Sphingolipid Signature of Human Feto-Placental Vasculature in Preeclampsia
Abstract
1. Introduction
2. Results
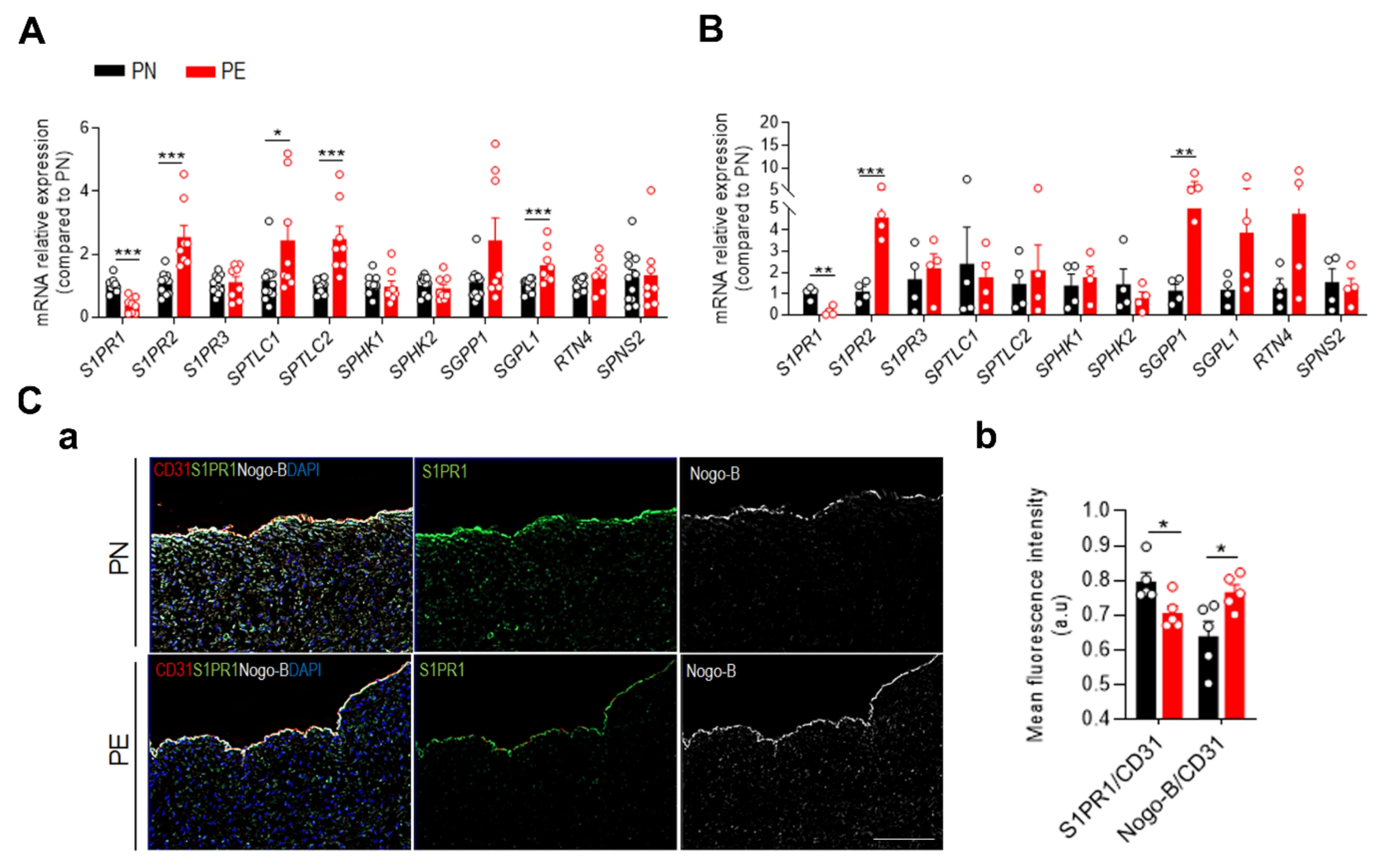
2.1. Sphingolipid Profile of Placental Chorionic Arteries from PE and Normotensive Subjects
2.2. PE Impairs S1P Signaling at the Feto-Placental Vasculature
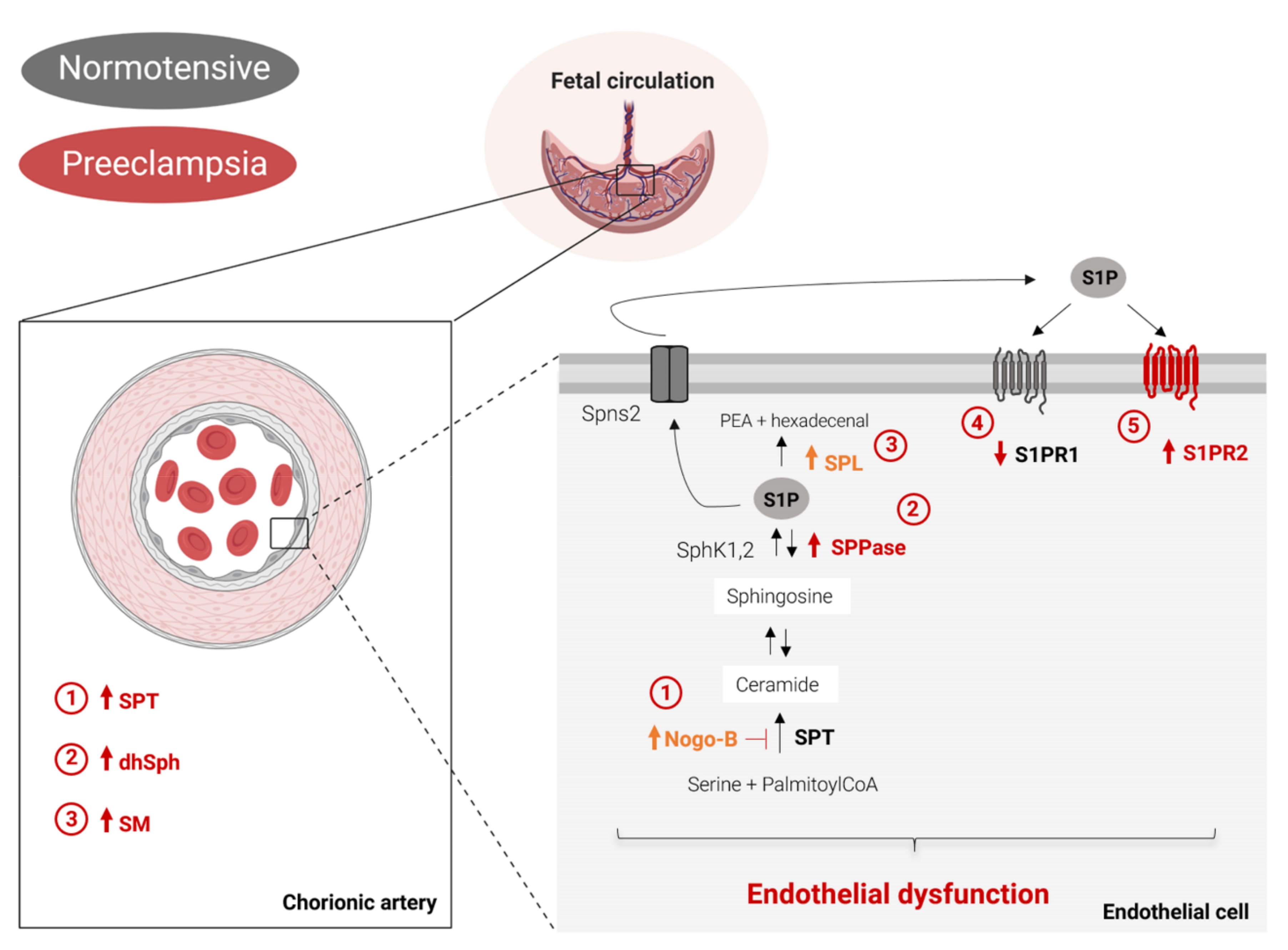
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Isolation of Arterial Chorionic Vessels and Primary Human Placental Arterial Endothelial Cells (fPAEC)
4.3. Sphingolipid Analysis by LC-MS/MS
4.4. SPT Activity Assay
4.5. Quantitative Real-Time PCR (qPCR)
4.6. Immunostaining
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| S1P | Sphingosisne-1-phosphate |
| S1PR | Sphingosisne-1-phosphate receptor |
| Cer | Ceramide |
| SM | Sphingomyelin |
| UCA | Umbilical cord artery |
| fPAEC | Fetal placental arterial endothelial cells |
| SPT | Serine palmitoyltranferase |
| SPTLC1 | Serine palmitoyltranferase long chain base subunit 1 |
| SPTLC2 | Serine palmitoyltranferase long chain base subunit 2 |
| SPNS2 | Spinster transporter 2 |
| SPHK1 | Sphingosine kinase 1 |
| SPHK2 | Sphingosine kinase 2 |
| SGPP1 | Sphingosisne-1-phosphate phosphatase 1 |
| SGPL1 | Sphingosisne-1-phosphate lyase 1 |
| Nogo-B | Reticulon-4B |
References
- Irgens, H.U.; Reisaeter, L.; Irgens, L.M.; Lie, R.T.; Lie, R.T. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 2001, 323, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The intrauterine origins of cardiovascular and obstructive lung disease in adult life. The Marc Daniels Lecture 1990. J. R. Coll. Physicians Lond. 1991, 25, 129–133. [Google Scholar] [PubMed]
- Lawlor, D.A.; Macdonald-Wallis, C.; Fraser, A.; Nelson, S.M.; Hingorani, A.; Davey Smith, G.; Sattar, N.; Deanfield, J. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: Findings from the Avon Longitudinal Study of Parents and Children. Eur. Heart J. 2012, 33, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Wallis, A.B.; Saftlas, A.F.; Hsia, J.; Atrash, H.K. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am. J. Hypertens. 2008, 21, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Wojcik-Baszko, D.; Charkiewicz, K.; Laudanski, P. Role of dyslipidemia in preeclampsia—A review of lipidomic analysis of blood, placenta, syncytiotrophoblast microvesicles and umbilical cord artery from women with preeclampsia. Prostaglandins Other Lipid Mediat. 2018, 139, 19–23. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Venkataraman, K.; Lee, Y.M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnstrom, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef]
- Dantas, A.P.V.; Igarashi, J.; Michel, T. Sphingosine 1-phosphate and control of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2045–H2052. [Google Scholar] [CrossRef]
- Kimura, T.; Watanabe, T.; Sato, K.; Kon, J.; Tomura, H.; Tamama, K.; Kuwabara, A.; Kanda, T.; Kobayashi, I.; Ohta, H.; et al. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem. J. 2000, 348 Pt 1, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; Lisowska, A.; Zabielski, P.; Musiał, W.; Baranowski, M. Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat. 2013, 106, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sattler, K.; Lehmann, I.; Gräler, M.; Bröcker-Preuss, M.; Erbel, R.; Heusch, G.; Levkau, B. HDL-Bound Sphingosine 1-Phosphate (S1P) Predicts the Severity of Coronary Artery Atherosclerosis. Cell. Physiol. Biochem. 2014, 34, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-S.; Hu, Y.; Noh, H.-L.; Drosatos, K.; Okajima, K.; Buchanan, J.; Tuinei, J.; Homma, S.; Jiang, X.-C.; Abel, E.D.; et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 2008, 49, 2101–2112. [Google Scholar] [CrossRef]
- Zhang, Q.-J.; Holland, W.L.; Wilson, L.; Tanner, J.M.; Kearns, D.; Cahoon, J.M.; Pettey, D.; Losee, J.; Duncan, B.; Gale, D.; et al. Ceramide Mediates Vascular Dysfunction in Diet-Induced Obesity by PP2A-Mediated Dephosphorylation of the eNOS-Akt Complex. Diabetes 2012, 61, 1848–1859. [Google Scholar] [CrossRef]
- Slotte, P.J. Molecular properties of various structurally defined sphingomyelins—Correlation of structure with function. Prog. Lipid Res. 2013, 52, 206–219. [Google Scholar] [CrossRef]
- Kikas, P.; Chalikias, G. Cardiovascular Implications of Sphingomyelin Presence in Biological Membranes. Eur. Cardiol. Rev. 2018, 13, 42. [Google Scholar] [CrossRef]
- Romanowicz, L.; Bańkowski, E. Preeclampsia-associated alterations in sphingolipid composition of the umbilical cord artery. Clin. Biochem. 2009, 42, 1719–1724. [Google Scholar] [CrossRef]
- Melland-Smith, M.; Ermini, L.; Chauvin, S.; Craig-Barnes, H.; Tagliaferro, A.; Todros, T.; Post, M.; Caniggia, I. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 2015, 11, 653–669. [Google Scholar] [CrossRef]
- Dobierzewska, A.; Soman, S.; Illanes, S.E.; Morris, A.J. Plasma cross-gestational sphingolipidomic analyses reveal potential first trimester biomarkers of preeclampsia. PLoS ONE 2017, 12, e0175118. [Google Scholar] [CrossRef]
- Cantalupo, A.; Zhang, Y.; Kothiya, M.; Galvani, S.; Obinata, H.; Bucci, M.; Giordano, F.J.; Jiang, X.-C.; Hla, T.; Di Lorenzo, A. Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat. Med. 2015, 21, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, S.; Jiang, M.; Zhu, Y.; Ding, L.; Shi, H.; Dong, P.; Yang, J.; Yang, Y. Atherosclerotic dyslipidemia revealed by plasma lipidomics on ApoE −/− mice fed a high-fat diet. Atherosclerosis 2017, 262, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [PubMed]
- Donati, C.; Bruni, P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: Implications in its biological response. Biochim. Biophys. Acta 2006, 1758, 2037–2048. [Google Scholar] [CrossRef]
- Jung, B.; Obinata, H.; Galvani, S.; Mendelson, K.; Ding, B.; Skoura, A.; Kinzel, B.; Brinkmann, V.; Rafii, S.; Evans, T.; et al. Flow-Regulated Endothelial S1P Receptor-1 Signaling Sustains Vascular Development. Dev. Cell 2012, 23, 600–610. [Google Scholar] [CrossRef]
- Borodzicz, S.; Czarzasta, K.; Kuch, M.; Cudnoch-Jedrzejewska, A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015, 14, 55. [Google Scholar] [CrossRef]
- Bolz, S.-S.; Vogel, L.; Sollinger, D.; Derwand, R.; Boer, C.; Pitson, S.M.; Spiegel, S.; Pohl, U. Sphingosine Kinase Modulates Microvascular Tone and Myogenic Responses Through Activation of RhoA/Rho Kinase. Circulation 2003, 108, 342–347. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Cantalupo, A.; Azevedo, P.S.; Siragusa, M.; Bielawski, J.; Giordano, F.J.; Di Lorenzo, A. Endothelial Nogo-B regulates sphingolipid biosynthesis to promote pathological cardiac hypertrophy during chronic pressure overload. JCI Insight 2016, 1. [Google Scholar] [CrossRef]
- Ikeda, M.; Kihara, A.; Igarashi, Y. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5′-phosphate binding domain exposed to the cytosol. Biochem. Biophys. Res. Commun. 2004, 325, 338–343. [Google Scholar] [CrossRef]
- Cantalupo, A.; Gargiulo, A.; Dautaj, E.; Liu, C.; Zhang, Y.; Hla, T.; Di Lorenzo, A. S1PR1 (Sphingosine-1-Phosphate Receptor 1) Signaling Regulates Blood Flow and Pressure. Hypertension 2017, 70, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Galvani, S.; Sanson, M.; Blaho, V.A.; Swendeman, S.L.; Obinata, H.; Conger, H.; Dahlback, B.; Kono, M.; Proia, R.L.; Smith, J.D.; et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 2015, 8, ra79. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Frej, C.; Holmér, A.; Guo, L.J.; Tran, S.; Dahlbäck, B. High-Density Lipoprotein–Associated Apolipoprotein M Limits Endothelial Inflammation by Delivering Sphingosine-1-Phosphate to the Sphingosine-1-Phosphate Receptor 1. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Kitada, Y.; Kajita, K.; Taguchi, K.; Mori, I.; Yamauchi, M.; Ikeda, T.; Kawashima, M.; Asano, M.; Kajita, T.; Ishizuka, T.; et al. Blockade of Sphingosine 1-Phosphate Receptor 2 Signaling Attenuates High-Fat Diet-Induced Adipocyte Hypertrophy and Systemic Glucose Intolerance in Mice. Endocrinology 2016, 157, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.A.; Summers, S.A. A Ceramide-Centric View of Insulin Resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Li, C.; Olivera, A.; Bielawski, J.; Bielawska, A.; Deng, C.-X.; Proia, R.L. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Invest. 2007, 117, 2993–3006. [Google Scholar] [CrossRef]
- Johnstone, E.D.; Chan, G.; Sibley, C.P.; Davidge, S.T.; Lowen, B.; Guilbert, L.J. Sphingosine-1-phosphate inhibition of placental trophoblast differentiation through a G i -coupled receptor response. J. Lipid Res. 2005, 46, 1833–1839. [Google Scholar] [CrossRef]
- Dunlap, K.A.; Kwak, H.; Burghardt, R.C.; Bazer, F.W.; Magness, R.R.; Johnson, G.A.; Bayless, K.J. The Sphingosine 1-Phosphate (S1P) Signaling Pathway Is Regulated During Pregnancy in Sheep1. Biol. Reprod. 2010, 82, 876–887. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Blachnio-Zabielska, A.; Raba, G.; Sakowicz, A.; Kalinka, J.; Chabowski, A.; Laudanski, P. Sphingolipids as a new factor in the pathomechanism of preeclampsia—Mass spectrometry analysis. PLoS ONE 2017, 12, e0177601. [Google Scholar] [CrossRef]
- Trudinger, B.J.; Giles, W.B.; Cook, C.M. Flow velocity waveforms in the maternal uteroplacental and fetal umbilical placental circulations. Am. J. Obstet. Gynecol. 1985, 152, 155–163. [Google Scholar] [CrossRef]
- Romanowicz, L.; Bańkowski, E. Sphingolipids of human umbilical cord vein and their alteration in preeclampsia. Mol. Cell. Biochem. 2010, 340, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, B.W. The long and the short of ceramides. J. Biol. Chem. 2018, 293, 9922–9923. [Google Scholar] [CrossRef] [PubMed]
- Young, M.M.; Kester, M.; Wang, H.-G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Grösch, S.; Schiffmann, S.; Geisslinger, G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012, 51, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Völzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Dobierzewska, A.; Palominos, M.; Sanchez, M.; Dyhr, M.; Helgert, K.; Venegas-Araneda, P.; Tong, S.; Illanes, S.E. Impairment of Angiogenic Sphingosine Kinase-1/Sphingosine-1-Phosphate Receptors Pathway in Preeclampsia. PLoS ONE 2016, 11, e0157221. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; Sun, M.; Lin, L.; Miao, L.; Ge, Z.; Yang, B. Comprehensive metabonomic analysis of heart tissue from isoproterenol-induced myocardial infarction rat based on reversed-phase and hydrophilic interaction chromatography coupled to mass spectrometry. J. Sep. Sci. 2017, 40, 2198–2206. [Google Scholar] [CrossRef]
- Kurek, K.; Mikłosz, A.; Łukaszuk, B.; Chabowski, A.; Górski, J.; Żendzian-Piotrowska, M. Inhibition of Ceramide De Novo Synthesis Ameliorates Diet Induced Skeletal Muscles Insulin Resistance. J. Diabetes Res. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Testai, F.D.; Kilkus, J.P.; Berdyshev, E.; Gorshkova, I.; Natarajan, V.; Dawson, G. Multiple sphingolipid abnormalities following cerebral microendothelial hypoxia. J. Neurochem. 2014, 131, 530–540. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef]
- Baig, S.; Lim, J.Y.; Fernandis, A.Z.; Wenk, M.R.; Kale, A.; Su, L.L.; Biswas, A.; Vasoo, S.; Shui, G.; Choolani, M. Lipidomic analysis of human placental Syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta 2013, 34, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Korkes, H.A.; Sass, N.; Moron, A.F.; Câmara, N.O.S.; Bonetti, T.; Cerdeira, A.S.; Da Silva, I.D.C.G.; De Oliveira, L. Lipidomic Assessment of Plasma and Placenta of Women with Early-Onset Preeclampsia. PLoS ONE 2014, 9, e110747. [Google Scholar] [CrossRef] [PubMed]
- Ermini, L.; Ausman, J.; Melland-Smith, M.; Yeganeh, B.; Rolfo, A.; Litvack, M.L.; Todros, T.; Letarte, M.; Post, M.; Caniggia, I. A Single Sphingomyelin Species Promotes Exosomal Release of Endoglin into the Maternal Circulation in Preeclampsia. Sci. Rep. 2017, 7, 12172. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, P.L.; Makley, A.T.; Hoehn, R.S.; Edwards, M.J.; Pritts, T.A. The role of sphingolipids in endothelial barrier function. Biol. Chem. 2015, 396, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Mulders, A.C.; Jongsma, M.; Alewijnse, A.E.; Peters, S.L. Vascular effects of sphingolipids. Acta Paediatr. 2007, 96, 44–48. [Google Scholar] [CrossRef]
- Cantalupo, A.; Di Lorenzo, A. S1P Signaling and De Novo Biosynthesis in Blood Pressure Homeostasis. J. Pharmacol. Exp. Ther. 2016, 358, 359–370. [Google Scholar] [CrossRef]
- Lee, M.-J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular Endothelial Cell Adherens Junction Assembly and Morphogenesis Induced by Sphingosine-1-Phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef]
- Igarashi, J.; Bernier, S.G.; Michel, T. Sphingosine 1-Phosphate and Activation of Endothelial Nitric-oxide Synthase. J. Biol. Chem. 2001, 276, 12420–12426. [Google Scholar] [CrossRef]
- Skaznik-Wikiel, M.E.; Kaneko-Tarui, T.; Kashiwagi, A.; Pru, J.K. Sphingosine-1-Phosphate Receptor Expression and Signaling Correlate with Uterine Prostaglandin-Endoperoxide Synthase 2 Expression and Angiogenesis During Early Pregnancy1. Biol. Reprod. 2006, 74, 569–576. [Google Scholar] [CrossRef]
- Del Gaudio, I.; Sreckovic, I.; Zardoya-Laguardia, P.; Bernhart, E.; Christoffersen, C.; Frank, S.; Marsche, G.; Illanes, S.E.; Wadsack, C. Circulating cord blood HDL-S1P complex preserves the integrity of the feto-placental vasculature. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158632. [Google Scholar] [CrossRef]
- Liu, W.; Liu, B.; Liu, S.; Zhang, J.; Lin, S. Sphingosine-1-phosphate receptor 2 mediates endothelial cells dysfunction by PI3K-Akt pathway under high glucose condition. Eur. J. Pharmacol. 2016, 776, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, L.; Kim, G.S.; Ryan, K.; Lu, S.; O’Donnell, R.K.; Spokes, K.; Shapiro, N.; Aird, W.C.; Kluk, M.J.; et al. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood 2013, 122, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, W.S.; Pitt, B.R.; McVerry, B.J. S1P 2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. Am. J. Physiol. Cell. Mol. Physiol. 2010, 299, L137–L145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Signorelli, P.; Avagliano, L.; Reforgiato, M.R.; Toppi, N.; Casas, J.; Fabriàs, G.; Marconi, A.M.; Ghidoni, R.; Caretti, A. De novo ceramide synthesis is involved in acute inflammation during labor. Biol. Chem. 2016, 397, 147–155. [Google Scholar] [CrossRef]
- Samad, F.; Hester, K.D.; Yang, G.; Hannun, Y.A.; Bielawski, J. Altered Adipose and Plasma Sphingolipid Metabolism in Obesity. Diabetes 2006, 55, 2579–2587. [Google Scholar] [CrossRef]
- Lang, I.; Schweizer, A.; Hiden, U.; Ghaffari-Tabrizi, N.; Hagendorfer, G.; Bilban, M.; Pabst, M.A.; Korgun, E.T.; Dohr, G.; Desoye, G. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation 2008, 76, 1031–1043. [Google Scholar] [CrossRef]
- Bielawski, J.; Pierce, J.S.; Snider, J.; Rembiesa, B.; Szulc, Z.M.; Bielawska, A. Sphingolipid Analysis by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS). In Sphingolipids as Signaling and Regulatory Molecules; Springer: New York, NY, USA, 2010; pp. 46–59. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Williams, R.D.; Wang, E.; Merrill, A.H. Enzymology of long-chain base synthesis by liver: Characterization of serine palmitoyltransferase in rat liver microsomes. Arch. Biochem. Biophys. 1984, 228, 282–291. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Gaudio, I.; Sasset, L.; Di Lorenzo, A.; Wadsack, C. Sphingolipid Signature of Human Feto-Placental Vasculature in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 1019. https://doi.org/10.3390/ijms21031019
Del Gaudio I, Sasset L, Di Lorenzo A, Wadsack C. Sphingolipid Signature of Human Feto-Placental Vasculature in Preeclampsia. International Journal of Molecular Sciences. 2020; 21(3):1019. https://doi.org/10.3390/ijms21031019
Chicago/Turabian StyleDel Gaudio, Ilaria, Linda Sasset, Annarita Di Lorenzo, and Christian Wadsack. 2020. "Sphingolipid Signature of Human Feto-Placental Vasculature in Preeclampsia" International Journal of Molecular Sciences 21, no. 3: 1019. https://doi.org/10.3390/ijms21031019
APA StyleDel Gaudio, I., Sasset, L., Di Lorenzo, A., & Wadsack, C. (2020). Sphingolipid Signature of Human Feto-Placental Vasculature in Preeclampsia. International Journal of Molecular Sciences, 21(3), 1019. https://doi.org/10.3390/ijms21031019





