.; Logan, J.G.; Mollat, P.; Sparatore, A.; Del Soldato, P.; Ralston, S.H.; Idris, A.I. Hydrogen sulphide-releasing diclofenac derivatives inhibit breast cancer-induced osteoclastogenesis in vitro and prevent osteolysis ex vivo. Br. J. Pharmacol. 2012, 165, 1914–1925. [Google Scholar] [CrossRef]
- Tang, G.; Wu, L.; Liang, W.; Wang, R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005, 68, 1757–1764. [Google Scholar] [CrossRef]
- Sen, U.; Mishra, P.K.; Tyagi, N.; Tyagi, S.C. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem. Biophys. 2010, 57, 49–58. [Google Scholar] [CrossRef]
- Kimura, H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013, 63, 492–497. [Google Scholar] [CrossRef]
- Dawe, G.S.; Han, S.P.; Bian, J.S.; Moore, P.K. Hydrogen sulphide in the hypothalamus causes an ATP-sensitive K+ channel-dependent decrease in blood pressure in freely moving rats. Neuroscience 2008, 152, 169–177. [Google Scholar] [CrossRef]
- Eto, K.; Asada, T.; Arima, K.; Makifuchi, T.; Kimura, H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 293, 1485–1488. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Mard, S.A.; Neisi, N.; Solgi, G.; Hassanpour, M.; Darbor, M.; Maleki, M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig. Dis. Sci. 2012, 57, 1496–1503. [Google Scholar] [CrossRef]
- Ju, Y.; Zhang, W.; Pei, Y.; Yang, G. H(2)S signaling in redox regulation of cellular functions. Can. J. Physiol. Pharmacol. 2013, 91, 8–14. [Google Scholar] [CrossRef]
- Dief, A.E.; Mostafa, D.K.; Sharara, G.M.; Zeitoun, T.H. Hydrogen sulfide releasing naproxen offers better anti-inflammatory and chondroprotective effect relative to naproxen in a rat model of zymosan induced arthritis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1537–1546. [Google Scholar]
- Ekundi-Valentim, E.; Mesquita, F.P.; Santos, K.T.; de Paula, M.A.; Florenzano, J.; Zanoni, C.I.; Rodrigues, L.; de Nucci, G.; Teixeira, S.A.; Ferreira, H.H.; et al. A comparative study on the anti-inflammatory effects of single oral doses of naproxen and its hydrogen sulfide (H2S)-releasing derivative ATB-346 in rats with carrageenan-induced synovitis. Med. Gas Res. 2013, 3, 24. [Google Scholar] [CrossRef]
- Wallace, J.L.; Nagy, P.; Feener, T.D.; Allain, T.; Ditroi, T.; Vaughan, D.J.; Muscara, M.N.; de Nucci, G.; Buret, A.G. A proof-of-concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vaughan, D.; Dicay, M.; MacNaughton, W.K.; de Nucci, G. Hydrogen Sulfide-Releasing Therapeutics: Translation to the Clinic. Antioxid. Redox Signal. 2018, 28, 1533–1540. [Google Scholar] [CrossRef]
- Kashfi, K.; Chattopadhyay, M.; Kodela, R. NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Redox Biol. 2015, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kodela, R.; Chattopadhyay, M.; Velazquez-Martinez, C.A.; Kashfi, K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 2015, 98, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Breschi, M.C.; Blandizzi, C.; Virdis, A.; Taddei, S.; Calderone, V. Hydrogen sulphide: Novel opportunity for drug discovery. Med. Res. Rev. 2012, 32, 1093–1130. [Google Scholar] [CrossRef]
- B Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Ghasemi, Z.; Roghani, M. S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur. J. Pharmacol. 2017, 794, 69–76. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, R.; Jin, X.; Tan, X. Antiarthritic Activity of Diallyl Disulfide against Freund’s Adjuvant-Induced Arthritic Rat Model. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 291–303. [Google Scholar] [CrossRef]
- Fiorucci, S.; Santucci, L. Hydrogen sulfide-based therapies: Focus on H2S releasing NSAIDs. Inflamm. Allergy Drug Targets 2011, 10, 133–140. [Google Scholar] [CrossRef]
- Muzaffar, S.; Jeremy, J.Y.; Sparatore, A.; Del Soldato, P.; Angelini, G.D.; Shukla, N. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: Role of protein kinases A and G. Br. J. Pharmacol. 2008, 155, 984–994. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Zhu, Y. Therapeutic application of hydrogen sulfide donors: The potential and challenges. Front. Med. 2016, 10, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; D’Amora, U.; Ravalli, S.; Ambrosio, L.; Di Rosa, M.; Musumeci, G. Functional Biomolecule Delivery Systems and Bioengineering in Cartilage Regeneration. Curr. Pharm. Biotechnol. 2019, 20, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudifar, N.; Doran, P.M. Chondrogenesis and cartilage tissue engineering: The longer road to technology development. Trends Biotechnol. 2012, 30, 166–176. [Google Scholar] [CrossRef]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011, 7, 463–477. [Google Scholar]
- Zhang, Q.; Liu, S.; Li, T.; Yuan, L.; Liu, H.; Wang, X.; Wang, F.; Wang, S.; Hao, A.; Liu, D.; et al. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget 2016, 7, 58089–58104. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, L.; Gong, Y.; Gao, C.; Shen, J. A polylactide/fibrin gel composite scaffold for cartilage tissue engineering: Fabrication and an in vitro evaluation. J. Mater. Sci. Mater. Med. 2009, 20, 135–143. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Kudva, A.K.; Saxena, N.S.; Roy, K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011, 32, 6946–6952. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, Y.; Xian, M.; Wang, Q. Biological thiols-triggered hydrogen sulfide releasing microfibers for tissue engineering applications. Acta Biomater. 2015, 27, 205–213. [Google Scholar] [CrossRef]
- Wang, C.C.; Yang, K.C.; Lin, K.H.; Liu, Y.L.; Liu, H.C.; Lin, F.H. Cartilage regeneration in SCID mice using a highly organized three-dimensional alginate scaffold. Biomaterials 2012, 33, 120–127. [Google Scholar] [CrossRef]
- Nettles, D.L.; Vail, T.P.; Morgan, M.T.; Grinstaff, M.W.; Setton, L.A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann. Biomed. Eng. 2004, 32, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Trattnig, S.; Ohel, K.; Mlynarik, V.; Juras, V.; Zbyn, S.; Korner, A. Morphological and compositional monitoring of a new cell-free cartilage repair hydrogel technology-GelrinC by MR using semi-quantitative MOCART scoring and quantitative T2 index and new zonal T2 index calculation. Osteoarthr. Cartil. 2015, 23, 2224–2232. [Google Scholar] [CrossRef]
- Mauretti, A.; Neri, A.; Kossover, O.; Seliktar, D.; Di Nardo, P.; Melino, S. Design of a Novel Composite H2 S-Releasing Hydrogel for Cardiac Tissue Repair. Macromol. Biosci. 2016, 16, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; He, C.; Kang, J.; Ye, J.; Xiao, Z.; Zhu, J.; Chen, A.; Feng, S.; Li, X.; et al. Novel H2S Releasing Nanofibrous Coating for In Vivo Dermal Wound Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 27474–27481. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2368. [Google Scholar] [CrossRef] [PubMed]
- Gambari, L.; Amore, E.; Raggio, R.; Bonani, W.; Barone, M.; Lisignoli, G.; Grigolo, B.; Motta, A.; Grassi, F. Hydrogen sulfide-releasing silk fibroin scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 471–482. [Google Scholar] [CrossRef]
- Liang, W.; Chen, J.; Li, L.; Li, M.; Wei, X.; Tan, B.; Shang, Y.; Fan, G.; Wang, W.; Liu, W. Conductive Hydrogen Sulfide-Releasing Hydrogel Encapsulating ADSCs for Myocardial Infarction Treatment. ACS Appl. Mater. Interfaces 2019, 11, 14619–14629. [Google Scholar] [CrossRef]
- Carter, J.M.; Qian, Y.; Foster, J.C.; Matson, J.B. Peptide-based hydrogen sulphide-releasing gels. Chem. Commun. 2015, 51, 13131–13134. [Google Scholar] [CrossRef]
 ), such as the rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Furthermore, innate immunity is involved in chronic inflammation. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the differentiation of the macrophages. Monocytes and macrophages, as well as dendritic cells, seem to play a vital role in the pathogenesis of inflammatory arthritis, as antigen-presenting cells (APCs), and they can express several pro-inflammatory cytokines. In early stages of inflammation, hypoxia and the production of ROS (reactive oxygen species) and RNS (reactive nitrogen species) seems to play a role in the initiation of the inflammatory process and induction of angiogenesis. The new blood vessels further maximize the recruitment of immune cells, amplifying the inflammatory process. The chronic inflammation finally perpetuates the production of pro-inflammatory cytokines (such as tumor necrosis factor-α (TNF-α)) and other mediators, such as prostaglandin E2, which ultimately generate vasodilation, infiltration of immune cells, and destruction of the cartilage.
), such as the rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Furthermore, innate immunity is involved in chronic inflammation. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the differentiation of the macrophages. Monocytes and macrophages, as well as dendritic cells, seem to play a vital role in the pathogenesis of inflammatory arthritis, as antigen-presenting cells (APCs), and they can express several pro-inflammatory cytokines. In early stages of inflammation, hypoxia and the production of ROS (reactive oxygen species) and RNS (reactive nitrogen species) seems to play a role in the initiation of the inflammatory process and induction of angiogenesis. The new blood vessels further maximize the recruitment of immune cells, amplifying the inflammatory process. The chronic inflammation finally perpetuates the production of pro-inflammatory cytokines (such as tumor necrosis factor-α (TNF-α)) and other mediators, such as prostaglandin E2, which ultimately generate vasodilation, infiltration of immune cells, and destruction of the cartilage.  , dendritic cell/APC;
, dendritic cell/APC;  , osteoclast;
, osteoclast;  , chondrocyte.
, chondrocyte.
 ), such as the rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Furthermore, innate immunity is involved in chronic inflammation. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the differentiation of the macrophages. Monocytes and macrophages, as well as dendritic cells, seem to play a vital role in the pathogenesis of inflammatory arthritis, as antigen-presenting cells (APCs), and they can express several pro-inflammatory cytokines. In early stages of inflammation, hypoxia and the production of ROS (reactive oxygen species) and RNS (reactive nitrogen species) seems to play a role in the initiation of the inflammatory process and induction of angiogenesis. The new blood vessels further maximize the recruitment of immune cells, amplifying the inflammatory process. The chronic inflammation finally perpetuates the production of pro-inflammatory cytokines (such as tumor necrosis factor-α (TNF-α)) and other mediators, such as prostaglandin E2, which ultimately generate vasodilation, infiltration of immune cells, and destruction of the cartilage.
), such as the rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Furthermore, innate immunity is involved in chronic inflammation. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the differentiation of the macrophages. Monocytes and macrophages, as well as dendritic cells, seem to play a vital role in the pathogenesis of inflammatory arthritis, as antigen-presenting cells (APCs), and they can express several pro-inflammatory cytokines. In early stages of inflammation, hypoxia and the production of ROS (reactive oxygen species) and RNS (reactive nitrogen species) seems to play a role in the initiation of the inflammatory process and induction of angiogenesis. The new blood vessels further maximize the recruitment of immune cells, amplifying the inflammatory process. The chronic inflammation finally perpetuates the production of pro-inflammatory cytokines (such as tumor necrosis factor-α (TNF-α)) and other mediators, such as prostaglandin E2, which ultimately generate vasodilation, infiltration of immune cells, and destruction of the cartilage.  , dendritic cell/APC;
, dendritic cell/APC;  , osteoclast;
, osteoclast;  , chondrocyte.
, chondrocyte.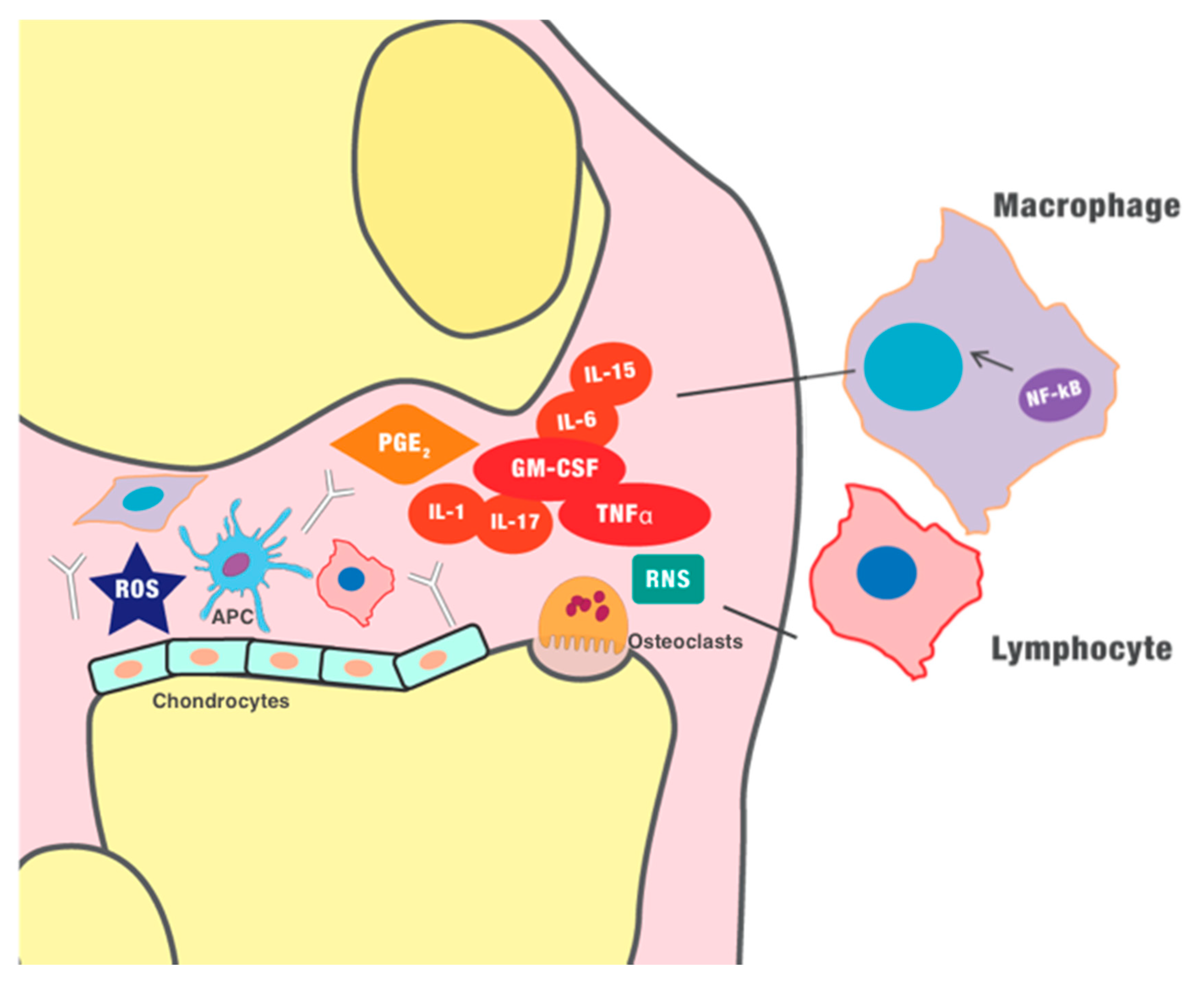
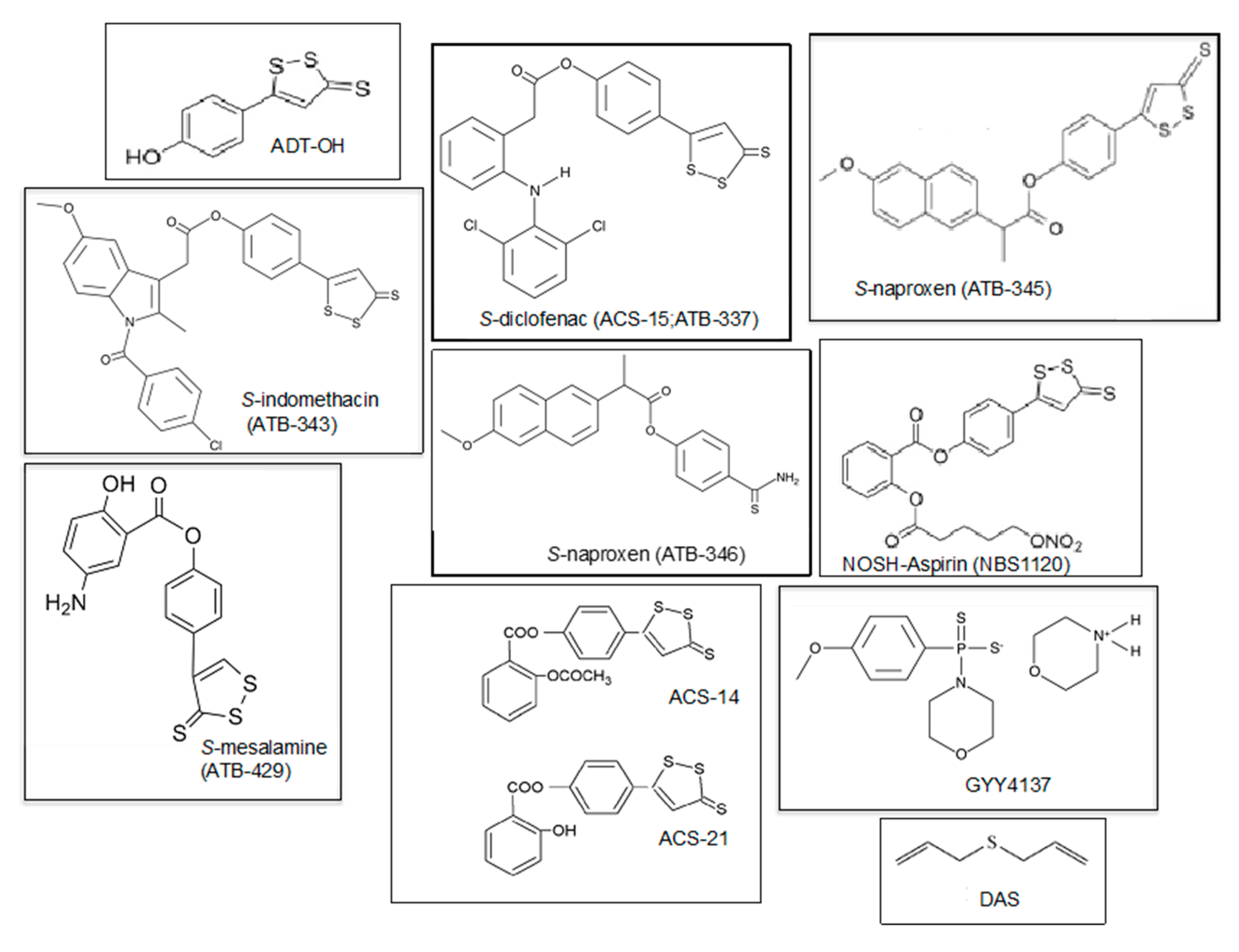
| H2S-Derivative Drug | Drug | Company | Clinical Phase | Clinical Applications | References |
|---|---|---|---|---|---|
| AVT-18A | Sulindac | Avicenna T. | Preclinical | Cancer, inflammation | [143] |
| NBS-1120 | Aspirin | Avicenna T. | Preclinical | Cancer, inflammation | [144] |
| ACS-14 | Aspirin | CTG Ph. | Preclinical | Inflammation, cardiac injury, Arthritis | [149] |
| ACS-21 | Aspirin | CTG Ph. | Preclinical | Inflammation, cardiac injury, Osteoarthritis | [149] |
| ACS-6 | Ketorolac | CTG Ph. | Preclinical | Arthritis Antioxidant | [149,150] |
| ATB-337/ACS-15 | Diclofenac | Antibe T. | Preclinical | Arthritis, inflammation | [149] |
| ATB-343 | Naproxen | Antibe T. | Preclinical | Inflammatory diseases, Alzheimer’s disease | [149] |
| ATB-346 | Naproxen | Antibe T. | Phase II | Osteoarthritis, inflammation | [102,141,149] |
| ATB-345 | Naproxen | Antibe T. | Preclinical | Inflammatory diseases | [102] |
| ATB-429 | Meselamine | Antibe T. | Preclinical | Cancer, inflammatory diseases, colitis | [75] |
| GYY4137 DAS/DADS | National Uni. of Singapore | Preclinical Preclinical | Inflammatory diseases, cancer, hypertension, arthritis Cancer, arthritis | [82,107,122,151] [147,148] |
| Scaffold | Characteristics and Effects | Type of Cells | Commercial Product | Phase of Study | References |
|---|---|---|---|---|---|
| PLLA/fibrin 1PLLA/chondrocyte/atelocollagen | Improved cell proliferation and expression of type I and type II collagen | Chondrocytes | PLA-based BioSeedR-C (BioTissue, AG, Zurich, Switzerland) | In vitro | [157] |
| PEG dyacrylate systems PEG/chitosan PEG/albumin | In situ photopolymerization and potential modulation of its mechanical properties, increasing of the expression of type I and II collagen and the amount of sulfated GAG | MSCs | In vitro | [158] | |
| Alginate | Increase in chondrocyte viability | Chondrocytes | In vivo (SCID mice) | [160] | |
| Hyaluronic acid/fibrin Hyaluronic acid/collagen type I | In situ photopolymerization, potential modulation of its mechanical properties, stimulation of ECM production and proteoglycan synthesis, and improved chondrocyte growth | Chondrocytes | Hyaluronic-based HyalograftR C autograft (Anika Therapeutics, Inc., Bedford, MA, USA) | In vivo (human) | [161] |
| PEG-DA/denatured human fibrinogen (DHF) | In situ photopolymerization, potential modulation of its mechanical properties, gradual resorption by the body being replaced by new cartilage tissue | Cell free | GelrinC (Regentis, Haifa, Israel) | Phase II | [163,164] |
| H2S-releasing scaffolds | |||||
| PCL/NSHD1 | Significant decrease in apoptosis in a model of tissue transplantation, protection from ROS damage, and increase in expression of collagen type I and type III | 3T3 | In vivo | [154] | |
| PFM/GaOS or DADS | Improved MSC proliferation and anti-microbial activity and protective effect against oxidative damage | hMSCs | In vitro | [166] | |
| TSTMBs-PFHy | In situ photopolymerization, potential modulation of its mechanical properties, induced spindled morphology of cells and cell proliferation | HFFs hCPCs | In vitro | [164] | |
| ALG-CHO/2-aminopyridine-5-thiocarboxamide/tetraaniline | Increase in ejection fraction value, reduction of the myocardial infarct size in rats | ADSCs | In vivo | [168] | |
| SATO/CaCl2 | Decrease in intimal hyperplasia in human veins | Endothelial cells | In vivo | [169] | |
| SF/GYY4137 | Significant increase in osteogenic differentiation of stem cells, upregulation of osteogenic and angiogenic genes and integrins | OBs, hMSC | In vitro | [167] | |
| HA or PCL/JK1 | H2S release in pH-dependent manner, improved cell proliferation. tissue regeneration, re-epithelialization, collagen deposition, angiogenesis | Raw 264.7 | In vivo (mouse Male C57BL) | [165] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunzini, F.; De Stefano, S.; Chimenti, M.S.; Melino, S. Hydrogen Sulfide as Potential Regulatory Gasotransmitter in Arthritic Diseases. Int. J. Mol. Sci. 2020, 21, 1180. https://doi.org/10.3390/ijms21041180
Sunzini F, De Stefano S, Chimenti MS, Melino S. Hydrogen Sulfide as Potential Regulatory Gasotransmitter in Arthritic Diseases. International Journal of Molecular Sciences. 2020; 21(4):1180. https://doi.org/10.3390/ijms21041180
Chicago/Turabian StyleSunzini, Flavia, Susanna De Stefano, Maria Sole Chimenti, and Sonia Melino. 2020. "Hydrogen Sulfide as Potential Regulatory Gasotransmitter in Arthritic Diseases" International Journal of Molecular Sciences 21, no. 4: 1180. https://doi.org/10.3390/ijms21041180
APA StyleSunzini, F., De Stefano, S., Chimenti, M. S., & Melino, S. (2020). Hydrogen Sulfide as Potential Regulatory Gasotransmitter in Arthritic Diseases. International Journal of Molecular Sciences, 21(4), 1180. https://doi.org/10.3390/ijms21041180






