Drinking Ice-Cold Water Reduces the Severity of Anticancer Drug-Induced Taste Dysfunction in Mice
Abstract
1. Introduction
2. Results
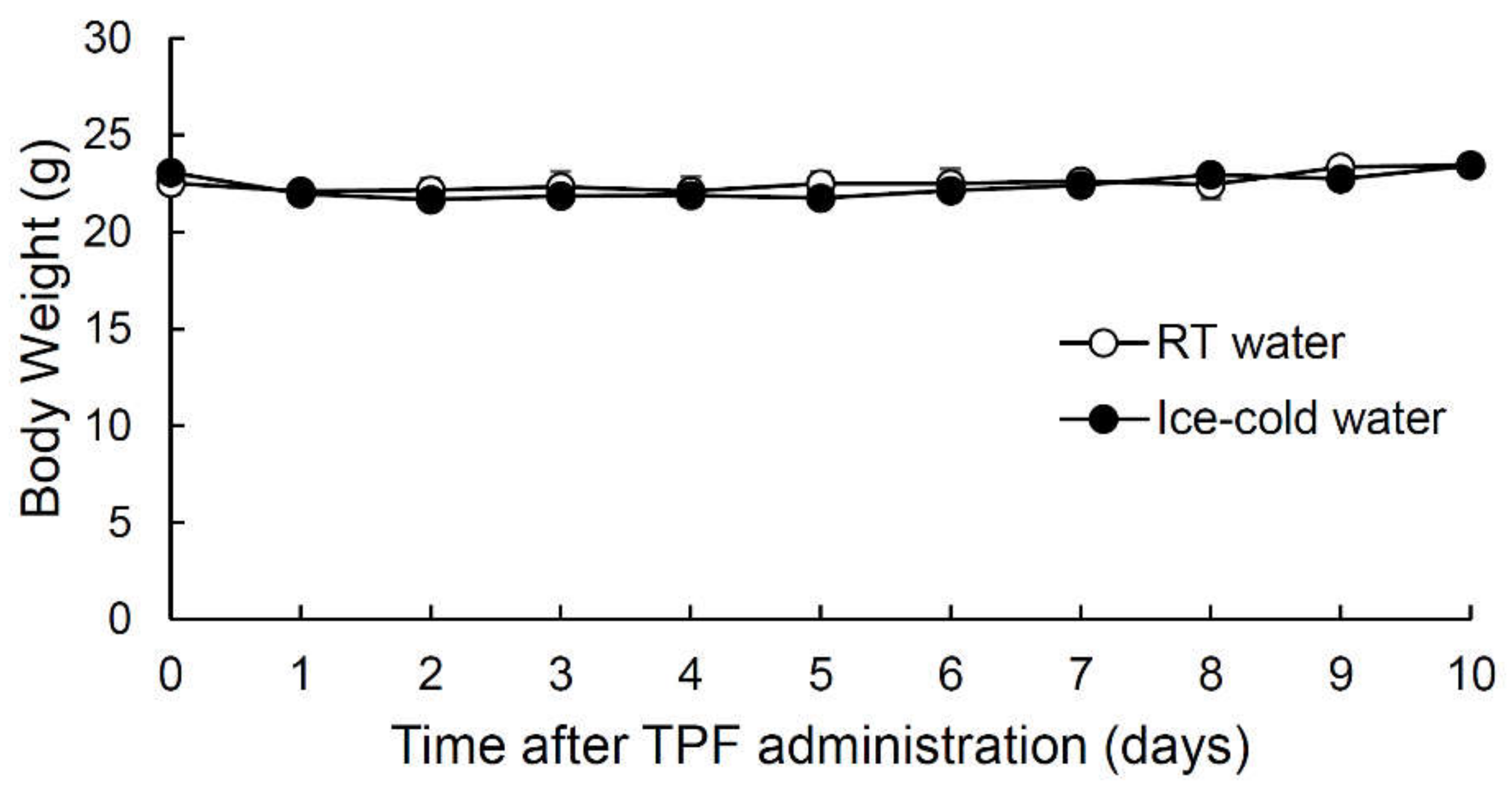
2.1. Intraperitoneal Injection of Anticancer Drugs (TPF) Did Not Affect Mouse Body Weight
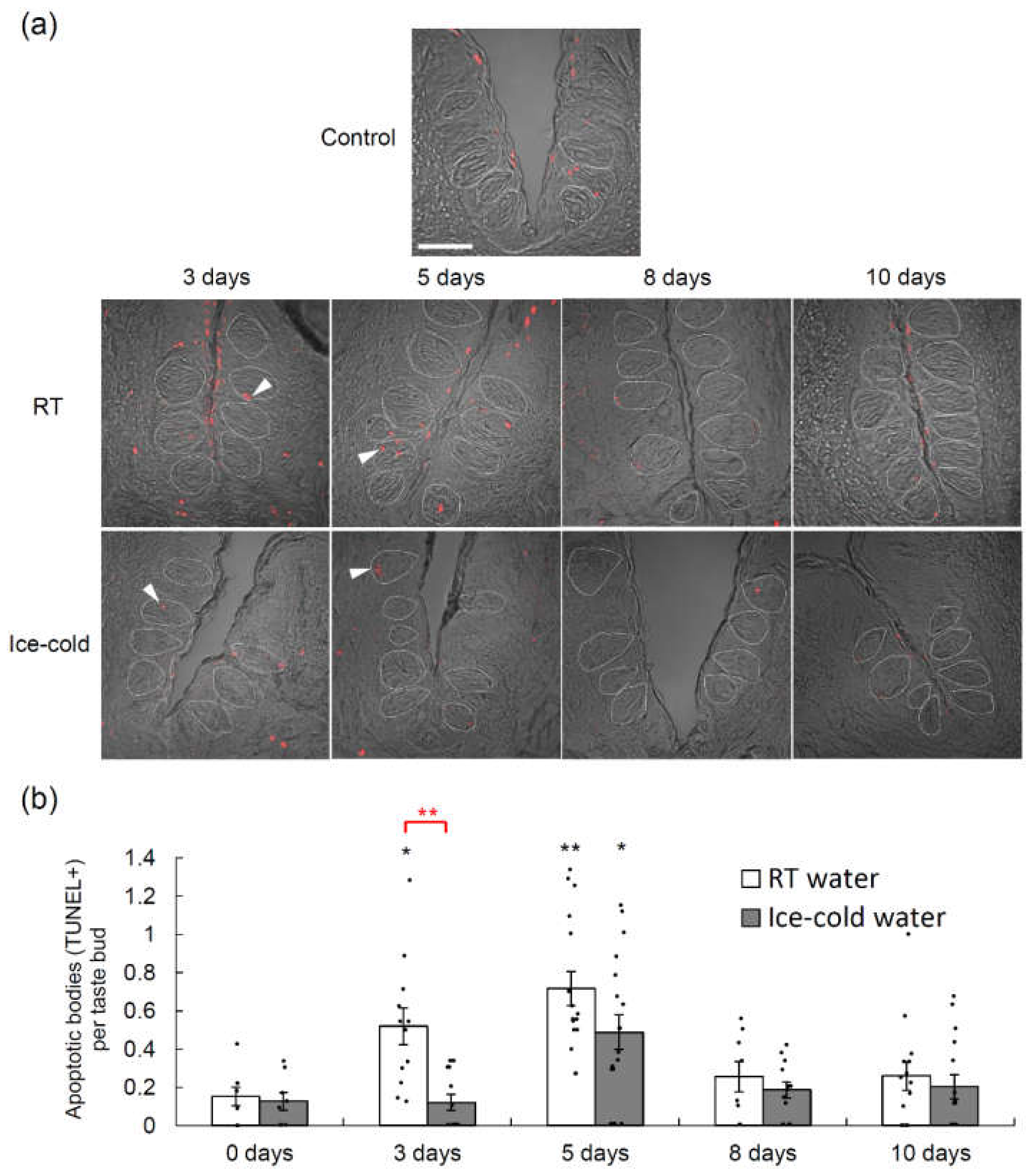
2.2. Taste Cell Apoptosis after TPF Administration Was Suppressed by the Drinking of Ice-Cold Water
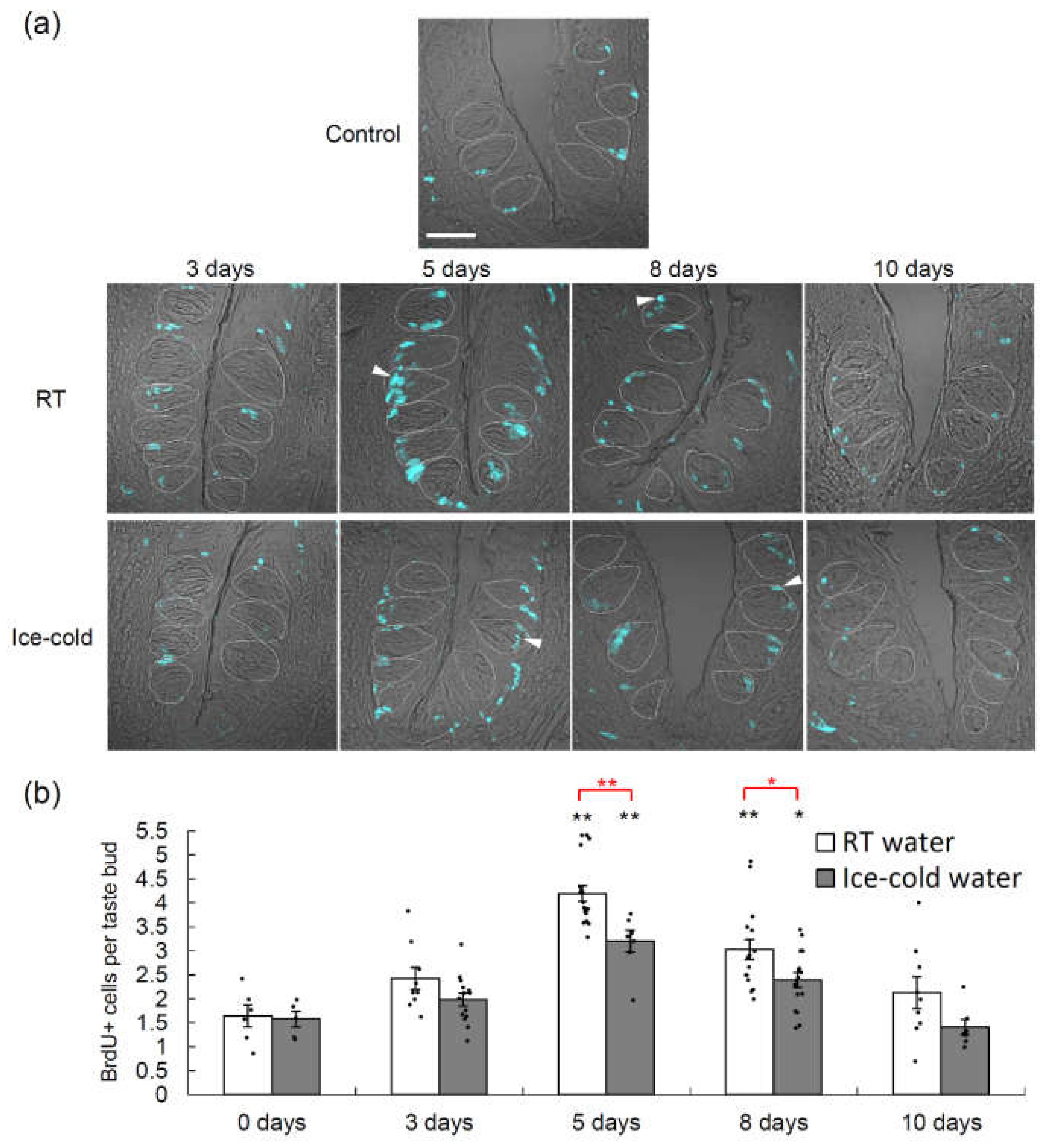
2.3. Taste Cell Proliferation after TPF Administration Was Suppressed by the Drinking of Ice-Cold Water
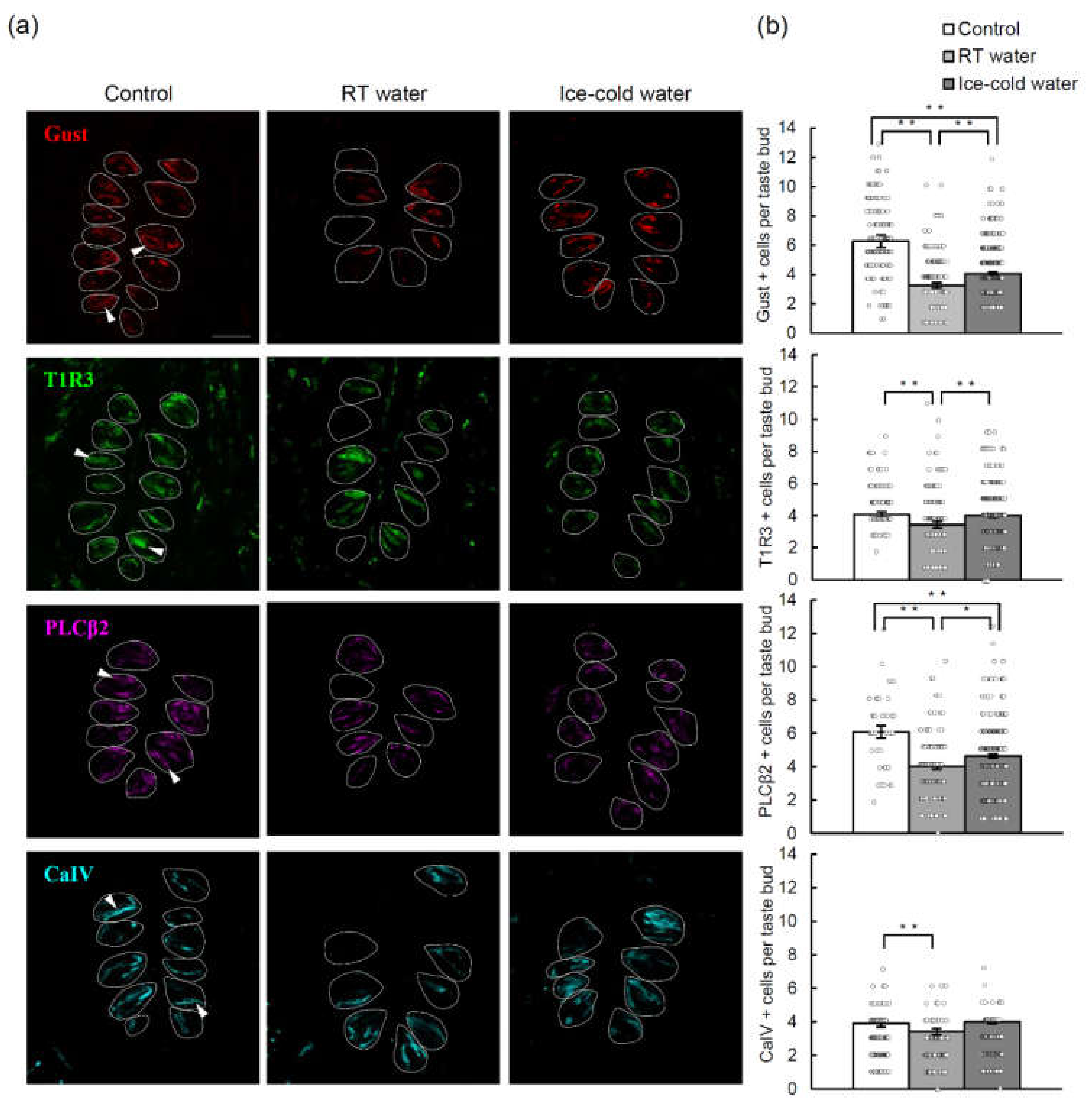
2.4. TPF-Induced Reductions in the Numbers of Cells Expressing Various Taste Cell Markers Were Suppressed by the Drinking of Ice-Cold Water
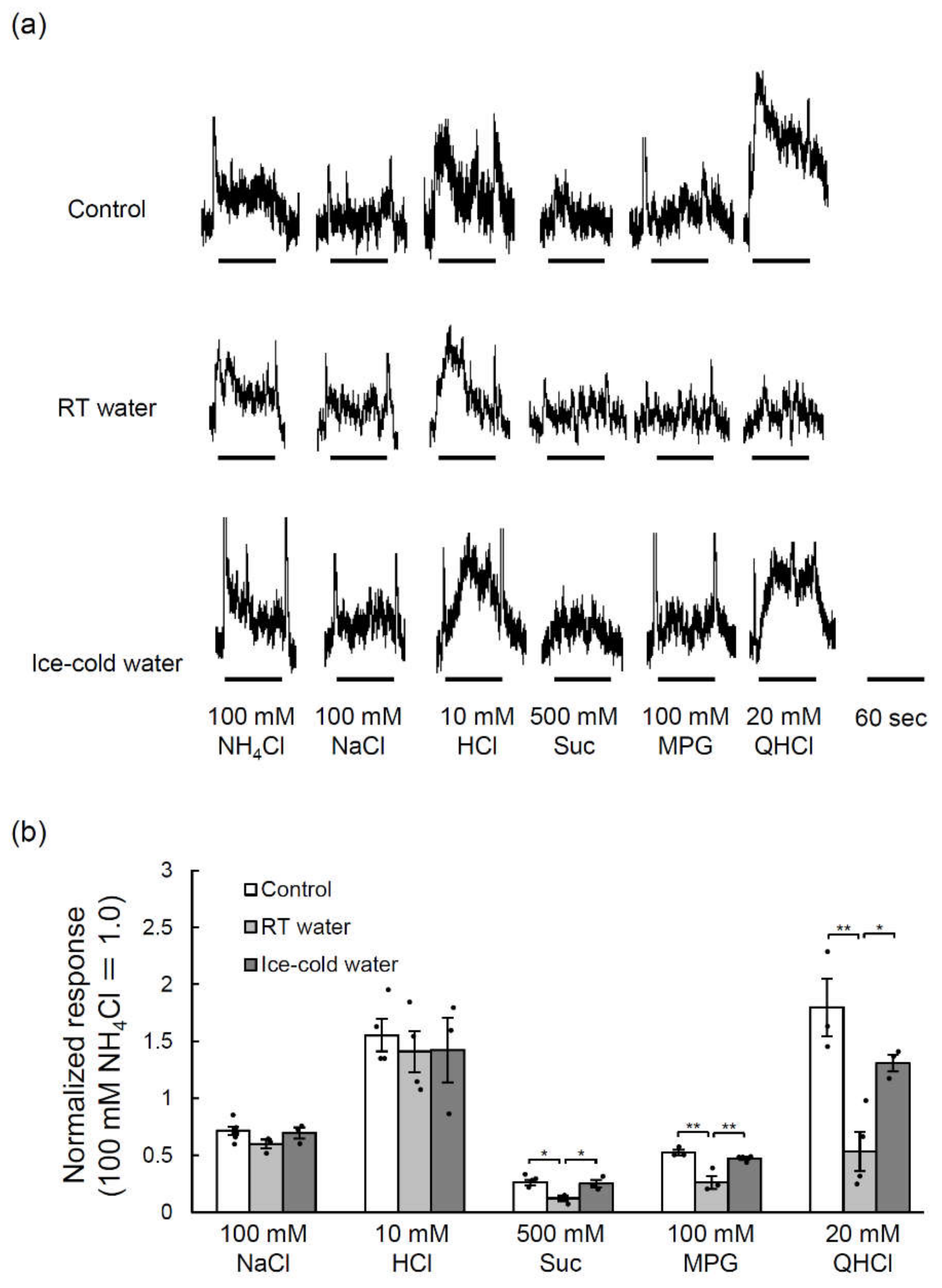
2.5. TPF-Induced Reductions in the Glossopharyngeal Nerve Responses to Taste Stimuli Were Suppressed by the Drinking of Ice-Cold Water
3. Discussions
4. Materials and Methods
4.1. Ethical Approval
4.2. Animals
4.3. Solutions
4.4. Cell Proliferation Assay
4.5. Cell Death Assay
4.6. Labeling of Differentiated Cells
4.7. Image Analysis and Counting of Labeled Taste Cells
4.8. Statistical Analysis of the Data for Taste Cell Marker Expression
4.9. Nerve Recordings
4.10. Statistical Analysis of the Nerve Recording Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| ADL | Activities of daily living |
| BrdU | Bromodeoxyuridine |
| CaIV | Carbonic anhydrase IV |
| CVP | Circumvallate papillae |
| ENaC | Epithelial sodium channel |
| Gli | Glioma-associated oncogene homolog |
| GPCR | G protein-coupled receptor |
| Gust | Gα-gustducin |
| Lgr | Leucine-rich repeat-containing G-protein coupled receptor |
| MPG | Monopotassium glutamate |
| Otop1 | Otopetrin-1 |
| PBS | Phosphate-buffered saline |
| PLCβ2 | Phospholipase C-beta 2 |
| QHCl | Quinine hydrochloride |
| QOL | Quality of life |
| RT | Room temperature |
| Shh | Sonic hedgehog |
| Smo | Smoothened |
| SNAP25 | Synaptosomal-associated protein 25 |
| Suc | Sucrose |
| T1R | Taste receptor type 1 member |
| T2R | Taste receptor type 2 |
| TNT | Tris-NaCl-Tween |
| TPF | Docetaxel, cisplatin and 5-fluorouracil |
| Trpm5 | Transient receptor potential cation channel M5 |
| TUNEL | Transferase-mediated dUTP nick end labeling |
References
- McQuade, R.M.; Stojanovska, V.; Donald, E.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Gastrointestinal dysfunction and enteric neurotoxicity following treatment with anticancer chemotherapeutic agent 5-fluorouracil. Neurogastroenterol. Motil. 2016, 28, 1861–1875. [Google Scholar] [CrossRef]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J.; et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N. Engl. J. Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef]
- Wickham, R.S.; Rehwaldt, M.; Kefer, C.; Shott, S.; Abbas, K.; Glynn-Tucker, E.; Potter, C.; Blendowski, C. Taste changes experienced by patients receiving chemotherapy. Oncol. Nurs. Forum 1999, 26, 697–706. [Google Scholar]
- Gamper, E.M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Wintner, L.M.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B.; Zabernigg, A. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: Prevalence, course of severity, and quality of life correlates. Acta Oncol. 2012, 51, 490–496. [Google Scholar] [CrossRef]
- Berteretche, M.V.; Dalix, A.M.; d’Ornano, A.M.C.; Bellisle, F.; Khayat, D.; Faurion, A. Decreased taste sensitivity in cancer patients under chemotherapy. Support. Care Cancer 2004, 12, 571–576. [Google Scholar] [CrossRef]
- Comeau, T.B.; Epstein, J.B.; Migas, C. Taste and smell dysfunction in patients receiving chemotherapy: A review of current knowledge. Support. Care Cancer 2001, 9, 575–580. [Google Scholar] [CrossRef]
- Epstein, J.B.; Phillips, N.; Parry, J.; Epstein, M.S.; Nevill, T.; Stevenson-Moore, P. Quality of life, taste, olfactory and oral function following high-dose chemotherapy and allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2002, 30, 785–792. [Google Scholar] [CrossRef]
- Spotten, L.E.; Corish, C.A.; Lorton, C.M.; Ui Dhuibhir, P.M.; O’Donoghue, N.C.; O’Connor, B.; Walsh, T.D. Subjective and objective taste and smell changes in cancer. Ann. Oncol. 2017, 28, 969–984. [Google Scholar] [CrossRef]
- Murtaza, B.; Hichami, A.; Khan, A.S.; Ghiringhelli, F.; Khan, N.A. Alteration in taste perception in cancer: Causes and strategies of treatment. Front. Physiol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef]
- Tu, Y.H.; Cooper, A.J.; Teng, B.; Chang, R.B.; Artiga, D.J.; Turner, H.N.; Mulhall, E.M.; Ye, W.; Smith, A.D.; Liman, E.R. An evolutionarily conserved gene family encodes proton-selective ion channels. Science 2018, 359, 1047–1050. [Google Scholar] [CrossRef]
- Shigemura, N.; Ninomiya, Y. Recent advances in molecular mechanisms of taste signaling and modifying. Int. Rev. Cell Mol. Biol. 2016, 323, 71–106. [Google Scholar] [CrossRef]
- Perea-Martinez, I.; Nagai, T.; Chaudhari, N. Functional cell types in taste buds have distinct longevities. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Mahood, D.J.; Dose, A.M.; Loprinzi, C.L.; Veeder, M.H.; Athmann, L.M.; Therneau, T.M.; Sorensen, J.M.; Gainey, D.K.; Mailliard, J.A.; Gusa, N.L.; et al. Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J. Clin. Oncol. 1991, 9, 449–452. [Google Scholar] [CrossRef]
- Davidson, W.; Teleni, L.; Muller, J.; Ferguson, M.; McCarthy, A.L.; Vick, J.; Isenring, E. Malnutrition and chemotherapy-induced nausea and vomiting: Implications for practice. Oncol. Nurs. Forum 2012, 39, E340–E345. [Google Scholar] [CrossRef]
- Kavoi, B.M.; Plendl, J.; Makanya, A.N.; Ochieng, S.; Kiama, S.G. Effects of anticancer drug docetaxel on the structure and function of the rabbit olfactory mucosa. Tissue Cell 2014, 46, 213–224. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Xiang, D.; Yang, J.; Liu, D.; Ren, X.; Zhang, C. Assessment of dose-response relationship of 5-fluorouracil to murine intestinal injury. Biomed. Pharmacother. 2018, 106, 910–916. [Google Scholar] [CrossRef]
- Bozec, A.; Sudaka, A.; Etienne-Grimaldi, M.-C.; Brunstein, M.-C.; Fischel, J.-L.; Milano, G. Antitumor activity of cetuximab associated with the taxotere–cisplatin–fluorouracil (TPF) combination on an orthotopic head and neck cancer model. Oral Oncol. 2011, 47, 940–945. [Google Scholar] [CrossRef]
- Shimada, I.S.; Peterson, B.M.; Spees, J.L. Isolation of locally derived stem/progenitor cells from the peri-infarct area that do not migrate from the lateral ventricle after cortical stroke. Stroke 2010, 41, e552–e560. [Google Scholar] [CrossRef]
- Miura, H.; Kato, H.; Kusakabe, Y.; Tagami, M.; Miura-Ohnuma, J.; Ninomiya, Y.; Hino, A. A strong nerve dependence of Sonic hedgehog expression in basal cells in mouse taste bud and an autonomous transcriptional control of genes in differentiated taste cells. Chem. Senses 2004, 29, 823–831. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Remenar, E.; van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef]
- Hall, J.M.; Bell, M.L.; Finger, T.E. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev. Biol. 2003, 255, 263–277. [Google Scholar] [CrossRef]
- Zabernigg, A.; Gamper, E.-M.; Giesinger, J.M.; Rumpold, G.; Kemmler, G.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B. Taste alterations in cancer patients receiving chemotherapy: A neglected side effect? Oncologist 2010, 15, 913–920. [Google Scholar] [CrossRef]
- Cohen, J.; Wakefield, C.E.; Laing, D.C. Smell and taste disorders resulting from cancer and chemotherapy. Curr. Pharm. Des. 2016, 22, 2253–2263. [Google Scholar] [CrossRef]
- Turcott, J.G.; Juárez-Hernández, E.; de la Torre-Vallejo, M.; Sánchez-Lara, K.; Luvian-Morales, J.; Arrieta, O. Value: Changes in the detection and recognition thresholds of three basic tastes in lung cancer patients receiving cisplatin and paclitaxel and its association with nutritional and quality of life parameters. Nutr. Cancer 2016, 68, 241–249. [Google Scholar] [CrossRef]
- Ponticelli, E.; Clari, M.; Frigerio, S.; de Clemente, A.; Bergese, I.; Scavino, E.; Bernardini, A.; Sacerdote, C. Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: A cross-sectional study. Eur. J. Cancer Care 2017, 26, e12633. [Google Scholar] [CrossRef]
- Rha, S.Y.; Lee, J. Symptom clusters during palliative chemotherapy and their influence on functioning and quality of life. Support. Care Cancer 2017, 25, 1519–1527. [Google Scholar] [CrossRef]
- IJpma, I.; Renken, R.J.; Gietema, J.A.; Slart, R.H.J.A.; Mensink, M.G.J.; Lefrandt, J.D.; Ter Horst, G.J.; Reyners, A.K.L. Changes in taste and smell function, dietary intake, food preference, and body composition in testicular cancer patients treated with cisplatin-based chemotherapy. Clin. Nutr. 2017, 36, 1642–1648. [Google Scholar] [CrossRef]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Chemosensory changes experienced by patients undergoing cancer chemotherapy: A qualitative interview study. J. Pain Symptom Manag. 2007, 34, 403–412. [Google Scholar] [CrossRef]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Self-reported taste and smell changes during cancer chemotherapy. Support. Care Cancer 2008, 16, 275–283. [Google Scholar] [CrossRef]
- Steinbach, S.; Hundt, W.; Schmalfeldt, B.; Böhner, C.; Berktold, S.; Wolf, P.; Harbeck, N. Effect of platinum-containing chemotherapy on olfactory, gustatory, and hearing function in ovarian cancer patients. Arch. Gynecol. Obstet. 2012, 286, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Boltong, A.; Aranda, S.; Keast, R.; Wynne, R.; Francis, P.A.; Chirgwin, J.; Gough, K. A prospective cohort study of the effects of adjuvant breast cancer chemotherapy on taste function, food liking, appetite and associated nutritional outcomes. PLoS ONE 2014, 9, e103512. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, M.S.; Sun, T.T.; Lavker, R.M. Strategies of epithelial repair: Modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 1998, 111, 2867–2875. [Google Scholar] [PubMed]
- Nguyen, H.M.; Reyland, M.E.; Barlow, L.A. Mechanisms of taste bud cell loss after head and neck irradiation. J. Neurosci. 2012, 32, 3474–3484. [Google Scholar] [CrossRef]
- Huang, A.L.; Chen, X.; Hoon, M.A.; Chandrashekar, J.; Guo, W.; Tränkner, D.; Ryba, N.J.P.; Zuker, C.S. The cells and logic for mammalian sour taste detection. Nature 2006, 442, 934–938. [Google Scholar] [CrossRef]
- Lossow, K.; Hermans-Borgmeyer, I.; Behrens, M.; Meyerhof, W. Genetic labeling of Car4-expressing cells reveals subpopulations of type III taste cells. Chem. Senses 2017, 42, 747–758. [Google Scholar] [CrossRef]
- Mukherjee, N.; Pal Choudhuri, S.; Delay, R.J.; Delay, E.R. Cellular mechanisms of cyclophosphamide-induced taste loss in mice. PLoS ONE 2017, 12, e0185473. [Google Scholar] [CrossRef]
- Mukherjee, N.; Carroll, B.L.; Spees, J.L.; Delay, E.R. Pre-Treatment with amifostine protects against cyclophosphamide-induced disruption of taste in mice. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Delay, E.R.; Socia, S.H.; Girardin, J.L.; Jewkes, B.C.; King, J.H.; Delay, R.J. Cyclophosphamide and the taste system: Effects of dose fractionation and amifostine on taste cell renewal. PLoS ONE 2019, 14, 1–23. [Google Scholar] [CrossRef]
- Liu, H.X.; MacCallum, D.K.; Edwards, C.; Gaffield, W.; Mistretta, C.M. Sonic hedgehog exerts distinct, stage-specific effects on tongue and taste papilla development. Dev. Biol. 2004, 276, 280–300. [Google Scholar] [CrossRef]
- Yang, H.; Cong, W.N.; Yoon, J.S.; Egan, J.M. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 2015, 4, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Ermilov, A.N.; Grachtchouk, M.; Dlugosz, A.A.; Allen, B.L.; Bradley, R.M.; Mistretta, C.M. Recovery of taste organs and sensory function after severe loss from Hedgehog/Smoothened inhibition with cancer drug sonidegib. Proc. Natl. Acad. Sci. USA 2017, 114, E10369–E10378. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.K.; Li, Y.; Redding, K.M.; Iwatsuki, K.; Margolskee, R.F.; Jiang, P. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells 2013, 31, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Ohmoto, M.; Narukawa, M.; Yoshihara, Y.; Abe, K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat. Neurosci. 2011, 14, 685–687. [Google Scholar] [CrossRef]
- Soares, P.M.G.; Mota, J.M.S.C.; Gomes, A.S.; Oliveira, R.B.; Assreuy, A.M.S.; Brito, G.A.C.; Santos, A.A.; Ribeiro, R.A.; Souza, M.H.L.P. Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother. Pharmacol. 2008, 63, 91–98. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Katranci, N.; Ovayolu, N.; Ovayolu, O.; Sevinc, A. Evaluation of the effect of cryotherapy in preventing oral mucositis associated with chemotherapy—A randomized controlled trial. Eur. J. Oncol. Nurs. 2012, 16, 339–344. [Google Scholar] [CrossRef]
- Vokurka, S.; Chvojkova, I.; Svoboda, T.; Brandejsova, R.; Jungova, A.; Bystricka, E.; Jindra, P. The impact of oral cryotherapy and oral and gastrointestinal mucositis after autologous stem cell transplantation. Eur. J. Oncol. Nurs. 2014, 18, 228–229. [Google Scholar] [CrossRef]
- Walladbegi, J.; Smith, S.A.; Grayson, A.K.; Murdoch, C.; Jontell, M.; Colley, H.E. Cooling of the oral mucosa to prevent adverse effects of chemotherapeutic agents: An in vitro study. J. Oral Pathol. Med. 2018, 47, 477–483. [Google Scholar] [CrossRef]
- Bauman, B.; Mick, R.; Martinez, E.; Lawless, T.M.; Zinck, L.; Sinclair, P.; Fuhrer, M.; O’Hara, M.; Schneider, C.J.; O’Dwyer, P.; et al. Efficacy of oral cryotherapy during oxaliplatin infusion in preventing oral thermal hyperalgesia: A randomized trial. Natl. Compr. Canc. Netw. 2019, 17, 358–364. [Google Scholar] [CrossRef]
- Hanai, A.; Ishiguro, H.; Sozu, T.; Tsuda, M.; Yano, I.; Nakagawa, T.; Imai, S.; Hamabe, Y.; Toi, M.; Arai, H.; et al. Effects of cryotherapy on objective and subjective symptoms of paclitaxel-induced neuropathy: Prospective self-controlled trial. J. Natl. Cancer Inst. 2018, 110, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Ohkuri, T.; Jyotaki, M.; Yasuo, T.; Horio, N.; Yasumatsu, K.; Sanematsu, K.; Shigemura, N.; Yamamoto, T.; Margolskee, R.F.; et al. Endocannabinoids selectively enhance sweet taste. Proc. Natl. Acad. Sci. USA 2010, 107, 935–939. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osaki, A.; Sanematsu, K.; Yamazoe, J.; Hirose, F.; Watanabe, Y.; Kawabata, Y.; Oike, A.; Hirayama, A.; Yamada, Y.; Iwata, S.; et al. Drinking Ice-Cold Water Reduces the Severity of Anticancer Drug-Induced Taste Dysfunction in Mice. Int. J. Mol. Sci. 2020, 21, 8958. https://doi.org/10.3390/ijms21238958
Osaki A, Sanematsu K, Yamazoe J, Hirose F, Watanabe Y, Kawabata Y, Oike A, Hirayama A, Yamada Y, Iwata S, et al. Drinking Ice-Cold Water Reduces the Severity of Anticancer Drug-Induced Taste Dysfunction in Mice. International Journal of Molecular Sciences. 2020; 21(23):8958. https://doi.org/10.3390/ijms21238958
Chicago/Turabian StyleOsaki, Ayana, Keisuke Sanematsu, Junichi Yamazoe, Fumie Hirose, Yu Watanabe, Yuko Kawabata, Asami Oike, Ayaka Hirayama, Yu Yamada, Shusuke Iwata, and et al. 2020. "Drinking Ice-Cold Water Reduces the Severity of Anticancer Drug-Induced Taste Dysfunction in Mice" International Journal of Molecular Sciences 21, no. 23: 8958. https://doi.org/10.3390/ijms21238958
APA StyleOsaki, A., Sanematsu, K., Yamazoe, J., Hirose, F., Watanabe, Y., Kawabata, Y., Oike, A., Hirayama, A., Yamada, Y., Iwata, S., Takai, S., Wada, N., & Shigemura, N. (2020). Drinking Ice-Cold Water Reduces the Severity of Anticancer Drug-Induced Taste Dysfunction in Mice. International Journal of Molecular Sciences, 21(23), 8958. https://doi.org/10.3390/ijms21238958




