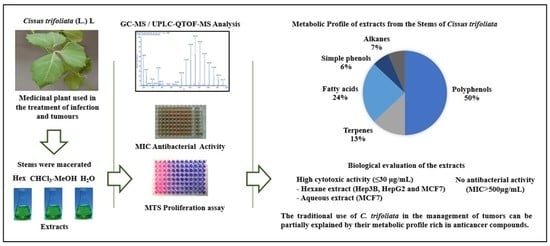
Metabolic Profile and Evaluation of Biological Activities of Extracts from the Stems of Cissus trifoliata
Abstract
1. Introduction
2. Results
2.1. GC-MS Analysis of Hexane Stem Extract of C. trifoliata
2.2. UPLC-QTOF-MS Analysis of CHCl3-MeOH Stems Extract of C. trifoliata
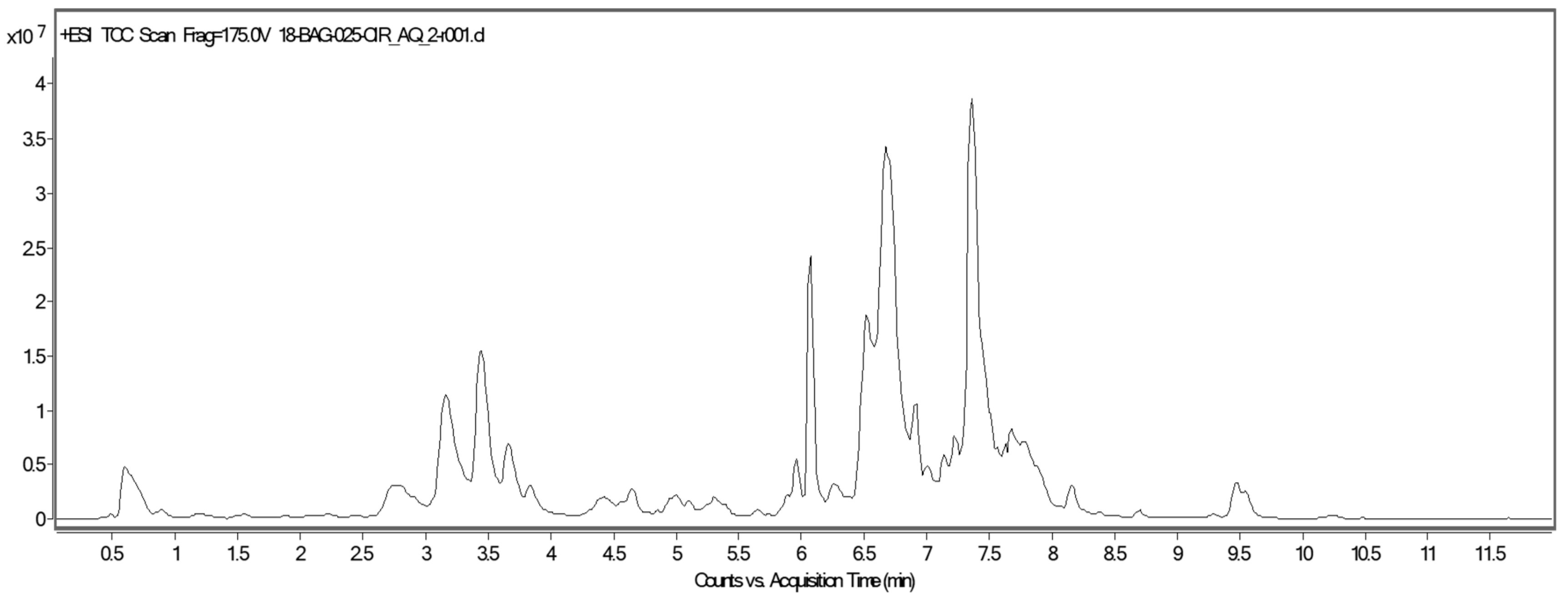
2.3. UPLC-QTOF-MS Analysis of Aqueous Stems Extract of C. trifoliata
2.4. Biological Evaluation of C. trifoliata Stem Extracts
2.4.1. Antibacterial Activity
2.4.2. Cytotoxic Activity
3. Discussion
3.1. Metabolic Profile of Stems Extracts from C. trifoliata
3.2. Antibacterial Activity
3.3. Cytotoxic Activity
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. GC-MS Analysis
4.3. UPLC-QTOF-MS Analysis
4.4. Antibacterial Activity
4.5. Cytotoxic Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A431 | Human epidermoid carcinoma in cell line |
| ATCC | American Type Culture Collection |
| Bcl2 | B-cell lymphoma 2 |
| CaCo-2 | Human colon caucasian colon adenocarcinoma |
| CDKs | Cyclin-dependent kinases |
| CFU | Colony forming units |
| ER | Estrogen receptor |
| ESI | Electrospray ionization |
| GC | Gas Chromatography |
| HeLa | Human cervix adenocarcinoma cell line |
| Hep3B | Human Hepatocellular Carcinoma cell line |
| HepG2 | Human Hepatocellular Carcinoma cell line |
| HMDB | Human Metabolome Database |
| LC | Liquid chromatography |
| MCF7 | Human breast carcinoma cell line |
| MIC | Minimum Inhibitory Concentration |
| MS | Mass spectrometry |
| MTS | [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| NDM-1 | New Delhi metallo-beta-lactamase |
| NIST | National Institute Standard and Technology |
| p21 | Cyclin-dependent kinase inhibitor |
| PC3 | Human prostate cancer cell line. |
| QTOF | Quadrupole Time of Flight |
| UPLC | Ultra High-Performance Liquid Chromatography |
References
- Banu, J. Medicinal properties of plants from the genus Cissus: A review. J. Med. Plant. Res. 2012, 6, 3080–3086. [Google Scholar]
- Chidambara, M.; Vanitha, A.; Mahadeva, M.; Ravishankar, G. Antioxidant and antimicrobial activity of Cissus quadrangularis L. J. Med Food. 2003, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bhujade, A.; Talmale, S.; Kumar, N.; Gupta, G.; Reddanna, P.; Patil, M. Evaluation of Cissus quadrangularis extracts as an inhibitor of COX, 5-LOX, and proinflammatory mediators. J. Ethnopharmacol. 2012, 141, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Lucena, F.; Almeida, E.; Aguiar, J.; Silva, T.; Souza, V.; Nascimento, S. Cytotoxic, antitumor and leukocyte migration activities of resveratrol and sitosterol present in the hidroalcoholic extract of Cissus sicyoides L., Vitaceae, leaves. Rev. Bras. de Farmacogn. 2010, 20, 729–733. [Google Scholar] [CrossRef]
- Beltrame, F.L.; Sartoretto, J.L.; Bazotte, R.B.; Cuman, R.N.; Cortez, D.; Fernandes, L.C.; Tchaikovski, O. Phytochemical study and evaluation of the antidiabetic potential of Cissus sicyoides L. (Vitaceae). Braz. Arch. Biol. Technol. 2001, 24, 783–785. [Google Scholar]
- Pathomwichaiwat, T.; Ochareon, P.; Soonthornchareonnon, N.; Ali, Z.; Khan, I.A.; Prathanturarug, S. Alkaline phosphatase activity-guided isolation of active compounds and new dammarane-type triterpenes from Cissus quadrangularis hexane extract. J. Ethnopharmacol. 2015, 160, 52–60. [Google Scholar] [CrossRef]
- Xu, F.; Matsuda, H.; Hata, H.; Sugawara, K.; Nakamura, S.; Yoshikawa, M. Structures of new flavonoids and benzofuran-type stilbene and degranulation inhibitors of rat basophilic leukemia cells from the Brazilian herbal medicine Cissus sicyoides. Chem. Pharm. Bull. 2009, 57, 1089–1095. [Google Scholar] [CrossRef][Green Version]
- Adesanya, S.A.; Nia, R.; Martin, M.; Boukamcha, N.; Montagnac, A.; Païs, M. Stilbene derivatives from Cissus quadrangularis. J. Nat. Prod. 1999, 62, 1694–1695. [Google Scholar] [CrossRef]
- Ahmadu, A.; Onanuga, A.; Aquino, R. Flavonoid glycosides from the leaves of Cissus ibuensis hook (vitaceae). Afr. J. Tradi. Complement. Altern. Med. 2010, 7, 225–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. Medicinal plants in Mexico: Healers’ consensus and cultural importance. Soc. Sci. Med. 1998, 47, 1859–1871. [Google Scholar] [CrossRef]
- Alvarado, V.; Moreno, L. Las Plantas útiles de Nuevo León. De la Lechuguilla a las Biopelículas Vegetales; Universidad Autónoma de Nuevo León: Monterrey, Nuevo León, Mexico, 2010; Volume 1, p. 650. [Google Scholar]
- Perez, R.; Pérez, S.; Perez, C.; Zavala, M. Anti-inflammatory activity of Cissus trifoliata. Fitotherapy 1993, 64, 103–107. [Google Scholar]
- Kashikar, N.; George, I. Antibacterial activity of Cissus quadrangularis Linn. Indian J. Pharm. Sci. 2006, 68, 245–247. [Google Scholar]
- Selvan, A.T.; Yezdhani, R.; Subramanian, N.S.; Devi, M.R.; Prasad, B.S.G.; Kumar, S.; Parimala, S. Antimicrobial activity of methanolic extract of Cissus pallida. Int. J. Pharmacog. 2014, 1, 592–595. [Google Scholar]
- Bhujade, A.; Gupta, G.; Talmale, S.; Das, S.; Patil, M. Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax–Bcl-2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food. Funct. 2013, 4, 338–346. [Google Scholar] [CrossRef]
- Opoku, A.; Geheeb-Keller, M.; Lin, J.; Terblanche, S.; Hutchings, A.; Chuturgoon, A.; Pillay, D. Preliminary screening of some traditional Zulu medicinal plants for antineoplastic activities versus the HepG2 cell line. Phytother. Res. 2000, 14, 534–537. [Google Scholar] [CrossRef]
- Sáenz, M.; Garcia, M.; Quilez, A.; Ahumada, M. Cytotoxic activity of Agave intermixta L. (Agavaceae) and Cissus sicyoides L. (Vitaceae). Phytother. Res. 2000, 14, 552–554. [Google Scholar] [CrossRef]
- Line-Edwige, M.; Raymond, F.G.; François, E.; Edouard, N. Antiproliferative effect of alcoholic extracts of some Gabonese medicinal plants on human colonic cancer cells. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 112–117. [Google Scholar] [CrossRef]
- Graziani, V.; Scognamiglio, M.; Belli, V.; Esposito, A.; Chambery, A.; Panella, M.; Ciardello, F. Metabolomic approach for a rapid identification of natural products with cytotoxic activity against human colorectal cancer cells. Sci. Rep. 2018, 8, 5309. [Google Scholar] [CrossRef]
- Farag, M.; Weigend, M.; Luebert, F.; Brokamp, G.; Wessjohann, L. Phytochemical, phylogenetic, and anti-inflammatory evaluation of 43 Urtica accessions (stinging nettle) based on UPLC–Q-TOF-MS metabolomic profiles. Phytochemistry 2013, 96, 170–183. [Google Scholar] [CrossRef]
- Sumner, L.; Amberg, A.; Barrett, D.; Beale, M.; Beger, R.; Daykin, C.; Viant, M. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.; Hammock, B. Mass spectrometry-based metabolomics. Mass. Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Vinaixa, M.; Schymanski, E.L.; Neumann, S.; Navarro, M.; Salek, R.M.; Yanes, O. Mass spectral databases for LC/MS-and GC/MS-based metabolomics. Trac. Trend. Anal. Chem. 2016, 78, 23–35. [Google Scholar] [CrossRef]
- Sana, T.; Roark, J.; Li, X.; Waddell, K.; Fischer, S. Molecular formula and METLIN Personal Metabolite Database matching applied to the identification of compounds generated by LC/TOF-MS. J. Biomol. Tech. 2008, 19, 258–266. [Google Scholar] [PubMed]
- Murray, P.R.; Baron, E.J.; Pfaller, M.A.; Tenover, F.C.; Myolken, R.H. National Committee for Clinical Laboratory Standards. Antibacterial Susceptibility Tests: Dilution and Disk Diffusion Methods. In Manual of Clinical Microbiology; Springer: Boston, MA, USA, 1999; pp. 1526–1543. [Google Scholar]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. In Seminars in Oncology; National Center for Biotechnology Information: Bethesda, MD, USA, 1992; pp. 622–638. [Google Scholar]
- Halket, J.; Waterman, D.; Przyborowska, A.; Patel, R.; Fraser, P.; Bramleym, P. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005, 56, 219–243. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant. Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Aggarwal, B.; Ali, M.; Singla, R. Isolation and Characterization of Phytoconstituents from the Stems of Ichnocarpus frutescens. Chin. J. Nat. Med. 2010, 8401–8404. [Google Scholar] [CrossRef]
- Vishnuthari, N.; Sripathi, S.K. GC-MS Analysis of Hexane Extract of Stems and Roots of the Ethnomedicinal Plant Cissus quadrangularis Linn. J. Chem. Bio. Phy. Sci. 2015, 5, 3954–3963. [Google Scholar]
- Kumar, T.S.; Anandan, A.; Jegadeesan, M. Identification of chemical compounds in Cissus quadrangularis L. Variant I of different samples using GC-MS analysis. Arch. Appl. Sci. Res. 2012, 4, 1782–1787. [Google Scholar]
- Shah, U.; Patel, S.M.; Patel, P.H.; Hingorani, L.; Jadhav, R.B. Development and Validation of a Simple Isocratic HPLC Method for Simultaneous Estimation of Phytosterols in Cissus quadrangularis. Indian. J. Pharm. Sci. 2010, 72, 753–758. [Google Scholar]
- Al-Said, M.; Khalifa, A.; Al-Azizi, M. Flavonoids from Cissus digitata. Int. J. Pharm. 1991, 29, 281–283. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Kumar, P.; Sakthi, S.; Meenaxshi, C. In vitro antioxidant and anticancer activity of flavonoid fraction from the aerial parts of Cissus quadrangularis linn against human breast carcinoma cell lines. J. Chem. 2013, 150675. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, Z.-K.; He, H.-P.; Wang, J.-S.; Zhou, H.; Ding, M.; Hao, X.-J. Stilbene C-glucosides from Cissus repens. J. Asian. Nat. Prod. Res. 2007, 9, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Tröndle, D.; Schröder, S.; Kassemeyer, H.H.; Kiefer, C.; Koch, M.A.; Nick, P. Molecular phylogeny of the genus Vitis (Vitaceae) based on plastid markers. Am. J. Bot. 2010, 97, 1168–1178. [Google Scholar] [CrossRef]
- Billet, K.; Houillé, B.; de Bernonville, T.D.; Besseau, S.; Oudin, A.; Courdavault, V.; Delanoue, G.; Guérin, L.; Clastre, M.; Giglioli-Guivarc’h, N. Field-based metabolomics of vitis vinifera l. stems provides new insights for genotype discrimination and polyphenol metabolism structuring. Front. Plant. Sci. 2018, 21, 798. [Google Scholar] [CrossRef]
- Kiselev, K.; Aleynova, O.; Grigorchuk, V.; Dubrovina, A.S. Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr. Planta 2017, 245, 151–159. [Google Scholar] [CrossRef]
- Shan, L.Y.; Thing, T.C.; Ping, T.S.; Awang, K.; Hashim, N.M.; Nafiah, M.A.; Ahmad, K. Cytotoxic, antibacterial and antioxidant activity of triterpenoids from Kopsia singapurensis Ridl. J. Chem. Pharm. Res. 2014, 6, 815–822. [Google Scholar]
- Awad, A.B.; Barta, S.L.; Fink, C.S.; Bradford, P.G. β-Sitosterol enhances tamoxifen effectiveness on breast cancer cells by affecting ceramide metabolism. Mol. Nutr. Food. Res. 2008, 252, 419–426. [Google Scholar] [CrossRef]
- Samita, F.; Ochieng, C.O.; Owuor, P.O.; Manguro, L.O.A.; Midiwo, J.O. Isolation of a new β-carboline alkaloid from aerial parts of Triclisia sacleuxii and its antibacterial and cytotoxicity effects. Nat. Prod. Res. 2017, 31, 529–536. [Google Scholar] [CrossRef]
- Awad, A.; Chinnam, M.; Fink, C.; Bradford, P. β-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine 2007, 14, 747–754. [Google Scholar] [CrossRef]
- Ju, Y.H.; Clausen, L.M.; Allred, K.F.; Almada, A.L.; Helferich, W.G. β-sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic mice. J. Nutr. 2004, 134, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Tahsin, T.; Wansi, J.D.; Al-Groshi, A.; Evans, A.; Nahar, L.; Martin, C.; Sarker, S.D. Cytotoxic Properties of the Stem Bark of Citrus reticulata Blanco (Rutaceae). Phytother. Res. 2017, 31, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Nigam, N.; Kalra, N.; Shukla, Y. Regulation of signaling pathways involved in lupeol induced inhibition of proliferation and induction of apoptosis in human prostate cancer cells. Mol. Carcinog. 2008, 47, 916–924. [Google Scholar] [CrossRef]
- El-Mowafy, A.M.; Alkhalaf, M. Resveratrol activates adenylyl-cyclase in human breast cancer cells: A novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis 2003, 24, 869–873. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 2, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, L.; Orazizadeh, M.; Niazvand, F.; Abbaspour, M.R.; Mansouri, E.; Khodadadi, A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl. Lek. Listy. 2017, 118, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N. HMDB 4.0: The human metabolome database for 2018. Nucleic. Acids. Res. 2017, 4, D608–D617. [Google Scholar] [CrossRef]
- Zgoda, J.; Porter, J. A convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
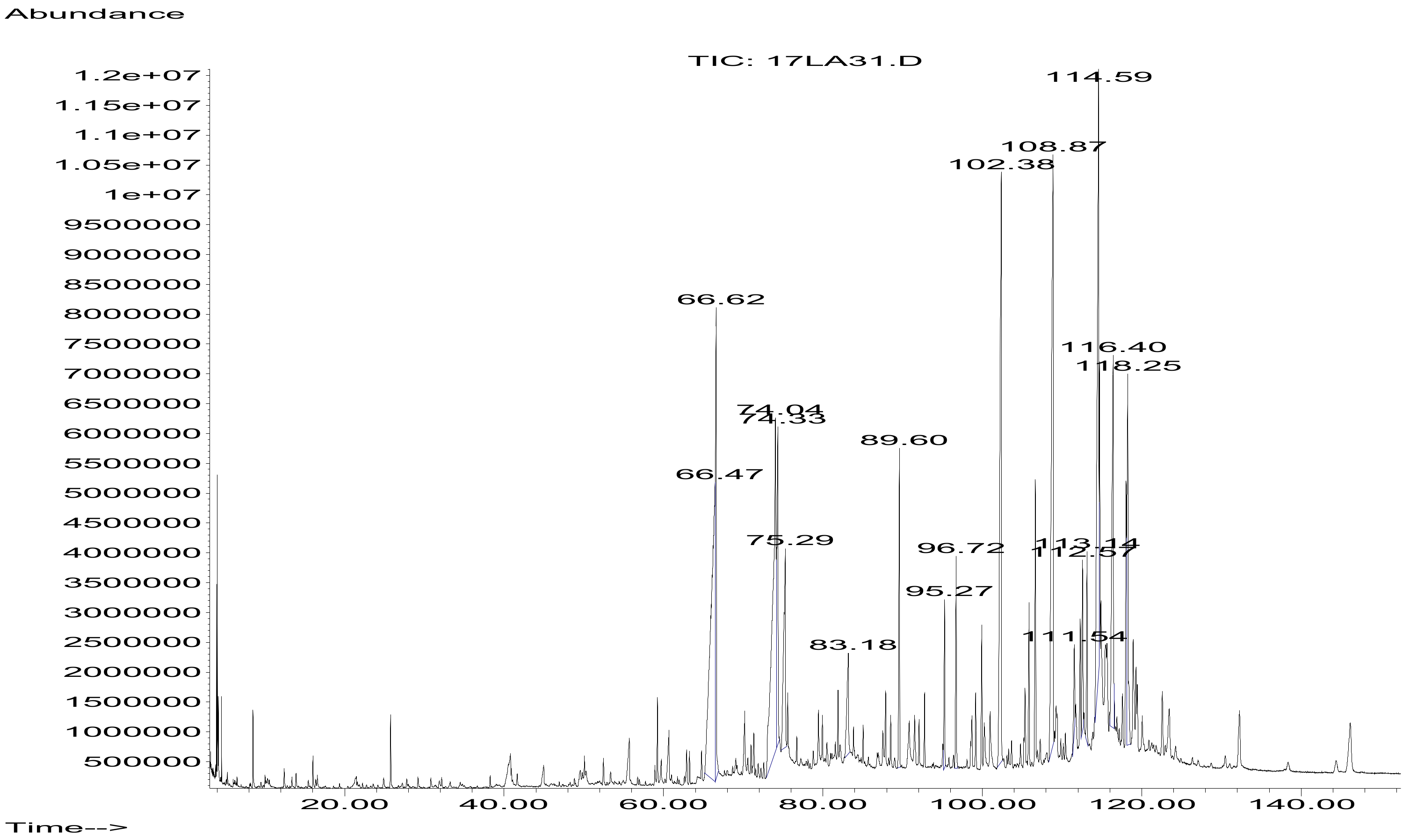

| RT (min) | Abundance (%) | Molecular Weight | Molecular Formula | Tentatively Identified Compound | Retention Index | Metabolite Class |
|---|---|---|---|---|---|---|
| 66.472 | 14.39 | 256.4241 | C16H32O2 | Hexadecanoic acid | 1964 | Fatty acid |
| 66.623 | 5.35 | 284.4772 | C18H36O2 | Hexadecanoic acid ethyl ester | 1994 | Fatty ester |
| 74.039 | 12.60 | 280.4455 | C18H32O2 | 9Z,12Z-Octadecadienoic acid | 1977 | Fatty acid |
| 74.328 | 4.63 | 282.4614 | C18H34O2 | 9Z-Octadecenoic acid | 2140 | Fatty acid |
| 75.294 | 4.42 | 284.4772 | C18H36O2 | Octadecanoic acid | 2188 | Fatty acid |
| 83.176 | 2.01 | 312.5304 | C20H40O2 | Eicosanoic acid | 2366 | Fatty acid |
| 89.609 | 1.94 | 394.7601 | C28H58 | Octacosane | 2800 | Alkane |
| 95.271 | 3.15 | 410.7180 | C30H50 | Squalene | 2847 | Triterpene |
| 102.377 | 10.45 | 408.7867 | C29H60 | Nonacosane | 2900 | Alkane |
| 108.871 | 12.82 | 436.8399 | C31H64 | Hentriacontane | 3100 | Alkane |
| 111.546 | 1.81 | 400.6801 | C28H48O | Campesterol | 3131 | Sterol |
| 112.571 | 1.91 | 412.6908 | C29H48O | Stigmasterol | 3170 | Sterol |
| 113.143 | 1.73 | 454.4749 | C30H62O2 | 1,30-Triacontanediol | 3241 | Alcohol |
| 114.588 | 11.23 | 414.7067 | C29H50O | β-sitosterol | 3187 | Sterol |
| 116.401 | 6.53 | 426.7174 | C30H50O | Lupeol | 3320 | Triterpene |
| 118.246 | 5.03 | 412.6908 | C29H48O | Stigmast-4-en-3-one | 3435 | Ketone |
| RT (min) | Experimental m/z [M–H]− | Theoretical Mass | Mass Error (ppm) | Molecular Formula | Tentatively Identified Compound | Metabolite Class |
|---|---|---|---|---|---|---|
| 0.612 | 593.1497 | 594.1590 | 1.69 | C27H30O15 | Kaempferol-O-α-rhamnosyl-glucopyranoside | Flavonoid |
| 2.419 | 625.1436 | 626.1488 | 1.60 | C27H30O17 | Myricetin 3-O-rutinoside | Flavonoid |
| 2.857 | 507.1147 | 508.1222 | 1.98 | C23H24O13 | Syringetin 3-O-galactoside | Flavonoid |
| 3.226 | 405.1198 | 406.1269 | 2.47 | C20H22O9 | Piceatannol glucoside | Stilbene |
| 3.547 | 595.1341 | 596.1382 | 1.68 | C26H28O16 | Quercetin 3-O-glucosyl-xyloside | Flavonoid |
| 3.774 | 310.2052 | - | - | - | Unknown | - |
| 4.042 | 315.0717 | 316.0799 | 3.18 | C13H16O9 | Protocatechuic acid hexoside | Phenolic |
| 4.807 | 433.1140 | 434.1218 | 2.32 | C21H22O10 | Dihydrokaempferol 3-O-rhamnoside | Flavonoid |
| 5.090 | 389.1249 | 390.1320 | 2.58 | C20H22O8 | Resveratrol 3-O-glucoside | Stilbene |
| 5.813 | 473.0362 | 474.0439 | 2.12 | C21H14O13 | Trigallic acid | Phenolic |
| 5.895 | 431.0939 | - | - | - | Unknown | - |
| 6.180 | 335.0403 | 336.0486 | 3.00 | C15H12O9 | Methyl digallate | Phenolic |
| 6.423 | 433.0760 | 434.0854 | 2.32 | C20H18O11 | Quercetin arabinoside | Flavonoid |
| 6.531 | 336.1840 | - | - | - | Unknown | - |
| 6.592 | 615.1869 | 616.1950 | 1.63 | C34H32O11 | Pallidol-3-O-glucoside | Stilbene |
| 6.763 | 447.0938 | 448.1011 | 2.24 | C21H20O11 | Kaempferol 3-O-galactoside | Flavonoid |
| 7.169 | 615.0988 | 616.1069 | 1.63 | C28H24O16 | Myricitrin O-gallate | Flavonoid |
| 7.191 | 297.3810 | - | - | - | Unknown | - |
| 7.417 | 253.2161 | 254.2251 | 3.96 | C16H30O2 | Hexadecenoic acid | Fatty acid |
| 7.534 | 279.2348 | 280.2407 | 3.58 | C18H32O2 | Octadecadienoic acid | Fatty acid |
| 7.595 | 255.2345 | 256.2407 | 3.92 | C16H32O2 | Palmitic acid | Fatty acid |
| 7.852 | 283.2649 | 284.2720 | 3.54 | C18H36O2 | Stearic acid | Fatty acid |
| 8.272 | 653.2235 | - | - | - | Unknown | - |
| 9.480 | 535.1650 | - | - | - | Unknown | - |
| RT (min) | Experimental m/z [M–H]− | Theoretical Mass | Mass Error (ppm) | Molecular Formula | Tentatively Identified Compound | Metabolite Class |
|---|---|---|---|---|---|---|
| 0.612 | 592.9786 | 594.1590 | 1.98 | C27H30O15 | Apigenin-6,8-di-C- glycoside | Flavonoid |
| 2.781 | 563.0218 | 564.1484 | 1.99 | C26H28O14 | Kaempferol rhamnosyl xyloside | Flavonoid |
| 3.180 | 405.1198 | 406.1269 | 2.47 | C20H22O9 | Piceatannol glucoside | Stilbene |
| 3.497 | 595.1341 | 596.1382 | 1.68 | C26H28O16 | Quercetin 3-O-glucosyl-xyloside | Flavonoid |
| 3.689 | 609.1451 | 610.1539 | 1.65 | C27H30O16 | Kaempferol 3,7-O-diglucoside | Flavonoid |
| 4.457 | 374.4914 | - | - | - | Unknown | - |
| 4.665 | 593.1497 | 594.1590 | 1.69 | C27H30O15 | Kaempferol-O-α-rhamnosyl-glucopyranoside | Flavonoid |
| 5.078 | 453.1356 | 454.1421 | 2.21 | C28H22O6 | E-Viniferin | Stilbene |
| 5.395 | 400.3705 | - | - | - | Unknown | - |
| 5.973 | 755.2030 | 756.2118 | 1.33 | C33H40O20 | Kaempferol 3-O-glucosyl-rhamnosyl-galactoside | Flavonoid |
| 6.179 | 594.1627 | - | - | - | Unknown | - |
| 6.423 | 433.0760 | 434.0854 | 2.32 | C20H18O11 | Quercetin arabinoside | Flavonoid |
| 6.779 | 448.1011 | 449.1089 | 2.24 | C21H21O11 | Cyanidin 3-O-galactoside | Flavonoid |
| 6.954 | 464.0960 | 465.1038 | 2.16 | C21H21O12 | Delphinidin 3-O-glucoside | Flavonoid |
| 7. 384 | 447.0930 | 448.1011 | 2.24 | C21H20O11 | Kaempferol hexoside | Flavonoid |
| 7.465 | 576.4380 | - | - | - | Unknown | - |
| 7.645 | 302.0060 | - | - | - | Unknown | - |
| 7.851 | 426.7290 | - | - | - | Unknown | - |
| Bacteria | Hexane | CHCl3-MeOH | Aqueous | Levofloxacin |
|---|---|---|---|---|
| S. aureus (ATCC, 29213) | ≥500 | ≥500 | ≥500 | 3.12 |
| S. epidermidis (ATCC, 14990) | ≥500 | ≥500 | ≥500 | 3.12 |
| E. faecium (ATCC, 2127) | ≥500 | ≥500 | ≥500 | 3.12 |
| E. coli (ATCC, 25922) | ≥500 | ≥500 | ≥500 | 3.12 |
| P. aeruginosa (ATCC, 27853) | ≥500 | ≥500 | ≥500 | 3.12 |
| K. pneumoniae (ATCC, 19606) | ≥500 | ≥500 | ≥500 | 3.12 |
| A. baumanni (ATCC, 13883) | ≥500 | ≥500 | ≥500 | 3.12 |
| Methicillin-resistant S.aureus (14-2095) | ≥500 | ≥500 | ≥500 | 12.5 |
| Linezolid-resistant S. epidermidis (14-583) | ≥500 | ≥500 | ≥500 | 6.25 |
| Vancomycin-resistant E. faecium (10-984) | ≥500 | ≥500 | ≥500 | 12.5 |
| ESBL- resistant E.coli (14-2081) | ≥500 | ≥500 | ≥500 | 25.0 |
| Carbapenem-resistant P. aeruginosa (13-1391) | ≥500 | ≥500 | ≥500 | 12.5 |
| Oxacillin-resistant K. pneumoniae (17-1692) | ≥500 | ≥500 | ≥500 | 6.25 |
| NDM-1+- resistant K. pneumoniae (14-3335) | ≥500 | ≥500 | ≥500 | 50.0 |
| Carbapenem-resistant A. baumannii (12-666) | ≥500 | ≥500 | ≥500 | 12.5 |
| Cell line | Hexane | CHCl3-MeOH | Aqueous | Paclitaxel |
|---|---|---|---|---|
| HepG2 | 26 ± 2 | 80 ± 8 | 79 ± 5 | 64.0 × 10−3 |
| Hep3B | 24 ± 2 | 81 ± 4 | 81 ± 7 | 33.0 × 10−3 |
| MCF7 | 30 ± 3 | 78 ± 5 | 30 ± 2 | 5.12 × 10−3 |
| HeLa | 35 ± 3 | 82 ± 4 | 90 ± 8 | 5.12 × 10−3 |
| A549 | 51 ± 4 | 85 ± 3 | 94 ± 6 | 4.27 × 10−3 |
| PC3 | 62 ± 3 | 61 ± 3 | 58 ± 4 | 79.4 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-López, L.F.; Garza-González, E.; Ríos, M.Y.; Ramírez-Cisneros, M.Á.; Alvarez, L.; González-Maya, L.; Sánchez-Carranza, J.N.; Camacho-Corona, M.d.R. Metabolic Profile and Evaluation of Biological Activities of Extracts from the Stems of Cissus trifoliata. Int. J. Mol. Sci. 2020, 21, 930. https://doi.org/10.3390/ijms21030930
Méndez-López LF, Garza-González E, Ríos MY, Ramírez-Cisneros MÁ, Alvarez L, González-Maya L, Sánchez-Carranza JN, Camacho-Corona MdR. Metabolic Profile and Evaluation of Biological Activities of Extracts from the Stems of Cissus trifoliata. International Journal of Molecular Sciences. 2020; 21(3):930. https://doi.org/10.3390/ijms21030930
Chicago/Turabian StyleMéndez-López, Luis Fernando, Elvira Garza-González, María Yolanda Ríos, M. Ángeles Ramírez-Cisneros, Laura Alvarez, Leticia González-Maya, Jessica N. Sánchez-Carranza, and María del Rayo Camacho-Corona. 2020. "Metabolic Profile and Evaluation of Biological Activities of Extracts from the Stems of Cissus trifoliata" International Journal of Molecular Sciences 21, no. 3: 930. https://doi.org/10.3390/ijms21030930
APA StyleMéndez-López, L. F., Garza-González, E., Ríos, M. Y., Ramírez-Cisneros, M. Á., Alvarez, L., González-Maya, L., Sánchez-Carranza, J. N., & Camacho-Corona, M. d. R. (2020). Metabolic Profile and Evaluation of Biological Activities of Extracts from the Stems of Cissus trifoliata. International Journal of Molecular Sciences, 21(3), 930. https://doi.org/10.3390/ijms21030930