Brain-Derived Neurotrophic Factor and Diabetes
Abstract
1. Diabetes Mellitus and Brain Function
2. Neurotrophins
3. Neurotrophins and Insulin Resistance
3.1. Experimental and Animal Studies
3.2. Type 2 Diabetes Mellitus
3.3. Type 1 Diabetes Mellitus
3.4. BDNF and Adipocytokines
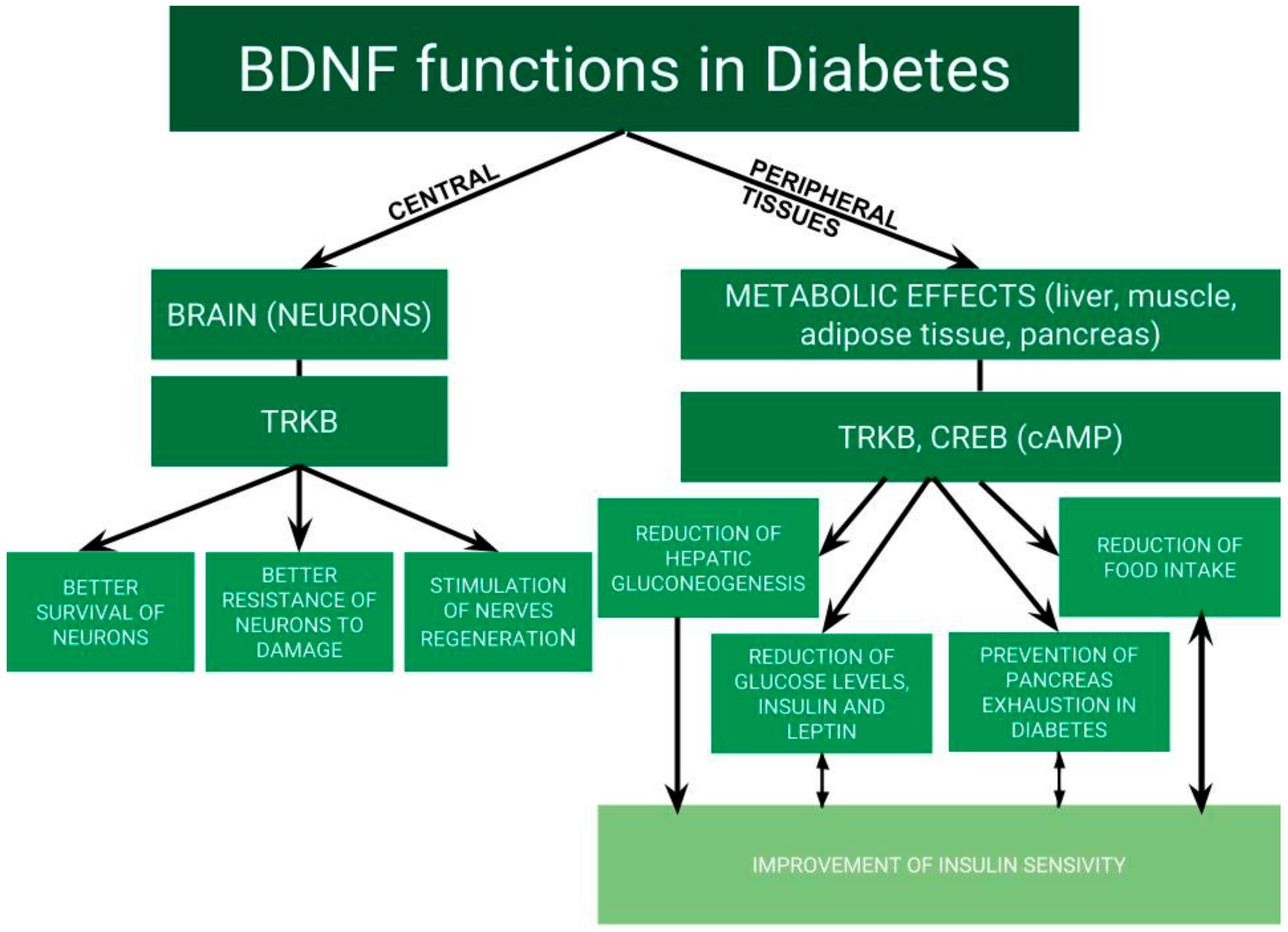
4. BDNF and Chronic Complications of Diabetes
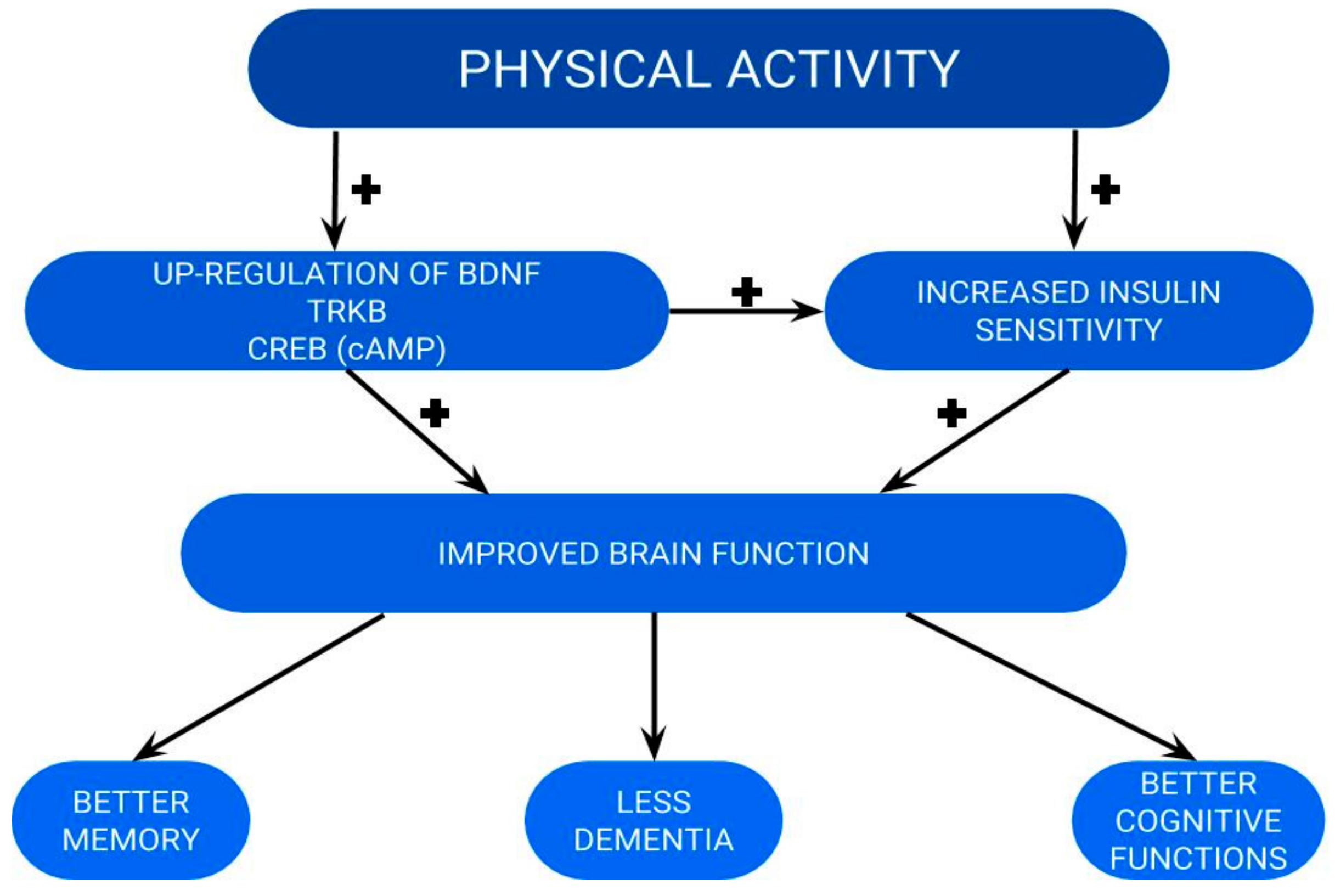
5. How Can We Increase BDNF Levels Behaviorally?
5.1. Experimental and Animal Studies
5.2. Healthy Population
5.3. Diabetes
6. Summary
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| HBA1C | Glycated Hemoglobin |
| NGF | Nerve Growth Factor |
| BDNF | Brain-Derived Neurotrophic Factor |
| NT | Neurotrophin |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| BMI | Body Mass Index |
| CRP | C-reactive protein |
| WBC | White blood cells |
| CT | Computed Tomography |
| IR | Insulin Resistance |
| G-CSF | Granulocyte-Colony Stimulating Factor |
| VO2max | Test for Maximal Oxygen Uptake |
| p75NTR | p75NTR p75 NT receptor |
| TrkB | receptor tyrosine kinase B |
| CREB | cAMP-response element binding protein |
References
- Zalecenia, P.T.D. 2017 Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clin. Diabet. 2017. [Google Scholar] [CrossRef]
- Garcia, M.E.; Lee, A.; Neuhaus, J.; Gonzalez, H.; To, T.M.; Haan, M.N. Diabetes Mellitus as a Risk Factor for Development of Depressive Symptoms in a Population-Based Cohort of Older Mexican Americans. J. Am. Geriatr. Soc. 2016, 64, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Clavarino, A.M.; Dingle, K.; Mamun, A.A.; Kairuz, T. Diabetes Mellitus and the Risk of Depressive and Anxiety Disorders in Australian Women: a Longitunal study. J. Womens Health (Larchmt). 2015, 24, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Butwicka, A.; Fendler, W.; Zalepa, A.; Szadkowska, A.; Zawodniak-Szalapska, M.; Gmitrowicz, A.; Mlynarski, W. Psychiatric Disorders and Health – Related Quality of life in Children With Type 1 Diabetes Mellitus. Psychosomatics 2016, 57, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Plastino, M.; Cristiano, D.; Colica, C.; Ermio, C.; De Bartolo, M.; Mungari, P.; Fonte, G.; Consoli, D.; Consoli, A.; et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J. Neurol. Sci. 2012, 315, 39–43. [Google Scholar] [CrossRef]
- An, Y.; Varma, V.R.; Varma, S.; Casanova, R.; Dammer, E.; Pletnikova, O.; Chia, C.W.; Egan, J.M.; Ferrucci, L.; Troncoso, J.; et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018, 14, 318–329. [Google Scholar] [CrossRef]
- De la Monte, S.M. Type 3 diabetes is sporadic Alzheimers disease: mini-review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef]
- Dmitrzak-Weglarz, M.; Skibinska, M.; Slopien, A.; Tyszkiewicz, M.; Pawlak, J.; Maciukiewicz, M.; Zaremba, D.; Rajewski, A.; Hauser, J. Serum neurotrophin concentrations in polish adolescent girls with anorexia nervosa. Neuropsychobiology 2013, 67, 25–32. [Google Scholar] [CrossRef]
- Apfel, S.C. Neurotrophic factor and diabetic peripherial neuropathy. Eur. Neurol. 1999, 41, 27–34. [Google Scholar] [CrossRef]
- Eyileten, C.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D.; Malek, L.; Postula, M. Antidiabetic Effect of Brain-Derived Neurotrophic Factor and Its Association with Inflammation in Type 2 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 2823671. [Google Scholar] [CrossRef]
- Babei, P.; Damirchi, A.; Mehdipoor, M.; Tehrani, B.S. Long term habitual exercise is associated with lower resting level of serum BDNF. Neurosci. Lett. 2014, 566, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bomberg, E.; Billington, C.; Levine, A.; Kotz, C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Morikawa, Y.; Nanjo, K.; Senba, E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience 2006, 139, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. (Lond) 2006, 110, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Liu, M.E.; Chou, K.H.; Yang, A.C.; Hung, C.C.; Hong, C.J.; Tsai, S.J.; Lin, C.P. Effect of BDNF Val66Met polymorphism on regional white matter hyperintensities and cognitive function in elderly males without dementia. Psychoneuroendocrinology 2014, 39, 94–103. [Google Scholar] [CrossRef]
- Manni, L.; Nikolova, V.; Vyagova, D.; Chaldakov, G.N.; Aloe, L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int. J. Cardiol. 2005, 102, 169–171. [Google Scholar] [CrossRef]
- Suwa, M.; Kishimoto, H.; Nofuji, Y.; Nakano, H.; Sasaki, H.; Radak, Z.; Kumagai, S. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism 2006, 55, 852–857. [Google Scholar] [CrossRef]
- Unger, T.J.; Calderon, G.A.; Bradley, L.C.; Sena-Esteves, M.; Rios, M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007, 27, 14265–14274. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Ono, M.; Ichihara, J.; Nonomura, T.; Itakura, Y.; Taiji, M.; Nakayama, C.; Noguchi, H. Brain- derived neurotrophic factor reduces blood glucose level in obese diabetic mice but not in normal mice. Biochem. Biophys. Res. Commun. 1997, 238, 633–637. [Google Scholar] [CrossRef]
- Śmieszek, A.; Stręk, Z.; Kornicka, K.; Grzesiak, J.; Weiss, C.; Marycz, K. Antioxidant and Anti-Senescence Effect of Metformin on Mouse Olfactory Ensheathing Cells (mOECs) May Be Associated with Increased Brain-Derived Neurotrophic Factor Levels—An Ex Vivo Study. Int. J. Mol. Sci. 2017, 18, 872. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Guo, Z.; Jiang, H.; Ware, M.; Mattson, M.P. Reversal of Behavioral and Metabolic Abnormalities, and Insulin Resistance Syndrome, by Dietetary Restriction in Brain-Derived Neurotrophic Factor. Endocrinology 2003, 144, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Itakura, Y.; Inoue, T.; Tsuchida, A.; Nakagawa, T.; Noguchi, H.; Taiji, M. Protective effect of brain-derived neurotrophic factor on pancreatic islets in obese diabetic mice. Metabolism 2006, 55, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, A.; Yamasaki, Y.; Matsuhisa, M.; Kubota, M.; Nakahara, I.; Nakatani, Y.; Hoshi, A.; Gorogawa, S.; Umayahara, Y.; Itakura, Y.; et al. Brain-derived Neurotrophic Factor Ameliorates Hepatic Insulin Resistnance in Zucker Fatty Rats. Metabolism 2003, 52, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Meek, T.H.; Wisse, B.E.; Thaler, J.P.; Guyenet, S.J.; Matsen, M.E.; Fischer, J.D.; Taborsky, G.J., Jr.; Schwartz, M.W.; Morton, G.J. BDNF action in the brain attenuates diabetic hyperglycemia via insulin-independent inhibition of hepatic glucose production. Diabetes 2013, 62, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, A.; Ohta, K.; Obayashi, H.; Fukui, M.; Hasegawa, G.; Nakamura, N.; Kozai, H.; Imai, S.; Ohta, M. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: Relationship to glucose metabolism and biomarkers of insulin resistance. Clin. Biochem. 2008, 41, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lang, N.; Cheng Z., F. Serum Levels of Brain-Derived Neurotrophic Factor Are Associated with Diabetes Risk, Complications, and Obesity: A Cohort Study from Chinese Patients with Type 2 Diabetes. Mol. Neurobiol. 2016, 53, 5492–5499. [Google Scholar] [CrossRef]
- Boyuk, B.; Degirmencioglu, S.; Atalay, H.; Guzel, S.; Acar, A.; Celebi, A.; Ekizoglu, I.; Simsek, C. Relationship between levels of brain-derieved neurotrophic factor and metabolic parameters In patients with typu 2 diabetes mellitus. J. Diabetes Res. 2014, 2014, 978143. [Google Scholar] [CrossRef]
- Lee, S.S.; Yoo, J.K.; Kang, S.; Woo, J.H.; Shin, K.O.; Kim, K.B.; Cho, S.Y.; Roh, H.T.; Kim, Y.I. The Effects of 12 Weeks Regular Aerobic Exercise on Brain-derived Neurotrophic Factor and Inflammatory Factors in Juvenile Obesity and Type 2 Diabetes Mellitus. J. Phys. Ther. Sci. 2014, 26, 1199–1204. [Google Scholar] [CrossRef]
- Toriya, M.; Maekawa, F.; Maejima, Y.; Onaka, T.; Fujiwara, K.; Nakagawa, T.; Nakata, M.; Yada, T. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2010, 22, 987–995. [Google Scholar] [CrossRef]
- Tonoli, C.; Heyman, E.; Buyse, L.; Roelands, B.; Piacentini, M.F.; Bailey, S.; Pattyn, N.; Berthoin, S.; Meeusen, R. Neurotrophins and cognitive functions in T1D compared with healthy controls: effects of a high-intensity exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Uruska, A.; Niedzwiecki, P.; Araszkiewicz, A.; Zozulinska-Ziolkiewicz, D. Brain-derived neurotrophic factor and insulin resistance during hyperinsulinaemic-euglycaemic clamp in type 1 diabetes patients in the PoProStu. Diabetes Metab. 2017, 43, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Hristova, M.G. Genotrophic effect of neurotrophins—Restart of β-cell regeneration in diabetes mellitus. Med. Hypotheses 2017, 107, 9–11. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Jang, E.H.; Kim, A.Y.; Fava, M.; Mischoulon, D.; Papakostas, G.I.; Na, E.J.; Jang, J.; Yu, H.Y.; Hong, J.P.; et al. Ratio of plasma BDNF to leptin levels are associated with treatment response in major depressive disorder but not in panic disorder: A 12-week follow-up study. J. Affect. Disord. 2019, 259, 349–354. [Google Scholar] [CrossRef]
- Eyileten, C.; Mirowska-Guzel, D.; Milanowski, L.; Zaremba, M.; Rosiak, M.; Cudna, A.; Kaplon-Cieslicka, A.; Opolski, G.; Filipiak, K.J.; Malek, L.; et al. Serum Brain-Derived Neurotrophic Factor is Related to Platelet Reactivity and Metformin Treatment in Adult Patients With Type 2 Diabetes Mellitus. Can. J. Diabetes 2019, 43, 19–26. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.I.; El-Asrar, A.A.; Abouammoh, M.; Alhomida, A.S. Reduced Levels of Brain Derived Neurotrophic Factor (BDNF) in the Serum of Diabetic Retinopathy Patients and in the Retina of Diabetic Rats. Cell. Mol. Neurobiol. 2013, 33, 359–367. [Google Scholar] [CrossRef]
- Kaviarasana, K.; Jithua, M.; Arif Mulla, M.; Sharmab, T.; Sivasankar, S.; Das, U.N.; Angayarkanni, N. Low blood and vitreal BDNF, LXA4 and altered Th1/Th2 cytokine balance are potential risk factors for diabetic retinopathy. Metabolism 2015, 64, 958–966. [Google Scholar] [CrossRef]
- Seki, M.; Tanaka, T.; Nawa, H.; Usui, T.; Fukuchi, T.; Ikeda, K.; Abe, H.; Takei, N. Involvement of Brain-Derived Neurotrophic Factor in Early Retinal Neuropathy of Streptozotocin-Induced Diabetes in Rats. Therapeutic Potential of Brain-Derived Neurotrophic Factor for Dopaminergic Amacrine Cells. Diabetes 2004, 53, 2412–2419. [Google Scholar] [CrossRef]
- Sun, Q.; Tang, D.D.; Yin, E.G.; Wei, L.L.; Chen, P.; Deng, S.P.; Tu, L.L. Diagnostic Significance of Serum Levels of Nerve Growth Factor and Brain Derived Neurotrophic Factor in Diabetic Peripheral Neuropathy. Med. Sci. Monit. 2018, 24, 5943–5950. [Google Scholar] [CrossRef]
- Li, L.; Yu, T.; Yu, L.; Li, H.; Liu, Y.; Wang, D. Exogenous brain-derived neurotrophic factor relieves pain symptoms of diabetic rats by reducing excitability of dorsal root ganglion neurons. Int. J. Neurosci. 2016, 126, 749–758. [Google Scholar] [CrossRef]
- Pate, R.R.; Taverno Ross, S.E.; Liese, A.D.; Dowda, M. Associations among physical activity, diet quality, and weight status in US adults. Med. Sci. Sports Exerc. 2015, 47, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Motahari-Tabari, N.; Ahmad Shirvani, M.; Shirzad-E-Ahoodashty, M.; Yousefi-Abdolmaleki, E.; Teimourzadeh, M. The Effect of 8 Weeks Aerobic Exercise on Insulin Resistance in Type 2 Diabetes: A Randomized Clinical Trial. Glob. J. Health Sci. 2014, 7, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Cancela, J.M.; Ayán, C.; Varela, S.; Seijo, M. Effects of long-term aerobic exercise intervention on institutionalized patients with dementia. J. Sci. Med. Sport 2016, 19, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Eslami, R.; Gharakhanlou, R.; Kazemi, A.; Dakhili, A.B.; Sorkhkamanzadeh, G.; Sheikhy, A. Does Endurance Training Compensate for Neurotrophin Deficiency Following Diabetic Neuropathy? Iran. Red Crescent Med. J. 2016, 18, e37757. [Google Scholar] [CrossRef][Green Version]
- Tang, L.; Kang, Y.T.; Yin, B.; Sun, L.J.; Fan, X.S. Effects of weight-bearing ladder and aerobic treadmill exercise on learning and memory ability of diabetic rats and its mechanism. Chin. J. Appl. Physiol. 2017, 33, 436–440. [Google Scholar] [CrossRef]
- Flöel, A.; Ruscheweyh, R.; Krüger, K.; Willemer, C.; Winter, B.; Völker, K.; Lohmann, H.; Zitzmann, M.; Mooren, F.; Breitenstein, C.; et al. Physical activity and memory functions: Are neurotrophins and cerebral Gray matter volume the missing link? NeuroImage 2010, 49, 2756–2763. [Google Scholar] [CrossRef]
- Cho, H.C.; Kim, J.; Kim, S.; Son, Y.H.; Lee, N.; Jung, S.H. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill V02max performance in healthy college men. Neurosci. Lett. 2012, 519, 78–83. [Google Scholar] [CrossRef]
- Tonoli, C.; Heyman, E.; Roelands, B.; Buyse, L.; Piacentini, F.; Berthoin, S.; Bailey, S.; Pattyn, N.; Meeusen, R. Glucose and Insulin during Continuous and Interval Exercise in Type 1 Diabetes. Int. J. Sports Med. 2015, 36, 955–959. [Google Scholar] [CrossRef]
- Brinkmann, C.; Schäfer, L.; Masoud, M.; Latsch, J.; Lay, D.; Bloch, W.; Brixius, K. Effects of Cycling and Exergaming on Neurotrophic Factors in Elderly Type 2 Diabetic Men—A Preliminary Investigation. Exp. Clin. Endocrinol. Diabetes 2017, 125, 436–440. [Google Scholar] [CrossRef]
- Miyamoto, T.; Iwakura, T.; Matsuoka, N.; Iwamoto, M.; Takenaka, M.; Akamatsu, Y.; Moritani, T. Impact of prolonged neuromuscular electrical stimulation on metabolic profile and cognition related blood parameters in type 2 diabetes: A randomized controlled cross-over trial. Diabetes Res. Clin. Pract. 2018, 142, 37–45. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. https://doi.org/10.3390/ijms21030841
Rozanska O, Uruska A, Zozulinska-Ziolkiewicz D. Brain-Derived Neurotrophic Factor and Diabetes. International Journal of Molecular Sciences. 2020; 21(3):841. https://doi.org/10.3390/ijms21030841
Chicago/Turabian StyleRozanska, Olga, Aleksandra Uruska, and Dorota Zozulinska-Ziolkiewicz. 2020. "Brain-Derived Neurotrophic Factor and Diabetes" International Journal of Molecular Sciences 21, no. 3: 841. https://doi.org/10.3390/ijms21030841
APA StyleRozanska, O., Uruska, A., & Zozulinska-Ziolkiewicz, D. (2020). Brain-Derived Neurotrophic Factor and Diabetes. International Journal of Molecular Sciences, 21(3), 841. https://doi.org/10.3390/ijms21030841




