Neuroimaging Biomarkers in SCA2 Gene Carriers
Abstract
1. Introduction
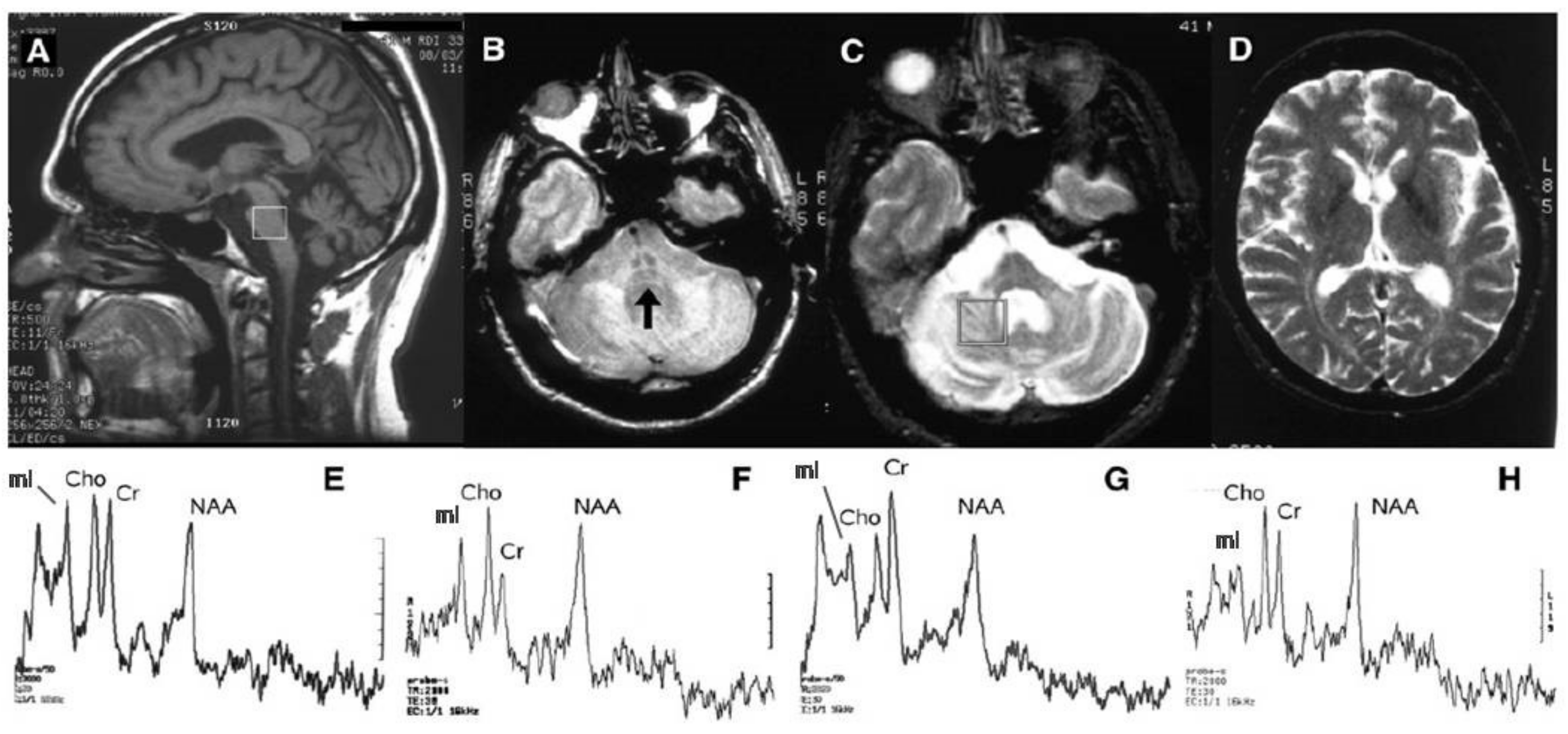
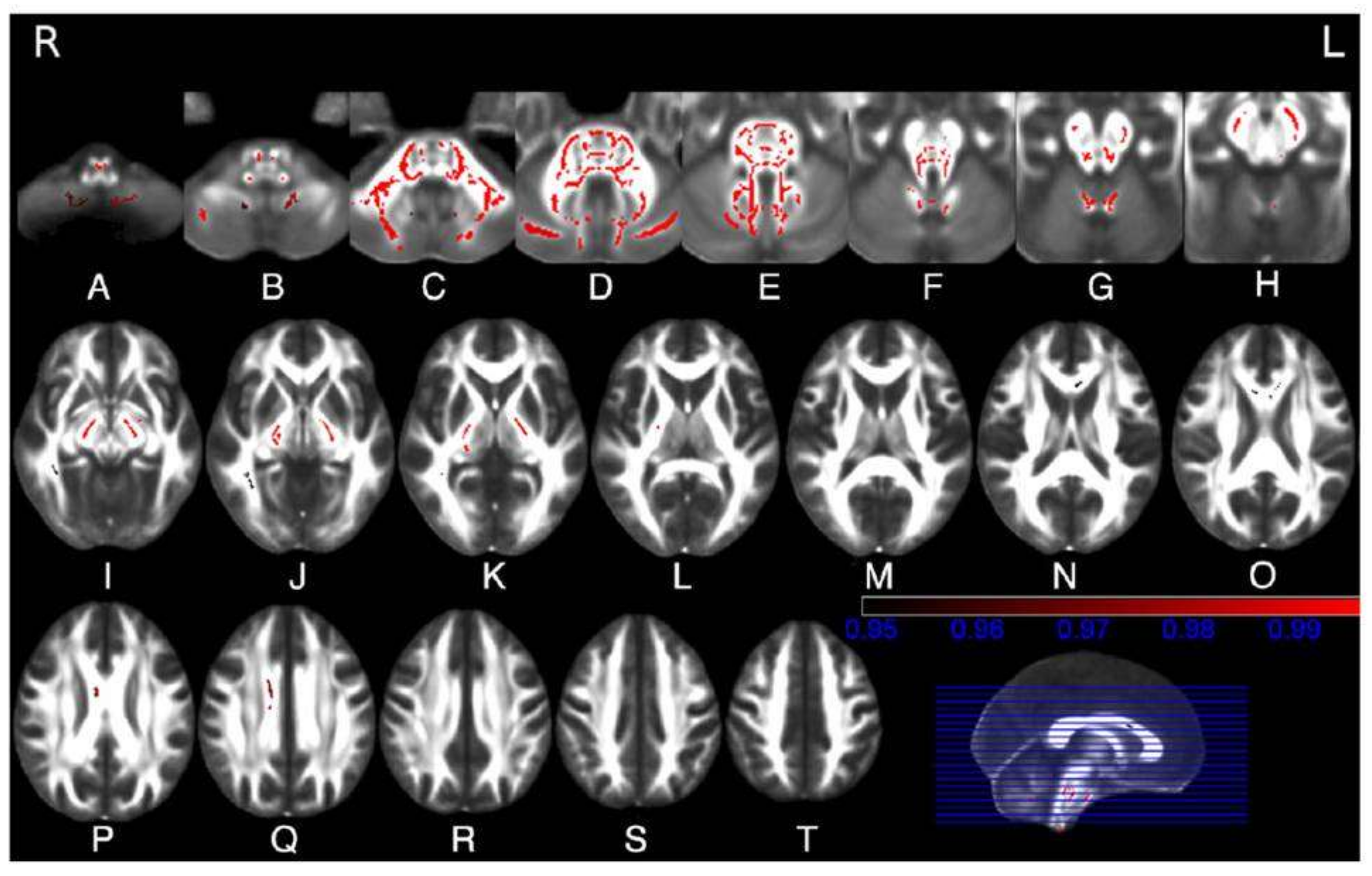
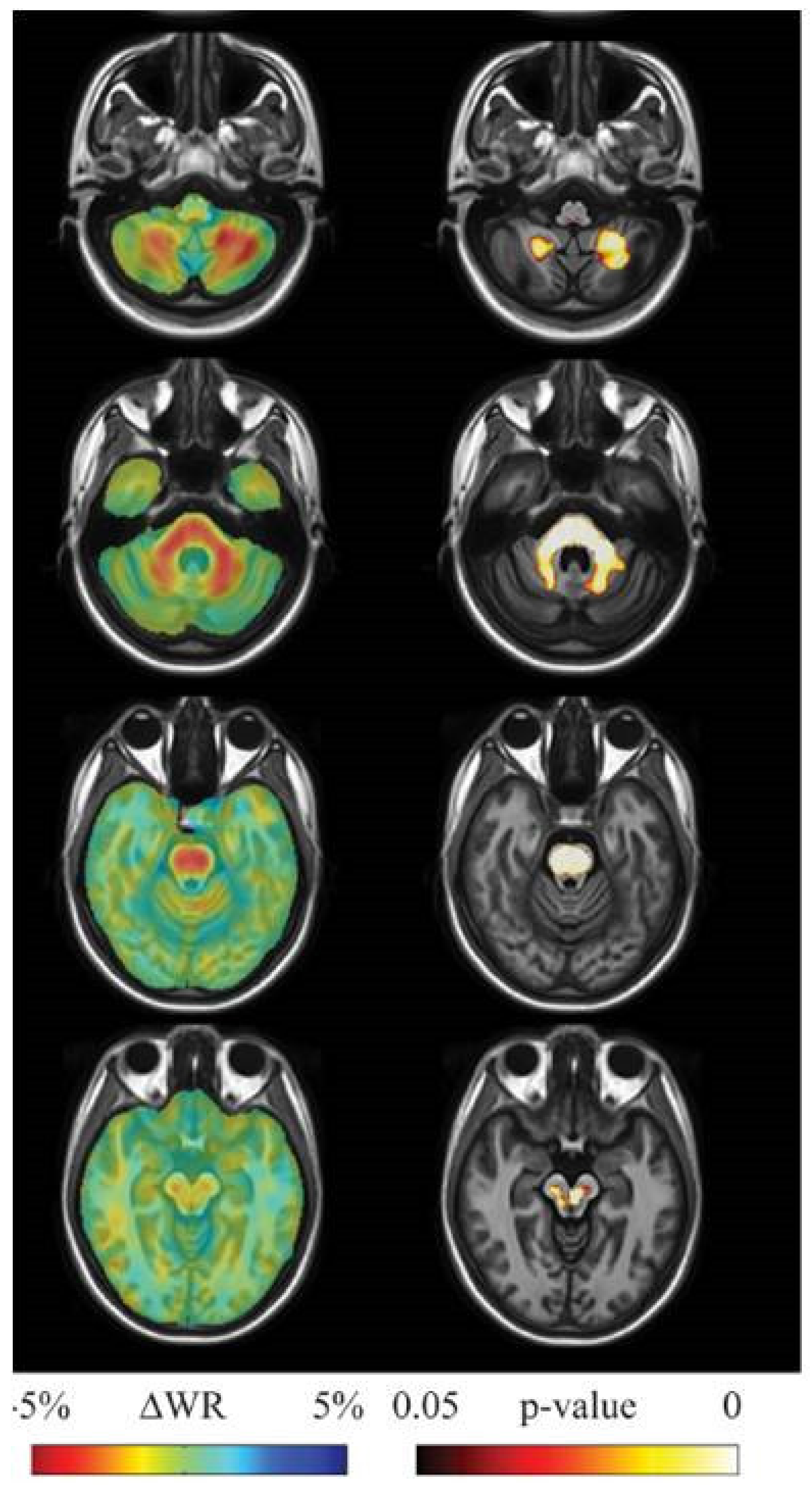
2. Structural and Microstructural MR Imaging
3. MR Spectroscopy
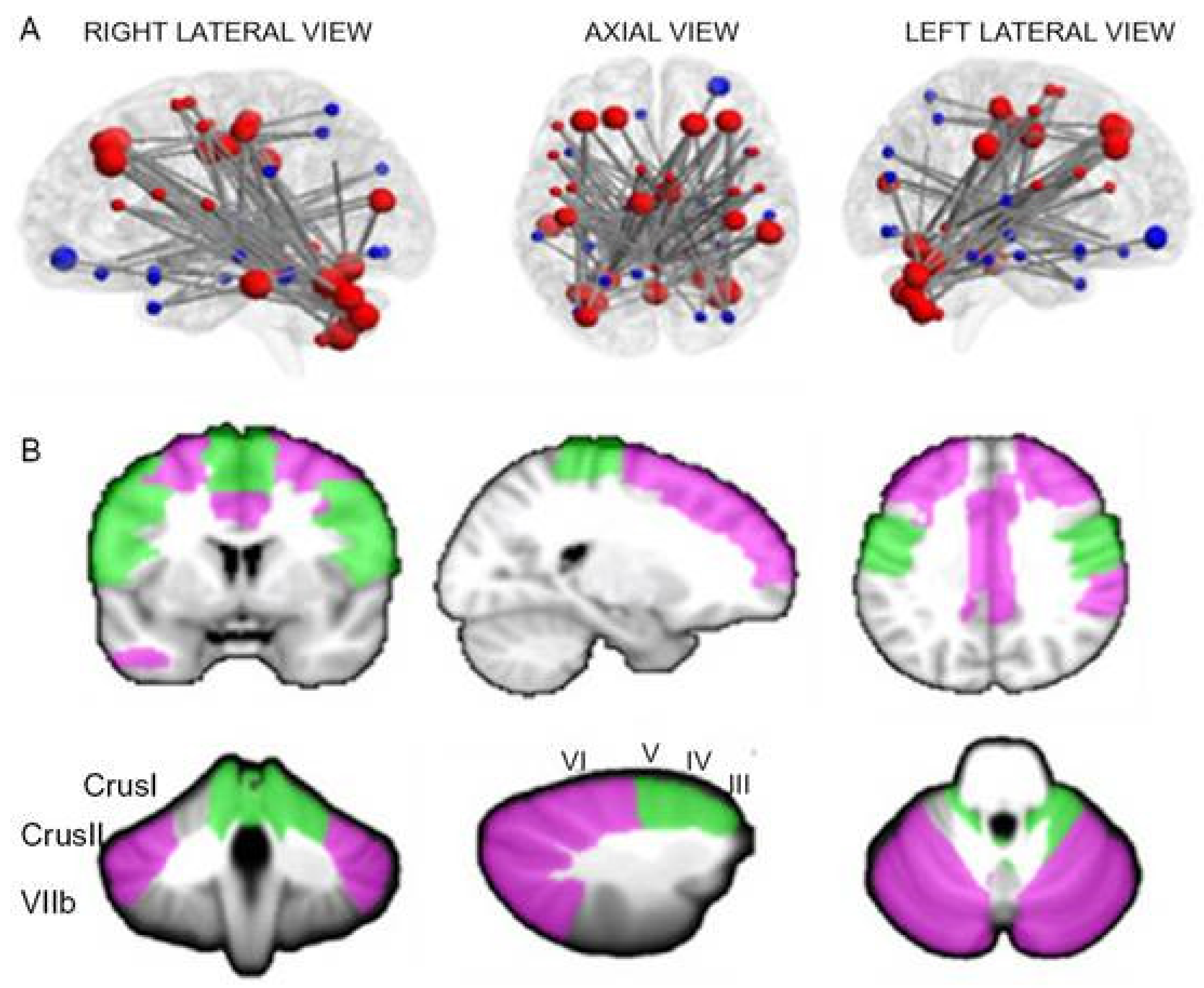
4. Functional MRI
5. Nuclear Medicine
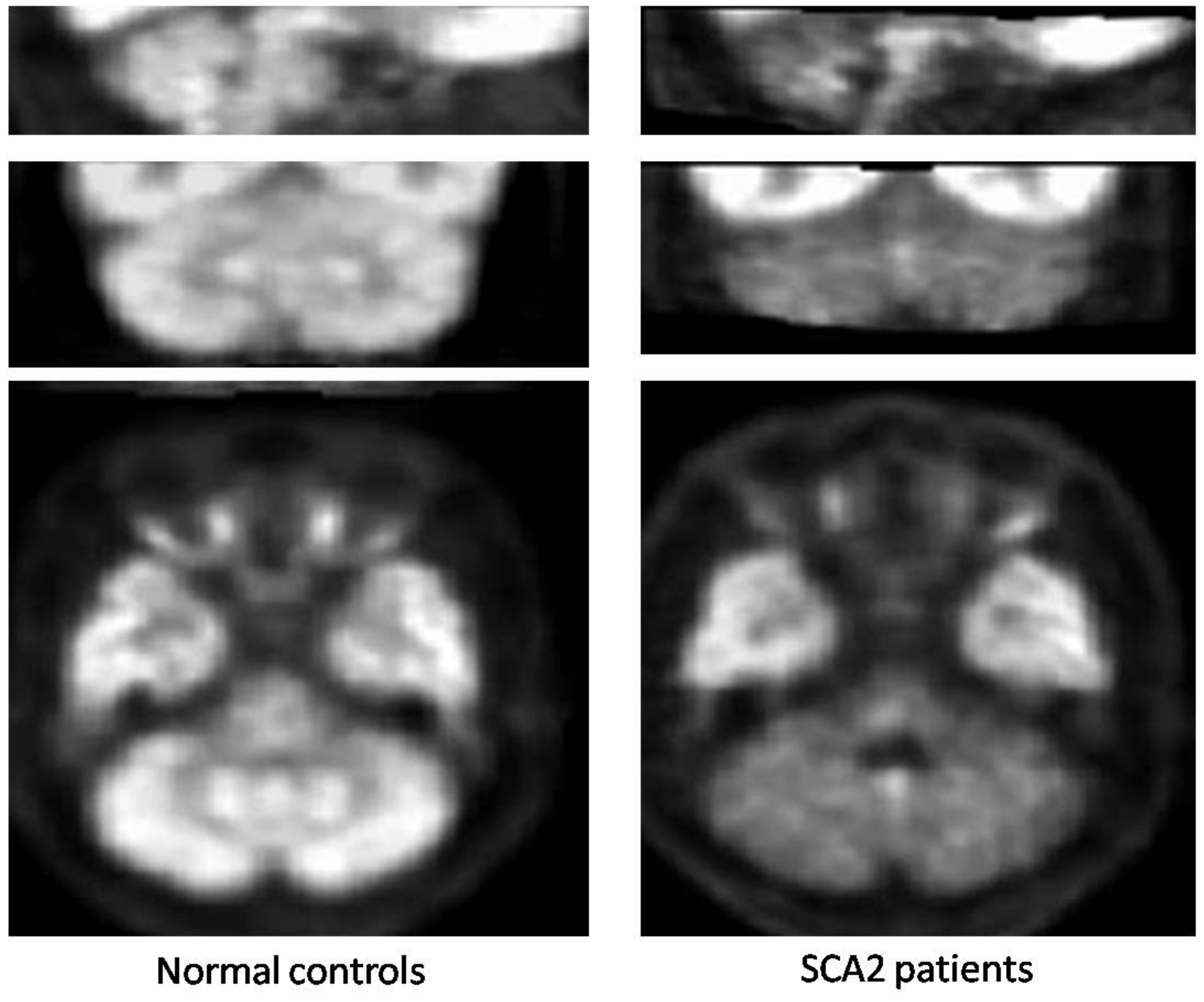
5.1. Glucose Metabolism
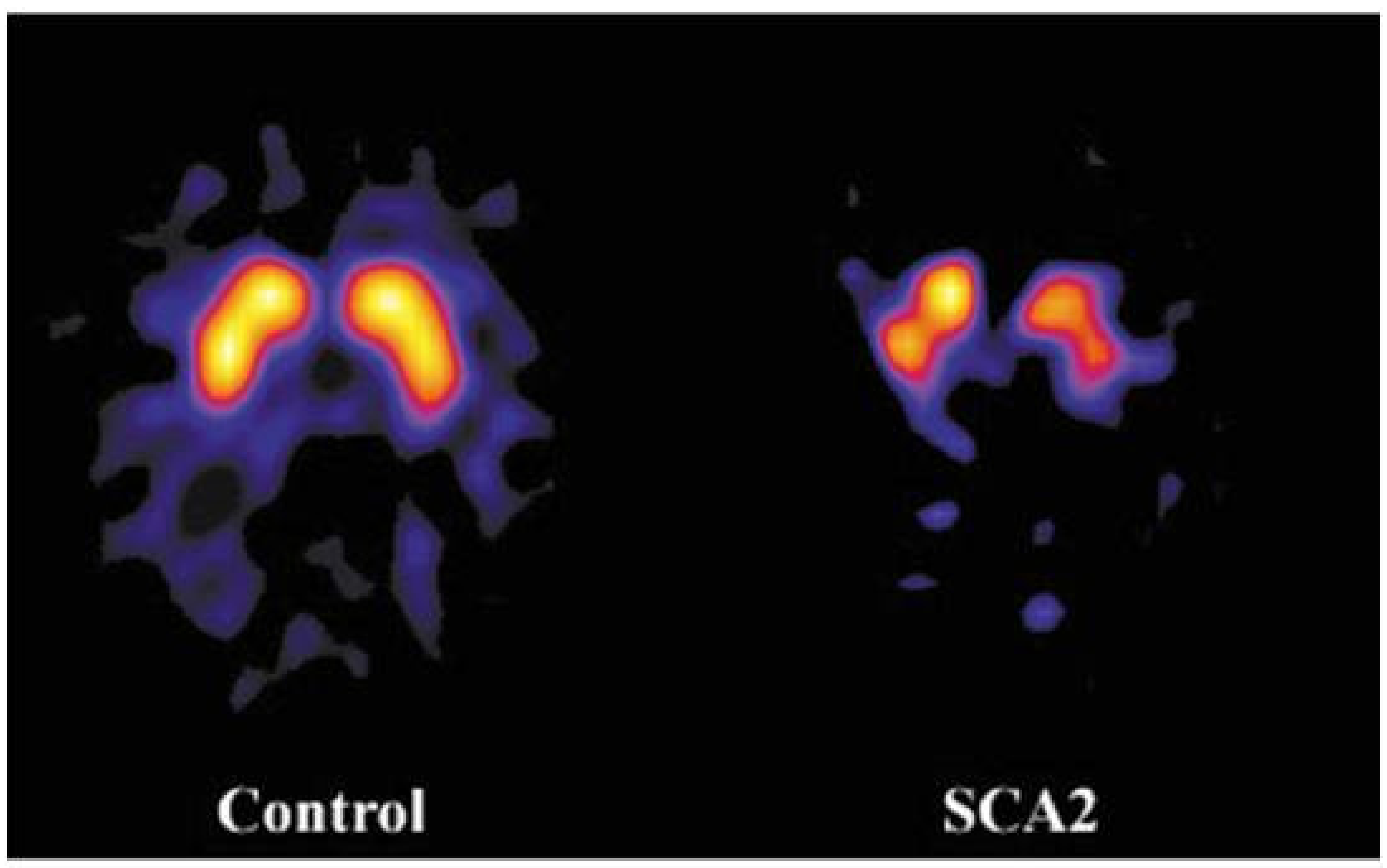
5.2. Nigrostriatal System
6. Limitations and Perspectives
- Data pooling with centralized analyses or re-analyses, as in the European Integrated Project on Spinocerebellar Ataxias (EUROSCA) (www.eurosca.org), the study of individuals at risk for SCA1, SCA2, SCA3 and SCA6 (RISCA) [15] and the ENIGMA project (http://enigma.ini.usc.edu/ongoing/enigma-ataxia).
7. Conclusions
Funding
Conflicts of Interest
References
- Stoyas, C.A.; La Spada, A.R. The CAG-polyglutamine repeat diseases: A clinical, molecular, genetic, and pathophysiologic nosology. Handb. Clin. Neurol. 2018, 147, 143–170. [Google Scholar] [PubMed]
- Lastres-Becker, I.; Rub, U.; Auburg, G. Spinocerebellar Ataxia 2 (SCA2). Cerebellum 2008, 7, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ishida, C.; Komai, K.; Yonezawa, K.; Sakajiri, K.; Nitta, E.; Kawashima, A.; Yamada, M. An autopsy case of an aged patient with spinocerebellar ataxia type 2. Neuropathology 2011, 31, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Rub, U.; Schols, L.; Paulson, H.; Auburger, G.; Kermer, P.; Jen, J.C.; Seidel, K.; Korf, H.W.; Deller, T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Progr. Neurobiol. 2013, 104, 38–66. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, A. The neuropathology of the adult cerebellum. Hand. Clin. Neurol. 2018, 154, 129–149. [Google Scholar]
- Baldaçara, L.; Currie, S.; Hadjivassiliou, M.; Hoggard, N.; Jack, A.; Jackowski, A.P.; Mascalchi, M.; Parazzini, C.; Reetz, J.; Righini, A.; et al. Consensus Paper: Radiological biomarkers of cerebellar diseases. Cerebellum 2015, 14, 175–196. [Google Scholar] [CrossRef]
- Mascalchi, M.; Vella, A. Neuroimaging applications in chronic ataxias. Int. Rev. Neurobiol. 2018, 143, 109–162. [Google Scholar]
- Burk, K.; Abele, M.; Fetter, M.; Dichgans, J.; Skalej, M.; Laccone, F.; Didierjean, O.; Brice, A.A.; Klockgether, T. Autosomal dominant cerebellar ataxia type I. Clinical features and MRI in families with SCA1, SCA2 andSCA3. Brain 1996, 119, 1497–1505. [Google Scholar] [CrossRef]
- Mandelli, M.L.; De Simone, T.; Minati, L.; Bruzzone, M.G.; Mariotti, C.; Fancellu, R.; Savoiardo, M.; Grisoli, M. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. AJNR Am. J. Neuroradiol. 2007, 28, 1996–2000. [Google Scholar] [CrossRef]
- Lee, Y.C.; Liu, C.S.; Wu, H.M.; Wang, P.S.; Chang, M.H.; Soong, B.W. The ‘hot cross bun’ sign in the patients with spinocerebellar ataxia. Eur. J. Neurol. 2009, 16, 513–516. [Google Scholar] [CrossRef]
- Mascalchi, M.; Vella, A. Magnetic resonance and nuclear medicine imaging in ataxias. Handb. Clin. Neurol. 2012, 103, 85–110. [Google Scholar] [PubMed]
- Klockgether, T.; Skalej, M.; Wedekind, D.; Luft, R.; Welte, D.; Schulz, J.B.; Abele, M.; Burk, K.; Laccone, F.; Brice, A.; et al. Autosomal Dominant Ataxia Type I. MRI-based volumetry of the posterior fossa structures and basal ganglia in spinocerebellar ataxias type 1, 2 and 3. Brain 1998, 121, 1687–1693. [Google Scholar] [CrossRef]
- Mascalchi, M.; Diciotti, S.; Giannelli, M.; Ginestroni, A.; Soricelli, A.; Nicolai, E.; Aiello, M.; Tessa, C.; Galli, L.; Dotti, M.T.; et al. Progression of brain atrophy in SCA2. A longitudinal TBM study. PLoS ONE 2014, 9, e89410. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Yang, J.; Xiong, H.; Shang, H. Voxel-based meta-analysis of gray and white matter volume abnormalities in spinocerebellar ataxia type 2. Brain Behav. 2018, 8, e01099. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, H.; Reetz, K.; du Montcel, S.T.; Bauer, P.; Mariotti, C.; Nanetti, L.; Rakowicz, M.; Sulek, A.; Durr, A.; Perrine, C.; et al. Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: Analysis of baseline data. Lancet Neurol. 2013, 12, 650–658. [Google Scholar] [CrossRef]
- Reetz, K.; Rodríguez-Labrada, R.; Dogan, I.; Mirzazade, S.; Romanzetti, S.; Schulz, J.B.; Cruz-Rivas, E.M.; Alvarez-Cuesta, J.A.; Aguilera Rodríguez, R.; Gonzalez Zaldivar, Y. Brain atrophy measures in preclinical and manifest spinocerebellar ataxia type 2. Ann. Clin. Transl. Neurol. 2018, 5, 128–137. [Google Scholar] [CrossRef]
- Della Nave, R.; Ginestroni, A.; Tessa, C.; Salvatore, E.; De Grandis, D.; Plasmati, R.; Salvi, F.; De Michele, G.; Dotti, M.T.; Piacentini, S.; et al. Brain white matter damage in SCA1 and SCA2. An in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics. NeuroImage 2008, 43, 10–19. [Google Scholar] [CrossRef]
- Marzi, C.; Ciulli, S.; Giannelli, M.; Ginestroni, A.; Tessa, T.; Mascalchi, M.; Diciotti, S. Structural complexity of the cerebellum and cerebral cortex is reduced in spinocerebellar ataxia type 2. J. Neuroimaging 2018, 28, 688–693. [Google Scholar] [CrossRef]
- Guerrini, L.; Lolli, F.; Ginestroni, A.; Belli, G.; Della Nave, R.; Tessa, C.; Foresti, S.; Cosottin, M.; Piacentini, S.; Salvi, F.; et al. Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A volumetric, diffusion and quantitative proton spectroscopy MR study. Brain 2004, 127, 1785–1795. [Google Scholar]
- Salvatore, E.; Tedeschi, E.; Mollica, C.; Tedeschi, E.; Mollica, C.; Vicidomini, C.; Varrone, A.; Coda, A.R.; Brunetti, A.; Salvatore, M.; et al. Supratentorial and infratentorial damage in spinocerebellar ataxia 2: A diffusion-weighted MRI study. Mov. Disord. 2014, 29, 780–786. [Google Scholar] [CrossRef]
- Hernandez-Castillo, C.R.; Galvez, V.; Mercadillo, R.; Diaz, R.; Campos-Romo, A.; Fernandez-Ruiz, J. Extensive white matter alterations and its correlations with ataxia severity in SCA 2 patients. PLoS ONE 2015, 10, e0135449. [Google Scholar] [CrossRef] [PubMed]
- Olivito, G.; Lupo, M.; Iacobacci, C.; Clausi, S.; Romano, S.; Masciullo, M.; Cercignani, M.; Bozzali, M.; Leggio, M. Microstructural MRI basis of the cognitive functions in patients with spinocerebellar ataxia type 2. Neuroscience 2017, 366, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Adanyeguh, I.M.; Perlbarg, V.; Henry, P.G.; Rinaldi, D.; Petit, E.; Valabregue, R.; Brice, A.; Durr, A.; Mochel, F. Autosomal dominant cerebellar ataxias: Imaging biomarkers with high effect sizes. NeuroImage Clin. 2018, 19, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Marzi, C.; Giannelli, M.; Ciulli, S.; Bianchi, A.; Ginestroni, A.; Tessa, C.; Nicolai, E.; Aiello, M.; Salvatore, E.; et al. Histogram analysis of DTI-derived indices reveals pontocerebellar degeneration and its progression in SCA2. PLoS ONE 2018, 13, e0200258. [Google Scholar] [CrossRef]
- Cousins, J.P. Perspective clinical MR Spectroscopy: Fundamentals, current applications, and future potential. AJR Am. J. Roentgenol. 1995, 164, 1337–1347. [Google Scholar] [CrossRef]
- Öz, G. Magnetic Resonance Spectroscopy of Degenerative Brain Diseases, 1st ed.; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Gill, S.S.; Small, R.K.; Thomas, D.G.T.; Patel, P.; Porteous, R.; Van Bruggen, N.; Gadian, D.G.; Kauppinen, R.A.; Williams, S.R. Brain metabolites as1H NMR markers of neuronal and glial disorders. NMR Biomed. 1989, 2, 196–200. [Google Scholar] [CrossRef]
- Miller, B.L. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-l-aspartate, creatine and choline. NMR Biomed. 1991, 4, 47–52. [Google Scholar] [CrossRef]
- Tsai, G.; Coylet, J.T. N-Acetylaspartate in neuropsychiatric disorders. Prog. Neurobiol. 1995, 46, 531–540. [Google Scholar] [CrossRef]
- Brand, A.; Richter-Landsberg, C.; Leibfritz, D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 1993, 15, 289–298. [Google Scholar] [CrossRef]
- Duarte, J.M.N.; Lei, H.; Mlynárik, V.; Gruetter, R. The neurochemical profile quantified by in vivo 1HNMR spectroscopy. Neuroimage 2012, 61, 342–362. [Google Scholar] [CrossRef]
- Mascalchi, M.; Brugnoli, R.; Guerrini, L.; Belli, G.; Nistri, M.; Politi, L.S.; Gavazzi, C.; Lolli, F.; Argenti, G.; Villari, N. Single voxel long TE MR spectroscopy of the normal brainstem and cerebellum. J. Magn. Reson. Imaging 2002, 16, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Boesch, S.M.; Schocke, M.; Bürk, K.; Hollosi, P.; Fornai, F.; Aichner, F.T.; Poewe, W.; Felber, S. Proton magnetic resonance spectroscopic imaging reveals differences in spinocerebellar ataxia types 2 and 6. J. Magn. Reson. Imaging 2001, 13, 553–559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Viau, M.; Marchand, L.; Bard, C.; Boulanger, Y. 1H magnetic resonance spectroscopy of autosomal ataxias. Brain Res. 2005, 1049, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Boesch, S.M.; Wolf, C.; Seppi, K.; Felber, S.; Wenning, G.K.; Schocke, M. Differentiation of SCA2 from MSA-C using proton magnetic resonance spectroscopic imaging. J. Magn. Reson. Imaging 2007, 25, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Belli, G.; Mazzoni, L.N.; Ginestroni, A.; Foresti, S.; Diciotti, S.; Della Nave, R.; Mascalchi, M. Impact of CSF contamination on brain metabolites evaluation with 1H-MR spectroscopy. A single voxel study of the cerebellar vermis in patients with degenerative ataxias. J. Magn. Reson. Imaging 2009, 30, 11–17. [Google Scholar] [CrossRef]
- Oz, G.; Iltis, I.; Hutter, D.; Thomas, W.; Bushara, K.O.; Gomez, C.M. Distinct neurochemical profiles of spinocerebellar ataxias 1, 2, 6, and cerebellar multiple system atrophy. Cerebellum 2011, 10, 208–217. [Google Scholar] [CrossRef]
- Lirng, J.F.; Wang, P.S.; Chen, H.C.; Soong, B.W.; Guo, W.Y.; Wu, H.M.; Chang, C.Y. Differences between spinocerebellar ataxias and multiple system atrophy-cerebellar type on proton magnetic resonance spectroscopy. PLoS ONE 2012, 7, e47925. [Google Scholar] [CrossRef]
- Wang, P.S.; Chen, H.C.; Wu, H.M.; Lirng, J.F.; Wu, Y.T.; Soong, B.W. Association between Proton Magnetic Resonance Spectroscopy measurements and CAG repeat number in patients with Spinocerebellar Ataxias 2, 3, or 6. PLoS ONE 2012, 7, e47479. [Google Scholar] [CrossRef]
- Chen, H.C.; Lirng, J.F.; Soong, B.W.; Guo, W.Y.; Wu, H.M.; Chen, C.C.; Chang, C.Y. The merit of proton magnetic resonance spectroscopy in the longitudinal assessment of spinocerebellar ataxias and multiple system atrophy-cerebellar type. Cerebellum Ataxias 2014, 1, 17. [Google Scholar] [CrossRef]
- Adanyeguh, I.M.; Henry, P.G.; Nguyen, T.M.; Rinaldi, D.; Jauffret, C.; Valabregue, R.; Emir, U.E.; Deelchand, D.K.; Brice, A.; Eberly, L.E.; et al. In vivo neurometabolic profiling in patients with spinocerebellar ataxia types 1, 2, 3, and 7. Mov. Disord. 2015, 30, 662–670. [Google Scholar] [CrossRef]
- Joers, J.M.; Deelchand, D.K.; Lyu, T.; Emir, U.E.; Hutter, D.; Gomez, C.; Bushara, K.O.; Eberly, L.E.; Öz, G. Neurochemical abnormalities in premanifest and early spinocerebellar ataxias. Ann. Neurol. 2018, 83, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Krahe, J.; Binkofski, F.; Schulz, J.B.; Reetz, K.; Romanzetti, S. Neurochemical profiles in hereditary ataxias: A meta-analysis of Magnetic Resonance Spectroscopy studies. Neurosci. Biobehav. Rev. 2019, 108, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Hohenfeld, C.; Werner, C.J.; Reetz, K. Resting-state connectivity in neurodegenerative disorders: Is there potential for an imaging biomarker? NeuroImage Clin. 2018, 18, 849–870. [Google Scholar] [CrossRef]
- Price, C.J.; Crinion, J.; Friston, K.J. Design and analysis of fMRI studies with neurologically impaired patients. J. Magn. Reson. Imaging 2006, 23, 816–826. [Google Scholar] [CrossRef]
- Wu, T.; Wang, C.; Wang, J.; Ha llett, M.; Zang, Y.; Chan, P. Preclinical and clinical neural network changes in SCA2 parkinsonism. Parkinsonism Relat. Disord. 2013, 19, 158–164. [Google Scholar] [CrossRef][Green Version]
- Cocozza, S.; Saccà, F.; Cervo, A.; Marsili, A.; Russo, C.V.; Giorgio, S.M.; De Michele, G.; Filla, A.; Brunetti, A.; Quarantelli, M. Modifications of resting state networks in spinocerebellar ataxia type 2. Mov. Disord. 2015, 30, 1382–1390. [Google Scholar] [CrossRef]
- Hernandez-Castillo, C.R.; Galvez, V.; Mercadillo, R.E.; Díaz, R.; Yescas, P.; Martinez, L.; Ochoa, A.; Velazquez-Perez, L.; Fernandez-Ruiz, J. Functional connectivity changes related to cognitive and motor performance in spinocerebellar ataxia type 2. Mov. Disord. 2015, 30, 1391–1399. [Google Scholar] [CrossRef]
- Olivito, G.; Cercignani, M.; Lupo, M.; Iacobacci, C.; Clausi, S.; Romano, S.; Masciullo, M.; Molinari, M.; Bozzali, M.; Leggio, M. Neural substrates of motor and cognitive dysfunctions in SCA2 patients: A network-based statistics analysis. NeuroImage Clin. 2017, 14, 719–725. [Google Scholar] [CrossRef]
- Mascalchi, M.; Vella, A.; Ceravolo, R. Movement disorders: Role of imaging in diagnosis. J. Magn. Reson. Imaging 2012, 35, 239–256. [Google Scholar] [CrossRef]
- Vella, A.; Mascalchi, M. Nuclear medicine of the cerebellum. Handb. Clin. Neurol. 2018, 154, 251–266. [Google Scholar]
- Wüllner, U.; Reimold, M.; Abele, M.; Bürk, K.; Minnerop, M.; Dohmen, B.M.; Machulla, H.J.; Bares, R.; Klockgether, T. Dopamine Transporter Positron Emission Tomography in Spinocerebellar Ataxias Type 1, 2, 3, and 6. Arch. Neurol. 2005, 62, 1280–1285. [Google Scholar] [CrossRef]
- Wang, P.S.; Liu, R.S.; Yang, B.H.; Soong, B.W. Regional patterns of cerebral glucose metabolism in spinocerebellar ataxia type 2, 3 and 6: A voxel-based FDG-positron emission tomography analysis. J. Neurol. 2007, 254, 838–845. [Google Scholar] [CrossRef]
- Oh, M.; Kim, J.S.; Oh, J.S.; Lee, C.S.; Chung, S.J. Different subregional metabolism patterns in patients with cerebellar ataxia by 18F-fluorodeoxyglucose positron emission tomography. PLoS ONE 2017, 12, e0173275. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, A.; Iida, A.; Matsubara, M.; Inagaki, H. Positron emission tomography and magnetic resonance imaging in spinocerebellar ataxia type 2: A study of symptomatic and asymptomatic individuals. Eur. J. Neurol. 2005, 12, 725–728. [Google Scholar] [CrossRef]
- Lu, C.S.; Wu Chou, Y.H.; Yen, T.C.; Tsai, C.H.; Chen, R.S.; Chang, H.C. Dopa-responsive parkinsonism phenotype of spinocerebellar ataxia type 2. Mov. Disord. 2002, 17, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Boesch, S.M.; Donnemiller, E.; Müller, J.; Seppi, K.; Weirich-Schwaiger, H.; Poewe, W.; Wenning, G.K. Abnormalities of dopaminergic neurotransmission in SCA2: A combined 123I-betaCIT and 123I-IBZM SPECT study. Mov. Disord. 2004, 19, 1320–1325. [Google Scholar] [CrossRef]
- Varrone, A.; Salvatore, E.; De Michele, G.; Barone, P.; Sansone, V.; Pellecchia, M.T.; Castaldo, I.; Coppola, G.; Brunetti, A.; Salvatore, M.; et al. Reduced striatal [123 I]FP-CIT binding in SCA2 patients without parkinsonism. Ann. Neurol. 2004, 55, 426–430. [Google Scholar] [CrossRef]
- Kim, J.M.; Hong, S.; Kim, G.P.; Choi, Y.J.; Kim, Y.K.; Park, S.S.; Kim, S.E.; Jeon, B.S. Importance of low-range CAG expansion and CAA interruption in SCA2 Parkinsonism. Arch. Neurol. 2007, 64, 1510–1518. [Google Scholar] [CrossRef]
- Shan, D.E.; Soong, B.W.; Sun, C.M.; Lee, S.J.; Liao, K.K.; Liu, R.S. Spinocerebellar ataxia type 2 presenting as familial levodopa-responsive parkinsonism. Ann. Neurol. 2001, 50, 812–815. [Google Scholar] [CrossRef]
- Furtado, S.; Farrer, M.; Tsuboi, Y.; Klimek, M.L.; de la Fuente-Fernández, R.; Hussey, J.; Lockhart, P.; Calne, D.B.; Suchowersky, O.; Stoessl, A.J.; et al. SCA-2 presenting as parkinsonism in an Alberta family: Clinical, genetic, and PET findings. Neurology 2002, 59, 1625–1627. [Google Scholar] [CrossRef]
- Schöls, L.; Reimold, M.; Seidel, K.; Globas, C.; Brockmann, K.; Hauser, T.K.; Auburger, G.; Bürk, K.; den Dunnen, W.; Reischl, G.; et al. No parkinsonism in SCA2 and SCA3 despite severe neurodegeneration of the dopaminergic substantia nigra. Brain 2015, 138, 3316–3326. [Google Scholar] [CrossRef] [PubMed]
- Scherfler, C.; Boesch, S.M.; Donnemiller, E.; Seppi, K.; Weirich-Schwaiger, H.; Goebel, G.; Virgolini, I.; Wenning, G.K.; Poewe, W. Topography of cerebral monoamine transporter availability in families with SCA2 mutations: A voxel-wise [123I]beta-CIT SPECT analysis. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Öz, G.; Paulson, H.L. Spinocerebellar ataxias: Prospects and challenges for therapy development. Nat. Rev. Neurol. 2018, 14, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Pirker, W.; Back, C.; Gerschlager, W.; Laccone, F.; Alesch, F. Chronic thalamic stimulation in a patient with spinocerebellar ataxia type 2. Mov. Disord. 2003, 18, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Pope, P.A.; Miall, R.C. Restoring cognitive functions using non-invasive brain stimulation techniques in patients with cerebellar disorders. Front. Psychiatry 2014, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.; Schols, L. The use of quantitative methods in clinical trials for spinocerebellar ataxia. Arch. Neurol. 2002, 59, 1044–1045. [Google Scholar]


| Common (Adult Phenotype) |
|---|
| Progressive cerebellar ataxia |
| Dysarthria |
| Dysphagia |
| Oculomotor dysfunction |
| Pyramidal signs |
| Signs of lower motor neuron degeneration |
| Extra-pyramidal features * |
| Sensory-motor peripheral neuropathy |
| Painful muscle cramps |
| Autonomic dysfunction |
| Olfactory deficit |
| Sleep disturbances |
| Cognitive decline |
| Psychiatric symptoms |
| Uncommon (Infantile Phenotype) |
| Developmental delay |
| Facial dysmorphism |
| Retinitis pigmentosa |
| Myoclonus-epilepsy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascalchi, M.; Vella, A. Neuroimaging Biomarkers in SCA2 Gene Carriers. Int. J. Mol. Sci. 2020, 21, 1020. https://doi.org/10.3390/ijms21031020
Mascalchi M, Vella A. Neuroimaging Biomarkers in SCA2 Gene Carriers. International Journal of Molecular Sciences. 2020; 21(3):1020. https://doi.org/10.3390/ijms21031020
Chicago/Turabian StyleMascalchi, Mario, and Alessandra Vella. 2020. "Neuroimaging Biomarkers in SCA2 Gene Carriers" International Journal of Molecular Sciences 21, no. 3: 1020. https://doi.org/10.3390/ijms21031020
APA StyleMascalchi, M., & Vella, A. (2020). Neuroimaging Biomarkers in SCA2 Gene Carriers. International Journal of Molecular Sciences, 21(3), 1020. https://doi.org/10.3390/ijms21031020




