The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium
Abstract
1. Intestinal Epithelium as a Selective Barrier
2. Tight Junction Protein Structure
Special Functions of Select Tight Junction Proteins
3. Acute Mechanisms of Repair in Injured Intestinal Epithelium
4. Regulation of Tight Junctions via Ion Channels/Transporters
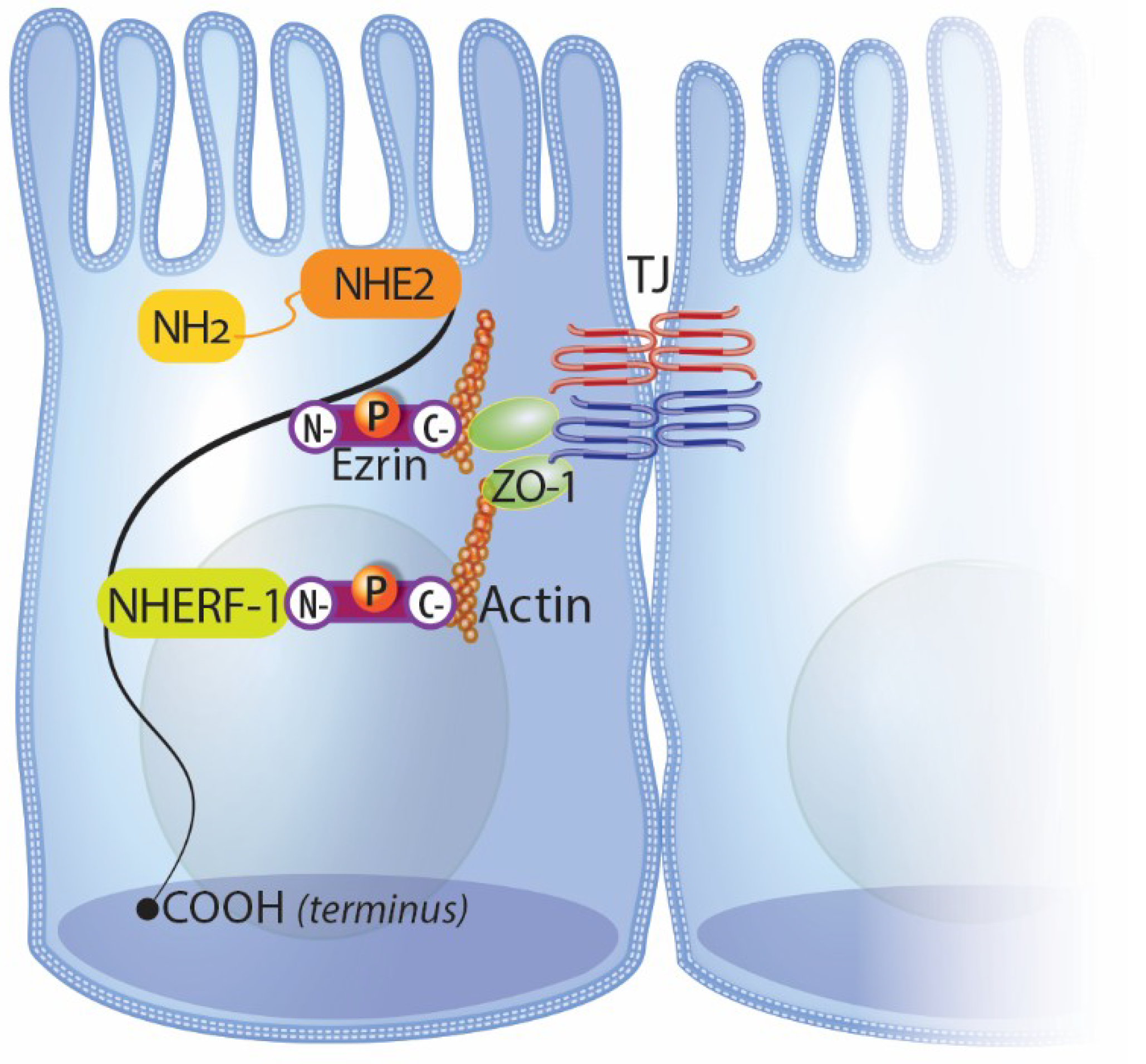
4.1. NHE2 and Intestinal Repair
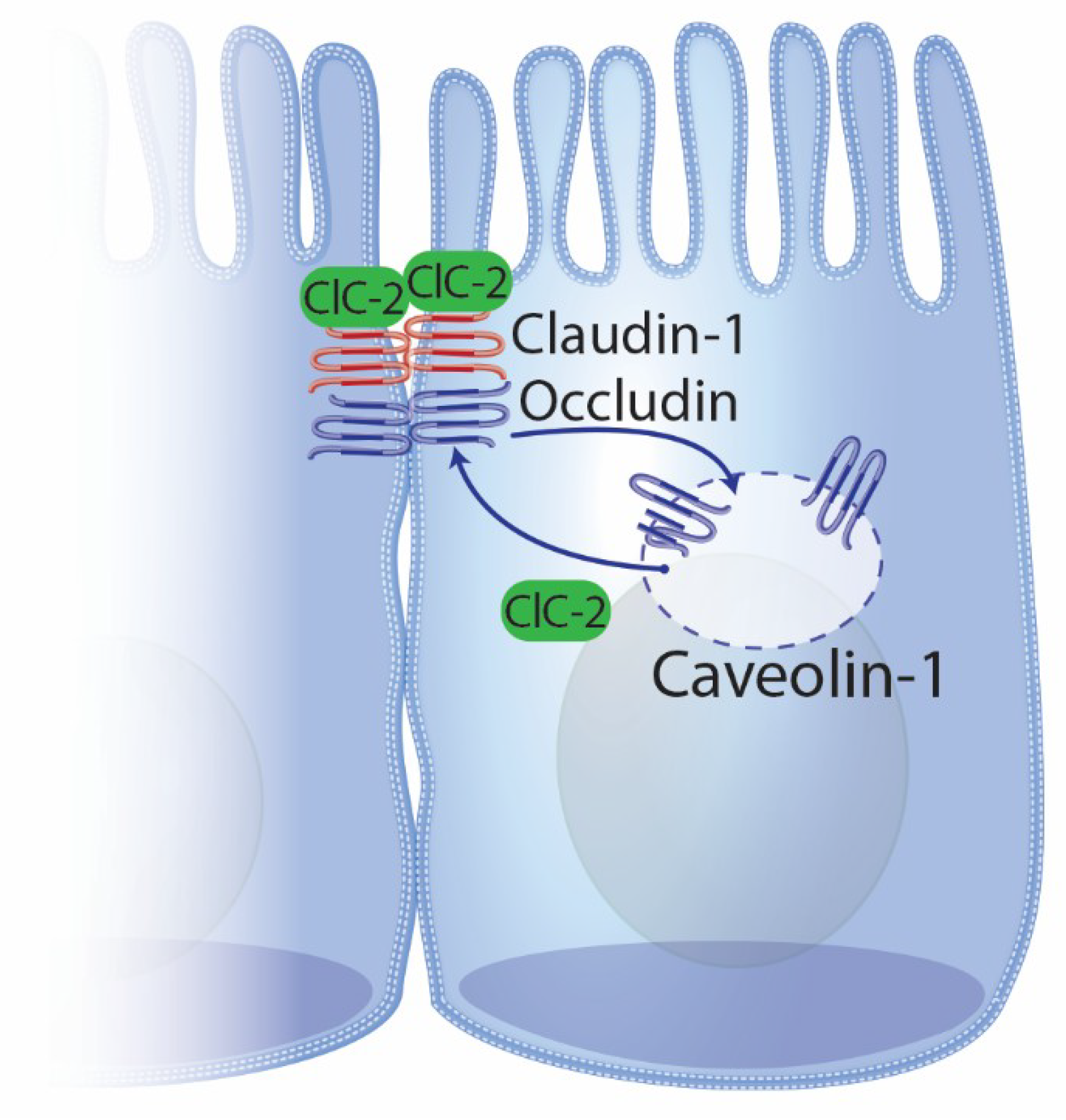
4.2. ClC-2 and Intestinal Repair
5. Conclusions
Funding
Conflicts of Interest
References
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.H.; Weber, C.R. Molecular aspects of tight junction barrier function. Curr. Opin. Pharmacol. 2014, 19, 84–89. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; Macdonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- El Asmar, R.; Panigrahi, P.; Bamford, P.; Berti, I.; Not, T.; Coppa, G.V.; Catassi, C.; Fasano, A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 2002, 123, 1607–1615. [Google Scholar] [CrossRef]
- Al-Sadi, R. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; Demeyere, K.; Berthiller, F.; Michlmayr, H.; Varga, E.; Adam, G.; Meyer, E.; Croubels, S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016, 95, 103–109. [Google Scholar] [CrossRef]
- Schumann, M.; Siegmund, B.; Schulzke, J.D.; Fromm, M. Celiac Disease: Role of the Epithelial Barrier. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 150–162. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, C.; Liu, X.; Qu, L.; Gu, L.; Li, N.; Li, J. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J. Cell. Mol. Med. 2009, 13, 4061–4076. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Blikslager, A.T.; Moeser, A.J.; Gookin, J.L.; Jones, S.L.; Odle, J. Restoration of Barrier Function in Injured Intestinal Mucosa. Physiol. Rev. 2007, 87, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Animal models of ischemia-reperfusion-induced intestinal injury: Progress and promise for translational research. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 308, G63–G75. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M. Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef]
- Chiba, H.; Osanai, M.; Murata, M.; Kojima, T.; Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta BBA Biomembr. 2008, 1778, 588–600. [Google Scholar] [CrossRef]
- Günzel, D.; Fromm, M. Claudins and Other Tight Junction Proteins. Compr. Physiol. 2012, 2, 1819–1852. [Google Scholar]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Phosphorylation of tight junction transmembrane proteins: Many sites, much to do. Tissue Barriers 2018, 6, e1382671. [Google Scholar] [CrossRef]
- Furuse, M. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef]
- Martin-Padura, I.; Lostaglio, S.; Schneemann, M.; Williams, L.; Romano, M.; Fruscella, P.; Panzeri, C.; Stoppacciaro, A.; Ruco, L.; Villa, A.; et al. Junctional Adhesion Molecule, a Novel Member of the Immunoglobulin Superfamily That Distributes at Intercellular Junctions and Modulates Monocyte Transmigration. J. Cell Biol. 1998, 142, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, D. Vascular Endothelial Junction-associated Molecule, a Novel Member of the Immunoglobulin Superfamily, Is Localized to Intercellular Boundaries of Endothelial Cells. J. Biol. Chem. 2000, 275, 19139–19145. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Chiu, H.H.; Gurney, A.; Sidle, A.; Tumas, D.B.; Schow, P.; Foster, J.; Klassen, T.; Dennis, K.; Demarco, R.A.; et al. Vascular endothelial-junctional adhesion molecule (VE-JAM)/JAM 2 interacts with T, NK, and dendritic cells through JAM 3. J. Immunol. 2002, 168, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Ebnet, K.; Aurrand-Lions, M.; Kiefer, F.; Butz, S.; Zander, K.; Zu Brickwedde, M.K.M.; Kuhn, A.; Suzuki, A.; Imhof, B.A.; Vestweber, D. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: A possible role for JAMs in endothelial cell polarity. J. Cell Sci. 2003, 116, 3879–3891. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Urbani, S.; Bradfield, P.F.; Imhof, B.A. Tight junction dynamics: The role of junctional adhesion molecules (JAMs). Cell Tissue Res. 2014, 355, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Zemans, R.L.; Colgan, S.P.; Downey, G.P. Transepithelial migration of neutrophils: Mechanisms and implications for acute lung injury. Am. J. Respir. Cell Mol. Biol. 2009, 40, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef]
- Gumbiner, B.; Lowenkopf, T.; Apatira, D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3460–3464. [Google Scholar] [CrossRef]
- Jesaitis, L.A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J. Cell Biol. 1994, 124, 949–961. [Google Scholar] [CrossRef]
- Balda, M.S. Assembly of the tight junction: The role of diacylglycerol. J. Cell Biol. 1993, 123, 293–302. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S.L.; Yin, H.; Havrilla, C.M.; Gao, L.; Morrow, J.D.; Porter, N.A.; Enck, A.H.; Lencer, W.I.; Schneeberger, E.E. Claudin-8 Expression in Madin-Darby Canine Kidney Cells Augments the Paracellular Barrier to Cation Permeation. J. Biol. Chem. 2003, 278, 17350–17359. [Google Scholar] [CrossRef]
- France, M.M.; Turner, J.R. The mucosal barrier at a glance. J. Cell Sci. 2017, 130, 307–314. [Google Scholar] [CrossRef]
- Luettig, J.; Rosenthal, R.; Barmeyer, C.; Schulzke, J. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 2015, 3, e977176. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Burgel, N.; Gunzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Escaffit, F.; Boudreau, F.; Beaulieu, J.F. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J. Cell. Physiol. 2005, 203, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Rahner, C.; Mitic, L.L.; Anderson, J.M. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 2001, 120, 411–422. [Google Scholar] [CrossRef]
- Fujita, H.; Chiba, H.; Yokozaki, H.; Sakai, N.; Sugimoto, K.; Wada, T.; Kojima, T.; Yamashita, T.; Sawada, N. Differential Expression and Subcellular Localization of Claudin-7, -8, -12, -13, and -15 Along the Mouse Intestine. J. Histochem. Cytochem. 2006, 54, 933–944. [Google Scholar] [CrossRef]
- Holmes, J.L.; Van Itallie, C.M.; Rasmussen, J.E.; Anderson, J.M. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr. Patterns 2006, 6, 581–588. [Google Scholar] [CrossRef]
- Pasternak, J.A.; Kent-Dennis, C.; Van Kessel, A.G.; Wilson, H.L. Claudin-4 Undergoes Age-Dependent Change in Cellular Localization on Pig Jejunal Villous Epithelial Cells, Independent of Bacterial Colonization. Mediat. Inflamm. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Lager, K.M.; Splichal, I.; Francis, D.; Kacskovics, I.; Sinkora, M.; Wertz, N.; Sun, J.; Zhao, Y.; Brown, W.R.; et al. The piglet as a model for B cell and immune system development. Vet. Immunol. Immunopathol. 2009, 128, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Amasheh, S.; Richter, J.F.; Milatz, S.; Günzel, D.; Westphal, J.K.; Huber, O.; Schulzke, J.D.; Fromm, M. Tricellulin Forms a Barrier to Macromolecules in Tricellular Tight Junctions without Affecting Ion Permeability. Mol. Biol. Cell 2009, 20, 3713–3724. [Google Scholar] [CrossRef]
- Ciarlet, M.; Conner, M.E.; Finegold, M.J.; Estes, M.K. Group A Rotavirus Infection and Age-Dependent Diarrheal Disease in Rats: A New Animal Model To Study the Pathophysiology of Rotavirus Infection. J. Virol. 2002, 76, 41–57. [Google Scholar] [CrossRef]
- Estes, M.K.; Kang, G.; Zeng, C.Q.; Crawford, S.E.; Ciarlet, M. Pathogenesis of Rotavirus Gastroenteritis. Novartis Found. Symp. 2001, 238, 82–96; discussion 96–100. [Google Scholar]
- Klunder, L.J.; Faber, K.N.; Dijkstra, G.; Van Ijzendoorn, S.C. Mechanisms of Cell Polarity–Controlled Epithelial Homeostasis and Immunity in the Intestine. Cold Spring Harb. Perspect. Biol. 2017, 9, a027888. [Google Scholar] [CrossRef]
- Moore, R.; Carlson, S.; Madara, J.L. Villus contraction aids repair of intestinal epithelium after injury. Am. J. Physiol. Liver Physiol. 1989, 257, G274–G283. [Google Scholar] [CrossRef]
- Gookin, J.L.; Galanko, J.A.; Blikslager, A.T.; Argenzio, R.A. PG-mediated closure of paracellular pathway and not restitution is the primary determinant of barrier recovery in acutely injured porcine ileum. Am. J. Physiol. Liver Physiol. 2003, 285, G967–G979. [Google Scholar] [CrossRef]
- Dignass, A.U. Mechanisms and Modulation of Intestinal Epithelial Repair. Inflamm. Bowel Dis. 2001, 7, 68–77. [Google Scholar] [CrossRef]
- Utech, M.; Mennigen, R.; Bruewer, M. Endocytosis and recycling of tight junction proteins in inflammation. J. Biomed. Biotechnol. 2010, 2010, 484987. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Johnson, A.M.; Sladojevic, N.; Keep, R.F.; Andjelkovic, A.V. Endocytosis of tight junction proteins and the regulation of degradation and recycling. Ann. N. Y. Acad. Sci. 2017, 1397, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Blikslager, A.T. Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury. Am. J. Physiol. Physiol. 2016, 311, C996–C1004. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R.; Black, E.D.; Ward, J.; Tse, C.M.; Uchwat, F.A.; Alli, H.A.; Donowitz, M.; Madara, J.L.; Angle, J.M. Transepithelial resistance can be regulated by the intestinal brush-border Na(+)/H(+) exchanger NHE3. Am. J. Physiol. Cell Physiol. 2000, 279, C1918–C1924. [Google Scholar] [CrossRef]
- Moeser, A.J.; Nighot, P.K.; Ryan, K.A.; Simpson, J.E.; Clarke, L.L.; Blikslager, A.T. Mice lacking the Na+/H+ exchanger 2 have impaired recovery of intestinal barrier function. Am. J. Physiol. Gastrointest Liver Physiol. 2008, 295, G791–G797. [Google Scholar] [CrossRef]
- Moeser, A.J.; Nighot, P.K.; Ryan, K.A.; Wooten, J.G.; Blikslager, A.T. Prostaglandin-mediated inhibition of Na+/H+ exchanger isoform 2 stimulates recovery of barrier function in ischemia-injured intestine. Am. J. Physiol. Gastrointest Liver Physiol. 2006, 291, G885–G894. [Google Scholar] [CrossRef]
- Moeser, A.J.; Nighot, P.K.; Engelke, K.J.; Ueno, R.; Blikslager, A.T. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am. J. Physiol. Liver Physiol. 2007, 292, G647–G656. [Google Scholar] [CrossRef]
- Feranchak, A.P. Ion channels in digestive health and disease. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 230–241. [Google Scholar] [CrossRef]
- Donowitz, M.; Ming Tse, C.; Fuster, D. SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol. Asp. Med. 2013, 34, 236–251. [Google Scholar] [CrossRef]
- Gurney, M.A.; Laubitz, D.; Ghishan, F.K.; Kiela, P.R. Pathophysiology of Intestinal Na(+)/H(+) exchange. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 27–40. [Google Scholar] [CrossRef]
- Kurashima, K.; D’Souza, S.; Szaszi, K.; Ramjeesingh, R.; Orlowski, J.; Grinstein, S. The apical Na(+)/H(+) exchanger isoform NHE3 is regulated by the actin cytoskeleton. J. Biol. Chem. 1999, 274, 29843–29849. [Google Scholar] [CrossRef] [PubMed]
- Donowitz, M.; Li, X. Regulatory Binding Partners and Complexes of NHE3. Physiol. Rev. 2007, 87, 825–872. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Cheng, L.; Forte, J.G. Biochemical Characterization of Ezrin-Actin Interaction. J. Biol. Chem. 1996, 271, 7224–7229. [Google Scholar] [CrossRef] [PubMed]
- Vaheri, A.; Carpén, O.; Heiska, L.; Helander, T.S.; Jääskeläinen, J.; Majander-Nordenswan, P.; Sainio, M.; Timonen, T.; Turunen, O. The ezrin protein family: Membrane-cytoskeleton interactions and disease associations. Curr. Opin. Cell Biol. 1997, 9, 659–666. [Google Scholar] [CrossRef]
- Bosk, S.; Braunger, J.A.; Gerke, V.; Steinem, C. Activation of F-Actin Binding Capacity of Ezrin: Synergism of PIP2 Interaction and Phosphorylation. Biophys. J. 2011, 100, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Brückner, B.R.; Pietuch, A.; Nehls, S.; Rother, J.; Janshoff, A. Ezrin is a Major Regulator of Membrane Tension in Epithelial Cells. Sci. Rep. 2015, 5, 14700. [Google Scholar] [CrossRef]
- Hayashi, H.; Tamura, A.; Krishnan, D.; Tsukita, S.; Suzuki, Y.; Kocinsky, H.S.; Aronson, P.S.; Orlowski, J.; Grinstein, S.; Alexander, R.T. Ezrin is Required for the Functional Regulation of the Epithelial Sodium Proton Exchanger, NHE3. PLoS ONE 2012, 8, e55623. [Google Scholar]
- Praetorius, J.; Andreasen, D.; Jensen, B.L.; Ainsworth, M.A.; Friis, U.G.; Johansen, T. NHE1, NHE2, and NHE3 contribute to regulation of intracellular pH in murine duodenal epithelial cells. Am. J. Physiol. Liver Physiol. 2000, 278, G197–G206. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. Tight junctions. Their structure, composition, and function. Circ. Res. 1984, 55, 723–733. [Google Scholar] [CrossRef]
- Barrett, K.E.; Keely, S.J. Chloride Secretion by the Intestinal Epithelium: Molecular Basis and Regulatory Aspects. Annu. Rev. Physiol. 2000, 62, 535–572. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, R.A.; Hanrahan, J.W. Physiology of Epithelial Chloride and Fluid Secretion. Cold Spring Harb. Perspect. Med. 2012, 2, a009563. [Google Scholar] [CrossRef] [PubMed]
- Poroca, D.R.; Pelis, R.M.; Chappe, V.M. ClC Channels and Transporters: Structure, Physiological Functions, and Implications in Human Chloride Channelopathies. Front. Pharmacol. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Gyomorey, K.; Yeger, H.; Ackerley, C.; Garami, E.; Bear, C.E. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am. J. Physiol. Physiol. 2000, 279, C1787–C1794. [Google Scholar] [CrossRef] [PubMed]
- Catalan, M.; Niemeyer, M.I.; Cid, L.; Sepúlveda, F.V. Basolateral ClC-2 chloride channels in surface colon epithelium: Regulation by a direct effect of intracellular chloride. Gastroenterology 2004, 126, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Nighot, P.K.; Blikslager, A.T. ClC-2 regulates mucosal barrier function associated with structural changes to the villus and epithelial tight junction. Am. J. Physiol. Liver Physiol. 2010, 299, G449–G456. [Google Scholar] [CrossRef] [PubMed]
- Nighot, P.; Young, K.; Nighot, M.; Rawat, M.; Sung, E.J.; Maharshak, N.; Plevy, S.E.; Ma, T.; Blikslager, A. Chloride channel ClC-2 is a key factor in the development of DSS-induced murine colitis. Inflamm. Bowel Dis. 2013, 19, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Nighot, P.K.; Leung, L.; Ma, T.Y. Chloride channel ClC- 2 enhances intestinal epithelial tight junction barrier function via regulation of caveolin-1 and caveolar trafficking of occludin. Exp. Cell Res. 2017, 352, 113–122. [Google Scholar] [CrossRef]
- Roman, R.M.; Smith, R.L.; Feranchak, A.P.; Clayton, G.H.; Doctor, R.B.; Fitz, J.G. ClC-2 chloride channels contribute to HTC cell volume homeostasis. Am. J. Physiol. Liver Physiol. 2001, 280, G344–G353. [Google Scholar] [CrossRef]
- Nighot, M.P.; Nighot, P.K.; Ma, T.Y.; Malinowska, D.H.; Shull, G.E.; Cuppoletti, J.; Blikslager, A.T. Genetic Ablation of the ClC-2 Cl- Channel Disrupts Mouse Gastric Parietal Cell Acid Secretion. PLoS ONE 2015, 10, e0138174. [Google Scholar] [CrossRef]
- Nighot, P.K.; Blikslager, A.T. Chloride channel ClC-2 modulates tight junction barrier function via intracellular trafficking of occludin. Am. J. Physiol. Physiol. 2012, 302, C178–C187. [Google Scholar] [CrossRef]
- Jin, Y.; Blikslager, A.T. ClC-2 regulation of intestinal barrier function: Translation of basic science to therapeutic target. Tissue Barriers 2015, 3, e1105906. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slifer, Z.M.; Blikslager, A.T. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. Int. J. Mol. Sci. 2020, 21, 972. https://doi.org/10.3390/ijms21030972
Slifer ZM, Blikslager AT. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. International Journal of Molecular Sciences. 2020; 21(3):972. https://doi.org/10.3390/ijms21030972
Chicago/Turabian StyleSlifer, Zachary M., and Anthony T. Blikslager. 2020. "The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium" International Journal of Molecular Sciences 21, no. 3: 972. https://doi.org/10.3390/ijms21030972
APA StyleSlifer, Z. M., & Blikslager, A. T. (2020). The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. International Journal of Molecular Sciences, 21(3), 972. https://doi.org/10.3390/ijms21030972




