Mutual Two-Way Interactions of Curcumin and Gut Microbiota
Abstract
1. Introduction
2. Search Criteria and Data Collection
3. The Effect of Curcumin on the Gut Microbiota
4. The Effect of Gut Microbiota on Curcumin
5. Conclusions
6. Outlook
Acknowledgments
Conflicts of Interest
References
- Pluta, R.; Bogucka-Kocka, A.; Ułamek-Kozioł, M.; Furmaga-Jabłońska, W.; Januszewski, S.; Brzozowska, J.; Jabłoński, M.; Kocki, J. Neurogenesis and neuroprotection in postischemic brain neurodegeneration with Alzheimer phenotype: Is there a role for the curcumin? Folia Neuropathol. 2015, 53, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Neuroprotective and neurological/cognitive enhancement effects of curcumin after brain ischemia injury with Alzheimer’s disease phenotype. Int. J. Mol. Sci. 2018, 19, E4002. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, E2147. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Dunbar, G.L. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective effects of indian spice curcumin against amyloid beta in Alzheimer’s disease. J. Alzheimers Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, Y.; Li, H.B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: Current progress, challenges, and perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Melchart, D. Therapeutic effects of curcumin—From traditional past to present and future clinical applications. Int. J. Mol. Sci. 2019, 20, 3757. [Google Scholar] [CrossRef]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Kosieniak, G.; Bijoch, L.; Sikora, E. The role of curcumin in the modulation of ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Benjamín Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of curcumin nanoparticles for brain diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, L.D. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Borges-Canha, M.; Portela-Cidade, J.P.; Dinis-Ribeiro, M.; Leite-Moreira, A.F.; Pimentel-Nunes, P. Role of colonic microbiota in colorectal carcinogenesis: A systematic review. Rev. Esp. Enferm. Dig. 2015, 107, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Intestinal microbiota and metabolic diseases: Pharmacological implications. Trends Pharmacol. Sci. 2016, 37, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.F. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, gut microbiota, and neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef]
- Greiner, A.K.; Papineni, R.V.L.; Umar, S. Chemoprevention in gastrointestinal physiology and disease. Natural products and microbiome. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G1–G15. [Google Scholar] [CrossRef]
- Schincaglia, G.P.; Hong, B.Y.; Rosania, A.; Barasz, J.; Thompson, A.; Sobue, T.; Panagakos, F.; Burleson, J.A.; Dongari-Bagtzoglou, A.; Diaz, P.I. Clinical, immune, and microbiome traits of gingivitis and periimplant mucositis. J. Dental. Res. 2017, 96, 47–55. [Google Scholar] [CrossRef]
- McFadden, R.M.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel. Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Rashmi, R.; Santhosh Kumar, T.R.; Karunagaran, D. Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and caspases. FEBS Lett. 2003, 538, 19–24. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Strier, L.; Kazanov, D.; Madar-Shapiro, L.; Dvory-Sobol, H.; Pinchuk, I.; Marian, B.; Lichtenberg, D.; Arber, N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin. Cancer Res. 2005, 11, 6738–6744. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, P.; Chen, Z.; Ni, C.; Zhang, J.; Hu, F.; Huang, J. Novel micelle formulation of curcumin for enhancing antitumor activity and inhibiting colorectal cancer stem cells. Int. J. Nanomed. 2012, 7, 4487–4497. [Google Scholar]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Johnson, J.J.; Mukhtar, H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007, 255, 170–181. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLOS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef]
- Lang, A.; Salomon, N.; Wu, J.C.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449. [Google Scholar] [CrossRef]
- Parian, A.; Limketkai, B.N. Dietary supplement therapies for inflammatory bowel disease: Crohn’s disease and ulcerative colitis. Curr. Pharm. Des. 2016, 22, 180–188. [Google Scholar] [CrossRef]
- Olszanecki, R.; Jawień, J.; Gajda, M.; Mateuszuk, L.; Gebska, A.; Korabiowska, M.; Chłopicki, S.; Korbut, R. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J. Physiol. Pharmacol. 2005, 56, 627–635. [Google Scholar]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Bie, J.H.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates Western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice - Role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of turmeric and curcumin dietary supplementation on human gut microbiota: A double-blind, randomized, placebo-controlled pilot study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients 2017, 9, E1146. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultraperformance liquid chromatography coupled with quadrupole time-offlight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2015, 985, 38–47. [Google Scholar] [CrossRef]
- Burapan, S.; Kim, M.; Han, J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. J. Agric. Food Chem. 2017, 65, 3305–3310. [Google Scholar] [CrossRef]
- Tan, S.; Calani, L.; Bresciani, L.; Dall’asta, M.; Faccini, A.; Augustin, M.A.; Gras, S.L.; Del Rio, D. The degradation of curcuminoids in a human faecal fermentation model. Int. J. Food Sci. Nutr. 2015, 66, 790–796. [Google Scholar] [CrossRef]
- Tan, S.; Rupasinghe, T.W.T.; Tull, D.L.; Boughton, B.; Oliver, C.; McSweeny, C.; Gras, S.L.; Augustin, M.A. Degradation of curcuminoids by in vitro pure culture fermentation. J. Agric. Food Chem. 2014, 62, 11005–11015. [Google Scholar] [CrossRef]
- An, C.Y.; Sun, Z.Z.; Shen, L.; Ji, H.F. Biotransformation of food spice curcumin by gut bacterium Bacillus megaterium DCMB-002 and its pharmacological implications. Food Nutr. Res. 2017, 61, 1412814. [Google Scholar] [CrossRef]
- Jazayeri, S.; Mustafa, D.S.; Manap, M.Y.; Ali, A.M.; Ismail, A. Survival of bifidobacteria and other selected intestinal bacteria in TPY medium supplemented with curcumin as assessed in vitro. Int. J. Probiotics Prebiotics 2009, 4, 15–22. [Google Scholar]
- Zhang, W.; Huang, J.; Wo, X.; Wang, P. Microbial transformation of curcumin to its derivatives with a novel pichia kudriavzevii ZJPH0802 strain. Appl. Biochem. Biotechnol. 2013, 170, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, M.; Li, R.; Yin, J.; Guo, D.A. Microbial transformation of curcumin by rhizopus chinensis. Biocatal. Biotransform. 2010, 28, 380–386. [Google Scholar] [CrossRef]
- Maehara, S.; Ikeda, M.; Haraguchi, H.; Kitamura, C.; Nagoe, T.; Ohashi, K.; Shibuya, H. Microbial conversion of curcumin into colorless hydroderivatives by the endophytic fungus diaporthe sp. Associated with curcuma longa. Chem. Pharm. Bull. Tokyo 2011, 59, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. Biofactors 2013, 39, 14–20. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Kawakishi, S.; Osawa, T. Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem. Pharmacol. 1996, 52, 519–525. [Google Scholar] [CrossRef]
- Wu, J.C.; Tsai, M.L.; Lai, C.S.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014, 1, 12–17. [Google Scholar] [CrossRef]
- Zhao, F.; Gong, Y.; Hu, Y.; Lu, M.; Wang, J.; Dong, J.; Chen, D.; Chen, L.; Fu, F.; Qiu, F. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: Translocation of nuclear factor-κB as potential target. Mol. Med. Rep. 2015, 11, 3087–3093. [Google Scholar] [CrossRef]
- Yan, F.S.; Sun, J.L.; Xie, W.H.; Shen, L.; Ji, H.F. Neuroprotective effects and mechanisms of curcumin-Cu(II) and -Zn(II) complexes systems and their pharmacological implications. Nutrients 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Substantiation for the use of curcumin during the development of neurodegeneration after brain ischemia. Int. J. Mol. Sci. 2020, 21, 517. [Google Scholar] [CrossRef]
- Lo Cascio, F.; Puangmalai, N.; Ellsworth, A.; Bucchieri, F.; Pace, A.; Palumbo Piccionello, A.; Kayed, R. Toxic tau oligomers modulated by novel curcumin derivatives. Sci. Rep. 2019, 9, 19011. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Malampati, S.; Zeng, Y.; Durairajan, S.S.K.; Yang, C.B.; Tong, B.C.; Iyaswamy, A.; Shang, W.B.; Sreenivasmurthy, S.G.; Zhu, Z.; et al. A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and tau pathology in Alzheimer’s disease models. Aging Cell. 2019, e13069. [Google Scholar] [CrossRef]
- Park, C.H.; Song, J.H.; Kim, S.N.; Lee, J.H.; Lee, H.J.; Kang, K.S.; Lim, H.H. Neuroprotective effects of tetrahydrocurcumin against glutamate-induced oxidative stress in hippocampal HT22 cells. Molecules 2019, 25, E144. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Marino Gammazza, A.; Lo Cascio, F.; Mocciaro, E.; Vitale, A.M.; Vergilio, G.; Pace, A.; Cappello, F.; Campanella, C. Palumbo Piccionello, A. Curcumin affects HSP60 folding activity and levels in neuroblastoma cells. Int. J. Mol. Sci. 2020, 21, 661. [Google Scholar] [CrossRef]
- Motawi, T.K.; Sadik, N.A.H.; Hamed, M.A.; Ali, S.A.; Khalil, W.K.B.; Ahmed, Y.R. Potential therapeutic effects of antagonizing adenosine A2A receptor, curcumin and niacin in rotenone-induced Parkinson’s disease mice model. Mol. Cell Biochem. 2020, 465, 89–102. [Google Scholar] [CrossRef]
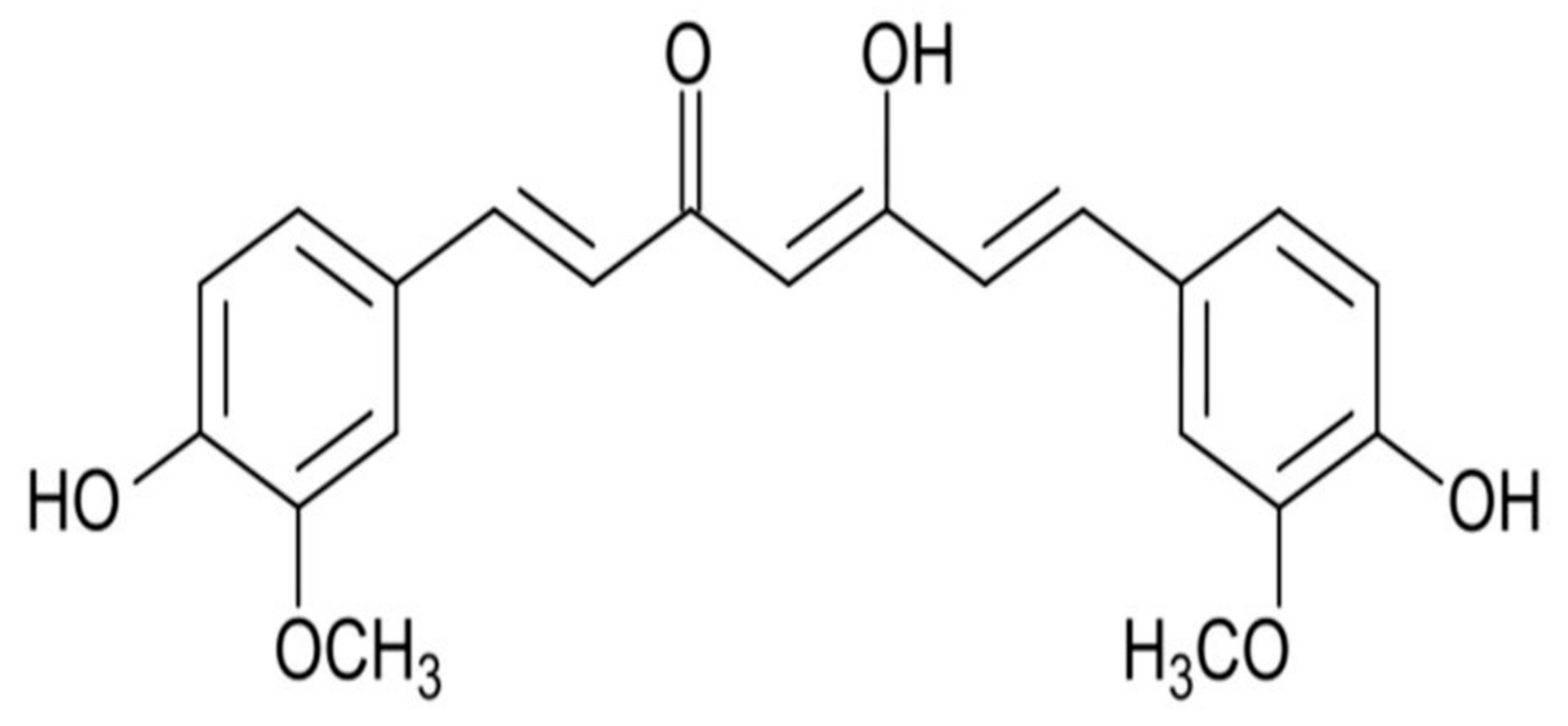
| Animal/Human | Pro-Health Bacteria | References |
|---|---|---|
| Animal | Prevotellaceae, Bacteroidaceae, Rikenellaceae | [16] |
| Animal/Human | Bifidobacterium, Lactobacilli | [13] |
| Animal | Bacteroides, Alistipes, Bacteroidaceae, Rikenellaceae | [14] |
| Animal | Lactobacillales | [19] |
| Animal | Lactobacilli, Bifidobacteria | [31] |
| Human | Clostridium spp., Bacteroides spp., Citrobacter spp., Cronobacter spp., Enterobacter spp., Enterococcus spp., Klebsiella spp., Parabacteroides spp., Pseudomonas spp. | [33] |
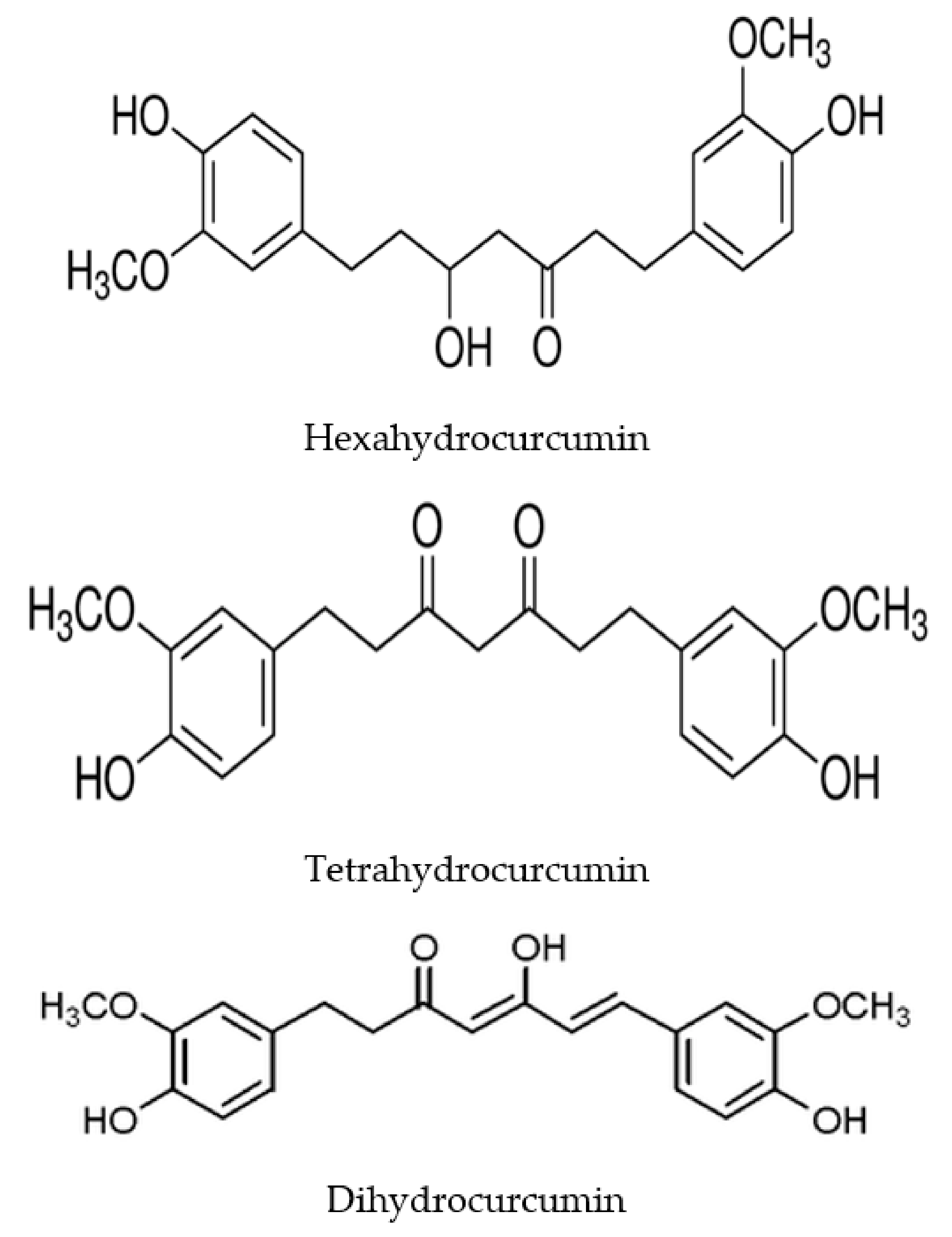
| Animal/Human | Material | Metabolites | References |
|---|---|---|---|
| Human | Human fecal primers | 1-(4-hydroxy-3-methoxyphenyl)-2-propanol, tetrahydrocurcumin, dihydroferulic acid | [35] |
| Human | Human fecal starters | Hexahydrocurcumin, tetrahydrocurcumin, octahydrocurcumin | [36] |
| Human | Intestinal Blautia sp. | Bisdemetylcurcumin, demethylcurcumin | [38] |
| Animal | Intestinal Escherichia fergusonii, Escherichia coli | Tetrahydrocurcumin, dihydrocurcumin, ferulic acid | [40] |
| Animal | Intestinal Pichia anomala | 5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)heptan-3-one, 5-hydroxy-1.7-bis(4-hydroxy-3-methoxyphenyl)heptan-3-one, 5-hydroxy-1,7-bis(4-hydroxyphenyl)heptane-3-one, 1,7-bis(4-hydroxy-3-methoxyphenyl)heptane-3,5-diol | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluta, R.; Januszewski, S.; Ułamek-Kozioł, M. Mutual Two-Way Interactions of Curcumin and Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 1055. https://doi.org/10.3390/ijms21031055
Pluta R, Januszewski S, Ułamek-Kozioł M. Mutual Two-Way Interactions of Curcumin and Gut Microbiota. International Journal of Molecular Sciences. 2020; 21(3):1055. https://doi.org/10.3390/ijms21031055
Chicago/Turabian StylePluta, Ryszard, Sławomir Januszewski, and Marzena Ułamek-Kozioł. 2020. "Mutual Two-Way Interactions of Curcumin and Gut Microbiota" International Journal of Molecular Sciences 21, no. 3: 1055. https://doi.org/10.3390/ijms21031055
APA StylePluta, R., Januszewski, S., & Ułamek-Kozioł, M. (2020). Mutual Two-Way Interactions of Curcumin and Gut Microbiota. International Journal of Molecular Sciences, 21(3), 1055. https://doi.org/10.3390/ijms21031055






