Targeting HGF/c-MET Axis in Pancreatic Cancer
Abstract
1. Introduction
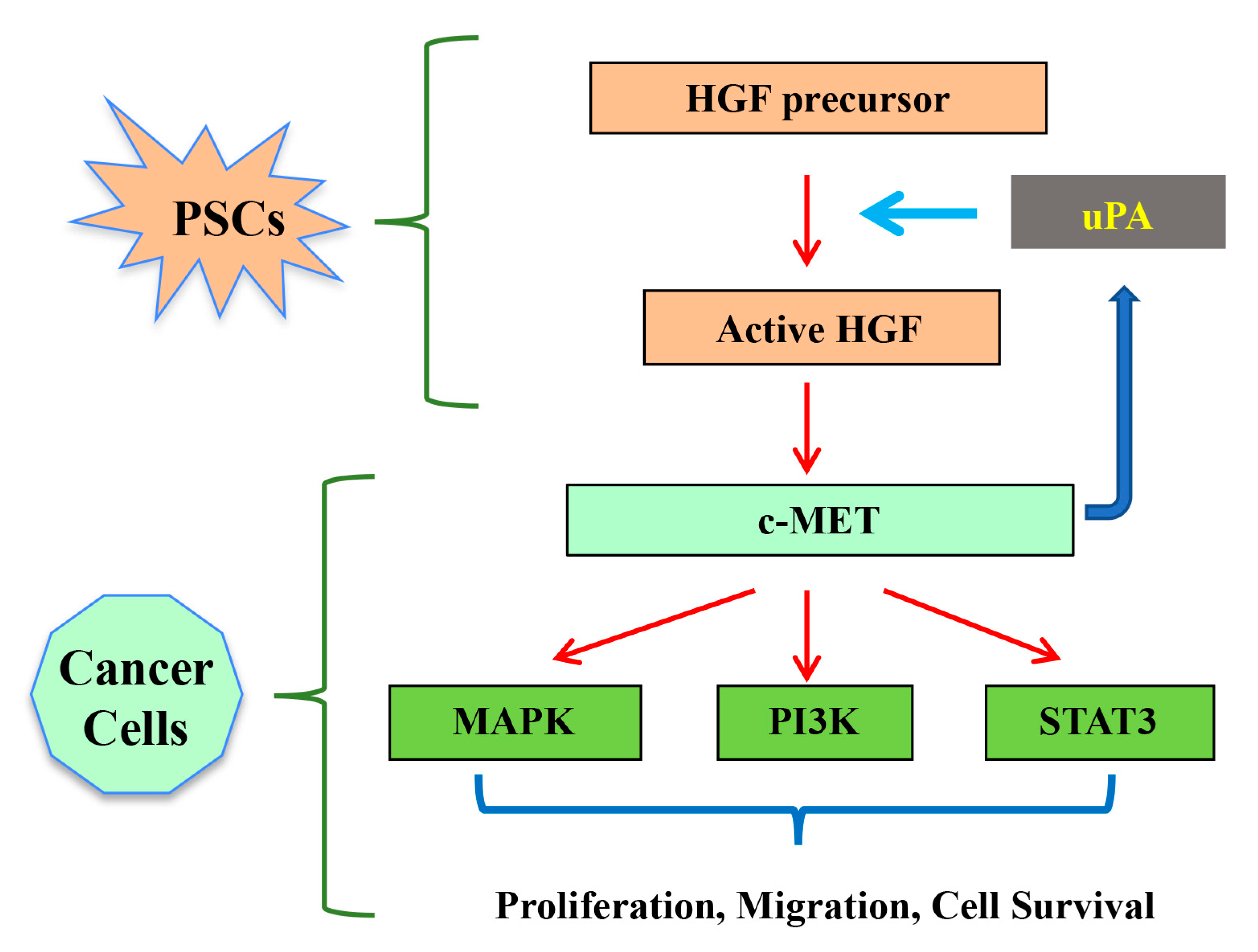
2. Hepatocyte Growth Factor (HGF)/c-MET Pathway
3. HGF/c-MET Pathway in Pancreatic Cancer
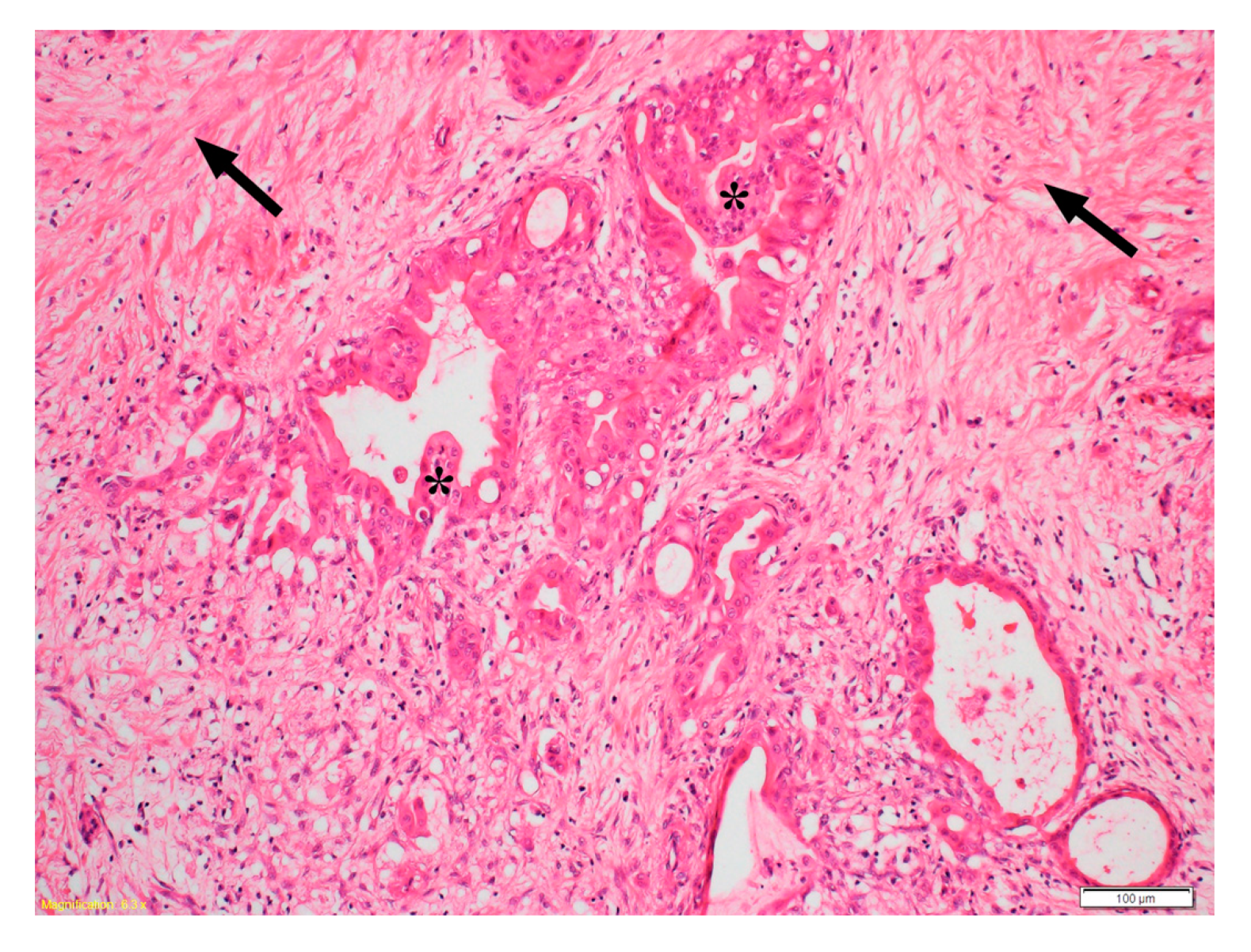
3.1. Role of HGF/c-MET Pathway in Hypoxia, Angiogenesis, Metastasis
3.2. HGF/c-MET and uPA Feed Forward Loop
3.3. HGF/c-MET and Microenvironment pH
3.4. HGF/c-MET and Treatment Resistance
3.5. Role of HGF as a Diagnostic Marker
4. Targeting HGF/c-MET Pathway
5. Conclusions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The Pancreas Cancer Microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef]
- Apte, M.V.; Xu, Z.; Pothula, S.; Goldstein, D.; Pirola, R.; Wilson, J. Pancreatic cancer: The microenvironment needs attention too! Pancreatology 2015, 15 (Suppl. S4), S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Park, S.; Phillips, P.A.; Santucci, N.; Goldstein, D.; Kumar, R.K.; Ramm, G.A.; Buchler, M.; Friess, H.; McCarroll, J.A.; et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Pothula, S.P.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Pancreatic stellate cells: Aiding and abetting pancreatic cancer progression. Pancreatology 2020, 20, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.F.; Moore, T.; Arumugam, T.; Ramachandran, V.; Amos, K.D.; Rivera, A.; Ji, B.; Evans, D.B.; Logsdon, C.D. Cancer-Associated Stromal Fibroblasts Promote Pancreatic Tumor Progression. Cancer Res. 2008, 68, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Pothula, S.P.; Xu, Z.; Goldstein, D.; Biankin, A.V.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Hepatocyte growth factor inhibition: A novel therapeutic approach in pancreatic cancer. Br. J. Cancer 2016, 114, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, A.; Joshi, S.; Qu, C.; Phillips, P.A.; Xu, Z.; Parker, N.R.; Toi, C.S.; Pirola, R.C.; Wilson, J.S.; Goldstein, D.; et al. Pancreatic Stellate Cells: Partners in Crime with Pancreatic Cancer Cells. Cancer Res. 2008, 68, 2085–2093. [Google Scholar] [CrossRef]
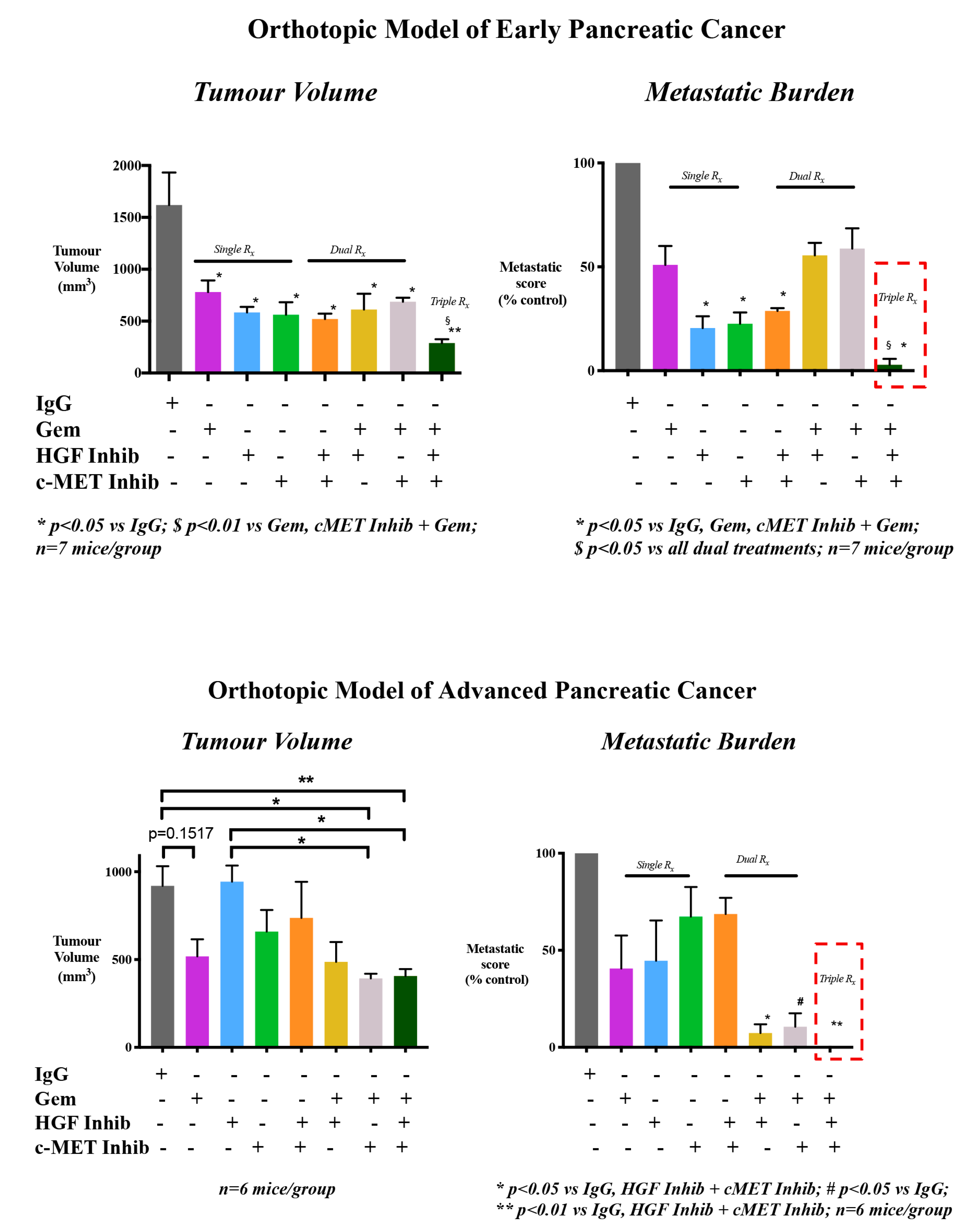
- Xu, Z.; Pang, T.C.Y.; Liu, A.C.; Pothula, S.P.; Mekapogu, A.R.; Perera, C.J.; Murakami, T.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; et al. Targeting the HGF/c-MET pathway in advanced pancreatic cancer: A key element of treatment that limits primary tumour growth and eliminates metastasis. Br. J. Cancer 2020, 122, 1486–1495. [Google Scholar] [CrossRef]
- Xu, Z.; Vonlaufen, A.; Phillips, P.A.; Fiala-Beer, E.; Zhang, X.; Yang, L.; Biankin, A.V.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; et al. Role of Pancreatic Stellate Cells in Pancreatic Cancer Metastasis. Am. J. Pathol. 2010, 177, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Mekapogu, A.R.; Pothula, S.P.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Multifunctional role of pancreatic stellate cells in pancreatic cancer. Ann. Pancreat. Cancer 2019, 2, 10. [Google Scholar] [CrossRef]
- Masamune, A.; Kikuta, K.; Watanabe, T.; Satoh, K.; Hirota, M.; Shimosegawa, T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am. J. Physiol. Liver Physiol. 2008, 295, G709–G717. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, G.; Zhou, S.; Henne-Bruns, D.; Bachem, M.; Kornmann, M. Interactions of pancreatic cancer and stellate cells are mediated by FGFR1-III isoform expression. Hepatogastroenterology 2012, 59, 1604–1608. [Google Scholar] [PubMed]
- Brammer, R.D.; Bramhall, S.R.; Eggo, M.C. Endostatin expression in pancreatic tissue is modulated by elastase. Br. J. Cancer 2004, 92, 89–93. [Google Scholar] [CrossRef]
- Garg, B.; Giri, B.; Modi, S.; Sethi, V.; Castro, I.; Umland, O.; Ban, Y.; Lavanis, S.; Dawra, R.; Banerjee, S. NFkappaB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-regulation of CXCL12. Gastroenterology 2018, 155, 880–891.e8. [Google Scholar] [CrossRef]
- Linde, N.; Lederle, W.; Depner, S.; Van Rooijen, N.; Gutschalk, C.M.; Mueller, M.M. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J. Pathol. 2012, 227, 17–28. [Google Scholar] [CrossRef]
- Uutela, M.; Wirzenius, M.; Paavonen, K.; Rajantie, I.; He, Y.; Karpanen, T.; Lohela, M.; Wiig, H.; Salven, P.; Pajusola, K.; et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood 2004, 104, 3198–3204. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Shi, J.; He, R.; Yang, W.; Habtezion, A.; Niu, N.; Lu, P.; Xue, J. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. EBioMedicine 2020, 58, 102920. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Ma, Q.; Xu, Q.; Liu, H.; Duan, W.; Lei, J.; Ma, J.; Wang, X.; Lv, S.; et al. Sonic Hedgehog Paracrine Signaling Activates Stromal Cells to Promote Perineural Invasion in Pancreatic Cancer. Clin. Cancer Res. 2014, 20, 4326–4338. [Google Scholar] [CrossRef]
- Han, L.; Ma, J.; Duan, W.; Zhang, L.; Yu, S.; Xu, Q.; Lei, J.; Li, X.; Wang, Z.; Wu, Z.; et al. Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway. Oncotarget 2016, 7, 18146–18158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lokker, N.; Mark, M.; Luis, E.; Bennett, G.; Robbins, K.; Baker, J.; Godowski, P. Structure-function analysis of hepatocyte growth factor: Identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992, 11, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Ponzetto, C.; Di Renzo, M.F.; Cooper, C.S.; Comoglio, P.M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nat. Cell Biol. 1989, 339, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, P.M.; Trusolino, L. Invasive growth: From development to metastasis. J. Clin. Investig. 2002, 109, 857–862. [Google Scholar] [CrossRef]
- Modica, C.; Tortarolo, D.; Comoglio, P.M.; Basilico, C.; Vigna, E. MET/HGF Co-Targeting in Pancreatic Cancer: A Tool to Provide Insight into the Tumor/Stroma Crosstalk. Int. J. Mol. Sci. 2018, 19, 3920. [Google Scholar] [CrossRef]
- Tamagnone, L.; Comoglio, P.M. Control of invasive growth by hepatocyte growth factor (HGF) and related scatter factors. Cytokine Growth Factor Rev. 1997, 8, 129–142. [Google Scholar] [CrossRef]
- Montesano, R.; Matsumoto, K.; Nakamura, T.; Orci, L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991, 67, 901–908. [Google Scholar] [CrossRef]
- Chmielowiec, J.; Borowiak, M.; Morkel, M.; Stradal, T.; Munz, B.; Werner, S.; Wehland, J.; Birchmeier, C.; Birchmeier, W. c-Met is essential for wound healing in the skin. J. Cell Biol. 2007, 177, 151–162. [Google Scholar] [CrossRef]
- Gherardi, E.; Birchmeier, W.; Birchmeier, C.; Woude, G.V. Targeting MET in cancer: Rationale and progress. Nat. Rev. Cancer 2012, 12, 89–103. [Google Scholar] [CrossRef]
- Jiang, W.G.; Martin, T.A.; Parr, C.; Davies, G.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit. Rev. Oncol. 2005, 53, 35–69. [Google Scholar] [CrossRef]
- Birchmeier, C.; Gherardi, E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998, 8, 404–410. [Google Scholar] [CrossRef]
- Bean, J.; Brennan, C.; Shih, J.-Y.; Riely, G.; Viale, A.; Wang, L.; Chitale, D.; Motoi, N.; Szoke, J.; Broderick, S.; et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. USA 2007, 104, 20932–20937. [Google Scholar] [CrossRef] [PubMed]
- Houldsworth, J.; Cordon-Cardo, C.; Ladanyi, M.; Kelsen, D.P.; Chaganti, R.S. Gene amplification in gastric and esophageal adenocarcinomas. Cancer Res. 1990, 50, 6423–6429. [Google Scholar]
- Schmidt, L.; Duh, F.M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubenshy, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y. Growth Factors and their receptors in cancer metastases. Front. Biosci. 2011, 16, 531–538. [Google Scholar] [CrossRef]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; Woude, G.F.V. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef]
- Cañadas, I.; Rojo, F.; Arumí-Uría, M.; Rovira, A.; Albanell, J.; Arriola, E. C-MET as a new therapeutic target for the development of novel anticancer drugs. Clin. Transl. Oncol. 2010, 12, 253–260. [Google Scholar] [CrossRef]
- Gibbons, A.V.; Lin, J.E.; Kim, G.W.; Marszalowicz, G.P.; Li, P.; Stoecker, B.A.; Blomain, E.S.; Rattan, S.; Snook, A.E.; Schulz, S.; et al. Intestinal GUCY2C prevents TGF-beta secretion coordinating desmoplasia and hyperproliferation in colorectal cancer. Cancer Res. 2013, 73, 6654–6666. [Google Scholar] [CrossRef]
- Patel, M.B.; Pothula, S.P.; Xu, Z.; Lee, A.K.; Goldstein, D.; Pirola, R.C.; Apte, M.V.; Wilson, J.S. The role of the hepatocyte growth factor/c-MET pathway in pancreatic stellate cell-endothelial cell interactions: Antiangiogenic implications in pancreatic cancer. Carcinogenesis 2014, 35, 1891–1900. [Google Scholar] [CrossRef]
- Kemik, O.; Purisa, S.; Kemik, A.S.; Tuzun, S. Increase in the circulating level of hepatocyte growth factor in pancreatic cancer patients. Bratisl Lek List. 2009, 110, 627–629. [Google Scholar]
- Ueda, T.; Takeyama, Y.; Hori, Y.; Nishikawa, J.; Yamamoto, M.; Saitoh, Y. Hepatocyte growth factor in assessment of acute pancreatitis: Comparison with C-reactive protein and interleukin-6. J. Gastroenterol. 1997, 32, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Huang, C.; Qiu, Z.J.; Liu, J.; Zhang, Z.H.; Zhao, N.; Fong, Z.-Z.; Lv, X.-H. Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Dig. Dis. Sci. 2011, 56, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kim, M.A.; Ryu, J.K.; Yoon, Y.B.; Kim, S.-W.; Han, H.-S.; Kang, G.H.; Kim, H.; Hwang, J.-H.; Kim, Y.T. Postoperative Prognostic Predictors of Pancreatic Ductal Adenocarcinoma: Clinical Analysis and Immunoprofile on Tissue Microarrays. Ann. Surg. Oncol. 2012, 19, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Rausch, V.; Giese, N.; Giese, T.; Schonsiegel, F.; Labsch, S.; Nwaeburu, C.; Mattern, J.; Gladkich, J.; Herr, I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013, 4, e627. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Somcio, R.J.; Fan, F.; Liu, W.; Johnson, M.; Lesslie, D.P.; Evans, U.B.; Gallick, G.; Ellis, L.M. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol. Cancer Ther. 2006, 5, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.S.; Gážiová, I.; Harrigal, L.; Guerra, Y.A.; Qiu, S.; Sastry, S.K.; Arumugam, T.; Logsdon, C.D.; Elferink, C.J. Met Receptor Tyrosine Kinase Signaling Induces Secretion of the Angiogenic Chemokine Interleukin-8/CXCL8 in Pancreatic Cancer. PLoS ONE 2012, 7, e40420. [Google Scholar] [CrossRef]
- Avan, A.; Caretti, V.; Funel, N.; Galvani, E.; Maftouh, M.; Honeywell, R.J.; Lagerweij, T.; Van Tellingen, O.; Campani, D.; Fuchs, D.; et al. Crizotinib inhibits metabolic inactivation of gemcitabine in c-Met-driven pancreatic carcinoma. Cancer Res. 2013, 73, 6745–6756. [Google Scholar] [CrossRef]
- Christensen, J.; Schreck, R.; Burrows, J.; Kuruganti, P.; Chan, E.; Le, P.; Chen, J.; Wang, X.; Ruslim, L.; Blake, R.; et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003, 63, 7345–7355. [Google Scholar]
- Jin, H.; Yang, R.; Zheng, Z.; Romero, M.; Ross, J.; Bou-Reslan, H.; Carano, R.A.D.; Kasman, I.; Mai, E.; Young, J.; et al. MetMAb, the One-Armed 5D5 Anti-c-Met Antibody, Inhibits Orthotopic Pancreatic Tumor Growth and Improves Survival. Cancer Res. 2008, 68, 4360–4368. [Google Scholar] [CrossRef]
- Ucar, D.A.; Magis, A.T.; He, D.-H.; Lawrence, N.J.; Sebti, S.M.; Kurenova, E.; Zajac-Kaye, M.; Zhang, J.; Hochwald, S.N. Inhibiting the Interaction of cMET and IGF-1R with FAK Effectively Reduces Growth of Pancreatic Cancer Cells in vitro and in vivo. Anti-Cancer Agents Med. Chem. 2013, 13, 595–602. [Google Scholar] [CrossRef]
- Mariani, M.; McHugh, M.; Petrillo, M.; Sieber, S.; He, S.; Andreoli, M.; Wu, Z.; Fiedler, P.; Scambia, G.; Shahabi, S.; et al. HGF/c-Met axis drives cancer aggressiveness in the neo-adjuvant setting of ovarian cancer. Oncotarget 2014, 5, 4855–4867. [Google Scholar] [CrossRef] [PubMed]
- Rizwani, W.; Allen, A.E.; Trevino, J.G. Hepatocyte Growth Factor from a Clinical Perspective: A Pancreatic Cancer Challenge. Cancers 2015, 7, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Pothula, S.P.; Xu, Z.; Goldstein, D.; Merrett, N.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Targeting the HGF/c-MET pathway: Stromal remodelling in pancreatic cancer. Oncotarget 2017, 8, 76722–76739. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Xu, Z.; Mekapogu, A.; Pothula, S.; Goldstein, D.; Pirola, R.; Wilson, J.; Apte, M. A Novel Adjuvant Treatment Approach in Pancreatic Cancer via Hepatocyte Growth Factor (HGF)/c-MET Inhibition; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019. [Google Scholar]
- Basilico, C.; Modica, C.; Maione, F.; Vigna, E.; Comoglio, P.M. Targeting the MET oncogene by concomitant inhibition of receptor and ligand via an antibody-“decoy” strategy. Int. J. Cancer 2018, 143, 1774–1785. [Google Scholar] [CrossRef]
- Pennacchietti, S.; Michieli, P.; Galluzzo, M.; Mazzone, M.; Giordano, S.; Comoglio, P.M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003, 3, 347–361. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ide, T.; Ohtsuka, T.; Miyazaki, K. Induction of hepatocyte growth factor activator gene expression under hypoxia activates the hepatocyte growth factor/c-Met system via hypoxia inducible factor-1 in pancreatic cancer. Cancer Sci. 2008, 99, 1341–1347. [Google Scholar] [CrossRef]
- Ding, S.; Merkulova-Rainon, T.; Han, Z.; Tobelem, G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood 2003, 101, 4816–4822. [Google Scholar] [CrossRef]
- Boccaccio, C.; Comoglio, P.M. Invasive growth: A MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer 2006, 6, 637–645. [Google Scholar] [CrossRef]
- Comoglio, P.M.; Trusolino, L.; Boccaccio, C. Known and novel roles of the MET oncogene in cancer: A coherent approach to targeted therapy. Nat. Rev. Cancer 2018, 18, 341–358. [Google Scholar] [CrossRef]
- Kubota, T.; Matsumura, A.; Taiyoh, H.; Izumiya, Y.; Fujiwara, H.; Okamoto, K.; Ichikawa, D.; Shiozaki, A.; Komatsu, S.; Nakanishi, M.; et al. Interruption of the HGF paracrine loop by NK4, an HGF antagonist, reduces VEGF expression of CT26 cells. Oncol. Rep. 2013, 30, 567–572. [Google Scholar] [CrossRef]
- Jiang, W.G.; Martin, T.A.; Matsumoto, K.; Nakamura, T.; Mansel, R.E. Hepatocyte growth factor/scatter factor decreases the expression of occludin and transendothelial resistance (TER) and increases paracellular permeability in human vascular endothelial cells. J. Cell. Physiol. 1999, 181, 319–329. [Google Scholar] [CrossRef]
- Li, H.; Shimura, H.; Aoki, Y.; Date, K.; Matsumoto, K.; Nakamura, T.; Tanaka, M. Hepatocyte growth factor stimulates the invasion of gallbladder carcinoma cell lines in vitro. Clin. Exp. Metastasis 1998, 16, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, O.; Murakami, K.; Yamaura, T.; Fujiuchi, Y.; Murata, J.; Fuse, H.; Saiki, I. Expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) on prostate cancer cell lines. Cancer Lett. 2000, 155, 173–179. [Google Scholar] [CrossRef]
- Jianga, Y.; Xua, W.; Lub, J.; Hea, F.; Yang, X. Invasiveness of Hepatocellular Carcinoma Cell Lines: Contribution of Hepatocyte Growth Factor, c-met, and Transcription Factor Ets-1. Biochem. Biophys. Res. Commun. 2001, 286, 1123–1130. [Google Scholar] [CrossRef]
- Nan, L.; Qin, T.; Xiao, Y.; Qian, W.; Li, J.; Wang, Z.; Ma, J.; Ma, Q.; Wu, Z. Pancreatic Stellate Cells Facilitate Perineural Invasion of Pancreatic Cancer via HGF/c-Met Pathway. Cell Transplant. 2019, 28, 1289–1298. [Google Scholar] [CrossRef]
- Paciucci, R.; Vilà, M.R.; Adell, T.; Díaz, V.M.; Torà, M.; Nakamura, T.; Real, F.X. Activation of the Urokinase Plasminogen Activator/Urokinase Plasminogen Activator Receptor System and Redistribution of E-Cadherin Are Associated with Hepatocyte Growth Factor-Induced Motility of Pancreas Tumor Cells Overexpressing Met. Am. J. Pathol. 1998, 153, 201–212. [Google Scholar] [CrossRef]
- Vilá, M.R.; Nakamura, T.; Real, F.X. Hepatocyte growth factor is a potent mitogen for normal human pancreas cells in vitro. Lab. Investig. 1995, 73, 409–418. [Google Scholar]
- Qian, L.-W.; Mizumoto, K.; Maehara, N.; Ohuchida, K.; Inadome, N.; Saimura, M.; Nagai, E.; Matsumoto, K.; Nakamura, T.; Tanaka, M. Co-cultivation of pancreatic cancer cells with orthotopic tumor-derived fibroblasts: Fibroblasts stimulate tumor cell invasion via HGF secretion whereas cancer cells exert a minor regulative effect on fibroblasts HGF production. Cancer Lett. 2003, 190, 105–112. [Google Scholar] [CrossRef]
- Ide, T.; Kitajima, Y.; Miyoshi, A.; Ohtsuka, T.; Mitsuno, M.; Ohtaka, K.; Koga, Y.; Miyazaki, K. Tumor-stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. Int. J. Cancer 2006, 119, 2750–2759. [Google Scholar] [CrossRef]
- Bauer, T.W.; Liu, W.; Fan, F.; Camp, E.R.; Yang, A.; Somcio, R.J.; Bucana, C.D.; Callahan, J.; Parry, G.C.; Evans, D.B.; et al. Targeting of Urokinase Plasminogen Activator Receptor in Human Pancreatic Carcinoma Cells Inhibits c-Met– and Insulin-like Growth Factor-I Receptor-Mediated Migration and Invasion and Orthotopic Tumor Growth in Mice. Cancer Res. 2005, 65, 7775–7781. [Google Scholar] [CrossRef]
- Buckley, B.J.; Aboelela, A.; Minaei, E.; Jiang, L.; Xu, Z.; Ali, U.; Fildes, K.; Cheung, C.-Y.; Cook, S.M.; Johnson, D.C.; et al. 6-Substituted Hexamethylene Amiloride (HMA) Derivatives as Potent and Selective Inhibitors of the Human Urokinase Plasminogen Activator for Use in Cancer. J. Med. Chem. 2018, 61, 8299–8320. [Google Scholar] [CrossRef] [PubMed]
- Putney, L.K.; Denker, S.P.; Barber, D.L. The changing face of the Na+/H+ exchanger, NHE1: Structure, regulation, and cellular actions. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 527–552. [Google Scholar] [CrossRef] [PubMed]
- Weinhouse, S.; Warburg, O.; Burk, D.; Schade, A.L. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Grinstein, S.; Rotin, D.; Mason, M.J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1989, 988, 73–97. [Google Scholar] [CrossRef]
- Harguindey, S.; Orive, G.; Pedraz, J.; Paradiso, A.; Alfarouk, K.O. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—one single nature. Biochim. Biophys. Acta (BBA) Bioenerg. 2005, 1756, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Seftor, E.A.; Seftor, R.E.B.; Chu, Y.-W.; Gillies, R.J.; Hendrix, M.J.C. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 1996, 14, 176–186. [Google Scholar] [CrossRef]
- Kaplan, D.L.; Boron, W.F. Long-term expression of c-H-ras stimulates Na-H and Na(+)-dependent Cl-HCO3 exchange in NIH-3T3 fibroblasts. J. Biol. Chem. 1994, 269, 4116–4126. [Google Scholar]
- Kaneko, A.; Hayashi, N.; Tanaka, Y.; Horimoto, M.; Ito, T.; Sasaki, Y.; Fusamoto, H.; Kamada, T. Activation of Na+/H+ exchanger by hepatocyte growth factor in hepatocytes. Hepatology 1995, 22, 629–636. [Google Scholar]
- Glunde, K.; Guggino, S.E.; Solaiyappan, M.; Pathak, A.P.; Ichikawa, Y.; Bhujwalla, Z.M. Extracellular Acidification Alters Lysosomal Trafficking in Human Breast Cancer Cells. Neoplasia 2003, 5, 533–545. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ohi, H.; Sugimura, M.; Shinohara, H.; Fujii, T.; Terao, T. Inhibition of in vitro ovarian cancer cell invasion by modulation of urokinase-type plasminogen activator and cathepsin B. Cancer Res. 1992, 52, 3610–3614. [Google Scholar]
- Steffan, J.J.; Williams, B.C.; Welbourne, T.; Cardelli, J.A. HGF-induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+-H+ exchangers. J. Cell Sci. 2010, 123, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, J.; Hynes, M.; Dosch, J.; Sarkar, B.; Welling, T.H.; Di Magliano, M.P.; Simeone, D.M. c-Met Is a Marker of Pancreatic Cancer Stem Cells and Therapeutic Target. Gastroenterology 2011, 141, 2218–2227.e5. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Summy, J.M.; Zhang, J.; Park, S.I.; Parikh, N.U.; Gallick, G. Development and Characterization of Gemcitabine-Resistant Pancreatic Tumor Cells. Ann. Surg. Oncol. 2007, 14, 3629–3637. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Konno, M.; Eguchi, H.; Kawamoto, K.; Mukai, R.; Nishida, N.; Koseki, J.; Wada, H.; Akita, H.; Satoh, T.; et al. c-Met affects gemcitabine resistance during carcinogenesis in a mouse model of pancreatic cancer. Oncol. Lett. 2018, 16, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Brandes, F.; Schmidt, K.; Wagner, C.; Redekopf, J.; Schlitt, H.J.; Geissler, E.K.; Lang, S.A. Targeting cMET with INC280 impairs tumour growth and improves efficacy of gemcitabine in a pancreatic cancer model. BMC Cancer 2015, 15. [Google Scholar] [CrossRef]
- Firuzi, O.; Che, P.P.; El Hassouni, B.; Buijs, M.; Coppola, S.; Löhr, J.-M.; Funel, N.; Heuchel, R.L.; Carnevale, I.; Schmidt, T.; et al. Role of c-MET Inhibitors in Overcoming Drug Resistance in Spheroid Models of Primary Human Pancreatic Cancer and Stellate Cells. Cancers 2019, 11, 638. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, J.; Kim, M.A.; Choi, J.; Kim, W.H. Increased HGF Expression Induces Resistance to c-MET Tyrosine Kinase Inhibitors in Gastric Cancer. Anticancer. Res. 2017, 37, 1127–1138. [Google Scholar] [CrossRef]
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernández-Pérez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; et al. Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018, 28, 848–865.e6. [Google Scholar] [CrossRef]
- Giannoni, P.; Pietra, G.; Travaini, G.; Quarto, R.; Shyti, G.; Benelli, R.; Ottaggio, L.; Mingari, M.C.; Zupo, S.; Cutrona, G.; et al. Chronic lymphocytic leukemia nurse-like cells express hepatocyte growth factor receptor (c-MET) and indoleamine 2,3-dioxygenase and display features of immunosuppressive type 2 skewed macrophages. Haematolpgy 2014, 99, 1078–1087. [Google Scholar] [CrossRef]
- Lee, L.S.; Banks, P.A.; Bellizzi, A.M.; Sainani, N.I.; Kadiyala, V.; Suleiman, S.; Conwell, D.L.; Paulo, J.A. Inflammatory protein profiling of pancreatic cyst fluid using EUS-FNA in tandem with cytokine microarray differentiates between branch duct IPMN and inflammatory cysts. J. Immunol. Methods 2012, 382, 142–149. [Google Scholar] [CrossRef]
- Cecchi, F.; Rabe, D.C.; Bottaro, D.P. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.R.; Tsao, M.S. c-MET as a potential therapeutic target and biomarker in cancer. Ther. Adv. Med. Oncol. 2011, 3 (Suppl. S1), S21–S35. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Senzer, N.; Mekhail, T.; Ganapathi, R.; Chai, F.; Savage, R.E.; Waghorne, C.; Abbadessa, G.; Schwartz, B.; Dreicer, R. A Phase I Dose-Escalation Study of Tivantinib (ARQ 197) in Adult Patients with Metastatic Solid Tumors. Clin. Cancer Res. 2011, 17, 7754–7764. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.S.; Sweeney, C.J.; Mendelson, D.S.; Eckhardt, S.G.; Anderson, A.; Beaupre, D.M.; Branstetter, D.; Burgess, T.L.; Coxon, A.; Deng, H.; et al. Safety, Pharmacokinetics, and Pharmacodynamics of AMG 102, a Fully Human Hepatocyte Growth Factor-Neutralizing Monoclonal Antibody, in a First-in-Human Study of Patients with Advanced Solid Tumors. Clin. Cancer Res. 2010, 16, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Adjei, A.A. In the clinic: Ongoing clinical trials evaluating c-MET-inhibiting drugs. Ther. Adv. Med Oncol. 2011, 3, S37–S50. [Google Scholar] [CrossRef] [PubMed]
- A Randomized Phase 2 Study of ARQ 197 Versus Gemcitabine in Treatment-Naïve Patients With. Unresectable Locally Advanced or Metastatic Pancreatic Adenocarcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00558207 (accessed on 30 November 2020).
- Catenacci, D.V.T.; Tebbutt, N.C.; David, C.; Murad, A.M.; Al-Batran, S.-E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482. [Google Scholar] [CrossRef]
- Spigel, D.R.; Edelman, M.J.; O’Byrne, K.; Paz-Ares, L.; Mocci, S.; Phan, S.; Shames, D.S.; Smith, D.; Yu, W.; Paton, V.E.; et al. Results From the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J. Clin. Oncol. 2017, 35, 412–420. [Google Scholar] [CrossRef]
- Papaccio, F.; Della Corte, C.; Viscardi, G.; Di Liello, R.; Esposito, G.; Sparano, F.; Ciardiello, F.; Morgillo, F. HGF/MET and the Immune System: Relevance for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3595. [Google Scholar] [CrossRef]
- Hughes, V.S.; Siemann, D.W. Have Clinical Trials Properly Assessed c-Met Inhibitors? Trends Cancer 2018, 4, 94–97. [Google Scholar] [CrossRef]
- Giordano, S.; Maffe, A.; Williams, T.A.; Artigiani, S.; Gual, P.; Bardelli, A.; Basilico, C.; Michieli, P.; Comoglio, P.M. Different point mutations in the met oncogene elicit distinct biological properties. FASEB J. 2000, 14, 399–406. [Google Scholar] [CrossRef]
- Michieli, P.; Basilico, C.; Pennacchietti, S.; Maffè, A.; Tamagnone, L.; Giordano, S.; Bardelli, A.; Comoglio, P.M. Mutant Met-mediated transformation is ligand-dependent and can be inhibited by HGF antagonists. Oncogene 1999, 18, 5221–5231. [Google Scholar] [CrossRef] [PubMed]
- Burgess, T.L.; Sun, J.; Meyer, S.; Tsuruda, T.S.; Elliott, G.; Chen, Q.; Haniu, M.; Barron, W.F.; Juan, T.; Zhang, K.; et al. Biochemical Characterization of AMG 102: A Neutralizing, Fully Human Monoclonal Antibody to Human and Nonhuman Primate Hepatocyte Growth Factor. Mol. Cancer Ther. 2010, 9, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, D.; Maehara, N.; Kuba, K.; Mizumoto, K.; Tanaka, M.; Matsumoto, K.; Nakamura, T. Inhibition of growth, invasion, and metastasis of human pancreatic carcinoma cells by NK4 in an orthotopic mouse model. Cancer Res. 2001, 61, 7518–7524. [Google Scholar] [PubMed]
- Qian, L.-W.; Mizumoto, K.; Inadome, N.; Nagai, E.; Sato, N.; Matsumoto, K.; Nakamura, T.; Tanaka, M. Radiation stimulates HGF receptor/c-Met expression that leads to amplifying cellular response to HGF stimulation via upregulated receptor tyrosine phosphorylation and MAP kinase activity in pancreatic cancer cells. Int. J. Cancer 2003, 104, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Burgess, T.; Coxon, A.; Meyer, S.; Sun, J.; Rex, K.; Tsuruda, T.; Chen, Q.; Ho, S.-Y.; Li, L.; Kaufman, S.; et al. Fully Human Monoclonal Antibodies to Hepatocyte Growth Factor with Therapeutic Potential against Hepatocyte Growth Factor/c-Met-Dependent Human Tumors. Cancer Res. 2006, 66, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Greenall, S.A.; Adams, T.E.; Johns, T.G. Incomplete target neutralization by the anti-cancer antibody rilotumumab. mAbs 2016, 8, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Hong, J.Y.; Park, S.H.; Park, J.O.; Park, Y.W.; Park, N.; Lee, H.; Hong, S.H.; Lee, S.-J.; Song, S.-W.; et al. First-in-human phase I trial of anti-hepatocyte growth factor antibody (YYB101) in refractory solid tumor patients. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef]
- You, W.-K.; Sennino, B.; Williamson, C.W.; Falcón, B.; Hashizume, H.; Yao, L.-C.; Aftab, D.T.; McDonald, D.M. VEGF and c-Met Blockade Amplify Angiogenesis Inhibition in Pancreatic Islet Cancer. Cancer Res. 2011, 71, 4758–4768. [Google Scholar] [CrossRef]
- Sennino, B.; Ishiguro-Oonuma, T.; Wei, Y.; Naylor, R.M.; Williamson, C.W.; Bhagwandin, V.; Tabruyn, S.P.; You, W.-K.; Chapman, H.A.; Christensen, J.G.; et al. Suppression of Tumor Invasion and Metastasis by Concurrent Inhibition of c-Met and VEGF Signaling in Pancreatic Neuroendocrine Tumors. Cancer Discov. 2012, 2, 270–287. [Google Scholar] [CrossRef]
- An Open-Label, Phase II Study of Cabozantinib (XL184) in Advanced Pancreatic Neuroendocrine and Carcinoid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT01466036 (accessed on 30 November 2020).
- Cabozantinib in Advanced Pancreatic Neuroendocrine and Carcinoid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT03375320 (accessed on 30 November 2020).
- Takiguchi, S.; Inoue, K.; Matsusue, K.; Furukawa, M.; Teramoto, N.; Iguchi, H. Crizotinib, a MET inhibitor, prevents peritoneal dissemination in pancreatic cancer. Int. J. Oncol. 2017, 51, 184–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, J.; Peng, F. Acquired resistance to crizotinib in advanced lung adenocarcinoma with MET exon 14 skipping. Lung Cancer 2017, 113, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Lai, A.; Ali, S.M.; Miller, V.A.; Raez, L.E. Mutation of MET Y1230 as an Acquired Mechanism of Crizotinib Resistance in NSCLC with MET Exon 14 Skipping. J. Thorac. Oncol. 2017, 12, e89–e90. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-H.I.; Young, L.; Schrock, A.B.; Johnson, A.; Klempner, S.J.; Zhu, V.W.; Miller, V.A.; Ali, S.M. Emergence of Preexisting MET Y1230C Mutation as a Resistance Mechanism to Crizotinib in NSCLC with MET Exon 14 Skipping. J. Thorac. Oncol. 2017, 12, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-J.; Li, P.; Wu, C.-L.; Zhou, X.-Y.; Lu, H.-J.; Zhou, T. Response and acquired resistance to crizotinib in Chinese patients with lung adenocarcinomas harboring MET Exon 14 splicing alternations. Lung Cancer 2016, 102, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, E.; Shen, H.; Wang, X.; Tang, T.; Zhang, X.; Xu, J.; Tang, Z.; Guo, C.; Bai, X.; et al. Targeting the HGF/MET Axis in Cancer Therapy: Challenges in Resistance and Opportunities for Improvement. Front. Cell Dev. Biol. 2020, 8, 152. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, W.; Wortinger, M.A.; Yan, S.B.; Cornwell, P.; Peek, V.L.; Stephens, J.R.; Tetreault, J.W.; Xia, J.; Manro, J.R.; et al. LY2875358, a Neutralizing and Internalizing Anti-MET Bivalent Antibody, Inhibits HGF-Dependent and HGF-Independent MET Activation and Tumor Growth. Clin. Cancer Res. 2014, 20, 6059–6070. [Google Scholar] [CrossRef]
- Rosen, L.S.; Goldman, J.W.; Algazi, A.P.; Turner, P.K.; Moser, B.; Hu, T.; Wang, X.A.; Tuttle, J.; Wacheck, V.; Wooldridge, J.E.; et al. A First-in-Human Phase I Study of a Bivalent MET Antibody, Emibetuzumab (LY2875358), as Monotherapy and in Combination with Erlotinib in Advanced Cancer. Clin. Cancer Res. 2017, 23, 1910–1919. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pothula, S.P.; Xu, Z.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Targeting HGF/c-MET Axis in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 9170. https://doi.org/10.3390/ijms21239170
Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV. Targeting HGF/c-MET Axis in Pancreatic Cancer. International Journal of Molecular Sciences. 2020; 21(23):9170. https://doi.org/10.3390/ijms21239170
Chicago/Turabian StylePothula, Srinivasa P., Zhihong Xu, David Goldstein, Romano C. Pirola, Jeremy S. Wilson, and Minoti V. Apte. 2020. "Targeting HGF/c-MET Axis in Pancreatic Cancer" International Journal of Molecular Sciences 21, no. 23: 9170. https://doi.org/10.3390/ijms21239170
APA StylePothula, S. P., Xu, Z., Goldstein, D., Pirola, R. C., Wilson, J. S., & Apte, M. V. (2020). Targeting HGF/c-MET Axis in Pancreatic Cancer. International Journal of Molecular Sciences, 21(23), 9170. https://doi.org/10.3390/ijms21239170




