Urinary Metabolomic Profiling in Streptozotocin-Induced Diabetic Mice after Treatment with Losartan
Abstract
1. Introduction
2. Results
2.1. Characteristics of Experimental Animals
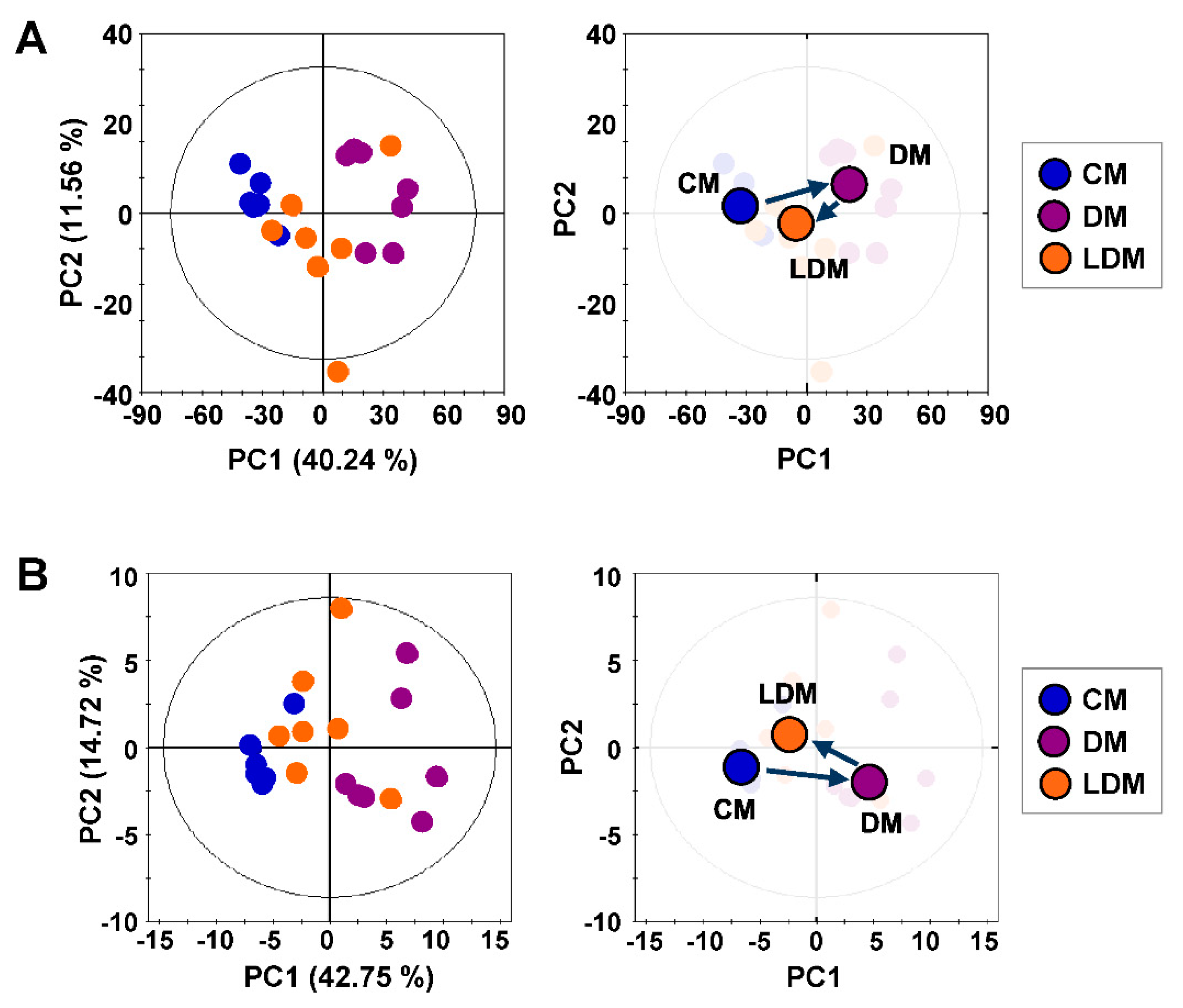
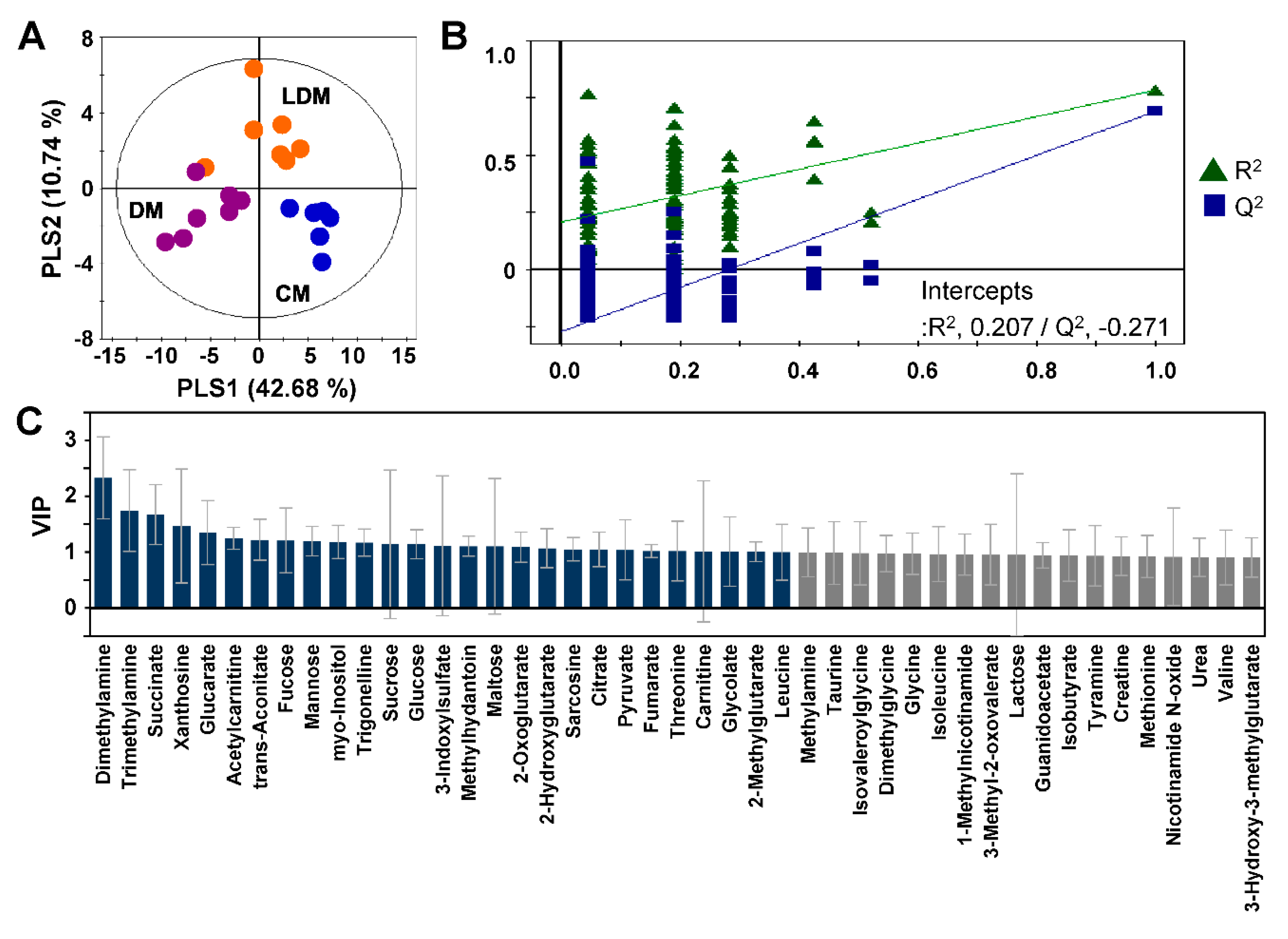
2.2. Metabolic Profiling and Pattern Recognition Analysis
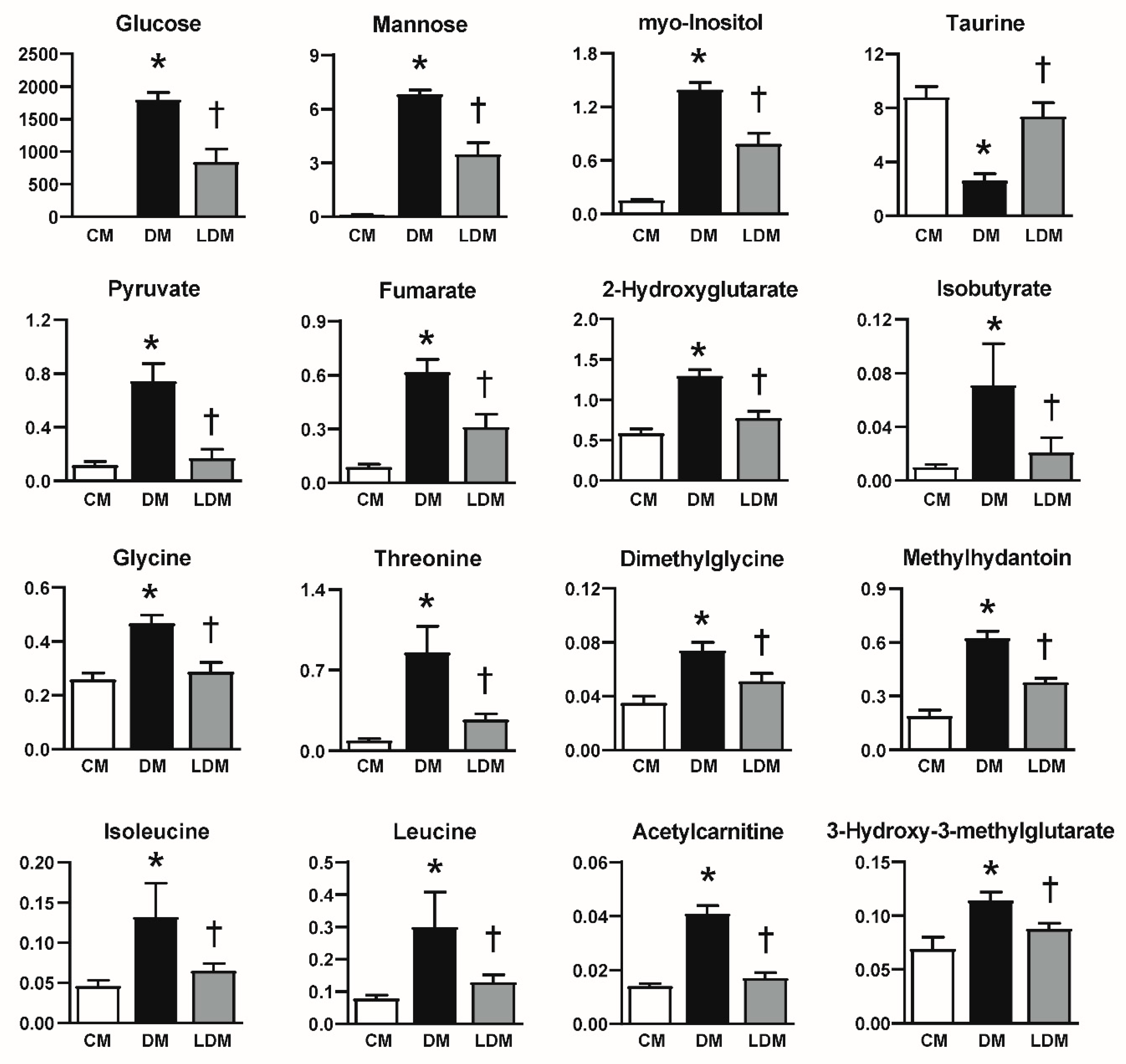
2.3. Changes in Urinary Metabolites in Response to Losartan Treatment
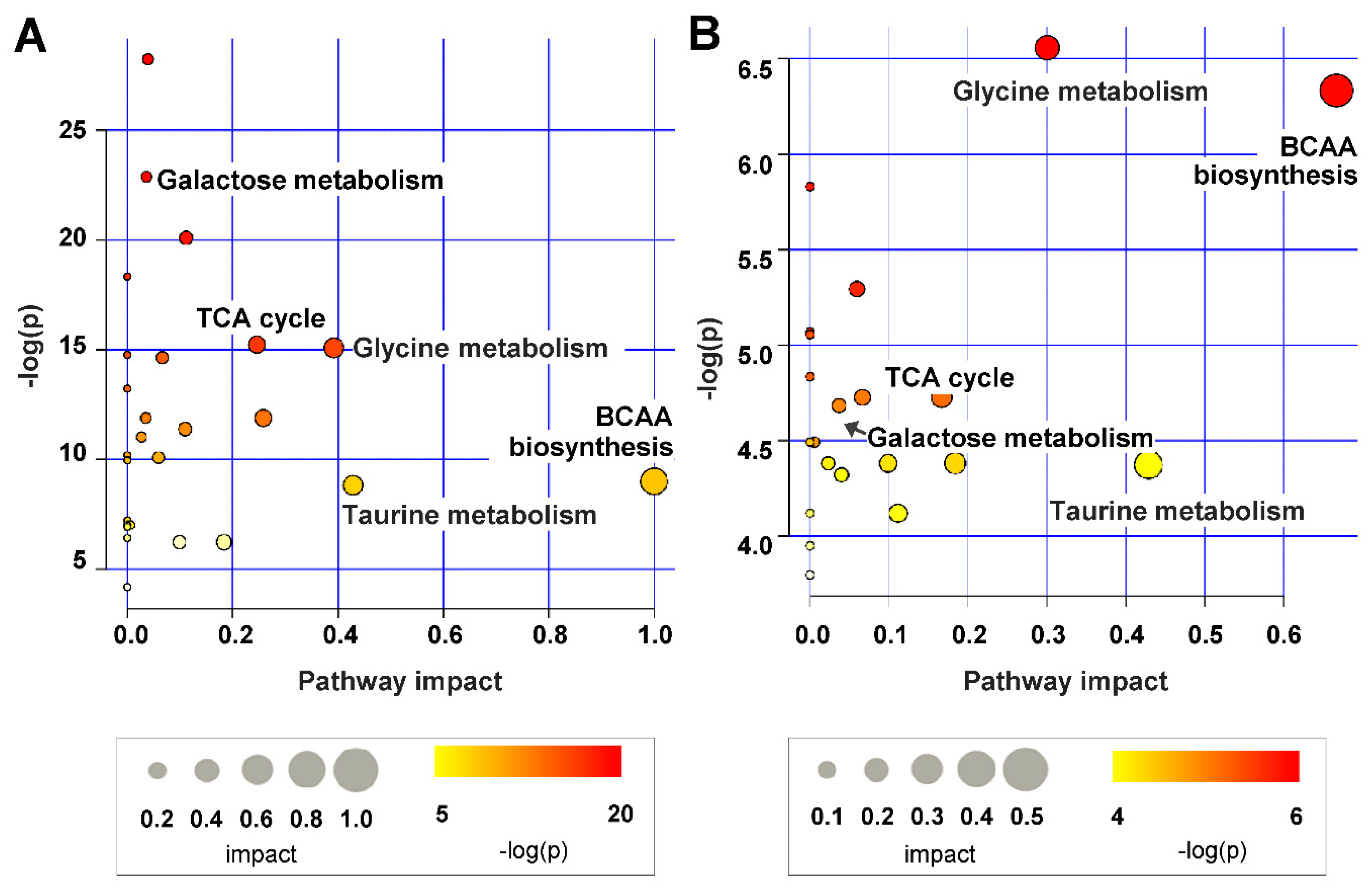
2.4. Metabolic Pathway Analysis
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Biochemical Analysis
4.3. 1H-NMR Samples and Experiments
4.4. NMR Spectral Preprocessing and Statistical Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014, 124, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Hocher, B.; Adamski, J. Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 319. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Uddin, M.J.; Lee, G.; Jiang, S.; Cho, A.; Lee, J.H.; Lee, S.R.; Bae, Y.S.; Moon, S.H.; Lee, S.J.; et al. A novel pan-Nox inhibitor, APX-115, protects kidney injury in streptozotocin-induced diabetic mice: Possible role of peroxisomal and mitochondrial biogenesis. Oncotarget 2017, 8, 74217. [Google Scholar] [CrossRef] [PubMed]
- Schievink, B.; Kröpelin, T.; Mulder, S.; Parving, H.H.; Remuzzi, G.; Dwyer, J.; Vemer, P.; de Zeeuw, D.; Lambers Heerspink, H.J. Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes. Metab. 2016, 18, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Adler, A.I.; Flyvbjerg, A.; Nelson, R.G.; So, W.-Y.; Wanner, C.; Kasiske, B.L.; Wheeler, D.C.; De Zeeuw, D.; Mogensen, C.E. Diabetic kidney disease: A clinical update from Kidney Disease: Improving Global Outcomes. Kidney Int. 2015, 87, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.H.; Kim, K. Metabolomics in the study of kidney diseases. Nat. Rev. Nephrol. 2012, 8, 22. [Google Scholar] [CrossRef]
- Mulder, S.; Hamidi, H.; Kretzler, M.; Ju, W. An integrative systems biology approach for precision medicine in diabetic kidney disease. Diabetes Obes. Metab. 2018, 20, 6–13. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, H.; Zhao, T.; Zhang, X.; Lu, J.; Yin, T.; Liang, Q.; Wang, Y.; Luo, G.; Lan, H.; et al. Intrarenal metabolomics reveals the association of local organic toxins with the progression of diabetic kidney disease. J. Pharm. Biomed. Anal. 2012, 60, 32–43. [Google Scholar] [CrossRef]
- Dorotea, D.; Cho, A.; Lee, G.; Kwon, G.; Lee, J.; Sahu, P.K.; Jeong, L.S.; Cha, D.R.; Ha, H. Orally active, species-independent novel A 3 adenosine receptor antagonist protects against kidney injury in db/db mice. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Saulnier, P.-J.; Darshi, M.; Wheelock, K.M.; Looker, H.C.; Fufaa, G.D.; Knowler, W.C.; Weil, E.J.; Tanamas, S.K.; Lemley, K.V.; Saito, R.; et al. Urine metabolites are associated with glomerular lesions in type 2 diabetes. Metabolomics 2018, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.F.; Wang, S.; Stothers, C.; Avance, J.; Denson, D.; Harris, R.; Voziyan, P. Alterations of urinary metabolite profile in model diabetic nephropathy. Biochem. Biophys. Res. Commun. 2015, 456, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Xie, L.; Diao, C.; Wang, N.; Hu, W.; Zheng, Y.; Jin, L.; Yan, Z.; Gao, H. Systemic perturbations of key metabolites in diabetic rats during the evolution of diabetes studied by urine metabonomics. PLoS ONE 2013, 8, e60409. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Hyeon, J.S.; Kim, N.H.; Cho, A.; Lee, G.; Jang, S.Y.; Kim, M.-K.; Lee, E.Y.; Chung, C.H.; Ha, H.; et al. Metabolic changes in urine and serum during progression of diabetic kidney disease in a mouse model. Arch. Biochem. Biophys. 2018, 646, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Vannini, P.; Marchesini, G.; Forlani, G.; Angiolini, A.; Ciavarella, A.; Zoli, M.; Pisi, E. Branched-chain amino acids and alanine as indices of the metabolic control in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1982, 22, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Van der Kloet, F.M.; Tempels, F.W.A.; Ismail, N.; Van der Heijden, R.; Kasper, P.T.; Rojas-Cherto, M.; Van Doorn, R.; Spijksma, G.; Koek, M.; Van der Greef, J.; et al. Discovery of early-stage biomarkers for diabetic kidney disease using ms-based metabolomics (FinnDiane study). Metabolomics 2012, 8, 109–119. [Google Scholar] [CrossRef]
- Koh, J.H.; Lee, E.S.; Hyun, M.; Kim, H.M.; Choi, Y.J.; Lee, E.Y.; Yadav, D.; Chung, C.H. Taurine alleviates the progression of diabetic nephropathy in type 2 diabetic rat model. Int. J. Endocrinol. 2014, 2014, 397307. [Google Scholar] [CrossRef]
- Posada-Ayala, M.; Zubiri, I.; Martin-Lorenzo, M.; Sanz-Maroto, A.; Molero, D.; Gonzalez-Calero, L.; Fernandez-Fernandez, B.; De La Cuesta, F.; Laborde, C.M.; Barderas, M.G.; et al. Identification of a urine metabolomic signature in patients with advanced-stage chronic kidney disease. Kidney Int. 2014, 85, 103–111. [Google Scholar] [CrossRef]
- Wen, H.; Yang, H.-J.; Choi, M.-J.; Kwon, H.-N.; Kim, M.-A.; Hong, S.-S.; Park, S.-H. Identification of urinary biomarkers related to cisplatin-induced acute renal toxicity using NMR-based metabolomics. Biomol. Ther. 2011, 19, 38–44. [Google Scholar] [CrossRef][Green Version]
- Skiba, W.E.; Taylor, M.P.; Wells, M.S.; Mangum, J.H.; Awad, W.M. Human hepatic methionine biosynthesis. Purification and characterization of betaine: Homocysteine S-methyltransferase. J. Biol. Chem. 1982, 257, 14944–14948. [Google Scholar] [PubMed]
- Slow, S.; McGregor, D.O.; Lever, M.; Lee, M.B.; George, P.M.; Chambers, S.T. Dimethylglycine supplementation does not affect plasma homocysteine concentrations in pre-dialysis chronic renal failure patients. Clin. Biochem. 2004, 37, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Wei, F.; Vaziri, N.D.; Cheng, X.-L.; Bai, X.; Lin, R.-C.; Zhao, Y.-Y. Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci. Rep. 2015, 5, 14472. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Mitochondrial hormesis and diabetic complications. Diabetes 2015, 64, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Gorin, Y.; Block, K.; Hernandez, J.; Bhandari, B.; Wagner, B.; Barnes, J.L.; Abboud, H.E. Nox4 NAD (P) H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J. Biol. Chem. 2005, 280, 39616–39626. [Google Scholar] [CrossRef]
- Qi, W.; Keenan, H.A.; Li, Q.; Ishikado, A.; Kannt, A.; Sadowski, T.; Yorek, M.A.; Wu, I.-H.; Lockhart, S.; Coppey, L.J.; et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat. Med. 2017, 23, 753. [Google Scholar] [CrossRef]
- You, Y.-H.; Quach, T.; Saito, R.; Pham, J.; Sharma, K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J. Am. Soc. Nephrol. 2016, 27, 466–481. [Google Scholar] [CrossRef]
- Gross, M.-L.; Dikow, R.; Ritz, E. Diabetic nephropathy: Recent insights into the pathophysiology and the progression of diabetic nephropathy. Kidney Int. 2005, 67, S50–S53. [Google Scholar] [CrossRef]
- Xiong, S.; Salazar, G.; San Martin, A.; Ahmad, M.; Patrushev, N.; Hilenski, L.; Nazarewicz, R.R.; Ma, M.; Ushio-Fukai, M.; Alexander, R.W. PGC-1α serine 570 phosphorylation and GCN5-mediated acetylation by angiotensin II drive catalase down-regulation and vascular hypertrophy. J. Biol. Chem. 2010, 285, 2474–2487. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lee, J.; Seo, J.; Hwang, G.S. Metabolite profiling study on the toxicological effects of polybrominated diphenyl ether in a rat model. Environ. Toxicol. 2017, 32, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
| CM (n = 6) | DM (n = 7) | LDM (n = 7) | |
|---|---|---|---|
| Body weight (g) | 26.6 (26.0, 26.9) | 22.6 (21.3, 23.1) *** | 25.3 (23.3, 25.9) |
| Blood glucose (mmol/L) | 192.5 (181.0, 223.0) | 519.0 (512.5, 543.0) *** | 509.0 (469.0, 522.0) |
| HbA1c (%) | 4.2 (4.1, 4.2) | 8.6 (8.4, 9.2) *** | 7.2 (6.6, 8.2) |
| Urine volume (mL/day) | 0.9 (0.6, 1.3) | 19.6 (17.6, 26.4) *** | 6.4 (4.3, 9.6) † |
| UACR (mg/mmol) | 22.1 (20.3, 26.7) | 102.2 (99.1, 112.9) ** | 36.9 (35.2, 49.6) †† |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyeon, J.S.; Jung, Y.; Lee, G.; Ha, H.; Hwang, G.-S. Urinary Metabolomic Profiling in Streptozotocin-Induced Diabetic Mice after Treatment with Losartan. Int. J. Mol. Sci. 2020, 21, 8969. https://doi.org/10.3390/ijms21238969
Hyeon JS, Jung Y, Lee G, Ha H, Hwang G-S. Urinary Metabolomic Profiling in Streptozotocin-Induced Diabetic Mice after Treatment with Losartan. International Journal of Molecular Sciences. 2020; 21(23):8969. https://doi.org/10.3390/ijms21238969
Chicago/Turabian StyleHyeon, Jin Seong, Youngae Jung, Gayoung Lee, Hunjoo Ha, and Geum-Sook Hwang. 2020. "Urinary Metabolomic Profiling in Streptozotocin-Induced Diabetic Mice after Treatment with Losartan" International Journal of Molecular Sciences 21, no. 23: 8969. https://doi.org/10.3390/ijms21238969
APA StyleHyeon, J. S., Jung, Y., Lee, G., Ha, H., & Hwang, G.-S. (2020). Urinary Metabolomic Profiling in Streptozotocin-Induced Diabetic Mice after Treatment with Losartan. International Journal of Molecular Sciences, 21(23), 8969. https://doi.org/10.3390/ijms21238969






