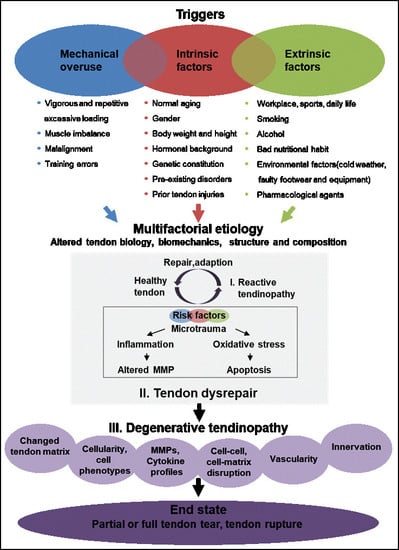
Spectrum of Tendon Pathologies: Triggers, Trails and End-State
Abstract
1. Terminology of Tendon Diseases
2. Tendon Structural and Functional Relationship

3. Tendinopathy: A Challenging Disease
4. Morbidity and Clinical Relevance of Tendinopathies
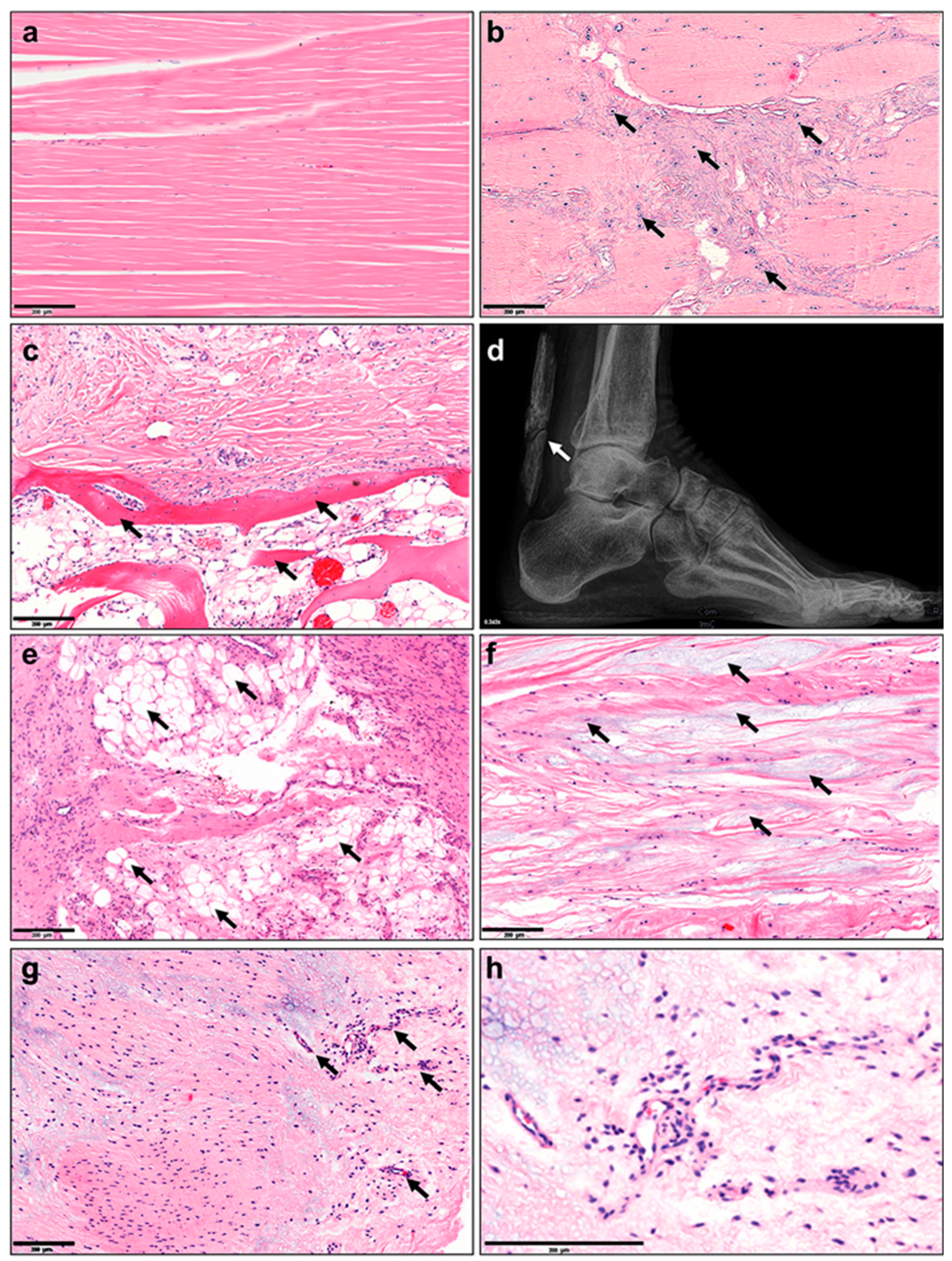
5. Tissue Changes: Histopathological, Structural, Cellular, Epigenetic, Transcriptomic, Proteomic and Metabolomic
6. Vasculature, Inflammation and Neurons
7. Biochemical and Biomechanical Alterations
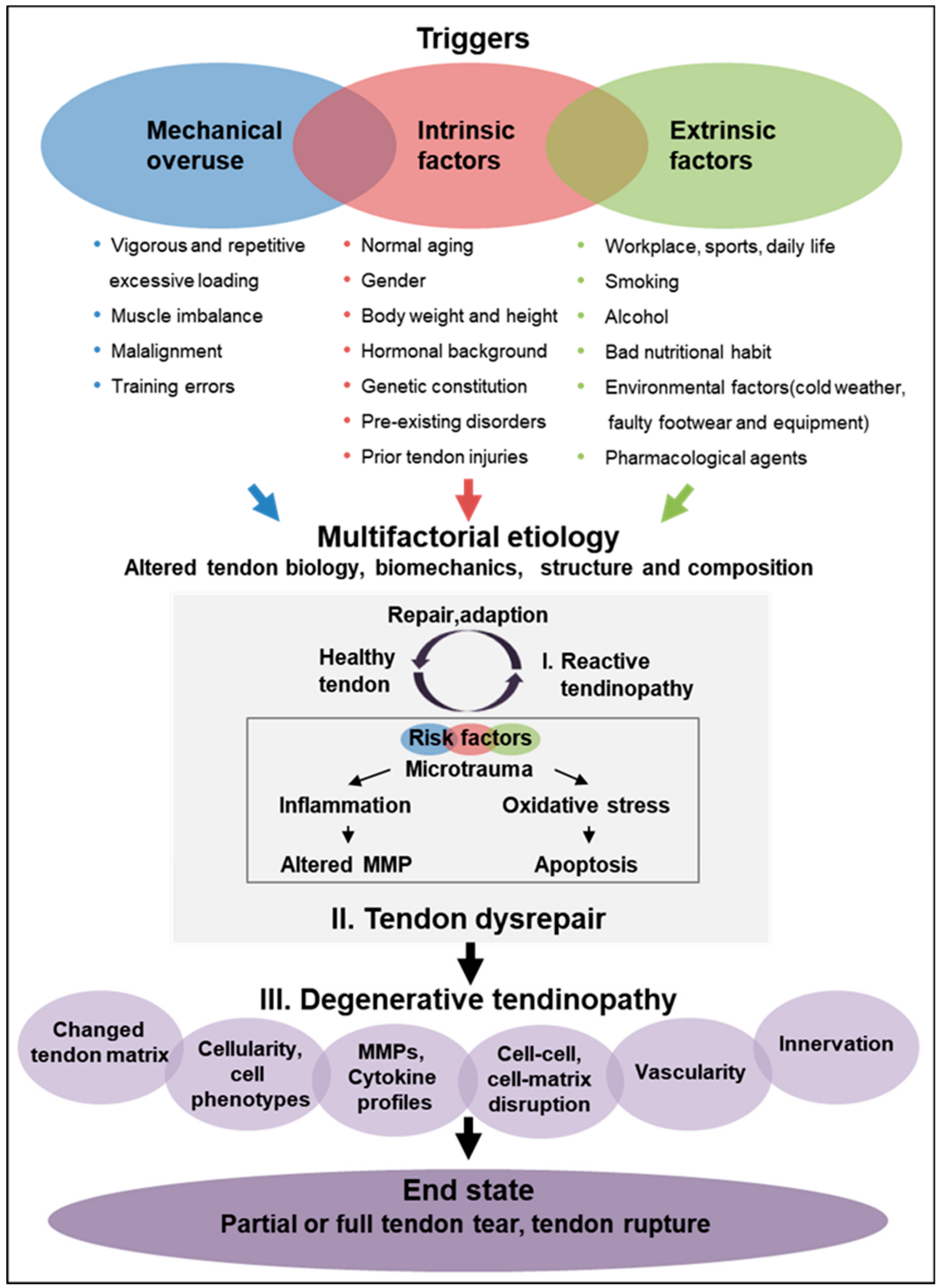
8. Tendinopathy: Variety of Etiological Factors and Disease Triggers, Trails and End-State
9. Current Tendinopathy Management Strategies
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACL | Anterior cruciate ligament |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| CpG | Cytosine nucleotide followed by a guanine in the DNA along its 5′ → 3′ direction |
| COX2 | Cyclooxygenase-2 |
| CRABP | Cellular retinoic acid-binding protein |
| DNA | Deoxyribonucleic acid |
| ECM | Extracellular matrix |
| GAGs | Glycosaminoglycans |
| IGF | Insulin growth factor |
| IL | Interleukin |
| MMP | Matrix metalloproteinases |
| NF-kB | Nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| NSAID | Non-steroidal anti-inflammatory drugs |
| PASTA | Partial articular supraspinatus tendon avulsion |
| PDGF | platelet derived growth factor |
| PGs | Proteoglycans |
| PRG4 | Proteoglycan 4 or lubricin |
| PRP | Platelet rich plasma |
| RNA | Ribonucleic acid |
| STAT6 | Signal transducer and activator of transcription 6 |
| TGF-β | Transforming growth factor β |
| TIMPs | Tissue inhibitor of metalloproteinases |
| TNFα | Tumor necrosis factor-α |
| TPPP3 | Tubulin Polymerization Promoting Protein Family Member 3 |
| TSPCs | Tendon stem progenitor cells |
| VEGF | Vascular endothelial growth factor |
References
- Khan, K.M.; Cook, J.L.; Kannus, P.; Maffulli, N.; Bonar, S.F. Time to abandon the “tendinitis” myth. Bmj 2002, 324, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Fu, S.C.; Chua, E.; Hu, X.; Rolf, C.; Mattila, V.M.; Qin, L.; Yung, P.S.; Chan, K.M. Critical review on the socio-economic impact of tendinopathy. Asia-Pacific J. Sports Med. Arthrosc. Rehabil. Technol. 2016, 4, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and epidemiology of tendinopathy. Clinics Sports Med. 2003, 22, 675–692. [Google Scholar] [CrossRef]
- Dakin, S.G.; Dudhia, J.; Smith, R.K. Resolving an inflammatory concept: The importance of inflammation and resolution in tendinopathy. Vet. Immunol. Immunopathol. 2014, 158, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Frankewycz, B.; Penz, A.; Weber, J.; da Silva, N.P.; Freimoser, F.; Bell, R.; Nerlich, M.; Jung, E.M.; Docheva, D.; Pfeifer, C.G. Achilles tendon elastic properties remain decreased in long term after rupture. Knee Surg. Sports Traumatol. Arthrosc: Official J. ESSKA 2018, 26, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, H.; Nerlich, M.; Pfeifer, C.G.; Docheva, D. Boosting tendon repair: Interplay of cells, growth factors and scaffold-free and gel-based carriers. J. Exp. Orthop. 2018, 5, 1. [Google Scholar] [CrossRef]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef]
- Schneider, M.; Docheva, D. Mysteries Behind the Cellular Content of Tendon Tissues. J. Am. Acad. Orthop. Surg. 2017, 25, e289–e290. [Google Scholar] [CrossRef]
- Docheva, D.; Muller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Jarvinen, M.; Jozsa, L.; Kannus, P.; Jarvinen, T.L.; Kvist, M.; Leadbetter, W. Histopathological findings in chronic tendon disorders. Scand. J. Med. Sci. Sports 1997, 7, 86–95. [Google Scholar] [CrossRef]
- Cook, J.L.; Purdam, C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Moosmayer, S.; Smith, H.J.; Tariq, R.; Larmo, A. Prevalence and characteristics of asymptomatic tears of the rotator cuff: An ultrasonographic and clinical study. J. Bone Jt. Surg. Br. Vol. 2009, 91, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Landry, S.C.; McKean, K.A.; Hubley-Kozey, C.L.; Stanish, W.D.; Deluzio, K.J. Neuromuscular and lower limb biomechanical differences exist between male and female elite adolescent soccer players during an unanticipated side-cut maneuver. Am. J. Sports Med. 2007, 35, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Andrew, G.; Jonathan, S. Comparison of achilles tendon loading between male and female recreational runners. J. Hum. Kinet. 2014, 44, 155–159. [Google Scholar] [CrossRef]
- Clayton, R.A.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344. [Google Scholar] [CrossRef]
- Gross, C.E.; Nunley, J.A., 2nd. Acute Achilles Tendon Ruptures. Foot. ankle Int. 2016, 37, 233–239. [Google Scholar] [CrossRef]
- Colvin, A.C.; Egorova, N.; Harrison, A.K.; Moskowitz, A.; Flatow, E.L. National trends in rotator cuff repair. J. Bone and Jt. Surg. Am. Vol. 2012, 94, 227–233. [Google Scholar] [CrossRef]
- Ganestam, A.; Kallemose, T.; Troelsen, A.; Barfod, K.W. Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013. A nationwide registry study of 33,160 patients. Knee Surg. Sports Traumatol. Arthrosc Off. J. ESSKA 2016, 24, 3730–3737. [Google Scholar] [CrossRef]
- Ahmad, J.; Repka, M.; Raikin, S.M. Treatment of myotendinous Achilles ruptures. Foot Ankle Int. 2013, 34, 1074–1078. [Google Scholar] [CrossRef]
- Abat, F.; Alfredson, H.; Cucchiarini, M.; Madry, H.; Marmotti, A.; Mouton, C.; Oliveira, J.M.; Pereira, H.; Peretti, G.M.; Romero-Rodriguez, D.; et al. Current trends in tendinopathy: Consensus of the ESSKA basic science committee. Part I: Biology, biomechanics, anatomy and an exercise-based approach. J. Exp. Orthop. 2017, 4, 18. [Google Scholar] [CrossRef]
- Buchbinder, R.; Green, S.E.; Struijs, P. Tennis elbow. BMJ Clin. Evid. 2008. [Google Scholar]
- May, T.; Garmel, G.M. Rotator Cuff Injury; StatPearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Nirschl, R.P.; Ashman, E.S. Elbow tendinopathy: Tennis elbow. Clin. sports Med. 2003, 22, 813–836. [Google Scholar] [CrossRef]
- Scott, A.; Ashe, M.C. Common tendinopathies in the upper and lower extremities. Curr. Sports Med. Rep. 2006, 5, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Jozsa, L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Jt. Surg. Am. Vol. 1991, 73, 1507–1525. [Google Scholar] [CrossRef]
- Dean, B.J.; Franklin, S.L.; Carr, A.J. The peripheral neuronal phenotype is important in the pathogenesis of painful human tendinopathy: A systematic review. Clin. Orthop. Relat. Res. 2013, 471, 3036–3046. [Google Scholar] [CrossRef]
- Andersson, G.; Danielson, P.; Alfredson, H.; Forsgren, S. Nerve-related characteristics of ventral paratendinous tissue in chronic Achilles tendinosis. Knee Surg. Sports Traumatol. Arthrosc: Off. J. ESSKA 2007, 15, 1272–1279. [Google Scholar] [CrossRef]
- Rickaby, R.; El Khoury, L.Y.; Samiric, T.; Raleigh, S.M. Epigenetic Status of The Human MMP11 Gene Promoter is Altered in Patellar Tendinopathy. J. Sports Sci. Med. 2019, 18, 155–159. [Google Scholar]
- El Khoury, L.Y.; Rickaby, R.; Samiric, T.; Raleigh, S.M. Promoter methylation status of the TIMP2 and ADAMTS4 genes and patellar tendinopathy. J. Sci. Med. Sport 2018, 21, 378–382. [Google Scholar] [CrossRef]
- Kohler, J.; Popov, C.; Klotz, B.; Alberton, P.; Prall, W.C.; Haasters, F.; Muller-Deubert, S.; Ebert, R.; Klein-Hitpass, L.; Jakob, F.; et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 2013, 12, 988–999. [Google Scholar] [CrossRef]
- Pease, L.I.; Clegg, P.D.; Proctor, C.J.; Shanley, D.J.; Cockell, S.J.; Peffers, M.J. Cross platform analysis of transcriptomic data identifies ageing has distinct and opposite effects on tendon in males and females. Sci. Reports 2017, 7, 14443. [Google Scholar] [CrossRef] [PubMed]
- Kendal, A.R.; Layton, T.; Al-Mossawi, H.; Brown, R.; Loizou, C.; Rogers, M.; Sharp, R.; Dakin, S.; Appletonr, L.; Carr, A. Identification of human tendon cell populations in healthy and diseased tissue using combined single cell transcriptomics and proteomics. bioRxiv 2019. [Google Scholar]
- Harvey, T.; Flamenco, S.; Fan, C.M. A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 2019, 21, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Hu, J.J.; Yang, L.; Zheng, Z.F.; An, C.R.; Wu, B.B.; Zhang, C.; Shen, W.L.; Liu, H.H.; Chen, J.L.; et al. Single-cell analysis reveals a nestin(+) tendon stem/progenitor cell population with strong tenogenic potentiality. Sci. Adv. 2016, 2, e1600874. [Google Scholar] [CrossRef]
- Plachel, F.; Heuberer, P.; Gehwolf, R.; Frank, J.; Tempfer, H.; Lehner, C.; Weissenbacher, N.; Wagner, A.; Weigl, M.; Moroder, P.; et al. MicroRNA Profiling Reveals Distinct Signatures in Degenerative Rotator Cuff Pathologies. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 202–211. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, X.; Xu, J.; Zhang, X.; Hu, G.; Fu, S.C.; Dai, K.; Chen, X.; Patrick, Y.S.; Zhang, X. Overexpression of mechanical sensitive miR-337-3p alleviates ectopic ossification in rat tendinopathy model via targeting IRS1 and Nox4 of tendon derived stem cells. J. Mol. Cell Biol. 2019. [Google Scholar] [CrossRef]
- Gibbon, A.; Saunders, C.J.; Collins, M.; Gamieldien, J.; September, A.V. Defining the molecular signatures of Achilles tendinopathy and anterior cruciate ligament ruptures: A whole-exome sequencing approach. PLoS ONE 2018, 13, e0205860. [Google Scholar] [CrossRef]
- Ge, H.; Shrestha, A.; Liu, C.; Wu, P.; Cheng, B. MicroRNA 148a-3p promotes Thrombospondin-4 expression and enhances angiogenesis during tendinopathy development by inhibiting Kruppel-like factor 6. Biochem. Biophys. Res. Commun. 2018, 502, 276–282. [Google Scholar] [CrossRef]
- Thankam, F.G.; Boosani, C.S.; Dilisio, M.F.; Agrawal, D.K. MicroRNAs associated with inflammation in shoulder tendinopathy and glenohumeral arthritis. Mol. Cell. Biochem. 2018, 437, 81–97. [Google Scholar] [CrossRef]
- Millar, N.L.; Gilchrist, D.S.; Akbar, M.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Murrell, G.A.C.; Liew, F.Y.; Kurowska-Stolarska, M.; McInnes, I.B. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat. Commun. 2015, 6, 6774. [Google Scholar] [CrossRef]
- Lu, Y.F.; Liu, Y.; Fu, W.M.; Xu, J.; Wang, B.; Sun, Y.X.; Wu, T.Y.; Xu, L.L.; Chan, K.M.; Zhang, J.F.; et al. Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-beta1 signaling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.J.; Thorpe, C.T.; Collins, J.A.; Eong, R.; Wei, T.K.; Screen, H.R.; Clegg, P.D. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J. Biol. Chem. 2014, 289, 25867–25878. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; Peffers, M.J.; Simpson, D.; Halliwell, E.; Screen, H.R.; Clegg, P.D. Anatomical heterogeneity of tendon: Fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci. Rep. 2016, 6, 20455. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, O.; Ternette, N.; Murphy, R.; Kessler, B.M.; Carr, A. A quantitative label-free analysis of the extracellular proteome of human supraspinatus tendon reveals damage to the pericellular and elastic fibre niches in torn and aged tissue. PloS one 2017, 12, e0177656. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, O.K.; Kwak, D.H.; Kim, Y.S. Metabolic profiling of serum and tissue from the rotator interval and anterior capsule in shoulder stiffness: A preliminary study. BMC Musculoskelet. Disord. 2019, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Izumi, S.; Otsuru, S.; Adachi, N.; Akabudike, N.; Enomoto-Iwamoto, M. Control of glucose metabolism is important in tenogenic differentiation of progenitors derived from human injured tendons. PloS ONE 2019, 14, e0213912. [Google Scholar] [CrossRef]
- Zhang, K.; Hast, M.W.; Izumi, S.; Usami, Y.; Shetye, S.; Akabudike, N.; Philp, N.J.; Iwamoto, M.; Nissim, I.; Soslowsky, L.J.; et al. Modulating Glucose Metabolism and Lactate Synthesis in Injured Mouse Tendons: Treatment With Dichloroacetate, a Lactate Synthesis Inhibitor, Improves Tendon Healing. Am. J. Sports Med. 2018, 46, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Wunderli, S.L.; Blache, U.; Beretta Piccoli, A.; Niederost, B.; Holenstein, C.N.; Passini, F.S.; Silvan, U.; Bundgaard, L.; Auf dem Keller, U.; Snedeker, J.G. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biol. J. Int. Soc. Matrix Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.H. Neovascularisation in tendinopathy: from eradication to stabilisation? Br. J. Sports Med. 2020. [Google Scholar] [CrossRef]
- Paavola, M.; Kannus, P.; Jarvinen, T.A.; Jarvinen, T.L.; Jozsa, L.; Jarvinen, M. Treatment of tendon disorders. Is there a role for corticosteroid injection? Foot Ankle Clin. 2002, 7, 501–513. [Google Scholar] [CrossRef]
- Millar, N.L.; Murrell, G.A.; McInnes, I.B. Inflammatory mechanisms in tendinopathy - towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.J.F.; Dakin, S.G.; Millar, N.L.; Carr, A.J. Review: Emerging concepts in the pathogenesis of tendinopathy. Surg. J. R. Coll. Surg. Edinb. Irel. 2017, 15, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Ewen, S.W.; Waterston, S.W.; Reaper, J.; Barrass, V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am. J. sports Med. 2000, 28, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.; Samiric, T.; Ilic, M.Z.; Cook, J.; Handley, C.J. Involvement of proteoglycans in tendinopathy. J. Musculoskelet. Neuronal Interact. 2011, 11, 86–93. [Google Scholar]
- Parkinson, J.; Samiric, T.; Ilic, M.Z.; Cook, J.; Feller, J.A.; Handley, C.J. Change in proteoglycan metabolism is a characteristic of human patellar tendinopathy. Arthritis Rheum. 2010, 62, 3028–3035. [Google Scholar] [CrossRef]
- Diniz-Fernandes, T.; Godoy-Santos, A.L.; Santos, M.C.; Pontin, P.; Pereira, C.A.A.; Jardim, Y.J.; Velosa, A.P.P.; Maffulli, N.; Teodoro, W.R.; Capelozzi, V.L. Matrix metalloproteinase-1 (MMP-1) and (MMP-8) gene polymorphisms promote increase and remodeling of the collagen III and V in posterior tibial tendinopathy. Histol. Histopathol. 2018, 33, 929–936. [Google Scholar]
- Baroneza, J.E.; Godoy-Santos, A.; Ferreira Massa, B.; Bocon de Araujo Munhoz, F.; Diniz Fernandes, T.; Leme Godoy dos Santos, M.C. MMP-1 promoter genotype and haplotype association with posterior tibial tendinopathy. Gene 2014, 547, 334–337. [Google Scholar] [CrossRef]
- Godoy-Santos, A.; Ortiz, R.T.; Mattar Junior, R.; Fernandes, T.D.; Santos, M.C. MMP-8 polymorphism is genetic marker to tendinopathy primary posterior tibial tendon. Scand. J. Med. Sci. Sports 2014, 24, 220–223. [Google Scholar] [CrossRef]
- Del Buono, A.; Oliva, F.; Osti, L.; Maffulli, N. Metalloproteases and tendinopathy. Muscles Ligaments Tendons J. 2013, 3, 51–57. [Google Scholar] [CrossRef]
- Jones, G.C.; Corps, A.N.; Pennington, C.J.; Clark, I.M.; Edwards, D.R.; Bradley, M.M.; Hazleman, B.L.; Riley, G.P. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006, 54, 832–842. [Google Scholar] [CrossRef]
- Raleigh, S.M.; van der Merwe, L.; Ribbans, W.J.; Smith, R.K.; Schwellnus, M.P.; Collins, M. Variants within the MMP3 gene are associated with Achilles tendinopathy: Possible interaction with the COL5A1 gene. Br. J. Sports Med. 2009, 43, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Lian, O.; Bahr, R.; Hart, D.A.; Duronio, V. VEGF expression in patellar tendinopathy: A preliminary study. Clin. Orthop. Relat. Res. 2008, 466, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Vasta, S.; Di Martino, A.; Zampogna, B.; Torre, G.; Papalia, R.; Denaro, V. Role of VEGF, Nitric Oxide, and Sympathetic Neurotransmitters in the Pathogenesis of Tendinopathy: A Review of the Current Evidences. Front. Aging Neurosci. 2016, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, S.; Saracino, M.; Castagna, A. Degenerative disease in rotator cuff tears: What are the biochemical and histological changes? Joints 2014, 2, 26–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schubert, T.E.; Weidler, C.; Lerch, K.; Hofstadter, F.; Straub, R.H. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann. Rheum. Dis. 2005, 64, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Corps, A.N.; Curry, V.A.; Harrall, R.L.; Dutt, D.; Hazleman, B.L.; Riley, G.P. Ciprofloxacin reduces the stimulation of prostaglandin E(2) output by interleukin-1beta in human tendon-derived cells. Rheumatology 2003, 42, 1306–1310. [Google Scholar] [CrossRef][Green Version]
- Eskildsen, S.M.; Berkoff, D.J.; Kallianos, S.A.; Weinhold, P.S. The use of an IL1-receptor antagonist to reverse the changes associated with established tendinopathy in a rat model. Scand. J. Med. Sci. Sports 2019, 29, 82–88. [Google Scholar] [CrossRef]
- Fu, S.C.; Wang, W.; Pau, H.M.; Wong, Y.P.; Chan, K.M.; Rolf, C.G. Increased expression of transforming growth factor-beta1 in patellar tendinosis. Clin. Orthop. Relat. Res. 2002, 40, 174–183. [Google Scholar] [CrossRef]
- Dolkart, O.; Liron, T.; Chechik, O.; Somjen, D.; Brosh, T.; Maman, E.; Gabet, Y. Statins enhance rotator cuff healing by stimulating the COX2/PGE2/EP4 pathway: An in vivo and in vitro study. Am. J. Sports Med. 2014, 42, 2869–2876. [Google Scholar] [CrossRef]
- Rolf, C.G.; Fu, B.S.; Pau, A.; Wang, W.; Chan, B. Increased cell proliferation and associated expression of PDGFRbeta causing hypercellularity in patellar tendinosis. Rheumatology 2001, 40, 256–261. [Google Scholar] [CrossRef]
- Scott, A.; Cook, J.L.; Hart, D.A.; Walker, D.C.; Duronio, V.; Khan, K.M. Tenocyte responses to mechanical loading in vivo: A role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007, 56, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, H.; Traweger, A. Tendon Vasculature in Health and Disease. Front. Physiol. 2015, 6, 330. [Google Scholar] [CrossRef]
- Yang, X.; Coleman, D.P.; Pugh, N.D.; Nokes, L.D. The volume of the neovascularity and its clinical implications in achilles tendinopathy. Ultrasound Med. & Biol. 2012, 38, 1887–1895. [Google Scholar]
- Millar, N.L.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Little, K.J.; Leach, W.J.; Rooney, B.P.; Murrell, G.A.; McInnes, I.B. Hypoxia: A critical regulator of early human tendinopathy. Ann. Rheum. Dis. 2012, 71, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Caceres, M.D.; Yan, Z.; Schieker, M.; Nerlich, M.; Docheva, D. Tenomodulin regulates matrix remodeling of mouse tendon stem/progenitor cells in an ex vivo collagen I gel model. Biochem. Biophys. Res. Commun. 2019, 512, 691–697. [Google Scholar] [CrossRef]
- Kiderlen, S.; Polzer, C.; Radler, J.O.; Docheva, D.; Clausen-Schaumann, H.; Sudhop, S. Age related changes in cell stiffness of tendon stem/progenitor cells and a rejuvenating effect of ROCK-inhibition. Biochem. Biophys. Res. Commun. 2019, 509, 839–844. [Google Scholar] [CrossRef]
- Riley, G. Tendinopathy--from basic science to treatment. Nat. Clin. Practice. Rheumatol. 2008, 4, 82–89. [Google Scholar] [CrossRef]
- Cook, J.L.; Rio, E.; Purdam, C.R.; Docking, S.I. Revisiting the continuum model of tendon pathology: What is its merit in clinical practice and research? British J. of Sports Med. 2016, 50, 1187–1191. [Google Scholar] [CrossRef]
- Rees, J.D.; Wilson, A.M.; Wolman, R.L. Current concepts in the management of tendon disorders. Rheumatology 2006, 45, 508–521. [Google Scholar] [CrossRef]
- Hess, G.W. Achilles tendon rupture: A review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010, 3, 29–32. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Tendinopathy and tendon injury: The future. Disabil. Rehabil. 2008, 30, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-function relationships in tendons: A review. J. Anat. 2008, 212, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Shearn, J.T.; Kinneberg, K.R.; Dyment, N.A.; Galloway, M.T.; Kenter, K.; Wylie, C.; Butler, D.L. Tendon tissue engineering: Progress, challenges, and translation to the clinic. J. Musculoskelet. Neuronal Interact. 2011, 11, 163–173. [Google Scholar]
- Leblanc, D.R.; Schneider, M.; Angele, P.; Vollmer, G.; Docheva, D. The effect of estrogen on tendon and ligament metabolism and function. J. Steroid Biochem. Mol. Biol. 2017, 172, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.; Magnusson, P.; Krogsgaard, M.; Boysen Moller, J.; Olesen, J.; Heinemeier, K.; Hansen, M.; Haraldsson, B.; Koskinen, S.; Esmarck, B.; et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J. Anat. 2006, 208, 445–450. [Google Scholar] [CrossRef]
- Kjaer, M.; Langberg, H.; Heinemeier, K.; Bayer, M.L.; Hansen, M.; Holm, L.; Doessing, S.; Kongsgaard, M.; Krogsgaard, M.R.; Magnusson, S.P. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports 2009, 19, 500–510. [Google Scholar] [CrossRef]
- Knobloch, K. Drug-Induced Tendon Disorders. Adv. Exp. Med. Biol. 2016, 920, 229–238. [Google Scholar]
- Van der Linden, P.D.; Sturkenboom, M.C.; Herings, R.M.; Leufkens, H.G.; Stricker, B.H. Fluoroquinolones and risk of Achilles tendon disorders: Case-control study. Bmj 2002, 324, 1306–1307. [Google Scholar] [CrossRef]
- Chimenti, R.L.; Cychosz, C.C.; Hall, M.M.; Phisitkul, P. Current Concepts Review Update: Insertional Achilles Tendinopathy. Foot Ankle Int. 2017, 38, 1160–1169. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.H. PRP Treatment Efficacy for Tendinopathy: A Review of Basic Science Studies. BioMed Res. Int. 2016, 2016, 9103792. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Platelet-rich Blood Derivatives for Tendon Regeneration. J. Am. Acad. Orthop. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Willers, C.; Xu, J.; Wang, A.; Zheng, M.H. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007, 13, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Mackie, K.; Breidahl, W.; Wang, T.; Zheng, M.H. Evidence for the Durability of Autologous Tenocyte Injection for Treatment of Chronic Resistant Lateral Epicondylitis: Mean 4.5-Year Clinical Follow-up. Am. J. Sports Med. 2015, 43, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.W.; Bauer, S.; Goonatillake, M.; Breidahl, W.; Zheng, M.H. Autologous tenocyte implantation, a novel treatment for partial-thickness rotator cuff tear and tendinopathy in an elite athlete. BMJ Case Reports 2013, 2013. [Google Scholar] [CrossRef]
- Aicale, R.; Tarantino, D.; Maffulli, N. Surgery in Tendinopathies. Sports Med. Arthrosc Rev. 2018, 26, 200–202. [Google Scholar] [CrossRef]
- Kaux, J.F.; Croisier, J.L.; Forthomme, B.; Crielaard, J.M. New conservative treatments of chronic tendinopathies. Revue Med. Liege 2015, 70, 507–510. [Google Scholar]
- Ma, R.; Schar, M.; Chen, T.; Wang, H.; Wada, S.; Ju, X.; Deng, X.H.; Rodeo, S.A. Use of Human Placenta-Derived Cells in a Preclinical Model of Tendon Injury. J. Bone Jt. Surg. Am. Vol. 2019, 101, e61. [Google Scholar] [CrossRef]
- Hsiao, M.Y.; Lin, A.C.; Liao, W.H.; Wang, T.G.; Hsu, C.H.; Chen, W.S.; Lin, F.H. Drug-loaded hyaluronic acid hydrogel as a sustained-release regimen with dual effects in early intervention of tendinopathy. Sci. Rep. 2019, 9, 4784. [Google Scholar] [CrossRef]
- Qiu, Y.; Lim, J.J.; Scott, L., Jr.; Adams, R.C.; Bui, H.T.; Temenoff, J.S. PEG-based hydrogels with tunable degradation characteristics to control delivery of marrow stromal cells for tendon overuse injuries. Acta Biomater. 2011, 7, 959–966. [Google Scholar] [CrossRef]
| Conservative Management | Surgical Management | |
|---|---|---|
| Biomechanical Therapies | Biological Therapies | Operative Therapies |
| Classical physiotherapy: Deep transverse friction massage Myofascial manipulation Controlled motion Ultrasound (0.75-3.0 MHz; pulsed or continuous) Ionophoresis Phonophoresis Acupuncture Electrical and laser stimulation: Pulsed electromagnetic fields Extracorporeal shock-wave therapy Laser treatment (pulsed or continuous) Stabilization and modification: Taping Splinting Bracing Straps Orthotic devices Modification of activity: Rest Eccentric exercises Thermic treatments: Cryotherapy (e.g., ice packs and baths) Thermotherapy (heat) | Pharmaceutical agents: Anti-inflammatory drugs (NSAIDs) Systemic corticosteroids Pain control (anesthetics) Antibody therapy (e.g., IL-17, IL-1β antagonist and BMP) Peritendinous (high volume) injections: Corticosteroid injection Saline injection Hyaluronic acid injection Botulinum toxin (BTA) injection MMP inhibitor injection (e.g., Aprotinin) Prolotherapy Topical glyceryl trinitrate therapy Polidocanol injection Glycosaminoglycan polysulfate injection Sclerosant injection Low-dose heparin Blood-based therapies: Platelet-rich plasma injection Autologous blood injection Actovegin (deproteinized extract of calf’s blood) Cell-based therapies: Autologous tenocyte implantation (Orthocell) | Arthroscopy Debridement and decompression Endoscopic/minimally invasive surgery Percutaneous longitudinal tenotomy Radiofrequency microtenotomy Stripping and destruction of neovessels Endoscopic tendon debridement Tenolysis Gastrocnemius recession Tendon replacement strategies after rupture: Tendon allografts Tendon transfer Tendon prosthesis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int. J. Mol. Sci. 2020, 21, 844. https://doi.org/10.3390/ijms21030844
Steinmann S, Pfeifer CG, Brochhausen C, Docheva D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. International Journal of Molecular Sciences. 2020; 21(3):844. https://doi.org/10.3390/ijms21030844
Chicago/Turabian StyleSteinmann, Sara, Christian G. Pfeifer, Christoph Brochhausen, and Denitsa Docheva. 2020. "Spectrum of Tendon Pathologies: Triggers, Trails and End-State" International Journal of Molecular Sciences 21, no. 3: 844. https://doi.org/10.3390/ijms21030844
APA StyleSteinmann, S., Pfeifer, C. G., Brochhausen, C., & Docheva, D. (2020). Spectrum of Tendon Pathologies: Triggers, Trails and End-State. International Journal of Molecular Sciences, 21(3), 844. https://doi.org/10.3390/ijms21030844