Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Lung Macrophages (LMs) in Chronic Obstructive Pulmonary Disease (COPD)
3. Origins of LMs
4. Normal Functions of LMs
5. Alveolar Macrophages (AMs) vs. Interstitial Macrophages (IMs)
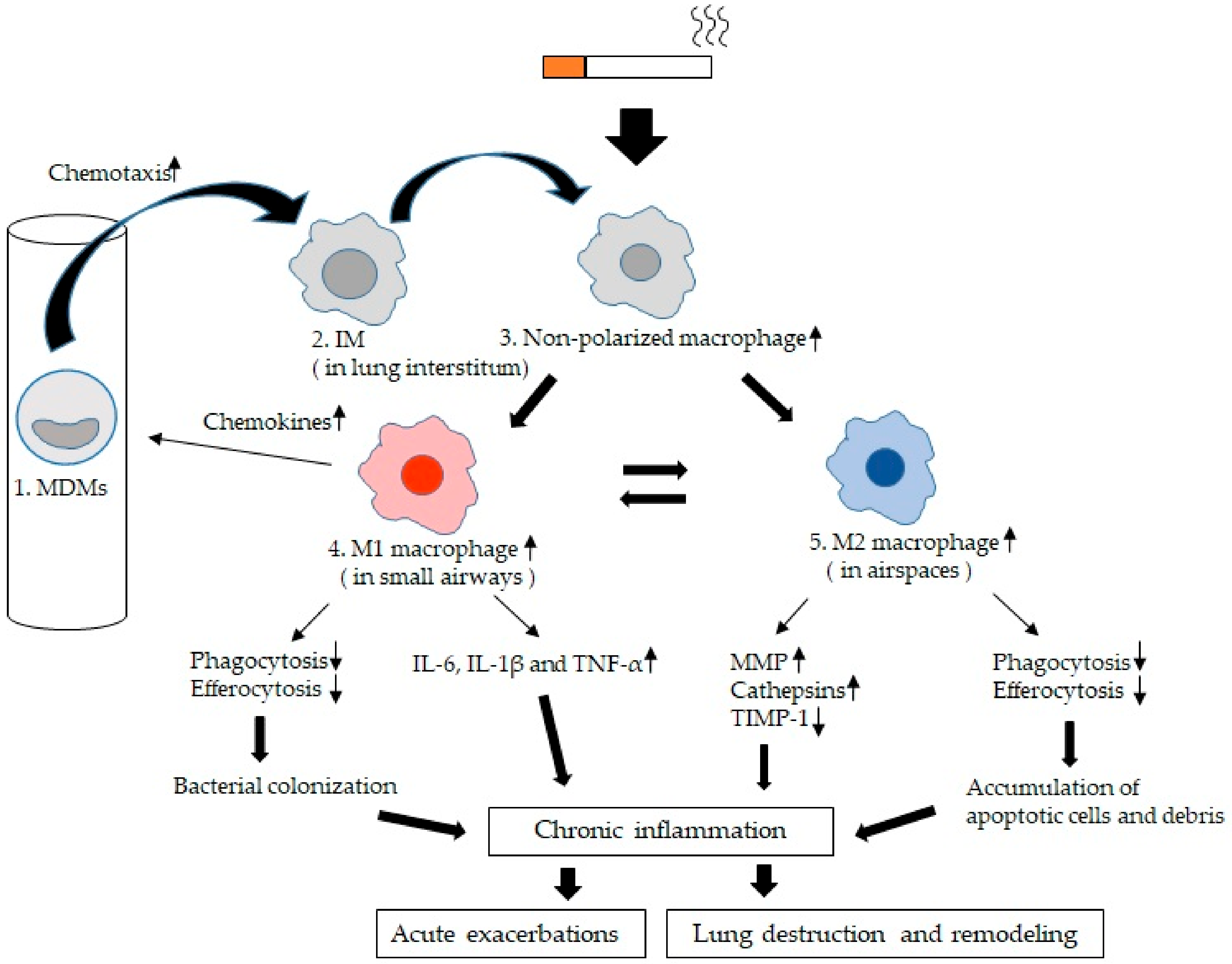
6. Functional Alterations in LMs of COPD
6.1. Chemotaxis
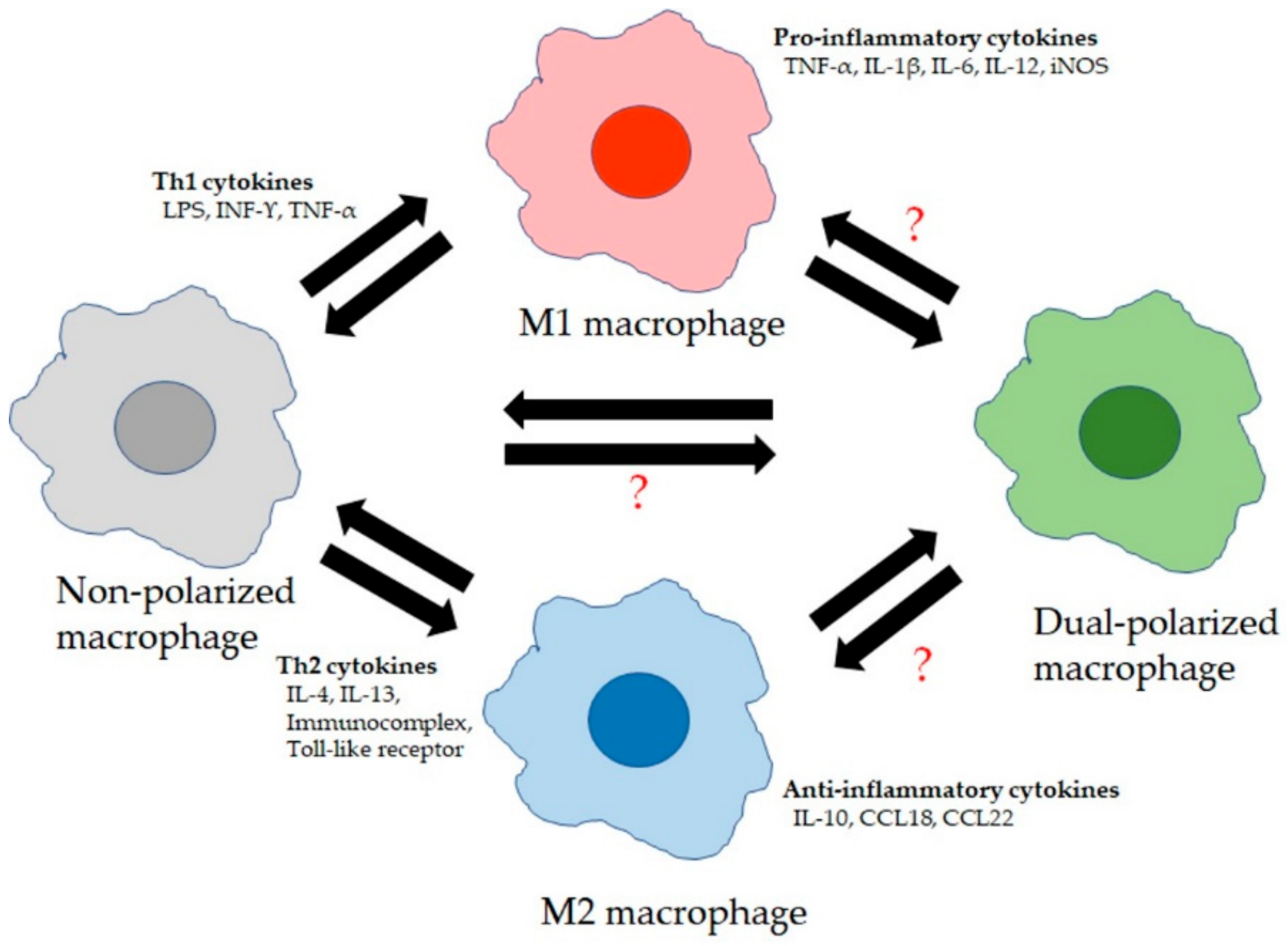
6.2. Mediator Production by LMs
6.3. Phagocytosis of LMs
7. Apoptosis in Lung
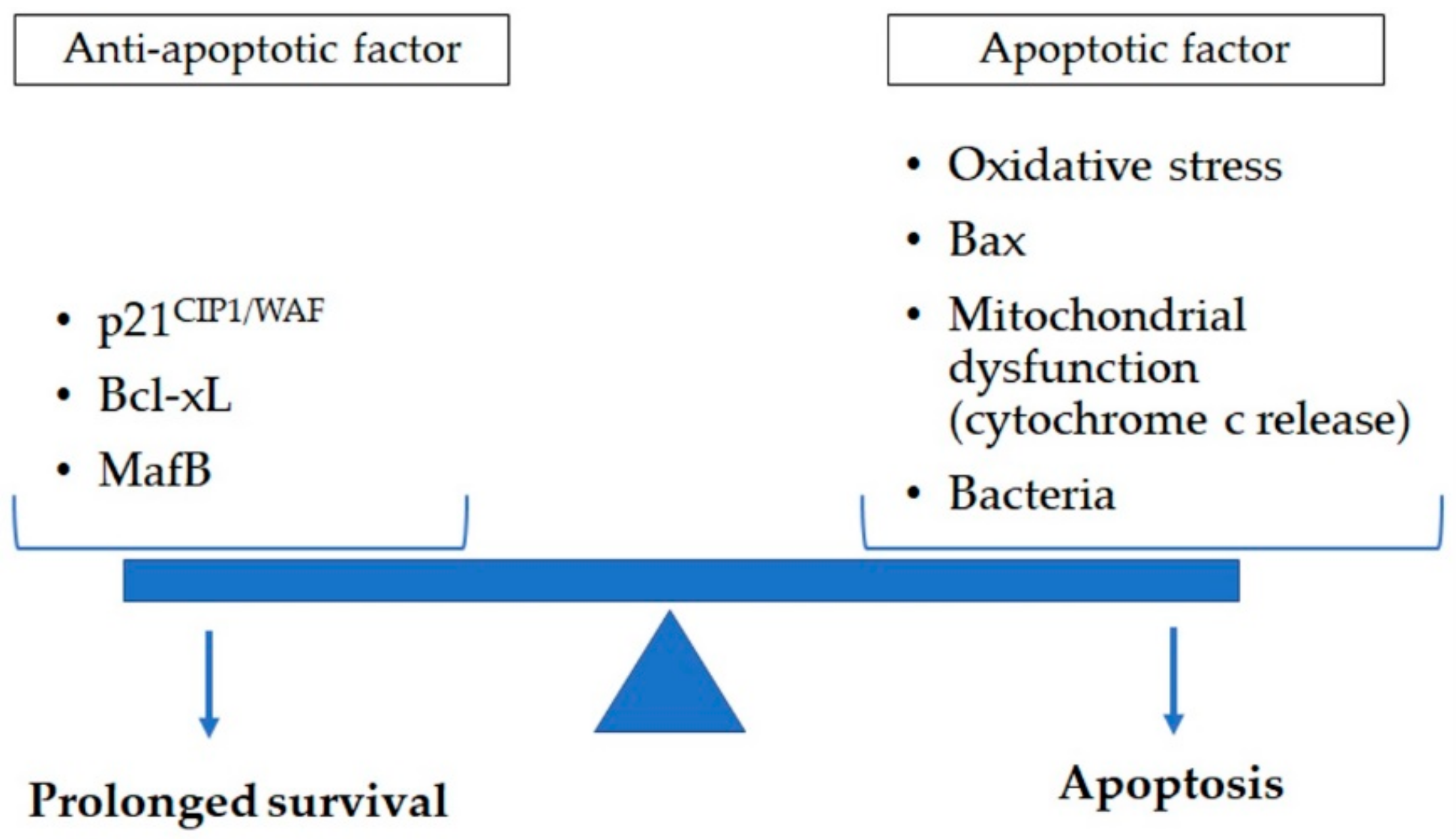
8. LMs and Apoptosis in COPD
8.1. LM Apotosis Accociated with Cigarette Smoke Exposure
8.2. LM Apoptosis Associated with Pathogen Exposure
9. LM Apoptosis vs. Senescence in COPD
10. Therapeutic Options to Improve Macrophage Function in COPD
10.1. Macrolides
10.2. Collectins
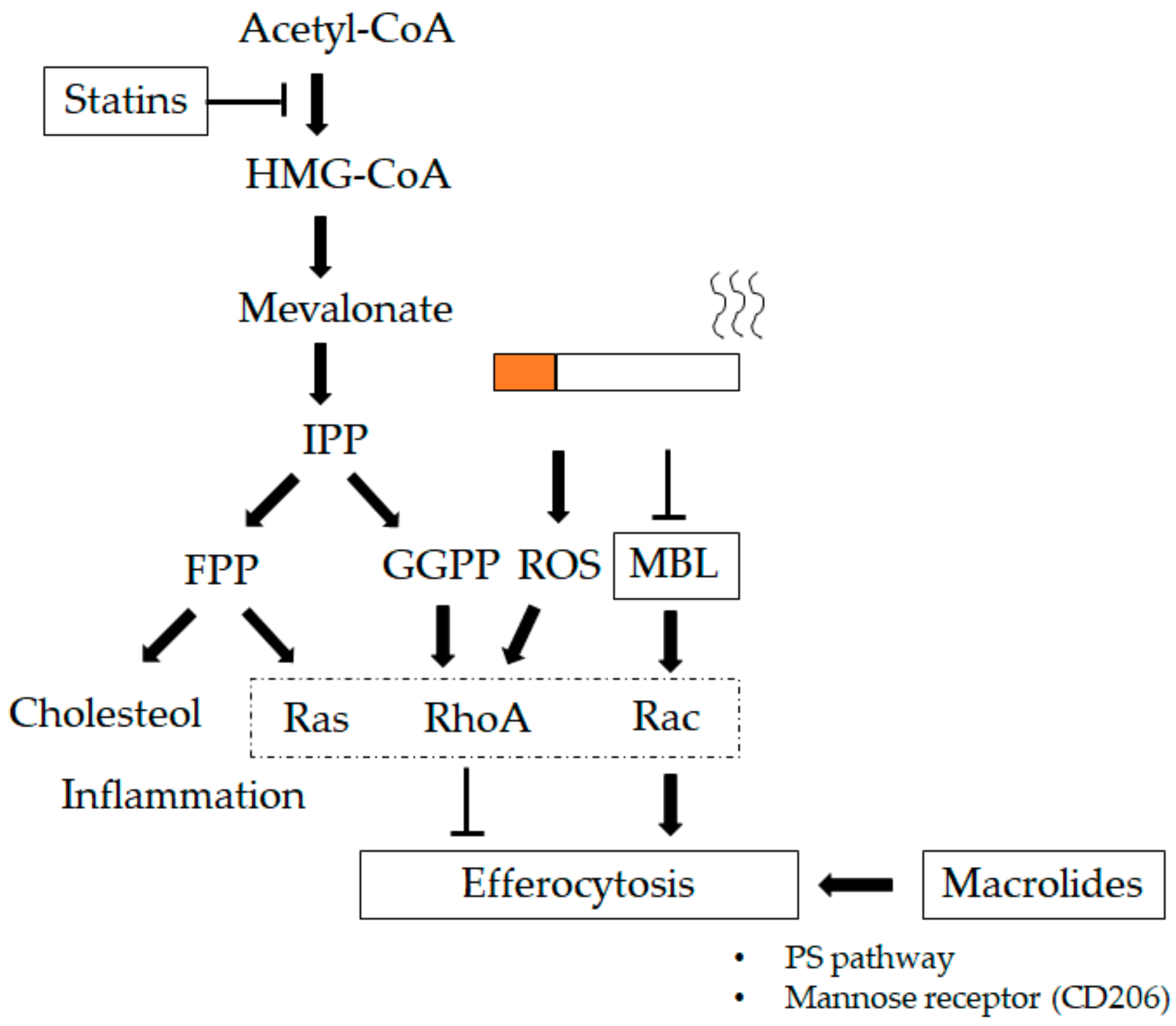
10.3. Statins
10.4. Phosphodiesterase (PDE) Inhibitors
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (accessed on 17 December 2019).
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Pothirat, C.; Chaiwong, W.; Liwsrisakun, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T.; Limsukon, A.; Tajarernmuang, P.; Phetsuk, N. Acute effects of air pollutants on daily mortality and hospitalizations due to cardiovascular and respiratory diseases. J. Thorac. Dis. 2019, 11, 3070–3083. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 2009, 4, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin. Chest. Med. 2014, 35, 71–86. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, S.F.; Sin, D.D. Chronic obstructive pulmonary disease: A chronic systemic inflammatory disease. Respiration 2008, 75, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharm. 2011, 257, 209–226. [Google Scholar] [CrossRef]
- Costabel, U.; Guzman, J. Effect of smoking on bronchoalveolar lavage constituents. Eur. Respir. J. 1992, 5, 776–779. [Google Scholar]
- Ando, M.; Sugimoto, M.; Nishi, R.; Suga, M.; Horio, S.; Kohrogi, H.; Shimazu, K.; Araki, S. Surface morphology and function of human pulmonary alveolar macrophages from smokers and non-smokers. Thorax 1984, 39, 850–856. [Google Scholar] [CrossRef]
- Traves, S.L.; Culpitt, S.V.; Russell, R.E.; Barnes, P.J.; Donnelly, L.E. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax 2002, 57, 590–595. [Google Scholar] [CrossRef]
- Barnes, P.J. Alveolar macrophages as orchestrators of COPD. Copd 2004, 1, 59–70. [Google Scholar] [CrossRef]
- Eapen, M.S.; Hansbro, P.M.; McAlinden, K.; Kim, R.Y.; Ward, C.; Hackett, T.L.; Walters, E.H.; Sohal, S.S. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci. Rep. 2017, 7, 13392. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Wang, Y.; Greter, M.; See, P.; Teo, P.; Malleret, B.; Leboeuf, M.; Low, D.; Oller, G.; Almeida, F.; et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012, 209, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, S.P.; McNelly, N.A.; Hoyt, R.F., Jr. CFU-rAM, the origin of lung macrophages, and the macrophage lineage. Am. J. Physiol. 1992, 263, L299–L307. [Google Scholar] [CrossRef]
- Sorokin, S.P.; Hoyt, R.F., Jr.; Grant, M.M. Development of macrophages in the lungs of fetal rabbits, rats, and hamsters. Anat. Rec. 1984, 208, 103–121. [Google Scholar] [CrossRef]
- van Furth, R.; Cohn, Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968, 128, 415–435. [Google Scholar] [CrossRef]
- Guilliams, M.; De Kleer, I.; Henri, S.; Post, S.; Vanhoutte, L.; De Prijck, S.; Deswarte, K.; Malissen, B.; Hammad, H.; Lambrecht, B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013, 210, 1977–1992. [Google Scholar] [CrossRef]
- Lavin, Y.; Mortha, A.; Rahman, A.; Merad, M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 2015, 15, 731–744. [Google Scholar] [CrossRef]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef]
- Tan, S.Y.; Krasnow, M.A. Developmental origin of lung macrophage diversity. Development 2016, 143, 1318–1327. [Google Scholar] [CrossRef]
- Todd, E.M.; Zhou, J.Y.; Szasz, T.P.; Deady, L.E.; D’Angelo, J.A.; Cheung, M.D.; Kim, A.H.; Morley, S.C. Alveolar macrophage development in mice requires L-plastin for cellular localization in alveoli. Blood 2016, 128, 2785–2796. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Nobs, S.P.; Kurrer, M.; Rehrauer, H.; Thiele, C.; Kopf, M. Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014, 15, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Finney-Hayward, T.K.; Quint, J.K.; Thomas, C.M.; Tudhope, S.J.; Wedzicha, J.A.; Barnes, P.J.; Donnelly, L.E. Defective macrophage phagocytosis of bacteria in COPD. Eur. Respir. J. 2010, 35, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. Pillars Article: M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000. 164: 6166-6173. J. Immunol. 2017, 199, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Byers, D.E.; Holtzman, M.J. Alternatively activated macrophages and airway disease. Chest 2011, 140, 768–774. [Google Scholar] [CrossRef]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Lavin, Y.; Winter, D.; Blecher-Gonen, R.; David, E.; Keren-Shaul, H.; Merad, M.; Jung, S.; Amit, I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014, 159, 1312–1326. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Hoppstadter, J.; Diesel, B.; Zarbock, R.; Breinig, T.; Monz, D.; Koch, M.; Meyerhans, A.; Gortner, L.; Lehr, C.M.; Huwer, H.; et al. Differential cell reaction upon Toll-like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respir. Res. 2010, 11, 124. [Google Scholar] [CrossRef]
- Fathi, M.; Johansson, A.; Lundborg, M.; Orre, L.; Skold, C.M.; Camner, P. Functional and morphological differences between human alveolar and interstitial macrophages. Exp. Mol. Pathol. 2001, 70, 77–82. [Google Scholar] [CrossRef]
- Gibbings, S.L.; Thomas, S.M.; Atif, S.M.; McCubbrey, A.L.; Desch, A.N.; Danhorn, T.; Leach, S.M.; Bratton, D.L.; Henson, P.M.; Janssen, W.J.; et al. Three Unique Interstitial Macrophages in the Murine Lung at Steady State. Am. J. Respir. Cell Mol. Biol. 2017, 57, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.; Perlman, H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef]
- Ferrari-Lacraz, S.; Nicod, L.P.; Chicheportiche, R.; Welgus, H.G.; Dayer, J.M. Human lung tissue macrophages, but not alveolar macrophages, express matrix metalloproteinases after direct contact with activated T lymphocytes. Am. J. Respir. Cell Mol. Biol. 2001, 24, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Kayama, H.; Nakama, T.; Hashimoto, T.; Umemoto, E.; Takeda, K. IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int. Immunol. 2016, 28, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Franke-Ullmann, G.; Pfortner, C.; Walter, P.; Steinmuller, C.; Lohmann-Matthes, M.L.; Kobzik, L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J. Immunol. 1996, 157, 3097–3104. [Google Scholar]
- Wizemann, T.M.; Laskin, D.L. Enhanced phagocytosis, chemotaxis, and production of reactive oxygen intermediates by interstitial lung macrophages following acute endotoxemia. Am. J. Respir. Cell Mol. Biol. 1994, 11, 358–365. [Google Scholar] [CrossRef]
- Toussaint, M.; Fievez, L.; Drion, P.V.; Cataldo, D.; Bureau, F.; Lekeux, P.; Desmet, C.J. Myeloid hypoxia-inducible factor 1alpha prevents airway allergy in mice through macrophage-mediated immunoregulation. Mucosal Immunol 2013, 6, 485–497. [Google Scholar] [CrossRef]
- Bedoret, D.; Wallemacq, H.; Marichal, T.; Desmet, C.; Quesada Calvo, F.; Henry, E.; Closset, R.; Dewals, B.; Thielen, C.; Gustin, P.; et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Invest. 2009, 119, 3723–3738. [Google Scholar] [CrossRef]
- Johansson, A.; Lundborg, M.; Skold, C.M.; Lundahl, J.; Tornling, G.; Eklund, A.; Camner, P. Functional, morphological, and phenotypical differences between rat alveolar and interstitial macrophages. Am. J. Respir. Cell Mol. Biol. 1997, 16, 582–588. [Google Scholar] [CrossRef]
- Lehnert, B.E.; Valdez, Y.E.; Holland, L.M. Pulmonary macrophages: Alveolar and interstitial populations. Exp. Lung Res. 1985, 9, 177–190. [Google Scholar] [CrossRef]
- Sabatel, C.; Radermecker, C.; Fievez, L.; Paulissen, G.; Chakarov, S.; Fernandes, C.; Olivier, S.; Toussaint, M.; Pirottin, D.; Xiao, X.; et al. Exposure to Bacterial CpG DNA Protects from Airway Allergic Inflammation by Expanding Regulatory Lung Interstitial Macrophages. Immunity 2017, 46, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sugimoto, C.; Arainga, M.; Alvarez, X.; Didier, E.S.; Kuroda, M.J. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: Implications for understanding lung disease in humans. J. Iimmunol. 2014, 192, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Bilyk, N.; Mackenzie, J.S.; Papadimitriou, J.M.; Holt, P.G. Functional studies on macrophage populations in the airways and the lung wall of SPF mice in the steady-state and during respiratory virus infection. Immunology 1988, 65, 417–425. [Google Scholar] [PubMed]
- Holt, P.G.; Warner, L.A.; Papadimitriou, J.M. Alveolar macrophages: Functional heterogeneity within macrophage populations from rat lung. Aust. J. Exp. Biol. Med. Sci. 1982, 60, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Summer, R.; Wilson, A.A.; Kotton, D.N.; Fine, A. The prolonged life-span of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2008, 38, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Maus, U.A.; Janzen, S.; Wall, G.; Srivastava, M.; Blackwell, T.S.; Christman, J.W.; Seeger, W.; Welte, T.; Lohmeyer, J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am. J. Respir. Cell Mol. Biol. 2006, 35, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Gotoh, H.; Watanabe, J.; Uetake, T.; Komuro, I.; Yuasa, K.; Watanabe, S.; Ieki, R.; Sakamaki, H.; Akiyama, H.; et al. Augmented proliferation of human alveolar macrophages after allogeneic bone marrow transplantation. Blood 1999, 93, 667–673. [Google Scholar] [CrossRef]
- Landsman, L.; Jung, S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J. Immunol. 2007, 179, 3488–3494. [Google Scholar] [CrossRef]
- Holt, P.G.; Degebrodt, A.; Venaille, T.; O’Leary, C.; Krska, K.; Flexman, J.; Farrell, H.; Shellam, G.; Young, P.; Penhale, J.; et al. Preparation of interstitial lung cells by enzymatic digestion of tissue slices: Preliminary characterization by morphology and performance in functional assays. Immunology 1985, 54, 139–147. [Google Scholar]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Russell, R.E.; Culpitt, S.V.; DeMatos, C.; Donnelly, L.; Smith, M.; Wiggins, J.; Barnes, P.J. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2002, 26, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Barbers, R.G.; Evans, M.J.; Gong, H., Jr.; Tashkin, D.P. Enhanced alveolar monocytic phagocyte (macrophage) proliferation in tobacco and marijuana smokers. Am. Rev. Respir. Dis. 1991, 143, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Bitterman, P.B.; Saltzman, L.E.; Adelberg, S.; Ferrans, V.J.; Crystal, R.G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J. Clin. Invest. 1984, 74, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.D.; Ramberg, R.E.; Sale, G.E.; Sparkes, R.S.; Golde, D.W. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science 1976, 192, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Godleski, J.J.; Brain, J.D. The origin of alveolar macrophages in mouse radiation chimeras. J. Exp. Med. 1972, 136, 630–643. [Google Scholar] [CrossRef]
- Russell, R.E.; Thorley, A.; Culpitt, S.V.; Dodd, S.; Donnelly, L.E.; Demattos, C.; Fitzgerald, M.; Barnes, P.J. Alveolar macrophage-mediated elastolysis: Roles of matrix metalloproteinases, cysteine, and serine proteases. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L867–L873. [Google Scholar] [CrossRef]
- Punturieri, A.; Filippov, S.; Allen, E.; Caras, I.; Murray, R.; Reddy, V.; Weiss, S.J. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J. Exp. Med. 2000, 192, 789–799. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Holmes, M.; Reynolds, P.N. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur. Respir. J. 2005, 25, 447–454. [Google Scholar] [CrossRef]
- Di Stefano, A.; Capelli, A.; Lusuardi, M.; Balbo, P.; Vecchio, C.; Maestrelli, P.; Mapp, C.E.; Fabbri, L.M.; Donner, C.F.; Saetta, M. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am. J. Respir. Crit. Care Med. 1998, 158, 1277–1285. [Google Scholar] [CrossRef]
- Finkelstein, R.; Fraser, R.S.; Ghezzo, H.; Cosio, M.G. Alveolar inflammation and its relation to emphysema in smokers. Am. J. Respir. Crit. Care Med. 1995, 152, 1666–1672. [Google Scholar] [CrossRef]
- Rottman, J.B. Key role of chemokines and chemokine receptors in inflammation, immunity, neoplasia, and infectious disease. Vet. Pathol. 1999, 36, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000, 18, 217–242. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Hayashi, S.; Hogg, J.C.; Fujii, T.; Goto, Y.; Sakamoto, N.; Mukae, H.; Vincent, R.; van Eeden, S.F. Alveolar macrophage-epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment. Respir. Res. 2005, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Hayashi, S.; Hogg, J.C.; Mukae, H.; Suwa, T.; Goto, Y.; Vincent, R.; van Eeden, S.F. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am. J. Respir. Cell Mol. Biol. 2002, 27, 34–41. [Google Scholar] [CrossRef]
- Costa, C.; Traves, S.L.; Tudhope, S.J.; Fenwick, P.S.; Belchamber, K.B.; Russell, R.E.; Barnes, P.J.; Donnelly, L.E. Enhanced monocyte migration to CXCR3 and CCR5 chemokines in COPD. Eur. Respir. J. 2016, 47, 1093–1102. [Google Scholar] [CrossRef]
- Ravi, A.K.; Plumb, J.; Gaskell, R.; Mason, S.; Broome, C.S.; Booth, G.; Catley, M.; Vestbo, J.; Singh, D. COPD monocytes demonstrate impaired migratory ability. Respir. Res. 2017, 18, 90. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Bonecchi, R.; Bianchi, G.; Bordignon, P.P.; D’Ambrosio, D.; Lang, R.; Borsatti, A.; Sozzani, S.; Allavena, P.; Gray, P.A.; Mantovani, A.; et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998, 187, 129–134. [Google Scholar] [CrossRef]
- Nieto, M.; Frade, J.M.; Sancho, D.; Mellado, M.; Martinez, A.C.; Sanchez-Madrid, F. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J. Exp. Med. 1997, 186, 153–158. [Google Scholar] [CrossRef]
- Frade, J.M.; Mellado, M.; del Real, G.; Gutierrez-Ramos, J.C.; Lind, P.; Martinez, A.C. Characterization of the CCR2 chemokine receptor: Functional CCR2 receptor expression in B cells. J. Immunol. 1997, 159, 5576–5584. [Google Scholar]
- Combadiere, C.; Ahuja, S.K.; Murphy, P.M. Cloning, chromosomal localization, and RNA expression of a human beta chemokine receptor-like gene. DNA Cell Biol. 1995, 14, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Capelli, A.; Di Stefano, A.; Gnemmi, I.; Balbo, P.; Cerutti, C.G.; Balbi, B.; Lusuardi, M.; Donner, C.F. Increased MCP-1 and MIP-1beta in bronchoalveolar lavage fluid of chronic bronchitics. Eur. Respir. J. 1999, 14, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Henrot, P.; Prevel, R.; Berger, P.; Dupin, I. Chemokines in COPD: From Implication to Therapeutic Use. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, S.F.; Yeung, A.; Quinlam, K.; Hogg, J.C. Systemic response to ambient particulate matter: Relevance to chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 61–67. [Google Scholar] [CrossRef]
- Park, E.J.; Roh, J.; Kim, Y.; Park, K.; Kim, D.S.; Yu, S.D. PM 2.5 collected in a residential area induced Th1-type inflammatory responses with oxidative stress in mice. Env. Res. 2011, 111, 348–355. [Google Scholar] [CrossRef]
- Mantecca, P.; Farina, F.; Moschini, E.; Gallinotti, D.; Gualtieri, M.; Rohr, A.; Sancini, G.; Palestini, P.; Camatini, M. Comparative acute lung inflammation induced by atmospheric PM and size-fractionated tire particles. Toxicol. Lett. 2010, 198, 244–254. [Google Scholar] [CrossRef]
- Wegesser, T.C.; Franzi, L.M.; Mitloehner, F.M.; Eiguren-Fernandez, A.; Last, J.A. Lung antioxidant and cytokine responses to coarse and fine particulate matter from the great California wildfires of 2008. Inhal. Toxicol. 2010, 22, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Alexis, N.E.; Lay, J.C.; Zeman, K.; Bennett, W.E.; Peden, D.B.; Soukup, J.M.; Devlin, R.B.; Becker, S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J. Allergy Clin. Immunol. 2006, 117, 1396–1403. [Google Scholar] [CrossRef]
- Jimenez, L.A.; Drost, E.M.; Gilmour, P.S.; Rahman, I.; Antonicelli, F.; Ritchie, H.; MacNee, W.; Donaldson, K. PM(10)-exposed macrophages stimulate a proinflammatory response in lung epithelial cells via TNF-alpha. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 282, L237–L248. [Google Scholar] [CrossRef]
- van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef]
- Bicknell, S.; van Eeden, S.; Hayashi, S.; Hards, J.; English, D.; Hogg, J.C. A non-radioisotopic method for tracing neutrophils in vivo using 5′-bromo-2′-deoxyuridine. Am. J. Respir. Cell Mol. Biol. 1994, 10, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Valledor, A.F.; Borras, F.E.; Cullell-Young, M.; Celada, A. Transcription factors that regulate monocyte/macrophage differentiation. J. Leukoc. Biol. 1998, 63, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ishii, H.; Hogg, J.C.; Shih, C.H.; Yatera, K.; Vincent, R.; van Eeden, S.F. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am. J. Respir. Crit. Care Med. 2004, 170, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.H.; Kluin-Nelemans, J.C.; Van Damme, J.; Wientjens, G.J.; Willemze, R.; Fibbe, W.E. Interleukin 6 is a permissive factor for monocytic colony formation by human hematopoietic progenitor cells. J. Exp. Med. 1992, 175, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Rosseau, S.; Hammerl, P.; Maus, U.; Walmrath, H.D.; Schutte, H.; Grimminger, F.; Seeger, W.; Lohmeyer, J. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L25–L35. [Google Scholar] [CrossRef] [PubMed]
- Pons, A.R.; Sauleda, J.; Noguera, A.; Pons, J.; Barcelo, B.; Fuster, A.; Agusti, A.G. Decreased macrophage release of TGF-beta and TIMP-1 in chronic obstructive pulmonary disease. Eur. Respir. J. 2005, 26, 60–66. [Google Scholar] [CrossRef]
- Abboud, R.T.; Vimalanathan, S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int. J. Tuberc. Lung. Dis. 2008, 12, 361–367. [Google Scholar]
- Kim, H.; Liu, X.; Kohyama, T.; Kobayashi, T.; Conner, H.; Abe, S.; Fang, Q.; Wen, F.Q.; Rennard, S.I. Cigarette smoke stimulates MMP-1 production by human lung fibroblasts through the ERK1/2 pathway. Copd 2004, 1, 13–23. [Google Scholar] [CrossRef]
- Yin, L.; Morita, A.; Tsuji, T. Alterations of extracellular matrix induced by tobacco smoke extract. Arch Derm. Res. 2000, 292, 188–194. [Google Scholar] [CrossRef]
- Janoff, A.; Carp, H.; Lee, D.K.; Drew, R.T. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science 1979, 206, 1313–1314. [Google Scholar] [CrossRef]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Carp, H.; Miller, F.; Hoidal, J.R.; Janoff, A. Potential mechanism of emphysema: Alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc. Natl. Acad. Sci. USA 1982, 79, 2041–2045. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Goodridge, H.S. Information processing during phagocytosis. Nat. Rev. Immunol. 2012, 12, 492–502. [Google Scholar] [CrossRef]
- Henson, P.M. Cell Removal: Efferocytosis. Annu. Rev. Cell Dev. Biol. 2017, 33, 127–144. [Google Scholar] [CrossRef]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 23, 962–978. [Google Scholar] [CrossRef]
- Martin, C.J.; Peters, K.N.; Behar, S.M. Macrophages clean up: Efferocytosis and microbial control. Curr. Opin. Microbiol. 2014, 17, 17–23. [Google Scholar] [CrossRef]
- deCathelineau, A.M.; Henson, P.M. The final step in programmed cell death: Phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003, 39, 105–117. [Google Scholar]
- Hoffmann, P.R.; deCathelineau, A.M.; Ogden, C.A.; Leverrier, Y.; Bratton, D.L.; Daleke, D.L.; Ridley, A.J.; Fadok, V.A.; Henson, P.M. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 2001, 155, 649–659. [Google Scholar] [CrossRef]
- Loov, C.; Mitchell, C.H.; Simonsson, M.; Erlandsson, A. Slow degradation in phagocytic astrocytes can be enhanced by lysosomal acidification. Glia 2015, 63, 1997–2009. [Google Scholar] [CrossRef]
- Monks, J.; Smith-Steinhart, C.; Kruk, E.R.; Fadok, V.A.; Henson, P.M. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol. Reprod. 2008, 78, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Flannagan, R.S.; Jaumouille, V.; Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Vandivier, R.W.; Fadok, V.A.; Hoffmann, P.R.; Bratton, D.L.; Penvari, C.; Brown, K.K.; Brain, J.D.; Accurso, F.J.; Henson, P.M. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 2002, 109, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Berenson, C.S.; Kruzel, R.L.; Wrona, C.T.; Mammen, M.J.; Sethi, S. Impaired Innate COPD Alveolar Macrophage Responses and Toll-Like Receptor-9 Polymorphisms. PloS ONE 2015, 10, e0134209. [Google Scholar] [CrossRef] [PubMed]
- Berenson, C.S.; Kruzel, R.L.; Eberhardt, E.; Sethi, S. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J. Infect. Dis. 2013, 208, 2036–2045. [Google Scholar] [CrossRef]
- Berenson, C.S.; Garlipp, M.A.; Grove, L.J.; Maloney, J.; Sethi, S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J. Infect. Dis. 2006, 194, 1375–1384. [Google Scholar] [CrossRef]
- Marti-Lliteras, P.; Regueiro, V.; Morey, P.; Hood, D.W.; Saus, C.; Sauleda, J.; Agusti, A.G.; Bengoechea, J.A.; Garmendia, J. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect. Immun. 2009, 77, 4232–4242. [Google Scholar] [CrossRef]
- Ferrara, F.; D’Adda, D.; Falchi, M.; Dall’Asta, L. The macrophagic activity of patients affected by pneumonia or chronic obstructive pulmonary disease. Int. J. Tissue React. 1996, 18, 109–114. [Google Scholar]
- Vecchiarelli, A.; Dottorini, M.; Puliti, M.; Todisco, T.; Cenci, E.; Bistoni, F. Defective candidacidal activity of alveolar macrophages and peripheral blood monocytes from patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1991, 143, 1049–1054. [Google Scholar] [CrossRef]
- Phipps, J.C.; Aronoff, D.M.; Curtis, J.L.; Goel, D.; O’Brien, E.; Mancuso, P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect. Immun. 2010, 78, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Minematsu, N.; Blumental-Perry, A.; Shapiro, S.D. Cigarette smoke inhibits engulfment of apoptotic cells by macrophages through inhibition of actin rearrangement. Am. J. Respir. Cell Mol. Biol. 2011, 44, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.A.; Spooner, G.; Rahman, I.; Rossi, A.G. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem. Biophys. Res. Commun. 2004, 318, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Eltboli, O.; Bafadhel, M.; Hollins, F.; Wright, A.; Hargadon, B.; Kulkarni, N.; Brightling, C. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm. Med. 2014, 14, 112. [Google Scholar] [CrossRef]
- Hodge, S.; Matthews, G.; Mukaro, V.; Ahern, J.; Shivam, A.; Hodge, G.; Holmes, M.; Jersmann, H.; Reynolds, P.N. Cigarette smoke-induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. Am. J. Respir. Cell Mol. Biol. 2011, 44, 673–681. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Smoking alters alveolar macrophage recognition and phagocytic ability: Implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2007, 37, 748–755. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Scicchitano, R.; Reynolds, P.N.; Holmes, M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 2003, 81, 289–296. [Google Scholar] [CrossRef]
- Droemann, D.; Goldmann, T.; Tiedje, T.; Zabel, P.; Dalhoff, K.; Schaaf, B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir. Res. 2005, 6, 68. [Google Scholar] [CrossRef]
- Todt, J.C.; Freeman, C.M.; Brown, J.P.; Sonstein, J.; Ames, T.M.; McCubbrey, A.L.; Martinez, F.J.; Chensue, S.W.; Beck, J.M.; Curtis, J.L. Smoking decreases the response of human lung macrophages to double-stranded RNA by reducing TLR3 expression. Respir. Res. 2013, 14, 33. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Jersmann, H.; Matthews, G.; Ahern, J.; Holmes, M.; Reynolds, P.N. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 178, 139–148. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Brozyna, S.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 2006, 28, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Pons, A.R.; Noguera, A.; Blanquer, D.; Sauleda, J.; Pons, J.; Agusti, A.G. Phenotypic characterisation of alveolar macrophages and peripheral blood monocytes in COPD. Eur. Respir. J. 2005, 25, 647–652. [Google Scholar] [CrossRef] [PubMed]
- McCubbrey, A.L.; Curtis, J.L. Efferocytosis and lung disease. Chest 2013, 143, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Ryter, S.W.; Choi, A.M. Functional significance of apoptosis in chronic obstructive pulmonary disease. Copd 2007, 4, 347–353. [Google Scholar] [CrossRef]
- Hogg, J.C. Why does airway inflammation persist after the smoking stops? Thorax 2006, 61, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Sauler, M.; Bazan, I.S.; Lee, P.J. Cell Death in the Lung: The Apoptosis-Necroptosis Axis. Annu. Rev. Physiol. 2019, 81, 375–402. [Google Scholar] [CrossRef]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef]
- Pryor, W.A.; Stone, K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 1993, 686, 12–27. [Google Scholar] [CrossRef]
- Stone, K.K.; Bermudez, E.; Pryor, W.A. Aqueous extracts of cigarette tar containing the tar free radical cause DNA nicks in mammalian cells. Env. Health Perspect. 1994, 102 (Suppl 10), 173–178. [Google Scholar]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Zou, H.; Henzel, W.J.; Liu, X.; Lutschg, A.; Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 1997, 90, 405–413. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Muzio, M.; Stockwell, B.R.; Stennicke, H.R.; Salvesen, G.S.; Dixit, V.M. An induced proximity model for caspase-8 activation. J. Biol. Chem. 1998, 273, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Muzio, M.; Chinnaiyan, A.M.; Kischkel, F.C.; O’Rourke, K.; Shevchenko, A.; Ni, J.; Scaffidi, C.; Bretz, J.D.; Zhang, M.; Gentz, R.; et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell 1996, 85, 817–827. [Google Scholar] [CrossRef]
- Tang, D.; Kidd, V.J. Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. J. Biol. Chem. 1998, 273, 28549–28552. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Bozinovski, S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front. Immunol. 2014, 5, 435. [Google Scholar] [CrossRef]
- Hiemstra, P.S. Altered macrophage function in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2013, 10, S180–S185. [Google Scholar] [CrossRef]
- Machiya, J.; Shibata, Y.; Yamauchi, K.; Hirama, N.; Wada, T.; Inoue, S.; Abe, S.; Takabatake, N.; Sata, M.; Kubota, I. Enhanced expression of MafB inhibits macrophage apoptosis induced by cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 2007, 36, 418–426. [Google Scholar] [CrossRef]
- Tomita, K.; Caramori, G.; Lim, S.; Ito, K.; Hanazawa, T.; Oates, T.; Chiselita, I.; Jazrawi, E.; Chung, K.F.; Barnes, P.J.; et al. Increased p21(CIP1/WAF1) and B cell lymphoma leukemia-x(L) expression and reduced apoptosis in alveolar macrophages from smokers. Am. J. Respir. Crit. Care Med. 2002, 166, 724–731. [Google Scholar] [CrossRef]
- Aoshiba, K.; Tamaoki, J.; Nagai, A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L1392–L1401. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, S.; Ishii, T.; Yamamoto, H.; Yamaguchi, Y.; Ouchi, Y. Apoptosis of circulating neutrophils and alveolar macrophages in COPD. Chest 2005, 127, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Voisin, C.; Aerts, C.; Fournier, E.; Firlik, M. Acute effects of tobacco smoke on alveolar macrophages cultured in gas phase. Eur. J. Respir. Dis. Suppl. 1985, 139, 76–81. [Google Scholar] [PubMed]
- Stuart, R.S.; Higgins, W.H.; Brown, P.W. In vitro toxicity of tobacco smoke solutions to rabbit alveolar macrophages. Arch. Env. Health 1978, 33, 135–140. [Google Scholar] [CrossRef]
- Singh, R.; Mackay, A.J.; Patel, A.R.; Garcha, D.S.; Kowlessar, B.S.; Brill, S.E.; Donnelly, L.E.; Barnes, P.J.; Donaldson, G.C.; Wedzicha, J.A. Inflammatory thresholds and the species-specific effects of colonising bacteria in stable chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 114. [Google Scholar] [CrossRef]
- Sapey, E.; Stockley, R.A. COPD exacerbations. 2: Aetiology. Thorax 2006, 61, 250–258. [Google Scholar] [CrossRef]
- Lundborg, M.; Johard, U.; Lastbom, L.; Gerde, P.; Camner, P. Human alveolar macrophage phagocytic function is impaired by aggregates of ultrafine carbon particles. Env. Res 2001, 86, 244–253. [Google Scholar] [CrossRef]
- Donnelly, L.E.; Barnes, P.J. Defective phagocytosis in airways disease. Chest 2012, 141, 1055–1062. [Google Scholar] [CrossRef]
- Hersh, D.; Monack, D.M.; Smith, M.R.; Ghori, N.; Falkow, S.; Zychlinsky, A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 1999, 96, 2396–2401. [Google Scholar] [CrossRef]
- Hilbi, H.; Moss, J.E.; Hersh, D.; Chen, Y.; Arondel, J.; Banerjee, S.; Flavell, R.A.; Yuan, J.; Sansonetti, P.J.; Zychlinsky, A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 1998, 273, 32895–32900. [Google Scholar] [CrossRef]
- Gao, L.Y.; Abu Kwaik, Y. Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity. Infect. Immun. 1999, 67, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Weglarczyk, K.; Mysiak, M.; Guzik, K.; Ernst, M.; Flad, H.D.; Pryjma, J. Fas (CD95)-Fas ligand interactions are responsible for monocyte apoptosis occurring as a result of phagocytosis and killing of Staphylococcus aureus. Infect. Immun. 2001, 69, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Ojcius, D.M.; Souque, P.; Perfettini, J.L.; Dautry-Varsat, A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J. Immunol. 1998, 161, 4220–4226. [Google Scholar] [PubMed]
- Ruckdeschel, K.; Harb, S.; Roggenkamp, A.; Hornef, M.; Zumbihl, R.; Kohler, S.; Heesemann, J.; Rouot, B. Yersinia enterocolitica impairs activation of transcription factor NF-kappaB: Involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 1998, 187, 1069–1079. [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Yang, R.B.; Mark, M.R.; Suggett, S.; Devaux, B.; Radolf, J.D.; Klimpel, G.R.; Godowski, P.; Zychlinsky, A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 1999, 285, 736–739. [Google Scholar] [CrossRef]
- Dockrell, D.H.; Lee, M.; Lynch, D.H.; Read, R.C. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 2001, 184, 713–722. [Google Scholar] [CrossRef]
- Patel, I.S.; Seemungal, T.A.; Wilks, M.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wedzicha, J.A. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002, 57, 759–764. [Google Scholar] [CrossRef]
- Hayflick, L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Houben, J.M.; Mercken, E.M.; Ketelslegers, H.B.; Bast, A.; Wouters, E.F.; Hageman, G.J.; Schols, A.M. Telomere shortening in chronic obstructive pulmonary disease. Respir. Med. 2009, 103, 230–236. [Google Scholar] [CrossRef]
- Savale, L.; Chaouat, A.; Bastuji-Garin, S.; Marcos, E.; Boyer, L.; Maitre, B.; Sarni, M.; Housset, B.; Weitzenblum, E.; Matrat, M.; et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009, 179, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Morla, M.; Busquets, X.; Pons, J.; Sauleda, J.; MacNee, W.; Agusti, A.G. Telomere shortening in smokers with and without COPD. Eur. Respir. J. 2006, 27, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Aoshiba, K.; Nagai, A. Alveolar cell senescence in patients with pulmonary emphysema. Am. J. Respir. Crit. Care Med. 2006, 174, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Sung, B.; Jung, K.J.; Zou, Y.; Yu, B.P. The molecular inflammatory process in aging. Antioxid. Redox Signal. 2006, 8, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Senescence in COPD and Its Comorbidities. Annu. Rev. Physiol. 2017, 79, 517–539. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.; Sadaie, M.; Hoare, M.; Narita, M. Cellular senescence and its effector programs. Genes Dev. 2014, 28, 99–114. [Google Scholar] [CrossRef]
- Nyunoya, T.; Powers, L.S.; Yarovinsky, T.O.; Butler, N.S.; Monick, M.M.; Hunninghake, G.W. Hyperoxia induces macrophage cell cycle arrest by adhesion-dependent induction of p21Cip1 and activation of the retinoblastoma protein. J. Biol. Chem. 2003, 278, 36099–36106. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, B.; Rhee, D.K.; Pyo, S. Acrylamide Induces Senescence in Macrophages through a Process Involving ATF3, ROS, p38/JNK, and a Telomerase-Independent Pathway. Chem. Res. Toxicol. 2015, 28, 71–86. [Google Scholar] [CrossRef]
- Flanary, B.E.; Streit, W.J. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia 2004, 45, 75–88. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, L.; Li, C.; Chen, P.; Chen, Y. Long-term macrolide treatment for the prevention of acute exacerbations in COPD: A systematic review and meta-analysis. Int. J. Chron Obs. Pulmon Dis. 2018, 13, 3813–3829. [Google Scholar] [CrossRef] [PubMed]
- Feola, D.J.; Garvy, B.A.; Cory, T.J.; Birket, S.E.; Hoy, H.; Hayes, D., Jr.; Murphy, B.S. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob. Agents Chemother. 2010, 54, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Yamaryo, T.; Oishi, K.; Yoshimine, H.; Tsuchihashi, Y.; Matsushima, K.; Nagatake, T. Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob. Agents Chemother. 2003, 47, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Kobayashi, D.; Asano, Y.; Sakurai, T.; Kashimura, M.; Okuyama, S.; Yoneda, Y.; Shapiro, S.D.; Takayama, K. Clarithromycin prevents smoke-induced emphysema in mice. Am. J. Respir. Crit. Care Med. 2009, 179, 271–278. [Google Scholar] [CrossRef]
- Haller, T.; Dietl, P.; Pfaller, K.; Frick, M.; Mair, N.; Paulmichl, M.; Hess, M.W.; Furst, J.; Maly, K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 2001, 155, 279–289. [Google Scholar] [CrossRef]
- Wirtz, H.R.; Dobbs, L.G. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 1990, 250, 1266–1269. [Google Scholar] [CrossRef]
- Wong, C.J.; Akiyama, J.; Allen, L.; Hawgood, S. Localization and developmental expression of surfactant proteins D and A in the respiratory tract of the mouse. Pediatr. Res. 1996, 39, 930–937. [Google Scholar] [CrossRef]
- Walker, S.R.; Williams, M.C.; Benson, B. Immunocytochemical localization of the major surfactant apoproteins in type II cells, Clara cells, and alveolar macrophages of rat lung. J. Histochem. Cytochem. 1986, 34, 1137–1148. [Google Scholar] [CrossRef]
- Wright, J.R. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 2005, 5, 58–68. [Google Scholar] [CrossRef]
- Shepherd, V.L. Pulmonary surfactant protein D: A novel link between innate and adaptive immunity. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 282, L516–L517. [Google Scholar] [CrossRef]
- van Iwaarden, F.; Welmers, B.; Verhoef, J.; Haagsman, H.P.; van Golde, L.M. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1990, 2, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tenner, A.J.; Robinson, S.L.; Borchelt, J.; Wright, J.R. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J. Biol. Chem. 1989, 264, 13923–13928. [Google Scholar] [PubMed]
- Kuronuma, K.; Sano, H.; Kato, K.; Kudo, K.; Hyakushima, N.; Yokota, S.; Takahashi, H.; Fujii, N.; Suzuki, H.; Kodama, T.; et al. Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J. Biol. Chem. 2004, 279, 21421–21430. [Google Scholar] [CrossRef] [PubMed]
- Beharka, A.A.; Gaynor, C.D.; Kang, B.K.; Voelker, D.R.; McCormack, F.X.; Schlesinger, L.S. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J. Immunol. 2002, 169, 3565–3573. [Google Scholar] [CrossRef]
- Hodge, S.; Matthews, G.; Dean, M.M.; Ahern, J.; Djukic, M.; Hodge, G.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Therapeutic role for mannose-binding lectin in cigarette smoke-induced lung inflammation? Evidence from a murine model. Am. J. Respir. Cell Mol. Biol. 2010, 42, 235–242. [Google Scholar] [CrossRef]
- Blamoun, A.I.; Batty, G.N.; DeBari, V.A.; Rashid, A.O.; Sheikh, M.; Khan, M.A. Statins may reduce episodes of exacerbation and the requirement for intubation in patients with COPD: Evidence from a retrospective cohort study. Int. J. Clin. Pr. 2008, 62, 1373–1378. [Google Scholar] [CrossRef]
- Alexeeff, S.E.; Litonjua, A.A.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Statin use reduces decline in lung function: VA Normative Aging Study. Am. J. Respir. Crit. Care Med. 2007, 176, 742–747. [Google Scholar] [CrossRef]
- Rezaie-Majd, A.; Maca, T.; Bucek, R.A.; Valent, P.; Muller, M.R.; Husslein, P.; Kashanipour, A.; Minar, E.; Baghestanian, M. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arter. Thromb. Vasc. Biol. 2002, 22, 1194–1199. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Napoli, C.; Lerman, A. Statin effects beyond lipid lowering--are they clinically relevant? Eur. Heart J. 2003, 24, 225–248. [Google Scholar] [CrossRef]
- Hothersall, E.; McSharry, C.; Thomson, N.C. Potential therapeutic role for statins in respiratory disease. Thorax 2006, 61, 729–734. [Google Scholar] [CrossRef]
- Frost, F.J.; Petersen, H.; Tollestrup, K.; Skipper, B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest 2007, 131, 1006–1012. [Google Scholar] [CrossRef]
- Soyseth, V.; Brekke, P.H.; Smith, P.; Omland, T. Statin use is associated with reduced mortality in COPD. Eur. Respir. J. 2007, 29, 279–283. [Google Scholar] [CrossRef]
- Fessler, M.B.; Young, S.K.; Jeyaseelan, S.; Lieber, J.G.; Arndt, P.G.; Nick, J.A.; Worthen, G.S. A role for hydroxy-methylglutaryl coenzyme a reductase in pulmonary inflammation and host defense. Am. J. Respir. Crit. Care Med. 2005, 171, 606–615. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.S.; Kim, E.K.; Choe, K.H.; Oh, Y.M.; Shim, T.S.; Kim, S.E.; Lee, Y.S.; Lee, S.D. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am. J. Respir. Crit. Care Med. 2005, 172, 987–993. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.; Leung, B.P.; McInnes, I.B.; Thomson, N.C.; Liew, F.Y. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J. Immunol. 2004, 172, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Lee, Y.J.; Park, H.J.; Chong, Y.H.; Kang, J.L. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. Am. J. Respir. Crit. Care Med. 2010, 181, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Janssen, W.J.; Fessler, M.B.; McPhillips, K.A.; Borges, V.M.; Bowler, R.P.; Xiao, Y.Q.; Kench, J.A.; Henson, P.M.; Vandivier, R.W. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J. Immunol. 2006, 176, 7657–7665. [Google Scholar] [CrossRef]
- Tosello-Trampont, A.C.; Nakada-Tsukui, K.; Ravichandran, K.S. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J. Biol. Chem. 2003, 278, 49911–49919. [Google Scholar] [CrossRef]
- Leverrier, Y.; Ridley, A.J. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr. Biol. 2001, 11, 195–199. [Google Scholar] [CrossRef]
- deBakker, C.D.; Haney, L.B.; Kinchen, J.M.; Grimsley, C.; Lu, M.; Klingele, D.; Hsu, P.K.; Chou, B.K.; Cheng, L.C.; Blangy, A.; et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr. Biol. 2004, 14, 2208–2216. [Google Scholar] [CrossRef]
- Chimini, G.; Chavrier, P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2000, 2, E191–E196. [Google Scholar] [CrossRef] [PubMed]
- Richens, T.R.; Linderman, D.J.; Horstmann, S.A.; Lambert, C.; Xiao, Y.Q.; Keith, R.L.; Boe, D.M.; Morimoto, K.; Bowler, R.P.; Day, B.J.; et al. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am. J. Respir. Crit. Care Med. 2009, 179, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Asada, K.; Sasaki, S.; Suda, T.; Chida, K.; Nakamura, H. Antiinflammatory roles of peroxisome proliferator-activated receptor gamma in human alveolar macrophages. Am. J. Respir. Crit. Care Med. 2004, 169, 195–200. [Google Scholar] [CrossRef]
- Bellosta, S.; Via, D.; Canavesi, M.; Pfister, P.; Fumagalli, R.; Paoletti, R.; Bernini, F. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arter. Thromb. Vasc. Biol. 1998, 18, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Thanh Thuy, T.T.; Lee, J.H.; Ro, J.Y.; Bae, Y.A.; Kong, Y.; Ahn, J.Y.; Lee, D.S.; Oh, Y.M.; Lee, S.D.; et al. Simvastatin inhibits induction of matrix metalloproteinase-9 in rat alveolar macrophages exposed to cigarette smoke extract. Exp. Mol. Med. 2009, 41, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef]
- Houslay, M.D.; Adams, D.R. PDE4 cAMP phosphodiesterases: Modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 2003, 370, 1–18. [Google Scholar] [CrossRef]
- Sanz, M.J.; Cortijo, J.; Morcillo, E.J. PDE4 inhibitors as new anti-inflammatory drugs: Effects on cell trafficking and cell adhesion molecules expression. Pharm. 2005, 106, 269–297. [Google Scholar] [CrossRef]
- Matera, M.G.; Page, C.P.; Cazzola, M. Novel bronchodilators for the treatment of chronic obstructive pulmonary disease. Trends Pharm. Sci. 2011, 32, 495–506. [Google Scholar] [CrossRef]
- Fan Chung, K. Phosphodiesterase inhibitors in airways disease. Eur. J. Pharm. 2006, 533, 110–117. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akata, K.; van Eeden, S.F. Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 853. https://doi.org/10.3390/ijms21030853
Akata K, van Eeden SF. Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease. International Journal of Molecular Sciences. 2020; 21(3):853. https://doi.org/10.3390/ijms21030853
Chicago/Turabian StyleAkata, Kentaro, and Stephan F. van Eeden. 2020. "Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease" International Journal of Molecular Sciences 21, no. 3: 853. https://doi.org/10.3390/ijms21030853
APA StyleAkata, K., & van Eeden, S. F. (2020). Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease. International Journal of Molecular Sciences, 21(3), 853. https://doi.org/10.3390/ijms21030853




