Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission?
Abstract
1. Introduction
2. Pharmacological Targets for Major pCBs
2.1. Transient Receptor Potential Vanilloid Sub-Family
2.2. Opioid Receptors
2.3. G Protein-Coupled Receptors GPR55
2.4. Voltage Gated Calcium Channels
2.5. Glycine Receptors
2.6. Serotonin Receptors
2.7. Acetylcholine Receptors
2.8. Voltage Gated Sodium Channels
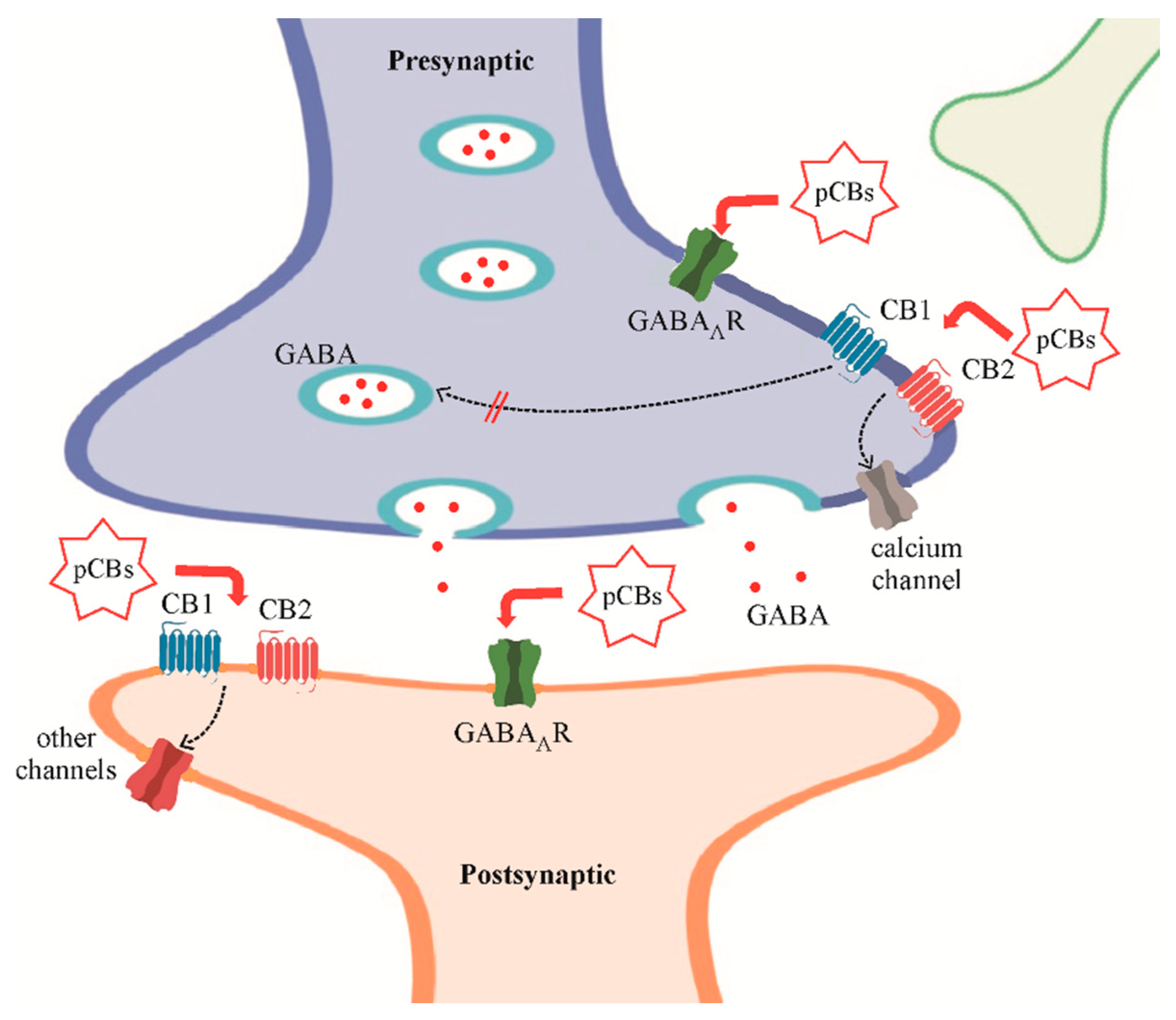
3. GABAA Receptors
Cannabinoids and GABAARs
4. Cannabinoids on Neurological Diseases with GABA Involvement
4.1. Parkinson Disease (PD) and Motor Functions Impairment
4.2. Alzheimer Disease (AD)
4.3. Amyotrophic Lateral Sclerosis (ALS)
4.4. Autism Spectrum Disorders (ASD)
4.5. Epilepsy
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sigel, E.; Steinmann, M.E. Structure, function, and modulation of GABAA receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [PubMed]
- Rabow, L.E.; Russek, S.J.; Farb, D.H. From ion currents to genomic analysis: Recent advances in GABAA receptor research. Synapse 1995, 21, 189–274. [Google Scholar] [CrossRef] [PubMed]
- Kasaragod, V.B.; Schindelin, H. Structure of Heteropentameric GABAA Receptors and Receptor-Anchoring Properties of Gephyrin. Front. Mol. Neurosci. 2019, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, J.; Sagredo, O.; Pazos, M.R.; Garcia, C.; Pertwee, R.; Mechoulam, R.; Martinez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; del Bel, E.A.; Guimaraes, F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3364–3378. [Google Scholar] [CrossRef]
- Pertwee, R. Handbook of Cannabis; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoyl-glycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commum. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.; Zuardi, A.W.; Crippa, J.A. Safety and side effects of cannabidiol: A Cannabis sativa constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Thomas, B.F.; Gilliam, A.F.; Burch, D.F.; Roche, M.J.; Seltzman, H.H. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J. Pharmacol. Exp. Ther. 1998, 285, 285–292. [Google Scholar]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.; Lanciego, J.L.; Nadal, X.; et al. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef]
- Jones, N.A.; Hill, A.J.; Smith, I.; Bevan, S.A.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J. Pharmacol. Exp. Ther. 2010, 332, 569–577. [Google Scholar] [CrossRef]
- Scuderi, C.; Filippis, D.D.; Iuvone, T.; Blasio, A.; Steardo, A.; Esposito, G. Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phytother. Res. 2009, 23, 597–602. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Puffenbarger, R.A.; Boothe, A.C.; Cabral, G.A. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia 2000, 29, 58–69. [Google Scholar] [CrossRef]
- Correa, F.; Docagne, F.; Mestre, L.; Clemente, D.; Hernangómez, M.; Loría, F.; Guaza, C. A role for CB2 receptors in anandamide signalling pathways involved in the regulation of IL-12 and IL-23 in microglial cells. Biochem. Pharmacol. 2009, 77, 86–100. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2019. [Google Scholar] [CrossRef]
- Oláh, A.; Szekanecz, Z.; Bíró, T. Targeting Cannabinoid Signaling in the Immune System: “High”-ly Exciting Questions, Possibilities, and Challenges. Front. Immunol. 2017, 8, 1487. [Google Scholar] [CrossRef]
- Cohen, K.; Weizman, A.; Weinstein, A. Modulatory effects of cannabinoids on brain neurotransmission. Eur. J. Neurosci. 2019, 50, 2322–2345. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Kathmann, M.; Flau, K.; Redmer, A.; Tränkle, C.; Schlicker, E. Cannabidiol is an allosteric modulator at mu- and deltaopioid receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 372, 354–361. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. [Google Scholar] [CrossRef]
- Xiong, W.; Cui, T.; Cheng, K.; Yang, F.; Chen, S.R.; Willenbring, D.; Guan, Y.; Pan, H.L.; Ren, K.; Xu, Y.; et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J. Exp. Med. 2012, 209, 1121–1134. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Resstel, L.B.M.; Tavares, R.F.; Lisboa, S.F.S.; Joca, S.R.L.; Corrêa, F.M.A.; Guimarães, F.S. 5-HT1A receptors are involved in the cannabidiol induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef]
- Soares Vde, P.; Campos, A.C.; Bortoli, V.C.; Zangrossi, H., Jr.; Guimarães, F.S.; Zuardi, A.W. Intra-dorsal periaqueductal gray administration of cannabidiol blocks panic-like response by activating 5-HT1A receptors. Behav. Brain Res. 2010, 213, 225–229. [Google Scholar] [CrossRef]
- Mahgoub, M.; Keun-Hang, S.Y.; Sydorenko, V.; Ashoor, A.; Kabbani, N.; Al Kury, L.; Sadek, B.; Howarth, C.F.; Isaev, D.; Galadari, S.; et al. Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2013, 720, 310–319. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, G.; Di Bonaventura, C.; Cifelli, P.; Roseti, C.; Fattouch, J.; Morano, A.; Limatola, C.; Aronica, E.; Palma, E.; Giallonardo, A.T. A novel action of lacosamide on GABAA currents sets the ground for a synergic interaction with levetiracetam in treatment of epilepsy. Neurobiol. Dis. 2018, 115, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Morano, A.; Cifelli, P.; Nencini, P.; Antonilli, L.; Fattouch, J.; Ruffolo, G.; Roseti, C.; Aronica, E.; Limatola, C.; Di Bonaventura, C.; et al. Cannabis in epilepsy: From clinical practice to basic research focusing on the possible role of cannabidivarin. Epilepsia Open 2016, 1, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Franco, V.; Perucca, E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 2019, 79, 1435–1454. [Google Scholar] [CrossRef] [PubMed]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef]
- Katona, I. Cannabis and Endocannabinoid Signaling in Epilepsy. Handb. Exp. Pharmacol. 2015, 231, 285–316. [Google Scholar]
- Ahrens, J.; Demir, R.; Leuwer, M.; de la Roche, J.; Krampfl, K.; Foadi, N.; Karst, M.; Haeseler, G. The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-Beta glycine receptor function. Pharmacology 2009, 83, 217–222. [Google Scholar] [CrossRef]
- Hill, A.J.; Jones, N.A.; Smith, I.; Hill, C.L.; Williams, C.M.; Stephens, G.J.; Whalley, B.J. Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci. Lett. 2014, 566, 269–274. [Google Scholar] [CrossRef]
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2012, 2, 795–816. [Google Scholar] [CrossRef]
- Minier, F.; Sigel, E. Positioning of the α-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 7769–7774. [Google Scholar] [CrossRef]
- Farrant, M.; Nusser, Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2015, 6, 215–229. [Google Scholar] [CrossRef]
- Mody, I.; Pearce, R.A. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004, 27, 569–575. [Google Scholar] [CrossRef]
- Chuang, S.H.; Reddy, D.S. Genetic and Molecular Regulation of Extrasynaptic GABA-A Receptors in the Brain: Therapeutic Insights for Epilepsy. J. Pharmacol. Exp. Ther. 2018, 364, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Laughton, D.L.; Walding, A.; Wolstenholme, A.J. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol. Immunol. 2006, 43, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.L.; Mody, I. GABA(A)R plasticity during pregnancy: Relevance to postpartum depression. Neuron 2008, 59, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.L.; Stell, B.M.; Rafizadeh, M.; Mody, I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 2005, 8, 797–804. [Google Scholar] [CrossRef]
- Palma, E.; Ruffolo, G.; Cifelli, P.; Roseti, C.; Vliet, E.A.V.; Aronica, E. Modulation of GABAA Receptors in the Treatment of Epilepsy. Curr. Pharm. Des. 2017, 23, 5563–5568. [Google Scholar] [CrossRef]
- Olsen, R.W.; Liang, J. Role of GABAA receptors in alcohol use disorders suggested by Chronic Intermittent Ethanol (CIE) rodent model. Mol. Brain 2017, 10, 45. [Google Scholar] [CrossRef]
- Holley, S.M.; Galvan, L.; Kamdjou, T.; Dong, A.; Levine, M.S.; Cepeda, C. Major Contribution of Somatostatin-Expressing Interneurons and Cannabinoid Receptors to Increased GABA Synaptic Activity in the Striatum of Huntington’s Disease Mice. Front. Synaptic Neurosci. 2019, 11, 14. [Google Scholar] [CrossRef]
- Bambico, F.R.; Li, Z.; Oliveira, C.; McNeill, S.; Diwan, M.; Raymond, R.; Nobrega, J.N. Rostrocaudal subregions of the ventral tegmental area are differentially impacted by chronic stress. Psychopharmacology (Berl) 2019, 236, 1917–1929. [Google Scholar] [CrossRef]
- Mazzuferi, M.; Palma, E.; Martinello, K.; Maiolino, F.; Roseti, C.; Fucile, S.; Fabene, P.F.; Schio, F.; Pellitteri, M.; Sperk, G.; et al. Enhancement of GABA(A)-current run-down in the hippocampus occurs at the first spontaneous seizure in a model of temporal lobe epilepsy. Proc. Natl. Acad. Sci. USA 2010, 107, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.E.; Zhang, G.; Celix, J.; Hsu, F.; Raol, R.H.; Telfeian, A.; Gallagher, P.R.; Coulter, D.A.; Brooks-Kayal, A.R. Heterogeneous GABAA receptor subunit expression in pediatric epilepsy patients. Neurobiol. Dis. 2005, 18, 484–491. [Google Scholar] [CrossRef]
- Ragozzino, D.; Palma, E.; Di Angelantonio, S.; Amici, M.; Mascia, A.; Arcella, A.; Giangaspero, F.; Cantore, G.; Di Gennaro, G.; Manfredi, M.; et al. Rundown of GABA type A receptors is a dysfunction associated with human drug-resistant mesial temporal lobe epilepsy. Proc. Natl. Acad. Sci. USA 2005, 102, 15219–15223. [Google Scholar] [CrossRef]
- Cifelli, P.; Palma, E.; Roseti, C.; Verlengia, G.; Simonato, M. Changes in the sensitivity of GABAA current rundown to drug treatments in a model of temporal lobe epilepsy. Front. Cell. Neurosci. 2013, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Spinelli, G.; Torchia, G.; Martinez-Torres, A.; Ragozzino, D.; Miledi, R.; Eusebi, F. Abnormal GABAA receptors from the human epileptic hippocampal subiculum microtransplanted to Xenopus oocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 2514–2518. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Ragozzino, D.A.; Di Angelantonio, S.; Spinelli, G.; Trettel, F.; Martinez-Torres, A.; Torchia, G.; Arcella, A.; Di Gennaro, G.; Quarato, P.P.; et al. Phosphatase inhibitors remove the run-down of gamma-aminobutyric acid type A receptors in the human epileptic brain. Proc. Natl. Acad. Sci. USA 2004, 101, 10183–10188. [Google Scholar] [CrossRef]
- Roseti, C.; Fucile, S.; Lauro, C.; Martinello, K.; Bertollini, C.; Esposito, V.; Mascia, A.; Catalano, M.; Aronica, E.; Limatola, C.; et al. Fractalkine/CX3CL1 modulates GABAA currents in human temporal lobe epilepsy. Epilepsia 2013, 54, 1834–1844. [Google Scholar] [CrossRef]
- Sigel, E.; Baur, R.; Rácz, I.; Marazzi, J.; Smart, T.G.; Zimmer, A.; Gertsch, J. The major central endocannabinoid directly acts at GABA(A) receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 18150–18155. [Google Scholar] [CrossRef] [PubMed]
- Lile, J.A.; Kelly, T.H.; Hays, L.R. Separate and combined effects of the GABAA positive allosteric modulator diazepam and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend. 2014, 143, 141–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruffolo, G.; Cifelli, P.; Roseti, C.; Thom, M.; van Vliet, E.A.; Limatola, C.; Aronica, E.; Palma, E. A novel GABAergic dysfunction in human Dravet syndrome. Epilepsia 2018, 59, 2106–2117. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Mercier, M.S.; Hill, T.D.; Glyn, S.E.; Jones, N.A.; Yamasaki, Y.; Futamura, T.; Duncan, M.; Stott, C.G.; Stephens, G.J.; et al. Cannabidivarin is anticonvulsant in mouse and rat. Br. J. Pharmacol. 2012, 167, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.; Cascio, M.G.; Romano, B.; Duncan, M.; Pertwee, R.G.; Williams, C.M.; Whalley, B.J.; Hill, A.J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 2013, 170, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Roseti, C.; Maiolino, F.; Fucile, S.; Martinello, K.; Mazzuferi, M.; Aronica, E.; Manfredi, M.; Esposito, V.; Cantore, G.; et al. GABA(A)-current rundown of temporal lobe epilepsy is associated with repetitive activation of GABA(A) “phasic” receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 20944–20948. [Google Scholar] [CrossRef]
- Szabó, G.G.; Lenkey, N.; Holderith, N.; Andrási, T.; Nusser, Z.; Hájos, N. Presynaptic calcium channel inhibition underlies CB₁ cannabinoid receptor-mediated suppression of GABA release. J. Neurosci. 2014, 34, 7958–7963. [Google Scholar] [CrossRef]
- Chiarlone, A.; Bellocchio, L.; Blázquez, C.; Resel, E.; Soria-Gómez, E.; Cannich, A.; Ferrero, J.J.; Sagredo, O.; Benito, C.; Romero, J.; et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc. Natl. Acad. Sci. USA 2014, 111, 8257–8262. [Google Scholar] [CrossRef]
- Maneuf, Y.P.; Crossman, A.R.; Brotchie, J.M. The cannabinoid receptor agonist WIN 55,212-2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson’s disease. Exp. Neurol. 1997, 148, 265–270. [Google Scholar] [CrossRef]
- Sieradzan, K.A.; Fox, S.H.; Hill, M.; Dick, J.P.; Crossman, A.R.; Brotchie, J.M. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: A pilot study. Neurology 2001, 57, 2108–2111. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Sancesario, A.; Morace, R.; Centonze, D.; Iezzi, E. Cannabinoids in Parkinson’s Disease. Cannabis Cannabinoid Res. 2017, 2, 21–29. [Google Scholar] [CrossRef]
- Sanchez-Mejias, E.; Nuñez-Diaz, C.; Sanchez-Varo, R.; Gomez-Arboledas, A.; Garcia-Leon, J.A.; Fernandez-Valenzuela, J.J.; Mejias-Ortega, M.; Trujillo-Estrada, L.; Baglietto-Vargas, D.; Moreno-Gonzalez, I.; et al. Distinct disease-sensitive GABAergic neurons in the perirhinal cortex of Alzheimer’s mice and patients. Brain Pathol. 2019. [Google Scholar] [CrossRef]
- Aso, E.; Andrés-Benito, P.; Ferrer, I. Delineating the Efficacy of a Cannabis-Based Medicine at Advanced Stages of Dementia in a Murine Model. J. Alzheimers Dis. 2016, 54, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimers Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Hobson, E.V.; McDermott, C.J. Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2016, 12, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, L.E.; Barbe, A.; Portalier, P.; Fritschy, J.M.; Bras, H. Differential expression of GABAA and glycine receptors in ALS-resistant vs. ALS-vulnerable motoneurons: Possible implications for selective vulnerability of motoneurons. Eur. J. Neurosci. 2006, 23, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Caioli, S.; Pieri, M.; Antonini, A.; Guglielmotti, A.; Severini, C.; Zona, C. Monocyte Chemoattractant Protein-1 upregulates GABA-induced current: Evidence of modified GABAA subunit composition in cortical neurons from the G93A mouse model of Amyotrophic Lateral Sclerosis. Neuropharmacology 2013, 73, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Bernardi, G.; Centonze, D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp. Neurol. 2010, 224, 92–102. [Google Scholar] [CrossRef]
- Coghlan, S.; Horder, J.; Inkster, B.; Mendez, M.A.; Murphy, D.G.; Nutt, D.J. GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neurosci. Biobehav. Rev. 2012, 36, 2044–2055. [Google Scholar] [CrossRef]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in autism spectrum disorder-a translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Parolaro, D. The Endocannabinoid System and Autism Spectrum Disorders: Insights from Animal Models. Int. J. Mol. Sci. 2017, 18, 1916. [Google Scholar] [CrossRef]
- Aran, A.; Eylon, M.; Harel, M.; Polianski, L.; Nemirovski, A.; Tepper, S.; Schnapp, A.; Cassuto, H.; Wattad, N.; Tam, J. Lower circulating endocannabinoid levels in children with autism spectrum disorder. Mol. Autism 2019, 10, 2. [Google Scholar] [CrossRef]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef] [PubMed]
- Poleg, S.; Golubchik, P.; Offen, D.; Weizman, A. Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 90–96. [Google Scholar] [CrossRef]
- Meldrum, B.S.; Fariello, R.G.; Puil, E.A.; Derouaux, M.; Naquet, R. Delta9-tetrahydrocannabinol and epilepsy in the photosensitive baboon, Papio papio. Epilepsia 1974, 15, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Perez-Reyes, M.; Wingfield, M. Letter: Cannabidiol and electroencephalographic epileptic activity. JAMA 1974, 230, 1635. [Google Scholar] [CrossRef] [PubMed]
- Ten Ham, M.; Loskota, W.J.; Lomax, P. Acute and chronic effects of beta9-tetrahydrocannabinol on seizures in the gerbil. Eur. J. Pharmacol. 1975, 31, 148–152. [Google Scholar] [PubMed]
- Consroe, P.F.; Wood, G.C.; Buchsbaum, H. Anticonvulsant nature of marihuana smoking. JAMA 1975, 234, 306–307. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves First drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe forms of Epilepsy. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms (accessed on 16 December 2019).
- GW Pharmaceuticals Receives European Commission Approval for Epidyolex for Seizures. Available online: https://www.thepharmaletter.com/article/gw-pharmaceuticals-receives-european-commission-approval-for-epidyolex-for-seizures (accessed on 16 December 2019).
- Elliott, J.; DeJean, D.; Clifford, T.; Coyle, D.; Potter, B.K.; Skidmore, B.; Alexander, C.; Repetski, A.E.; Shukla, V.; McCoy, B.; et al. Cannabis-based products for pediatric epilepsy: A systematic review. Epilepsia 2019, 60, 6–19. [Google Scholar] [CrossRef]
- Anderson, L.L.; Absalom, N.L.; Abelev, S.V.; Low, I.K.; Doohan, P.T.; Martin, L.J.; Chebib, M.; McGregor, I.S.; Arnold, J.C. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia 2019, 60, 2224–2234. [Google Scholar] [CrossRef]
- De Carvalho Reis, R.; Almeida, K.J.; da Silva Lopes, L.; de Melo Mendes, C.M.; Bor-Seng-Shu, E. Efficacy and adverse event profile of cannabidiol and medicinal cannabis for treatment-resistant epilepsy: Systematic review and meta-analysis. Epilepsy Behav. 2019, 102, 106635. [Google Scholar] [CrossRef]
- Gu, B.; Zhu, M.; Glass, M.R.; Rougié, M.; Nikolova, V.D.; Moy, S.S.; Carney, P.R.; Philpot, B.D. Cannabidiol attenuates seizures and EEG abnormalities in Angelman syndrome model mice. J. Clin. Investig. 2019, 129, 5462–5467. [Google Scholar] [CrossRef]
- Braat, S.; Kooy, R.F. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron 2015, 86, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, J.M. Blurred boundaries: The therapeutics and politics of medical marijuana. Mayo Clin. Proc. 2012, 87, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Burns, J.; Liu, C.K.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Cannabis Addiction and the Brain: A Review. J. Neuroimmune Pharmacol. 2018, 13, 438–452. [Google Scholar] [CrossRef] [PubMed]
| Target | pCBs Tested | Concentration | Experimental Model | References |
|---|---|---|---|---|
| Vanilloid receptor type 1 | CBD | 10 μM | human hembryonic kidney cells (HEK) | Bisogno et al, 2001 [23] |
| Opioid receptors | THC and CBD | 30 μM for both compounds | rat cerebral cortex membrane homogenates | Kathmann et al, 2006 [24] |
| G protein-coupled receptor GPR55 | THC and CBD | from 500 nM to 2.5 μM | human embryonic kidney (HEK293s) cells | Ryberg et al, 2007 [25] |
| voltage-gated calcium channels | THC and CBD | from 1 μM to 30 μM | human embryonic kidney (HEK293s) cells | Ross et al, 2007 [26] |
| Glycine receptors | CBD | from 1 μM to 100 μM | in vivo mice model of chronic pain | Xiong et al, 2012 [27] |
| Serotonin receptors | (1) CBD (2) CBD (3) CBD | (1) 16 μM (2) from 1 to 20 mg/Kg (3) from 30 to 60 nmol | (1) Chinese Hamster Ovary (CHO) cells (2) in vivo rat model of restrain (3) in vivo rat model of pain | (1) Russo et al, 2005 [28] (2) Resstel et al, 2007 [29] (3) Soares et al, 2010 [30] |
| Acetylcholine receptors | CBD | 10 μM | Xenopus oocytes | Mahgoub et al, 2010 [31] |
| Voltage gated sodium channels | THC and CBD | from 1 to 10 μM | human embryonic kidney cells (HEK) and human iPSC neurons | Ghovanloo et al, 2018 [32] |
| GABAA receptors | (1) THC and CBD (2) CBD (3) CBDV | (1) from 0.1 to 100 μM (2) 5 μM (3) 1 μM | (1) human cDNA in Xenopus oocytes (2) surgical human Dravet cortical tissue in Xenopus oocytes (3) surgical cortical human epileptic tissue | (1) Bakas et al, 2017 [33] (2) Ruffolo et al, 2018 [34] (3) Morano et al, 2016 [35] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifelli, P.; Ruffolo, G.; De Felice, E.; Alfano, V.; van Vliet, E.A.; Aronica, E.; Palma, E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? Int. J. Mol. Sci. 2020, 21, 723. https://doi.org/10.3390/ijms21030723
Cifelli P, Ruffolo G, De Felice E, Alfano V, van Vliet EA, Aronica E, Palma E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? International Journal of Molecular Sciences. 2020; 21(3):723. https://doi.org/10.3390/ijms21030723
Chicago/Turabian StyleCifelli, Pierangelo, Gabriele Ruffolo, Eleonora De Felice, Veronica Alfano, Erwin Alexander van Vliet, Eleonora Aronica, and Eleonora Palma. 2020. "Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission?" International Journal of Molecular Sciences 21, no. 3: 723. https://doi.org/10.3390/ijms21030723
APA StyleCifelli, P., Ruffolo, G., De Felice, E., Alfano, V., van Vliet, E. A., Aronica, E., & Palma, E. (2020). Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? International Journal of Molecular Sciences, 21(3), 723. https://doi.org/10.3390/ijms21030723





