Production of Extracellular Matrix Proteins in the Cytoplasm of E. coli: Making Giants in Tiny Factories
Abstract
1. Introduction
2. Results and Discussion
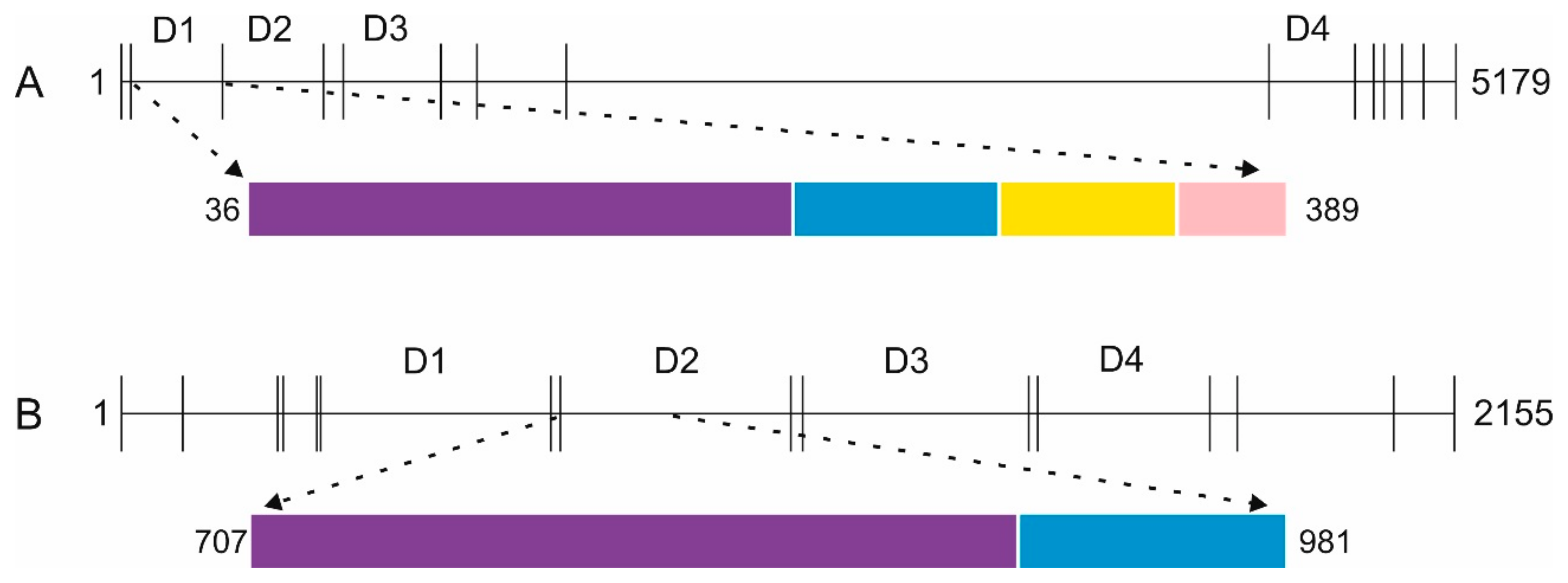
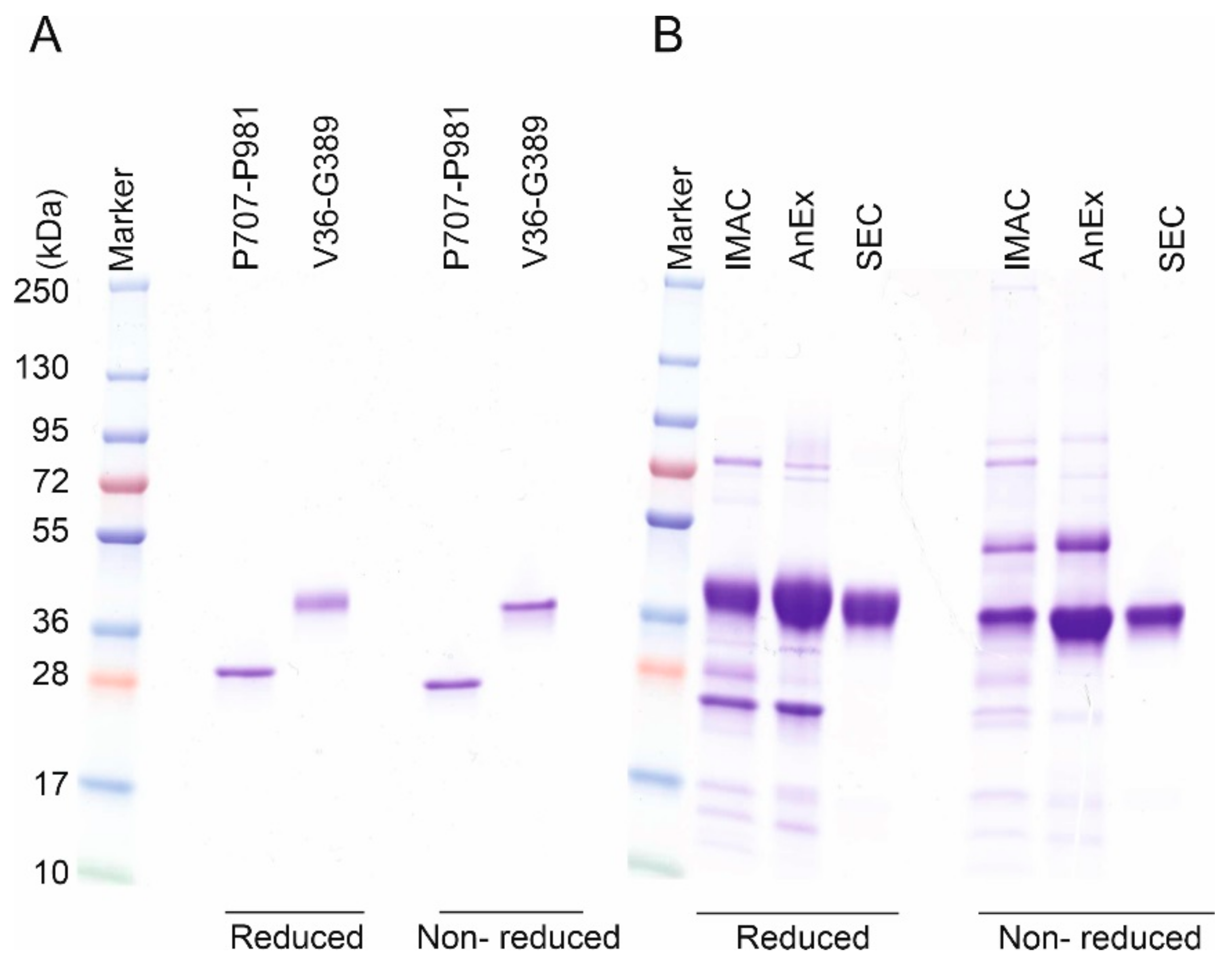
2.1. Disulfide-rich ECM Proteins as Model Proteins
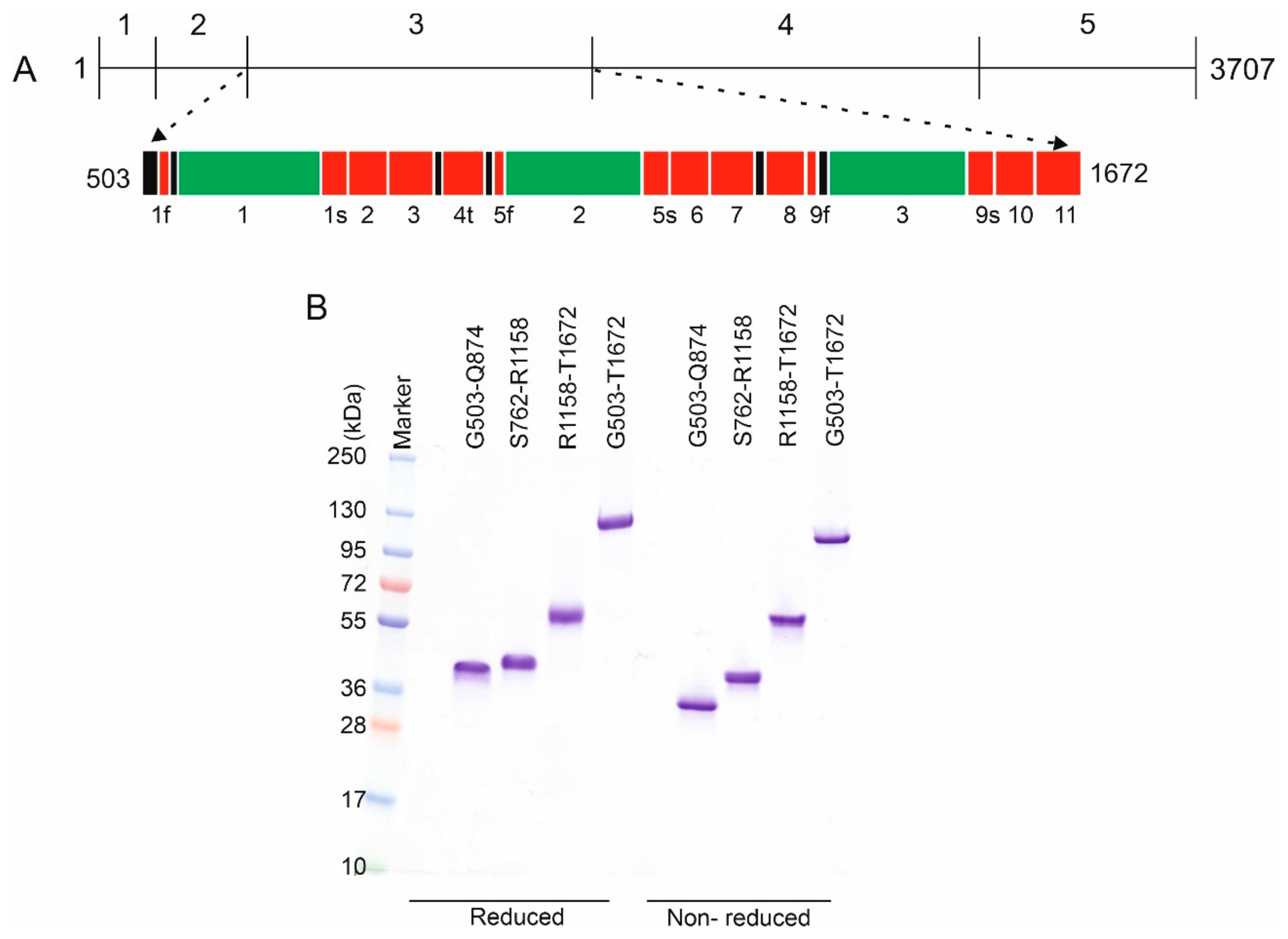
2.2. Perlecan as a Model Protein
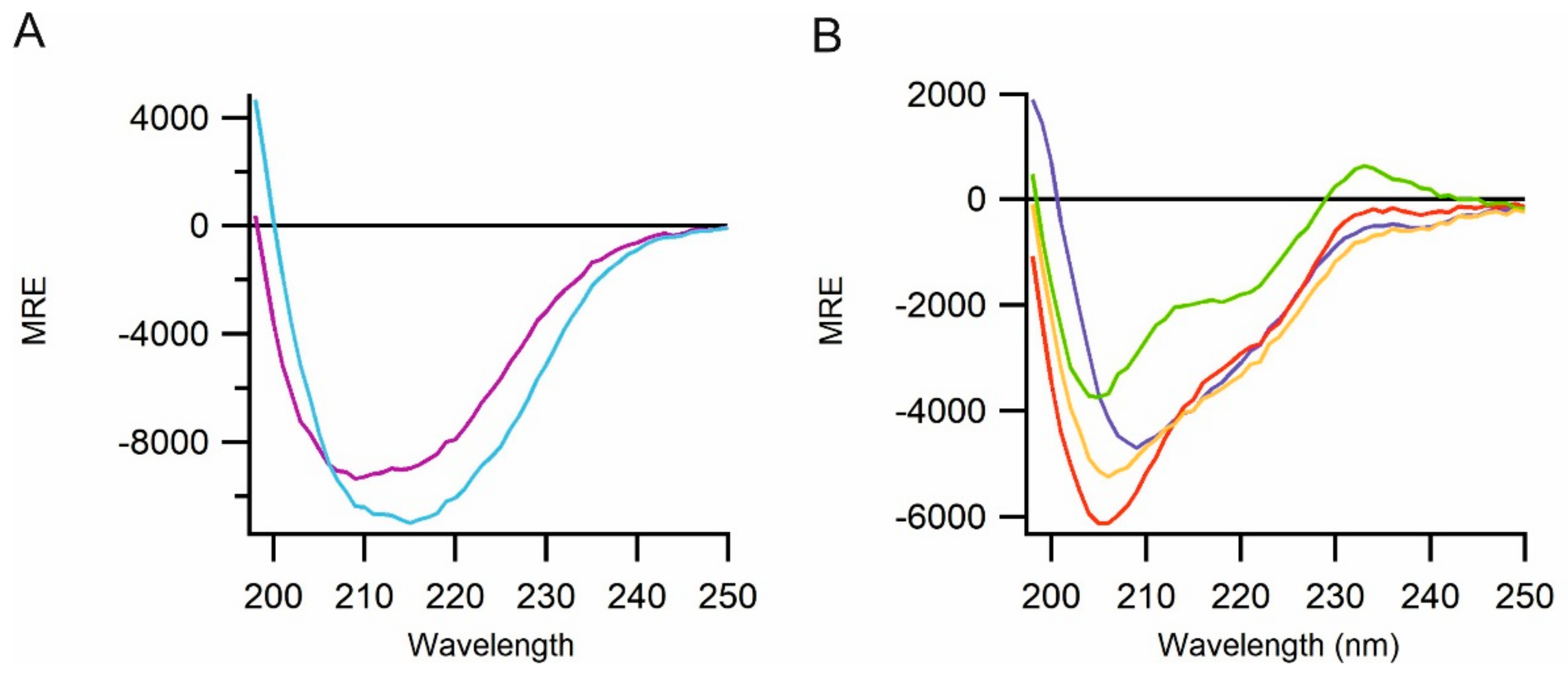
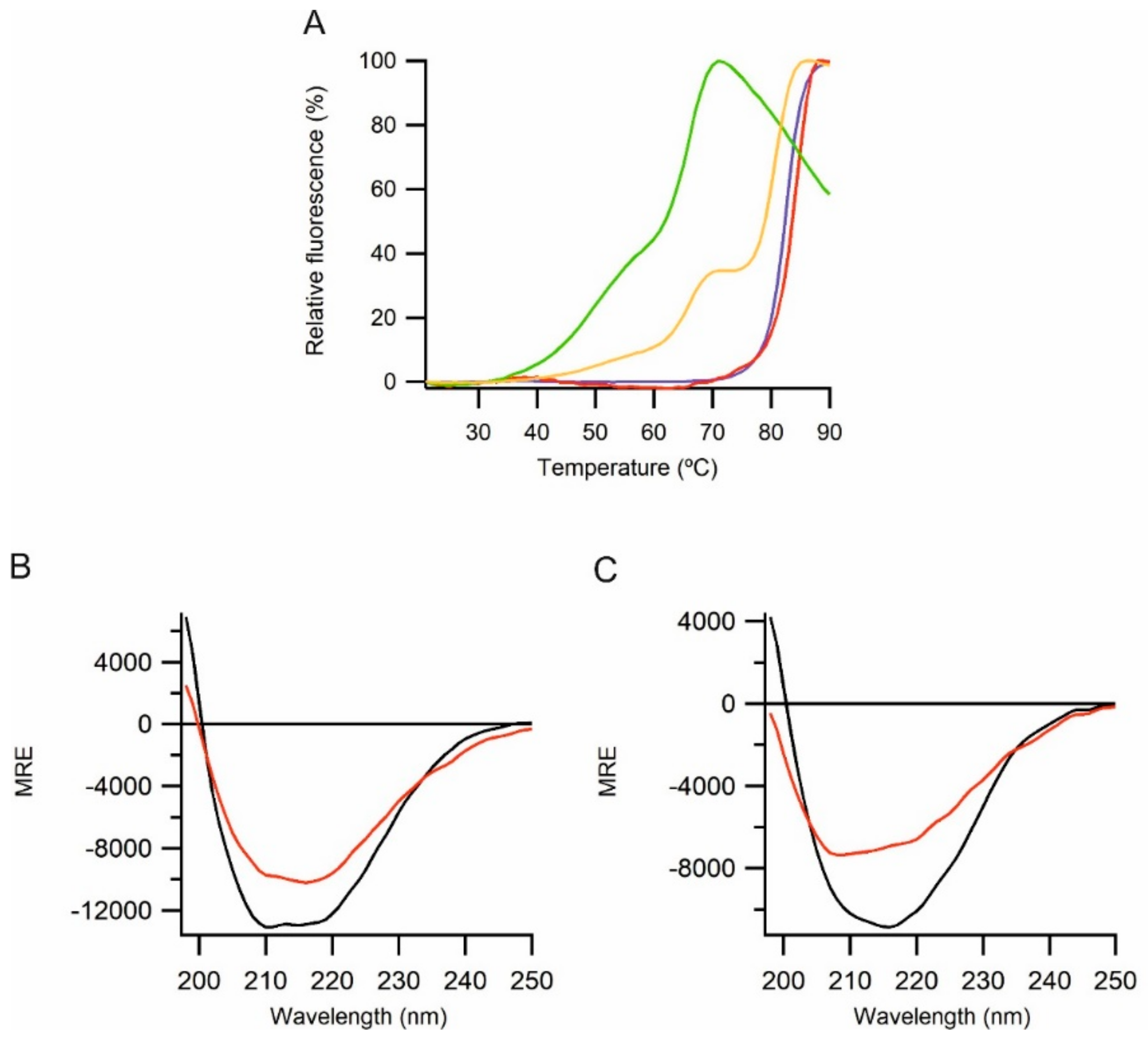
2.3. Biophysical Characterization of CyDisCo Produced ECM Proteins
3. Materials and Methods
3.1. Bioinformatics
3.2. Cloning
3.3. Expression
3.4. Protein Purification
3.5. Biophysical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riemer, J.; Bulleid, N.; Herrmann, J.M. Disulfide Formation in the ER and Mitochondria: Two Solutions to a Common Process. Science 2009, 324, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-F.; Bardwell, J.C.A. Oxidative protein folding in bacteria. Mol. Microbiol. 2002, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bulleid, N.J. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2012, 4, a013219. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M.; Köhl, R. Catch me if you can! Oxidative protein trapping in the intermembrane space of mitochondria. J. Cell Biol. 2007, 176, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Gleiter, S.; Bardwell, J.C.A. Disulfide bond isomerization in prokaryotes. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 530–534. [Google Scholar] [CrossRef]
- Thorpe, C.; Hoober, K.L.; Raje, S.; Glynn, N.M.; Burnside, J.; Turi, G.K.; Coppock, D.L. Sulfhydryl oxidases: Emerging catalysts of protein disulfide bond formation in eukaryotes. Arch. Biochem. Biophys. 2002, 405, 1–12. [Google Scholar] [CrossRef]
- Mamathambika, B.S.; Bardwell, J.C. Disulfide-Linked Protein Folding Pathways. Annu. Rev. Cell Dev. Biol. 2008, 24, 211–235. [Google Scholar] [CrossRef]
- Inaba, K. Disulfide bond formation system in Escherichia coli. J. Biochem. 2009, 146, 591–597. [Google Scholar] [CrossRef]
- Hatahet, F.; Ruddock, L.W. Protein disulfide isomerase: A critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 2009, 11, 2807–2850. [Google Scholar] [CrossRef]
- Sørensen, H.P.; Mortensen, K.K. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J. Biotechnol. 2005, 115, 113–128. [Google Scholar] [CrossRef]
- Francis, D.M.; Page, R. Strategies to Optimize Protein Expression in E. coli. Curr. Protoc. Protein Sci. 2010, 61, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Prinz, W.A.; Åslund, F.; Holmgren, A.; Beckwith, J. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J. Biol. Chem. 1997, 272, 15661–15667. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.L.; Beveridge, T.J.; Nanninga, N. Periplasmic space and the concept of the periplasm. Trends Biochem. Sci. 1991, 16, 328–329. [Google Scholar] [CrossRef]
- Schlegel, S.; Rujas, E.; Ytterberg, A.J.; Zubarev, R.A.; Luirink, J.; de Grier, J.W. Optimizing heterologous protein production in the periplasm of E. coli by regulating gene expression levels. Microb. Cell Fact. 2013, 12, 24. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Hatahet, F.; Salo, K.E.; Enlund, E.; Zhang, C.; Ruddock, L.W. Pre-expression of a sulfhydryl oxidase significantly increases the yields of eukaryotic disulfide bond containing proteins expressed in the cytoplasm of E. coli. Microb. Cell Fact. 2011, 10, 1. [Google Scholar] [CrossRef]
- Hatahet, F.; Nguyen, V.D.; Salo, K.E.H.; Ruddock, L.W. Disruption of reducing pathways is not essential for efficient disulfide bond formation in the cytoplasm of E. coli. Microb. Cell Fact. 2010, 9, 67. [Google Scholar] [CrossRef]
- Gąciarz, A.; Khatri, N.K.; Lourdes Velez-Suberbie, M.; Saaranen, M.J.; Uchida, Y.; Keshavarz-Moore, E.; Ruddock, L.W. Efficient soluble expression of disulfide bonded proteins in the cytoplasm of Escherichia coli in fed-batch fermentations on chemically defined minimal media. Microb. Cell Fact. 2017, 16, 108. [Google Scholar] [CrossRef]
- Gaciarz, A.; Veijola, J.; Uchida, Y.; Saaranen, M.J.; Wang, C.; Hörkkö, S.; Ruddock, L.W. Systematic screening of soluble expression of antibody fragments in the cytoplasm of E. coli. Microb. Cell Fact. 2016, 15, 22. [Google Scholar] [CrossRef]
- Saaranen, M.J.; Ruddock, L.W. Applications of catalyzed cytoplasmic disulfide bond formation. Biochem. Soc. Trans. 2019, 47, 1223–1231. [Google Scholar] [CrossRef]
- Swami, A.; Kaur, V. Von Willebrand Disease: A Concise Review and Update for the Practicing Physician. Clin. Appl. Thromb. 2017, 23, 900–910. [Google Scholar] [CrossRef]
- Funari, V.A.; Krakow, D.; Nevarez, L.; Chen, Z.; Funari, T.L.; Vatanavicharn, N.; Wilcox, W.R.; Rimoin, D.L.; Nelson, S.F.; Cohn, D.H. BMPER mutation in diaphanospondylodysostosis identified by ancestral autozygosity mapping and targeted high-throughput sequencing. Am. J. Hum. Genet. 2010, 87, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Balciuniene, J.; Dahl, N.; Jalonen, P.; Verhoeven, K.; Van Camp, G.; Borg, E.; Pettersson, U.; Jazin, E.E. Alpha-tectorin involvement in hearing disabilities: One gene – two phenotypes. Hum. Genet. 1999, 105, 211–216. [Google Scholar] [PubMed]
- Dong, X.; Leksa, N.C.; Chhabra, E.S.; Arndt, J.W.; Lu, Q.; Knockenhauer, K.E.; Peters, R.T.; Springer, T.A. The von Willebrand factor D’D3 assembly and structural principles for factor VIII binding and concatemer biogenesis. Blood 2019, 133, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodriguez-Piňeiro, A.M.; Nyström, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc 2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Johansson, M.E.; Larsson, J.M.; Hansson, G.C. The two mucus layers of the colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef]
- Zoeller, J.J.; McQuillan, A.; Whitelock, J.; Ho, S.Y.; Iozzo, R.V. A central function for perlecan in skeletal muscle and cardiovascular development. J. Cell Biol. 2008, 181, 381–394. [Google Scholar] [CrossRef]
- Lowe, D.A.; Lepori-Bui, N.; Fomin, P.V.; Sloofman, L.G.; Zhou, X.; Farach-Carson, M.C.; Wang, L.; Kirn-Safran, C.B. Deficiency in perlecan/HSPG2 during bone development enhances osteogenesis and decreases quality of adult bone in mice. Calcif. Tissue Int. 2014, 95, 29–38. [Google Scholar] [CrossRef]
- Iozzo, R.V. MATRIX PROTEOGLYCANS: From Molecular Design to Cellular Function. Annu. Rev. Biochem. 1998, 67, 609–652. [Google Scholar] [CrossRef]
- Martinez, J.R.; Dhawan, A.; Farach-Carson, M.C. Modular proteoglycan perlecan/HSPG2: Mutations, phenotypes and functions. Genes 2018, 6, 556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Couchman, J.R. Perlecan and Tumor Angiogenesis. J. Histochem. Cytochem. 2003, 51, 1393–1410. [Google Scholar] [CrossRef] [PubMed]
- Noonan, D.M.; Fulle, A.; Valente, P.; Cai, S.; Horigan, E.; Sasaki, M.; Yamada, Y.; Hassell, J.R. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J. Biol. Chem. 1991, 266, 22939–22947. [Google Scholar] [PubMed]
- Noonan, D.M.; Hassell, J.R. Perlecan, the large low-density proteoglycan of basement membranes: Structure and variant forms. Kidney Int. 1993, 43, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Cohen, I.R.; Grassel, S.; Murdoch, A.D. The biology of perlecan: The multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem. J. 1994, 302, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Farach-Carson, M.C.; Warren, C.R.; Harrington, D.A.; Carson, D.D. Border patrol: Insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol. 2014, 34, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Creighton, T.E. Intermediates in the refolding of reduced ribonuclease A. J. Mol. Biol. 1979, 129, 411–413. [Google Scholar] [CrossRef]
- Ruoppolo, M.; Freedman, R.B.; Pucci, P.; Marino, G. Glutathione-dependent pathways of refolding of RNase T1 by oxidation and disulfide isomerization: Catalysis by protein disulfide isomerase. Biochemistry 1996, 35, 13636–16346. [Google Scholar] [CrossRef]
- Saaranen, M.J.; Karala, A.R.; Lappi, A.K.; Ruddock, L.W. The role of dehydroascorabte in disulfide bond formation. Antioxid. Redox Signal. 2010, 12, 15–25. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Saaranen, M.J.; Karala, A.R.; Lappi, A.K.; Wang, L.; Raykhel, I.B.; Alanen, H.I.; Salo, K.E.; Wang, C.C.; Ruddock, L.W. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J. Mol. Biol. 2011, 406, 503–515. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Creighton, T.E. The disulfide folding pathway of BPTI. Science 1992, 256, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Weissman, J.S.; Kim, P.S. Re-examination of the folding pathway of BPTI: Predominance of native intermediates. Science 1992, 253, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-Y. Diverse pathways of oxidative folding of disulfide proteins: Underlying causes and folding models. Biochemistry 2011, 50, 3414–3431. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; Mayer, U.; Timpl, R.; Huber, R. Crystal structure of three consecutive laminin-type epidermal growth factor-like (LE) modules of laminin γ1 chain harboring the nidogen binding site. J. Mol. Biol. 1996, 257, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Yang, Y.; Liu, J.H.; Wang, J.H.; Springer, T.A. Complex between nidogen and laminin fragments, reveals a paradigmatic β-propeller interface. Nature 2003, 424, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, F.; Hussain, S.-A.; Hohenester, E. Crystal Structures of the Network-Forming Short-Arm Tips of the Laminin β1 and γ1 Chains. PLoS ONE 2012, 7, e42473. [Google Scholar] [CrossRef] [PubMed]
- Reuten, R.; Patel, T.R.; McDougall, M.; Rama, N.; Nikodemus, D.; Gibert, B.; Delcros, J.G.; Prein, C.; Meier, M.; Metzger, S.; et al. Structural decoding of netrin-4 reveals a regulatory function towards mature basement membranes. Nat. Commun. 2016, 7, 13515. [Google Scholar] [CrossRef]
- Finci, L.I.; Krüger, N.; Sun, X.; Zhang, J.; Chegkazi, M.; Wu, Y.; Schenk, G.; Mertens, H.D.T.; Svergun, D.I.; Zhang, Y.; et al. The Crystal Structure of Netrin-1 in Complex with DCC Reveals the Bifunctionality of Netrin-1 As a Guidance Cue. Neuron 2014, 83, 839–849. [Google Scholar] [CrossRef]
- Moran, T.; Gat, Y.; Fass, D. Laminin L4 domain structure resembles adhesion modules in ephrin receptor and other transmembrane glycoproteins. FEBS J. 2015, 282, 2746–2757. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Drozdetskiy, A.; Cole, C.; Procter, J.; Barton, G.J. JPred4: A protein secondary structure prediction server. Nucleic Acids Res. 2015, 43, W389–W394. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 0008. [Google Scholar] [CrossRef] [PubMed]
- Pósfai, G.; Plunkett, G.; Fehér, T.; Frisch, D.; Keil, G.M.; Umenhoffer, K.; Kolisnychenko, V.; Stahl, B.; Sharma, S.S.; De Arruda, M.; et al. Emergent properties of reduced-genome Escherichia coli. Science 2006, 312, 1044–1046. [Google Scholar]
- Moilanen, A.; Korhonen, K.; Saaranen, M.J.; Ruddock, L.W. Molecular analysis of human Ero1 reveals novel regulatory mechanisms for oxidative protein folding. Life Sci. Alliance 2018, 1, e201800090. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
| Construct | # of Cysteine | Mtheor (Da) | Mexp (Da) | Δ mass |
|---|---|---|---|---|
| Perlecan MH6M-(G503-Q874) | 26 | 41713 | 41688 | 25 |
| Perlecan MH6M-(S762-R1158) | 30 | 43712 | 43681 | 31 |
| Perlecan MH6M-(R1158-T1672) | 48 | 56079 | 56030 | 49 |
| Perlecan MH6M-(G503-T1672) | 88 | 127264 | 127173 | 91 |
| Construct | Tm (°C) |
|---|---|
| Mucin 2 (V36-G389) | Above 90 |
| Alpha tectorin (P707-P981) | Above 90 |
| Perlecan (G503-Q874) | 83 |
| Perlecan (S762-R1158) | 84 |
| Perlecan (R1158-T1672) | 49, 66 |
| Perlecan (G503-T1672) | 51, 66, 81 |
| Plasmid | Construct Details | Reference |
|---|---|---|
| pMG01 | MH6M-human mucin (V36-G389) | This study |
| pMG03 | MH6M-human alpha tectorin (P707-P981) | This study |
| pAS20 | MH6M-mouse perlecan (G503-Q874) | This study |
| pAS04 | MH6M-mouse perlecan (S762-R1158) | This study |
| pAS05 | MH6M-mouse perlecan (R1158-T1672) | This study |
| pAS39 | MH6M-mouse perlecan (G503-T1672) | This study |
| pMJS205 | CyDisCo: Erv1p and PDI | [18] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, A.A.; Gaikwad, M.; Khadka, P.; Saaranen, M.J.; Ruddock, L.W. Production of Extracellular Matrix Proteins in the Cytoplasm of E. coli: Making Giants in Tiny Factories. Int. J. Mol. Sci. 2020, 21, 688. https://doi.org/10.3390/ijms21030688
Sohail AA, Gaikwad M, Khadka P, Saaranen MJ, Ruddock LW. Production of Extracellular Matrix Proteins in the Cytoplasm of E. coli: Making Giants in Tiny Factories. International Journal of Molecular Sciences. 2020; 21(3):688. https://doi.org/10.3390/ijms21030688
Chicago/Turabian StyleSohail, Anil A., Madhuri Gaikwad, Prakash Khadka, Mirva J. Saaranen, and Lloyd W. Ruddock. 2020. "Production of Extracellular Matrix Proteins in the Cytoplasm of E. coli: Making Giants in Tiny Factories" International Journal of Molecular Sciences 21, no. 3: 688. https://doi.org/10.3390/ijms21030688
APA StyleSohail, A. A., Gaikwad, M., Khadka, P., Saaranen, M. J., & Ruddock, L. W. (2020). Production of Extracellular Matrix Proteins in the Cytoplasm of E. coli: Making Giants in Tiny Factories. International Journal of Molecular Sciences, 21(3), 688. https://doi.org/10.3390/ijms21030688





