Extremely Low Leakage Expression Systems Using Dual Transcriptional-Translational Control for Toxic Protein Production
Abstract
1. Introduction
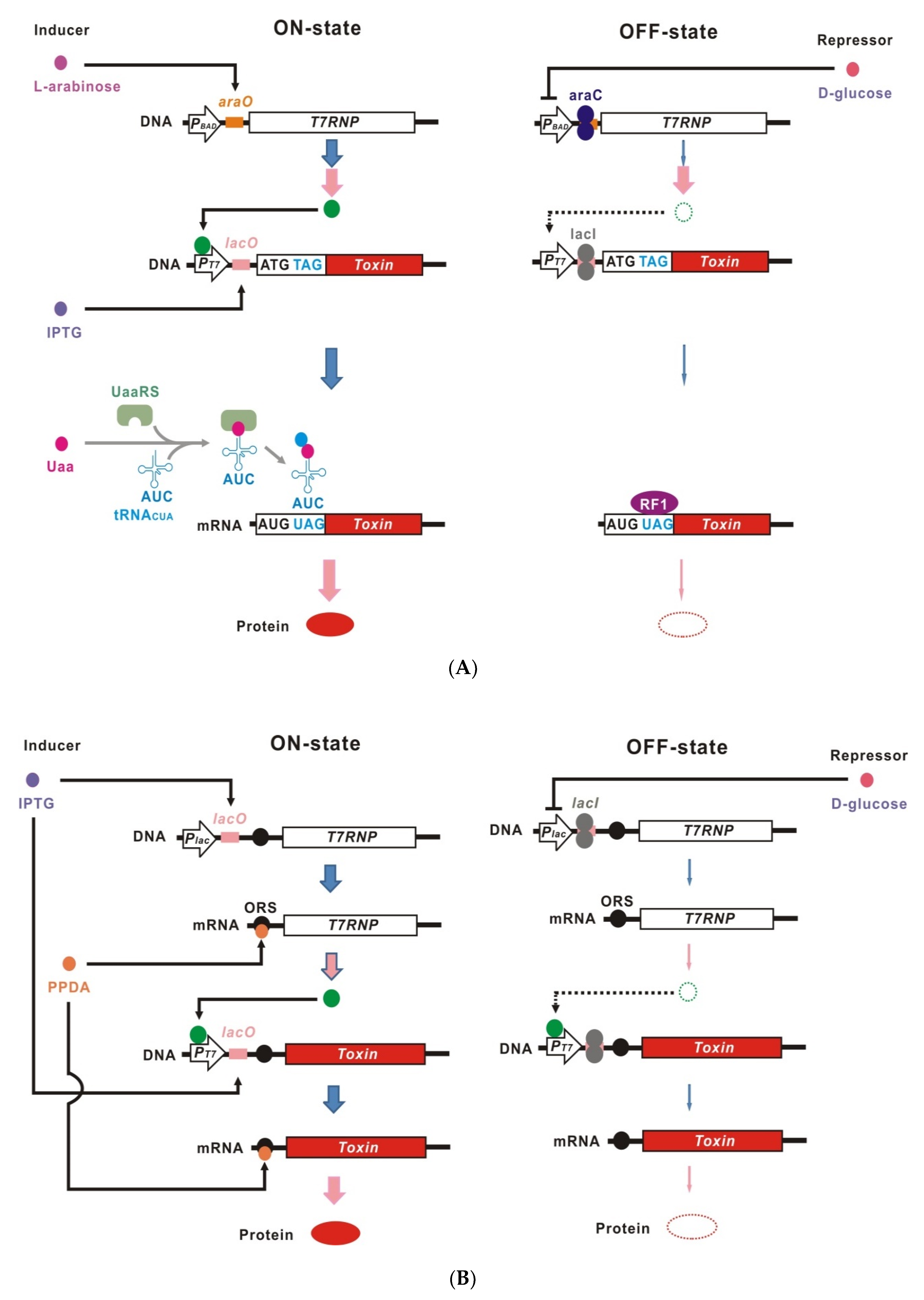
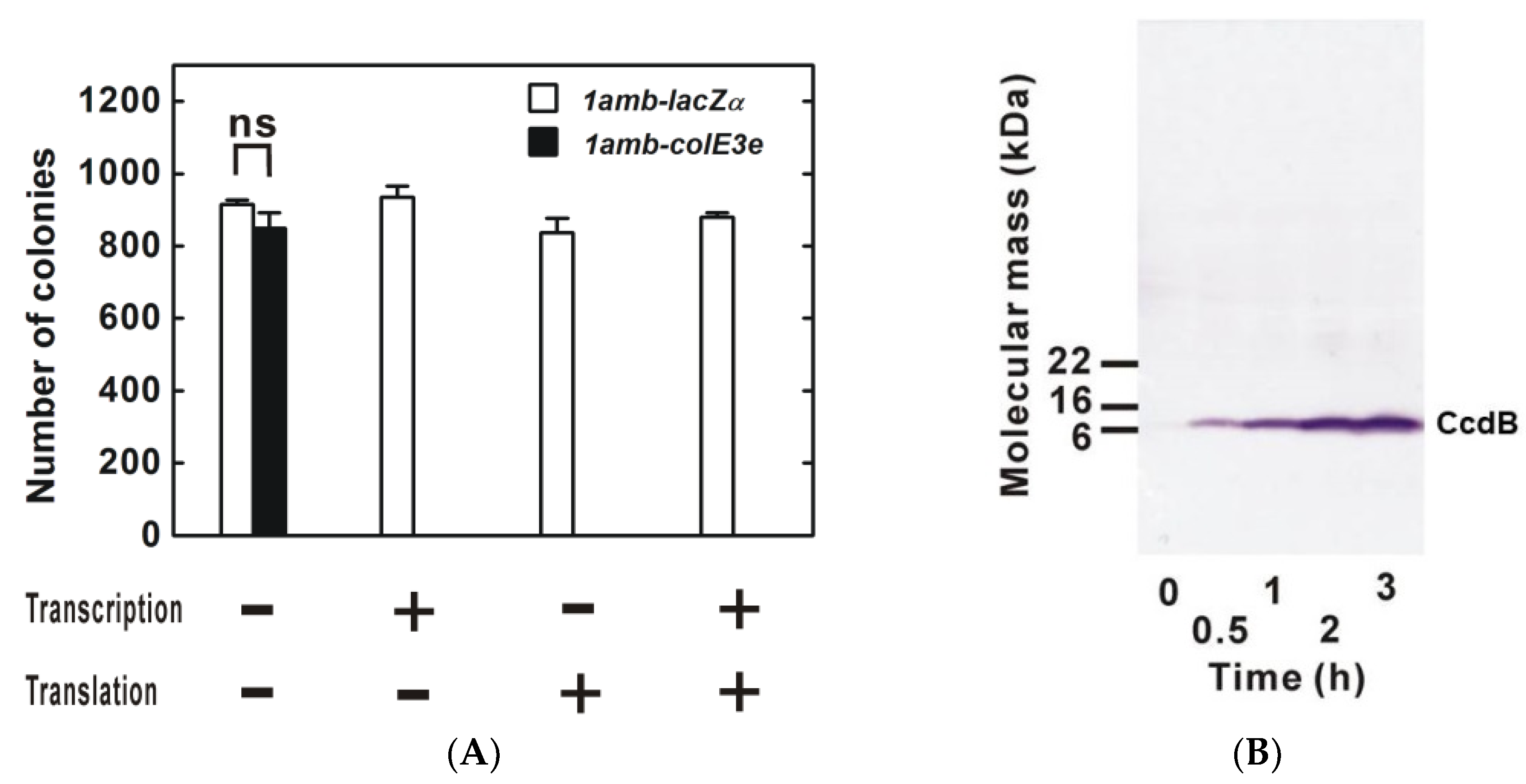
2. Advantages of Dual Transcriptional-Translational Control
3. Site-Specific Unnatural Amino Acid Incorporation
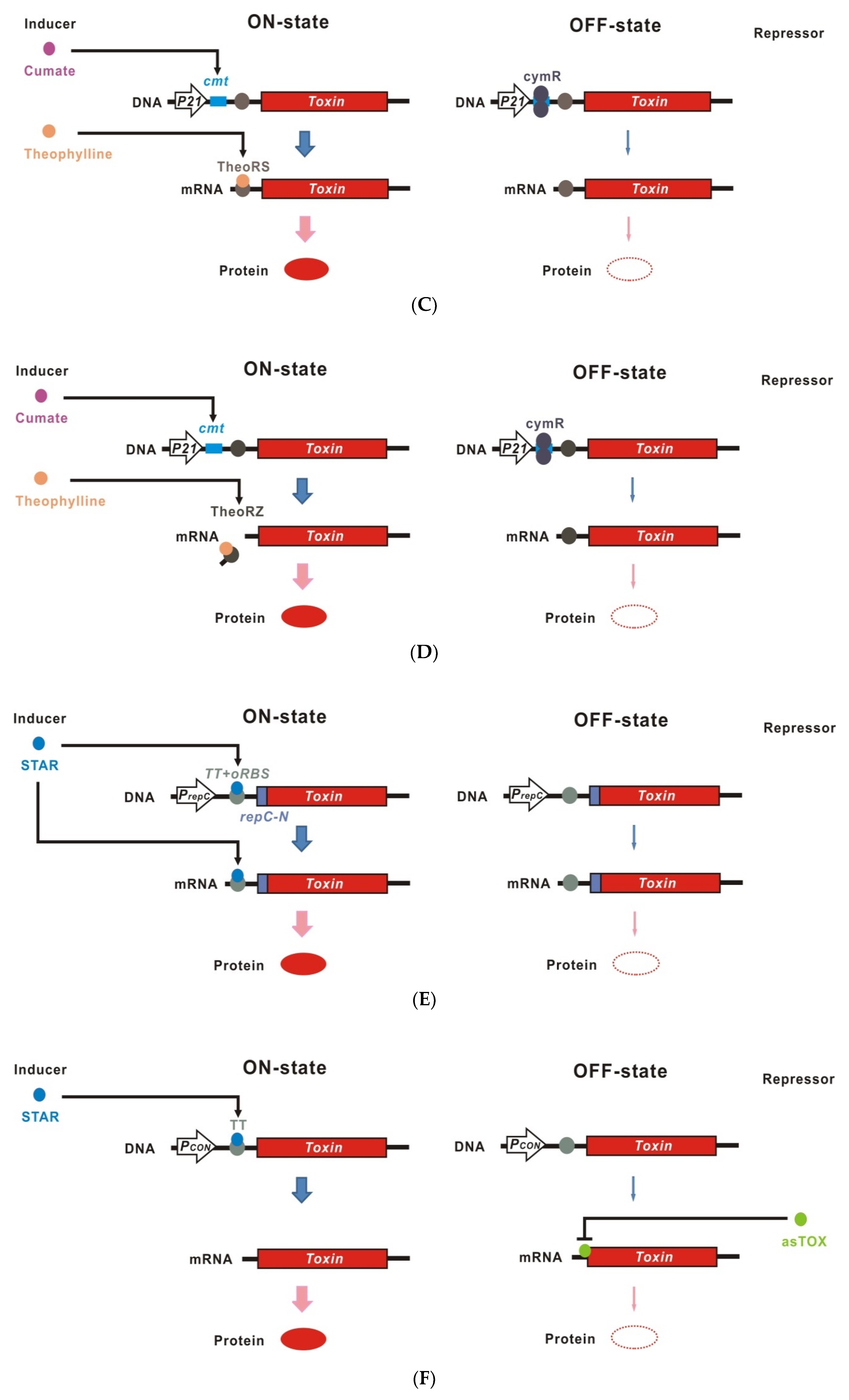
4. Riboswitches
5. Ribozymes
6. Antisense RNA
7. Conclusions
Funding
Conflicts of Interest
References
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Dermain, A.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Bacterial expression systems for recombinant protein production. E. coli and beyond. Biotechnol. Adv. 2012, 30, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Miralles, N.; Villaverde, A. Bacterial cell factories for recombinant protein production; expanding the catalogue. Microb. Cell Fact. 2013, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Saïda, F.; Uzan, M.; Odaert, B.; Bontems, F. Expression of highly toxic genes in E. coli: Special strategies and genetic tools. Curr. Prot. Pept. Sci. 2006, 7, 47–56. [Google Scholar] [CrossRef]
- Kitagawa, M.; Ara, T.; Arifuzzaman, M.; Ioka-Nakamichi, T.; Inamoto, E.; Toyonaga, H.; Mori, H. Complete set of ORF clones of Escherichia coli ASKA library (A Complete S et of E. coli K -12 ORF A rchive): Unique Resources for Biological Research. DNA Res. 2005, 12, 291–299. [Google Scholar] [CrossRef]
- Giacalone, M.J.; Gentitle, A.M.; Lovitt, B.T.; Berkly, N.L.; Gunderson, C.W.; Surber, M.W. Toxic protein expression in Escherichia coli using a rhamnose-based tightly regulated and tunable promoter system. BioTechniques 2006, 40, 355–364. [Google Scholar] [CrossRef]
- Ellefson, J.W.; Meyer, A.J.; Hughes, R.A.; Cannon, J.R.; Brodbelt, J.S.; Ellington, A.D. Directed evolution of genetic parts and circuits by compartmentalized partnered replication. Nat. Biotechnol. 2014, 32, 97–101. [Google Scholar] [CrossRef]
- Jacob, F.; Monod, J. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 1961, 3, 318–356. [Google Scholar] [CrossRef]
- Du, M.; Kodner, S.; Bai, L. Enhancement of LacI binding in vivo. Nucl. Acids Res. 2019, 47, 9609–9618. [Google Scholar] [CrossRef]
- Lanzer, M.; Bujard, H. Promoters largely determine the efficiency of repressor action. Proc. Natl. Acad. Sci. USA 1988, 85, 8973–8977. [Google Scholar] [CrossRef]
- Guzman, L.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Ohta, T. Colicin E3 and its immunity genes. J. Mol. Biol. 1985, 182, 217–227. [Google Scholar] [CrossRef]
- Lazzaroni, J.-C.; Dubuisson, J.-F.; Vianney, A. The Tol proteins of Escherichia coli and their involvement in the translocation of group A colicins. Biochimie 2002, 84, 391–397. [Google Scholar] [CrossRef]
- Anthony, L.C.; Suzuki, H.; Filutowicz, M. Tightly regulated vectors for the cloning and expression of toxic genes. J. Microbiol. Meth. 2004, 58, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Schultz, P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 2010, 79, 413–444. [Google Scholar] [CrossRef]
- Xiao, H.; Schultz, P.G. At the interface of chemical and biological synthesis: An expanded genetic code. CSH Perspect. Biol. 2016, 8, a023945. [Google Scholar] [CrossRef]
- Chin, J.W. Expanding and reprogramming the genetic code. Nature 2017, 550, 53–60. [Google Scholar] [CrossRef]
- Mukai, T.; Lajoie, M.J.; Englert, M.; Söll, D. Rewriting the genetic code. Annu. Rev. Microbiol. 2017, 71, 557–577. [Google Scholar] [CrossRef]
- Terasaka, N.; Iwane, Y.; Geiermann, A.S.; Goto, Y.; Suga, H. Recent developments of engineered translational machineries for the incorporation of non-canonical amino acids into polypeptides. Int. J. Mol. Sci. 2015, 16, 6513–6531. [Google Scholar] [CrossRef]
- Minaba, M.; Kato, Y. High-yield, zero-leakage expression system with a translational switch using site-specific unnatural amino acid incorporation. Appl. Environ. Microbiol. 2014, 80, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. Tunable translational control using site-specific unnatural amino acid incorporation in Escherichia coli. PeerJ 2015, 3, e904. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. Tight translational control using site-specific unnatural amino acid incorporation with positive feedback gene circuits. ACS Synth. Biol. 2018, 7, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. Translational Control using an Expanded Genetic Code. Int. J. Mol. Sci. 2019, 20, 887. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Murayama, K.; Oki, K.; Iraha, F.; KatoMurayama, M.; Takahashi, M.; Ohtake, K.; Kobayashi, T.; Kuramitsu, S.; Shirouzu, M.; et al. Genetic encoding of 3-iodo-L-tyrosine in Escherichia coli for single-wavelength anomalous dispersion phasing in protein crystallography. Structure 2009, 17, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Ishii, R.; Fukunaga, R.; Kobayashi, T.; Sakamoto, K.; Yokoyama, S. Multistep engineering of pyrrolysyl-tRNA synthetase to genetically encode Nε-(o-Azidobenzyloxycarbonyl)lysine for site-specific protein modification. Chem. Biol. 2008, 15, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Schultz, P.G. A strategy for creating organisms dependent on noncanonical amino acids. Angew. Chem. Int. Ed. 2017, 56, 9170–9173. [Google Scholar] [CrossRef]
- Volkwein, W.; Maier, C.; Krafczyk, R.; Jung, K.; Lassak, J. A versatile toolbox for the control of protein levels using Nɛ-acetyl-L-lysine dependent amber suppression. ACS Synth. Biol. 2017, 6, 1892–1902. [Google Scholar] [CrossRef]
- Pavlova, N.; Kaloudas, D.; Penchovsky, R. Riboswitch distribution, structure, and function in bacteria. Gene 2019, 708, 38–48. [Google Scholar] [CrossRef]
- McCown, P.J.; Corbino, K.A.; Stav, S.; Sherlock, M.E.; Breaker, R.R. Riboswitch diversity and distribution. RNA 2017, 23, 995–1011. [Google Scholar] [CrossRef]
- Hallberg, Z.F.; Su, Y.; Kitto, R.Z.; Hammond, M.C. Engineering and in vivo applications of riboswitches. Annu. Rev. Biochem. 2017, 86, 515–539. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Breaker, R.R. Adenine riboswitches and gene activation by disruption of a transcription terminator. Nat. Struct. Mol. Biol. 2004, 11, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Dixon, N.; Duncan, J.N.; Geerlings, T.; Dunstan, M.S.; McCarthy, J.E.; Leys, D.; Micklefield, J. Reengineering orthogonally selective riboswitches. Proc. Natl. Acad. Sci. USA 2010, 107, 2830–2835. [Google Scholar] [CrossRef] [PubMed]
- Dixon, N.; Robinson, C.J.; Geerlings, T.; Duncan, J.N.; Drummond, S.P.; Micklefeld, J. Orthogonal riboswitches for tuneable coexpression in bacteria. Angew. Chem. Int. Ed. Engl. 2012, 51, 3620–3624. [Google Scholar] [CrossRef]
- Robinson, C.J.; Vincent, H.A.; Wu, M.-C.; Lowe, P.T.; Dunstan, M.S.; Leys, D.; Micklefield, J. Modular riboswitch toolsets for synthetic genetic control in diverse bacterial species. J. Am. Chem. Soc. 2014, 136, 10615–10624. [Google Scholar] [CrossRef]
- Morra, R.; Shankar, J.; Robinson, C.J.; Halliwell, S.; Butler, L.; Upton, M.; Hay, S.; Micklefield, J.; Dixon, N. Dual transcriptional-translational cascade permits cellular level tuneable expression control. Nucl. Acids Res. 2016, 44, e21. [Google Scholar] [CrossRef]
- Horga, L.G.; Halliwell, S.; Castiñeiras, T.S.; Wyre, C.; Matos, C.F.R.O.; Yovcheva, D.S.; Kent, R.; Morra, R.; Williams, S.G.; Smith, D.C.; et al. Tuning recombinant protein expression to match secretion capacity. Microb. Cell Fact. 2018, 17, 199. [Google Scholar] [CrossRef]
- Sandiford, S.; Upton, M. Identifcation, characterization, and recombinant expression of epidermicin NI01, a novel unmodified bacteriocin produced by Staphylococcus epidermidis that displays potent activity against Staphylococci. Antimicrob. Agents Chemother. 2012, 56, 1539–1547. [Google Scholar] [CrossRef]
- Gay, P.; Le Coq, D.; Steinmetz, M.; Berkelman, T.; Kado, C.I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 1985, 164, 918–921. [Google Scholar] [CrossRef]
- Sharan, S.K.; Thomason, L.C.; Kuznetsov, S.G.; Court, D.L. Recombineering: A homologous recombination-based method of genetic engineering. Nat. Protoc. 2009, 4, 206–223. [Google Scholar] [CrossRef]
- Horbal, L.; Luzhetskyy, A. Dual control system—A novel scaffolding architecture of an inducible regulatory device for the precise regulation of gene expression. Metab. Eng. 2016, 37, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Horbal, L.; Fedorenko, V.; Luzhetskyy, A. 2014. Novel and tightly regulated resorcinol and cumate-inducible expression systems for Streptomyces and other actinobacteria. Appl. Microbiol. Biotechnol. 2014, 98, 8641–8655. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.M.; Vockenhuber, M.P.; Suess, B. Synthetic riboswitches for the conditional control of gene expression in Streptomyces coelicolor. Microbiology 2013, 159, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- McCann, P.A.; Pogell, B.M. Pamamycin: A new antibiotic and stimulator of aerial mycelia formation. J. Antibiot. 1979, 32, 673–678. [Google Scholar] [CrossRef]
- Rebets, Y.; Brötz, E.; Manderscheid, N.; Tokovenko, B.; Myronovskyi, M.; Metz, P.; Petzke, L.; Luzhetskyy, A. Insights into the pamamycin biosynthesis. Angew. Chem. Int. Ed. Engl. 2015, 54, 2280–2284. [Google Scholar] [CrossRef]
- Park, S.V.; Yang, J.S.; Jo, H.; Kang, B.; Oh, S.S.; Jung, G.Y. Catalytic RNA, ribozyme, and its applications in synthetic biology. Biotechnol. Adv. 2019, 37, 107452. [Google Scholar] [CrossRef]
- De la Peña, M.; García-Robles, I.; Cervera, A. The Hammerhead Ribozyme: A Long History for a Short RNA. Molecules 2017, 22, 78. [Google Scholar] [CrossRef]
- Wieland, M.; Hartig, J.S. Improved aptazyme design and in vivo screening enable riboswitching in bacteria. Angew. Chem. Int. Ed. Engl. 2008, 47, 2604–2607. [Google Scholar] [CrossRef]
- Lucks, J.B.; Qi, L.; Mutalik, V.K.; Wang, D.; Arkin, A.P. Versatile RNA-sensing transcriptional regulators for engineering genetic networks. Proc. Natl. Acad. Sci. USA 2011, 108, 8617–8622. [Google Scholar]
- Chappell, J.; Takahashi, M.K.; Lucks, J.B. Creating small transcription activating RNAs. Nat. Chem. Biol. 2015, 11, 214–220. [Google Scholar] [CrossRef]
- Green, A.A.; Silver, P.A.; Collins, J.J.; Yin, P. Toehold switches: De-novo-designed regulators of gene expression. Cell 2014, 159, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.; Yoo, S.M.; Yang, D.; Lee, S.Y. Broad-spectrum gene repression using scaffold engineering of synthetic sRNAs. ACS Synth. Biol. 2019, 8, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Thomason, M.K.; Storz, G. Bacterial antisense RNAs: How many are there, and what are they doing. Annu. Rev. Genet. 2010, 44, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, A.M.; Lucks, J.B. Achieving large dynamic range control of gene expression with a compact RNA transcription–translation regulator. Nucleic Acids Res. 2017, 45, 5614–5624. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P.; Iordanescu, S.; Projan, S.J.; Kornblum, J.; Edelman, I. pT181 plasmid replication is regulated by a countertranscript-driven transcriptional attenuator. Cell 1989, 59, 395–404. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.J.; Moon, T.S. Multilevel regulation of bacterial gene expression with the combined STAR and antisense RNA system. ACS Synth. Biol. 2018, 7, 853–865. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Zhang, F.; del Cardayre, S.B.; Keasling, J.D. Microbial engineering for the production of advanced biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef]
- Ho, J.M.L.; Bennett, M.R. Improved memory devices for synthetic cells. Science 2018, 360, 150–151. [Google Scholar] [CrossRef]
- Carothers, J.M.; Goler, J.A.; Juminaga, D.; Keasling, J.D. Model-driven engineering of RNA devices to quantitatively program gene expression. Science 2011, 334, 1716–1719. [Google Scholar]
- O’Connor, C.D.; Timmis, K.N. Highly repressible expression system for cloning genes that specify potentially toxic proteins. J. Bacteriol. 1987, 169, 4457–4462. [Google Scholar] [CrossRef]
- Studier, F.W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J. Mol. Biol. 1991, 219, 37–44. [Google Scholar] [CrossRef]
- Unnithan, S.; Green, L.; Morrissey, L.; Binkley, J.; Singer, B.; Karam, J.; Gold, L. Binding of the bacteriophage T4 regA protein to mRNA targets: An initiator AUG is required. Nucleic Acids Res. 1990, 18, 7083–7092. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, Y. Extremely Low Leakage Expression Systems Using Dual Transcriptional-Translational Control for Toxic Protein Production. Int. J. Mol. Sci. 2020, 21, 705. https://doi.org/10.3390/ijms21030705
Kato Y. Extremely Low Leakage Expression Systems Using Dual Transcriptional-Translational Control for Toxic Protein Production. International Journal of Molecular Sciences. 2020; 21(3):705. https://doi.org/10.3390/ijms21030705
Chicago/Turabian StyleKato, Yusuke. 2020. "Extremely Low Leakage Expression Systems Using Dual Transcriptional-Translational Control for Toxic Protein Production" International Journal of Molecular Sciences 21, no. 3: 705. https://doi.org/10.3390/ijms21030705
APA StyleKato, Y. (2020). Extremely Low Leakage Expression Systems Using Dual Transcriptional-Translational Control for Toxic Protein Production. International Journal of Molecular Sciences, 21(3), 705. https://doi.org/10.3390/ijms21030705





