P2Y2 and P2X4 Receptors Mediate Ca2+ Mobilization in DH82 Canine Macrophage Cells
Abstract
1. Introduction
2. Results
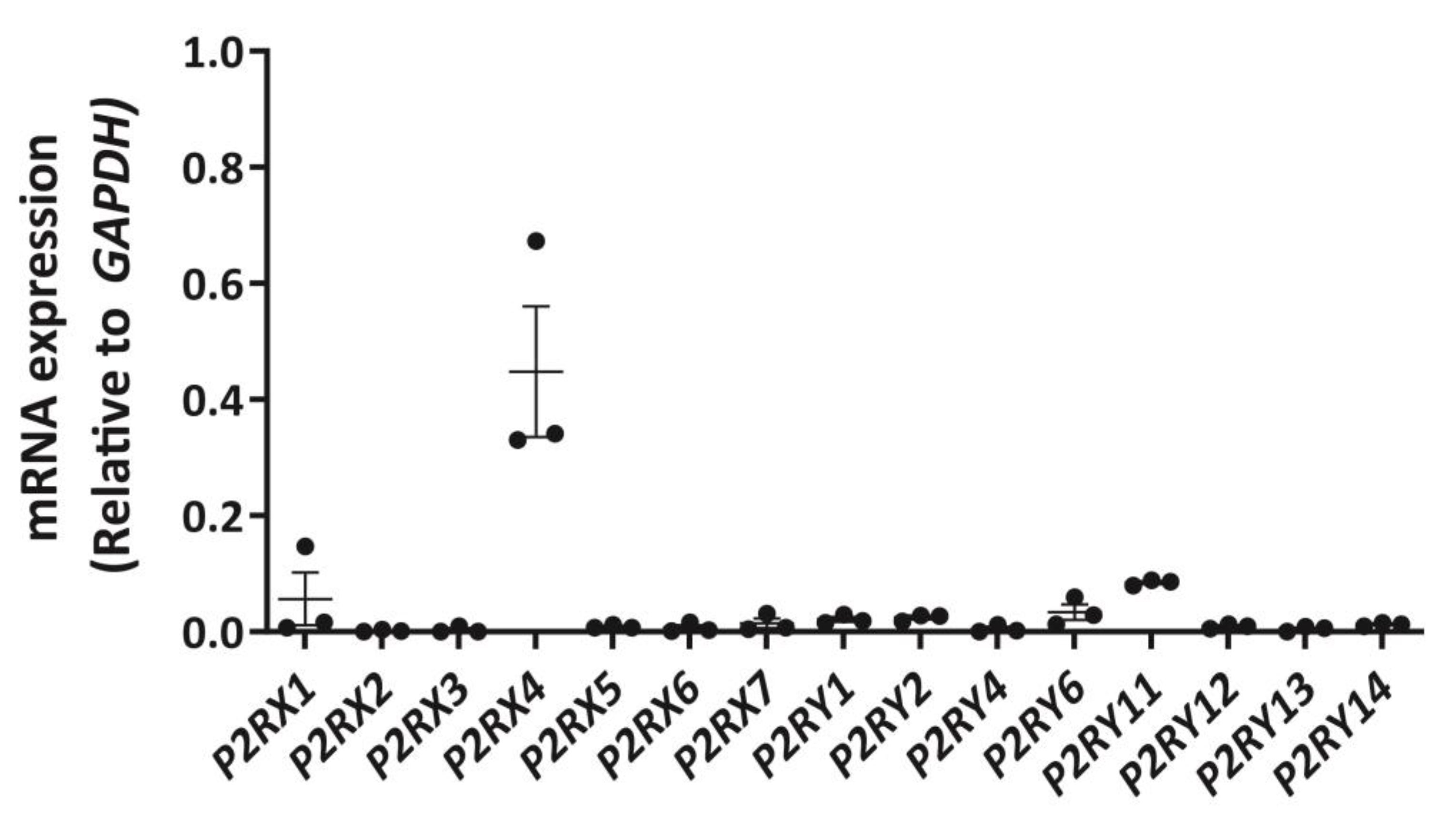
2.1. DH82 Cells Express Abundant P2RX4 mRNA Compared to Other P2 Receptors
2.2. Nucleotides Mediate Ca2+ Responses in DH82 Cells
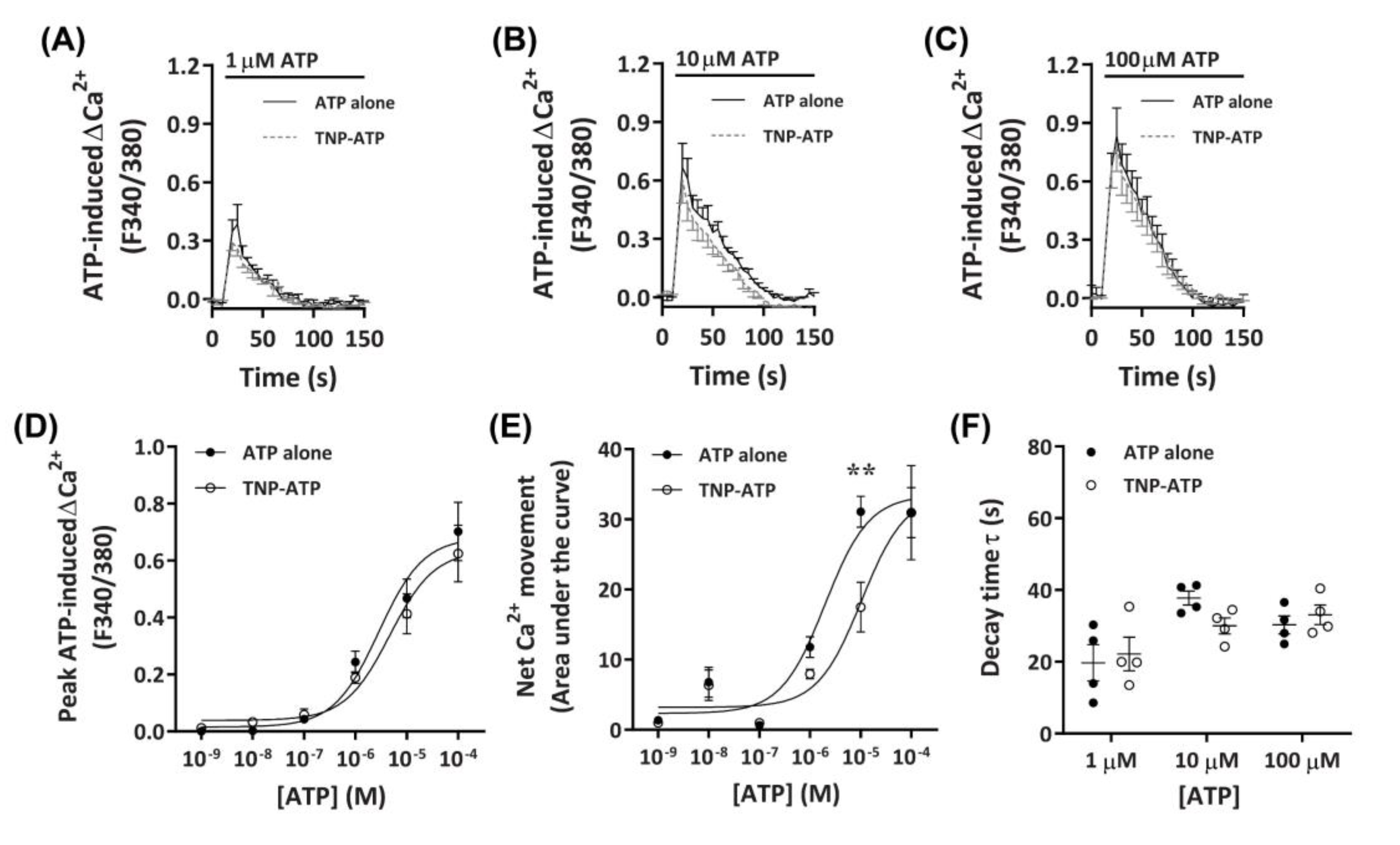
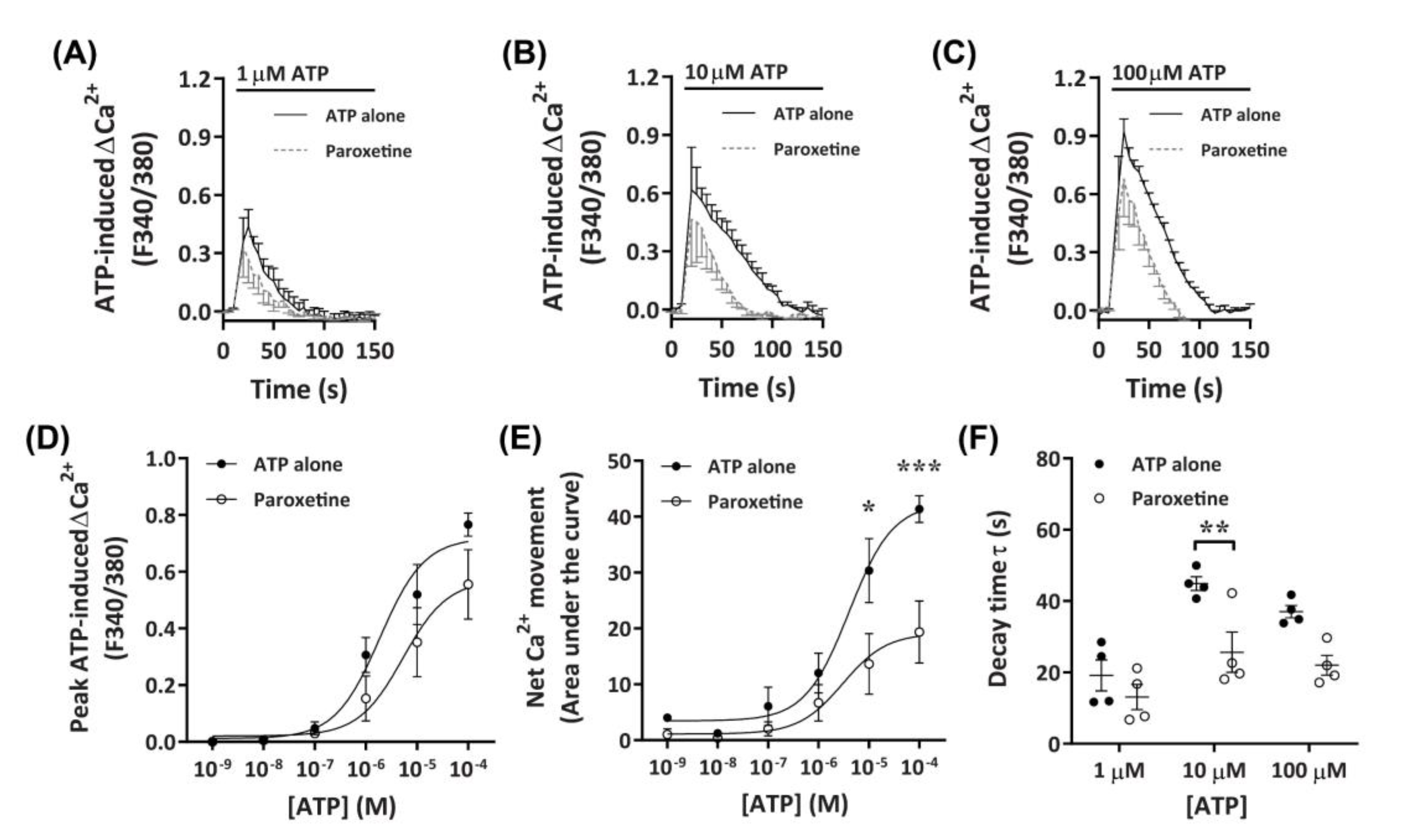
2.3. P2X4 Receptors Mediate Minor Changes in Intracellular Ca2+ in DH82 Cells
2.3.1. TNP-ATP Partially Reduces ATP-Induced Net Ca2+ Movement
2.3.2. Paroxetine Partially Reduces ATP-Induced Net Ca2+ Movement
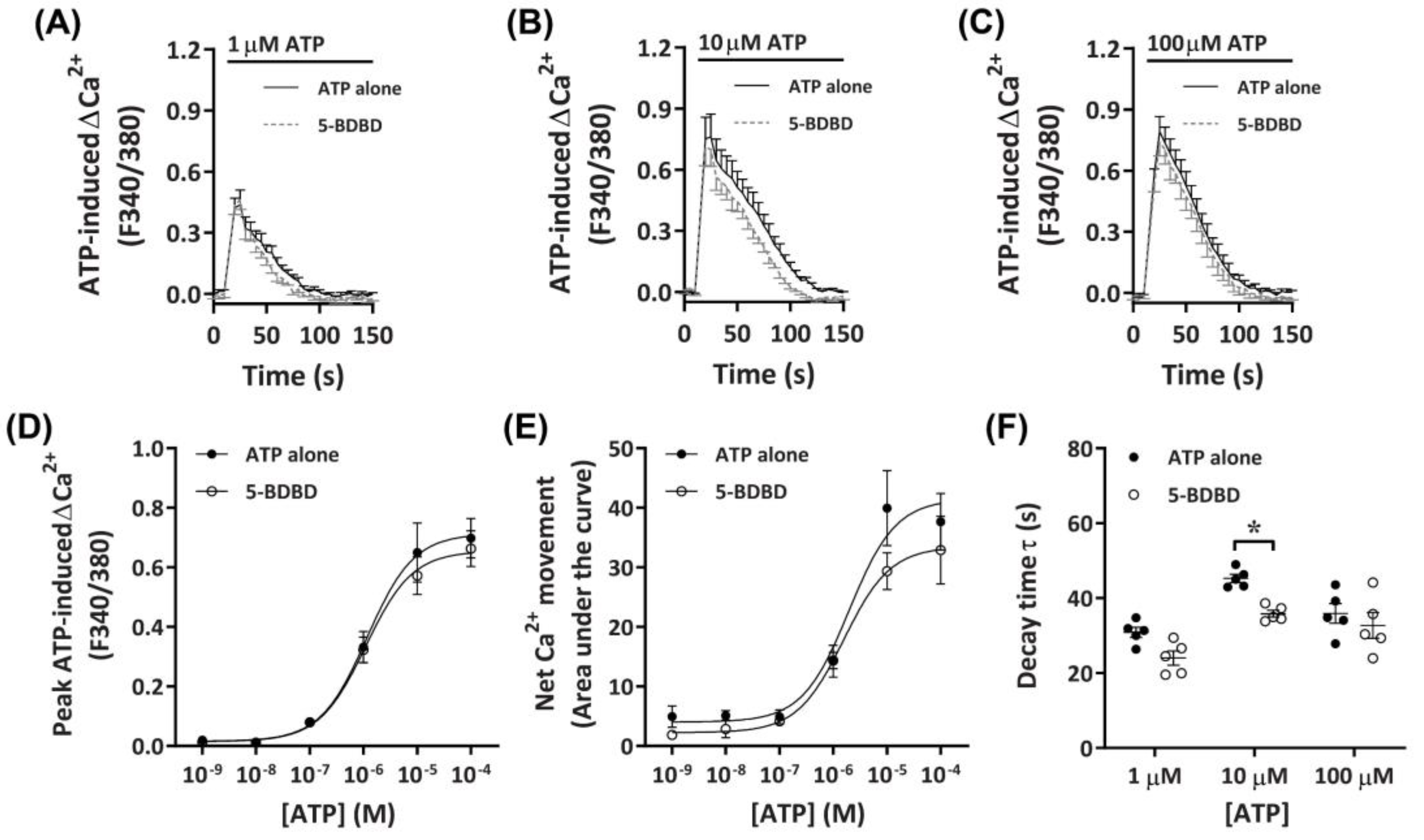
2.3.3. 5-BDBD Partially Reduces ATP-Induced Net Ca2+ Movement
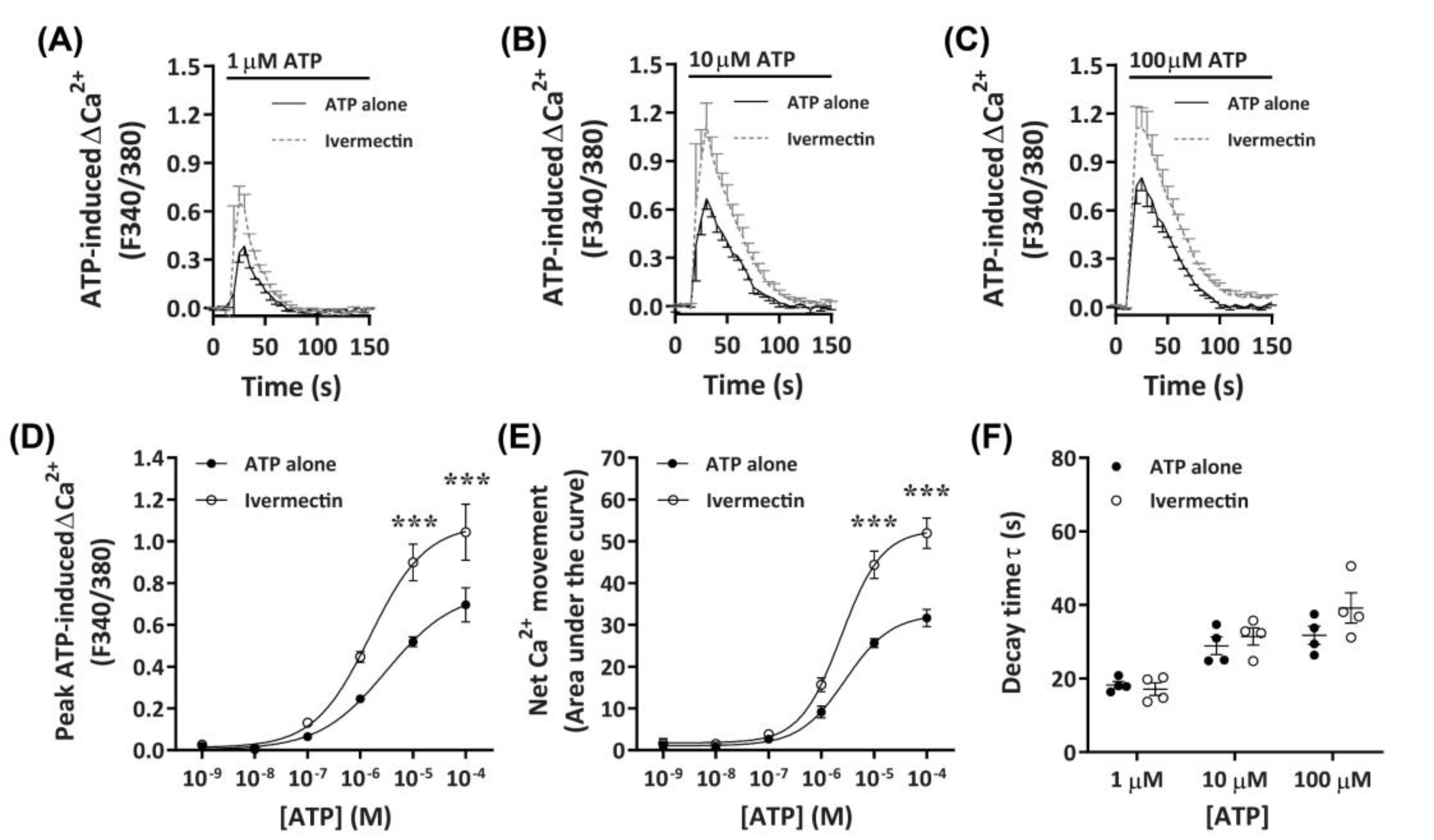
2.3.4. Ivermectin Positively Modulates ATP-Induced Net Ca2+ Movement
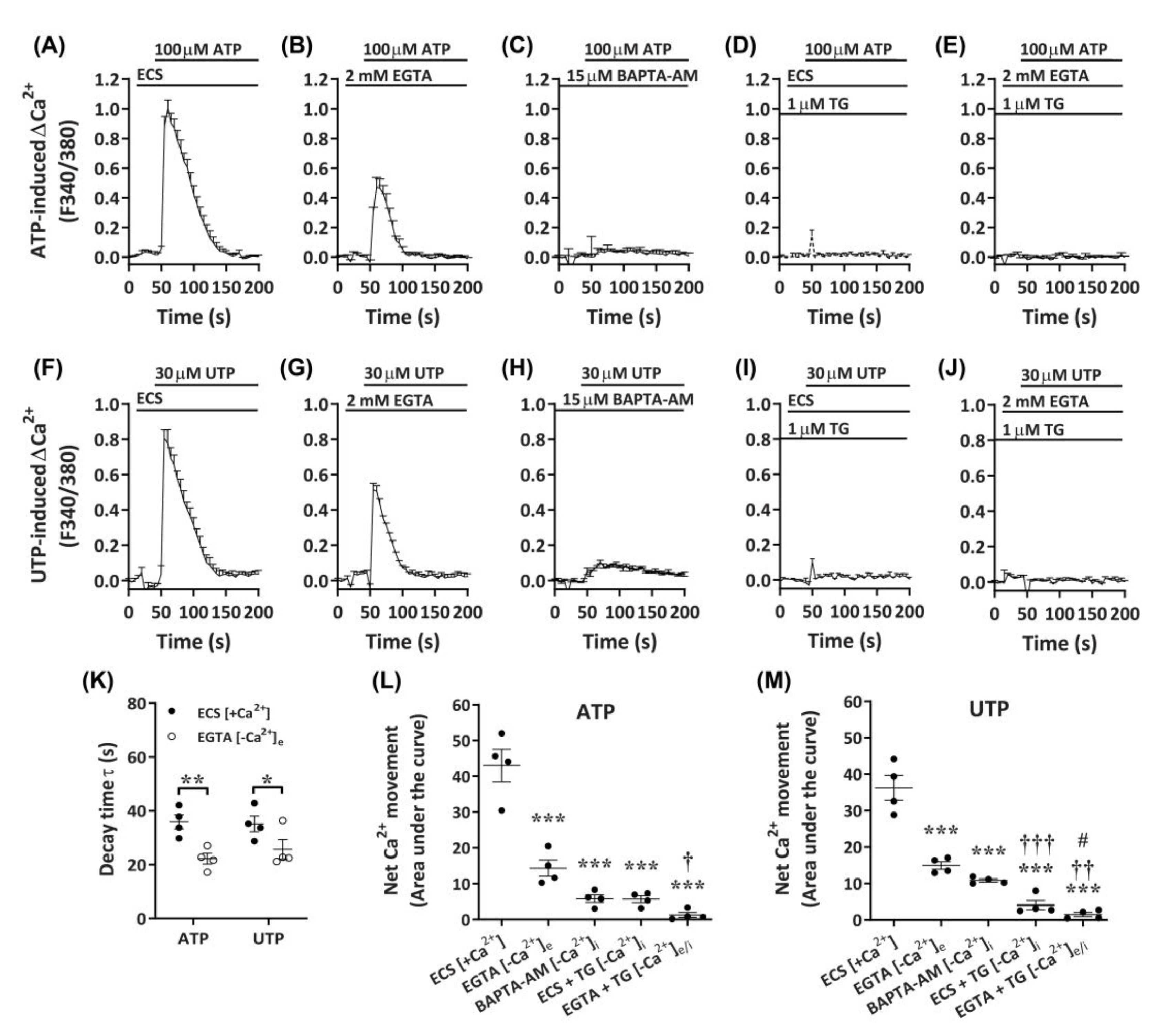
2.4. ATP and UTP Mediate Both Ca2+ Influx and Store-Operated Ca2+ Entry in DH82 Cells
2.5. P2Y2 Receptor Activation Mediates Ca2+ Mobilization in DH82 Cells
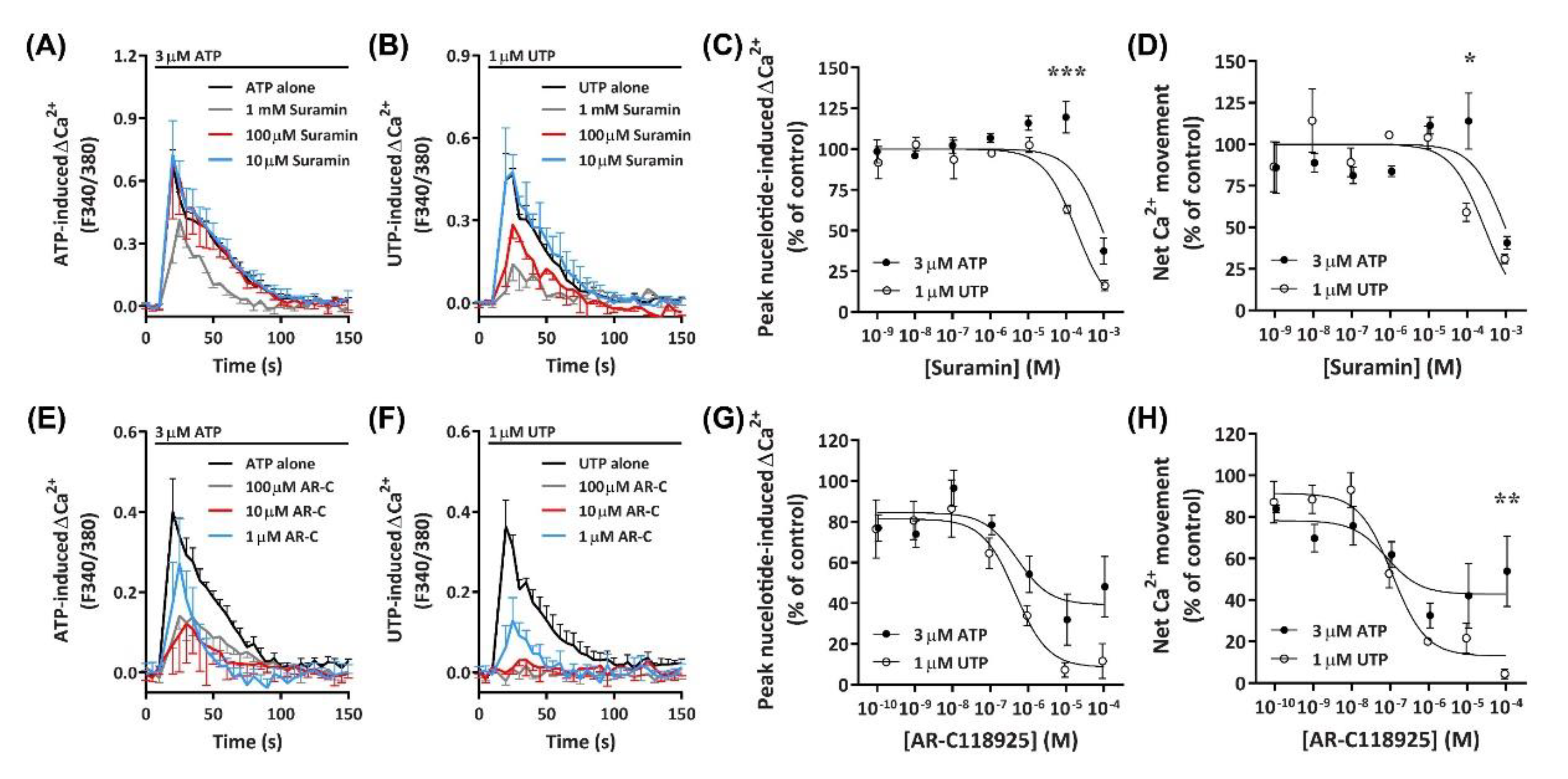
2.5.1. Suramin Reduces ATP- and UTP-Induced Ca2+ Mobilization
2.5.2. AR-C118925 Reduces ATP- and UTP-Induced Ca2+ Mobilization
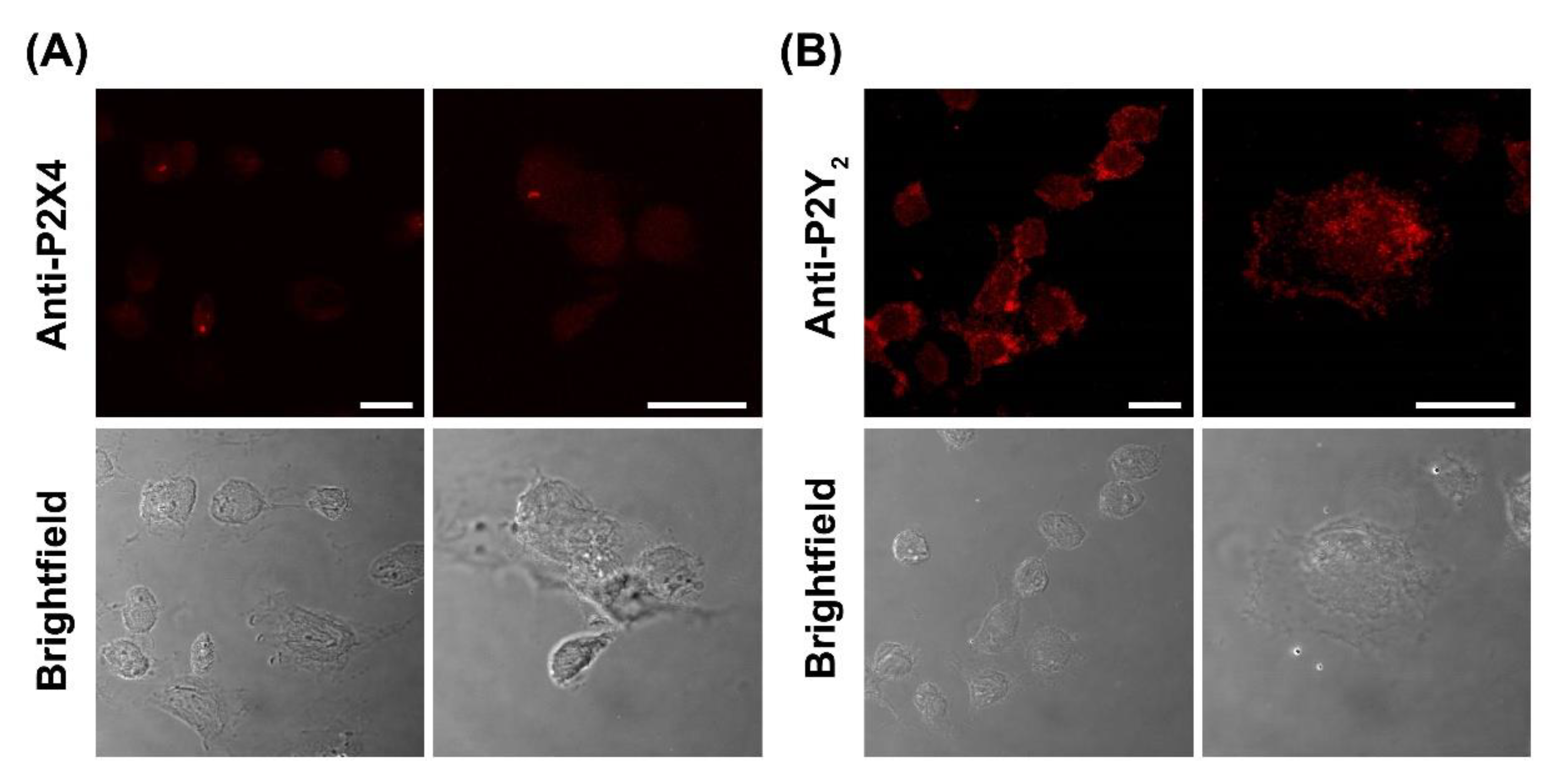
2.6. DH82 Canine Macrophages Predominantly Express Cell Surface P2Y2 Receptors
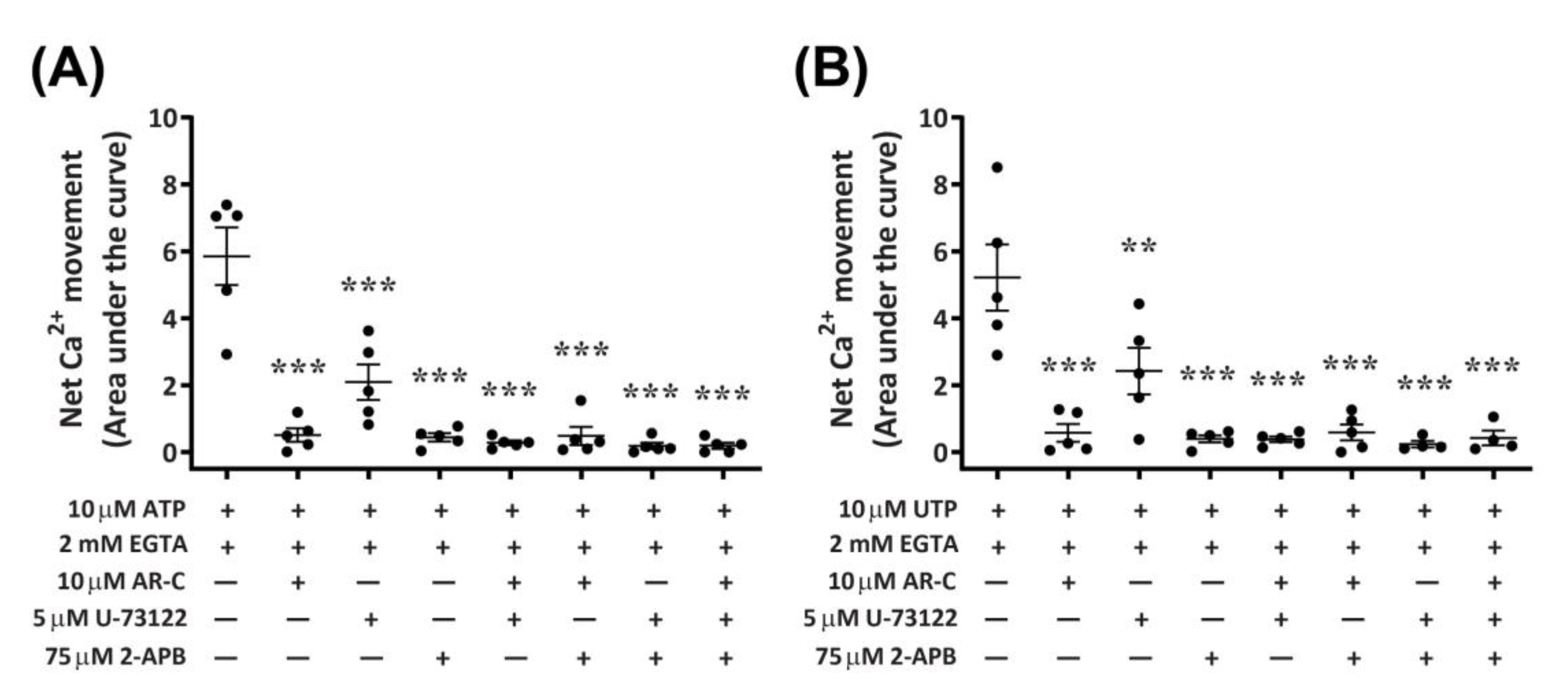
2.7. P2Y2 Receptor Activation Downstream Ca2+ Mobilization Is Coupled to the Phospholipase C/Inositol Triphosphate Signal Transduction Pathway in DH82 Cells
3. Discussion
4. Materials and Methods
4.1. Compounds and Reagents
4.2. Cells
4.3. RNA Isolation, cDNA Synthesis and RT-PCR
4.4. Measurement of Intracellular Ca2+
4.5. Immunocytochemistry and Confocal Microscopy
4.6. Data and Statistical Analysis
4.7. Nomenclature of Targets and Ligands
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-APB | 2-Aminoethoxydiphenyl borate |
| 2MeSADP | 2-Methylthio-ADP |
| ADP | Adenosine 5′-diphosphate |
| ATP | Adenosine 5′-triphosphate |
| BAPTA-AM | 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) |
| BzATP | 3’-O-(4-Benzoyl)benzoyl-ATP |
| CNS | Central nervous system |
| CCL2 | C-C chemokine ligand 2 |
| CCR2 | C-C chemokine receptor 2 |
| DMSO | Dimethyl sulfoxide |
| EC50 | Half maximal effective concentration |
| EGTA | Ethylene glycol tetraacetic acid |
| FBS | Fetal Bovine Serum |
| IC50 | Half-maximal inhibitory concentration |
| IL | Interleukin |
| IP3 | Inositol triphosphate |
| LPS | Lipopolysaccharide |
| MDCK | Madin Darby canine kidney |
| PLC | Phospholipase C |
| TNF | Tumor necrosis factor |
| TNP | 2′,3′-O-(2,4,6-Trinitrophenyl) |
| UDP | Uridine 5′-diphosphate |
| UTP | Uridine 5′-triphosphate |
References
- Ulmann, L.; Hatcher, J.P.; Hughes, J.P.; Chaumont, S.; Green, P.J.; Conquet, F.; Buell, G.N.; Reeve, A.J.; Chessell, I.P.; Rassendren, F. Up-Regulation of P2X4 Receptors in Spinal Microglia after Peripheral Nerve Injury Mediates BDNF Release and Neuropathic Pain. J. Neurosci. 2008, 28, 11263–11268. [Google Scholar] [CrossRef] [PubMed]
- Ulmann, L.; Hirbec, H.; Rassendren, F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010, 29, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yu, Y.; Zheng, L.; Wang, L.; Li, C.; Yu, J.; Wei, J.; Wang, C.; Zhang, J.; Xu, S.; et al. Chronic inflammatory pain upregulates expression of P2Y2 receptor in small-diameter sensory neurons. Metab. Brain Dis. 2015, 30, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, Z.Y.; Yu, L.H.; Burnstock, G.; Deng, X.M.; Ma, B. Inhibition of G protein-coupled P2Y2 receptor induced analgesia in a rat model of trigeminal neuropathic pain. Mol. Pain 2014, 10, 21. [Google Scholar] [CrossRef]
- Su, W.F.; Wu, F.; Jin, Z.H.; Gu, Y.; Chen, Y.T.; Fei, Y.; Chen, H.; Wang, Y.X.; Xing, L.Y.; Zhao, Y.Y.; et al. Overexpression of P2X4 receptor in Schwann cells promotes motor and sensory functional recovery and remyelination via BDNF secretion after nerve injury. Glia 2019, 67, 78–90. [Google Scholar] [CrossRef]
- Zabala, A.; Vazquez-Villoldo, N.; Rissiek, B.; Gejo, J.; Martin, A.; Palomino, A.; Perez-Samartín, A.; Pulagam, K.R.; Lukowiak, M.; Capetillo-Zarate, E.; et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 2018, 10, e8743. [Google Scholar] [CrossRef]
- Burnstock, G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 2006, 58, 58–86. [Google Scholar] [CrossRef]
- Stokes, L.; Surprenant, A. Purinergic P2Y2 receptors induce increased MCP-1/CCL2 synthesis and release from rat alveolar and peritoneal macrophages. J. Immunol. 2007, 179, 6016–6023. [Google Scholar] [CrossRef]
- Layhadi, J.A.; Fountain, S.J. ATP-Evoked Intracellular Ca2+ Responses in M-CSF Differentiated Human Monocyte-Derived Macrophage are Mediated by P2X4 and P2Y11 Receptor Activation. Int. J. Mol. Sci. 2019, 20, 5113. [Google Scholar] [CrossRef]
- Higgins, K.R.; Kovacevic, W.; Stokes, L. Nucleotides regulate secretion of the inflammatory chemokine CCL2 from human macrophages and monocytes. Mediators Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Layhadi, J.A.; Turner, J.; Crossman, D.; Fountain, S.J. ATP Evokes Ca2+ Responses and CXCL5 Secretion via P2X4 Receptor Activation in Human Monocyte-Derived Macrophages. J. Immunol. 2018, 200, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Bowler, J.W.; Bailey, R.J.; North, R.A.; Surprenant, A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br. J. Pharmacol. 2003, 140, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Stokes, L.; Surprenant, A. Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur. J. Immunol. 2009, 39, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Martínez, E.M.; Gómez-Coronado, K.S.; Espinosa-Luna, R.; Valdez-Morales, E.E.; Barrios-García, T.; Barajas-Espinosa, A.; Ochoa-Cortes, F.; Montaño, L.M.; Barajas-López, C.; Guerrero-Alba, R. Functional expression of P2X1, P2X4 and P2X7 purinergic receptors in human monocyte-derived macrophages. Eur. J. Pharmacol. 2020, 888, 173460. [Google Scholar] [CrossRef] [PubMed]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L.; et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Kuboyama, K.; Inoue, T.; Nagata, K.; Tozaki-Saitoh, H.; Inoue, K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol. Pain 2009, 5, 28. [Google Scholar] [CrossRef]
- Trang, T.; Beggs, S.; Wan, X.; Salter, M.W. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. 2009, 29. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424. [Google Scholar] [CrossRef]
- Wellman, M.L.; Krakowka, S.; Jacobs, R.M.; Kociba, G.J. A macrophage-monocyte cell line from a dog with malignant histiocytosis. In Vitro Cell. Dev. Biol. 1988, 24, 223–229. [Google Scholar] [CrossRef]
- Herrmann, I.; Gotovina, J.; Fazekas-Singer, J.; Fischer, M.B.; Hufnagl, K.; Bianchini, R.; Jensen-Jarolim, E. Canine macrophages can like human macrophages be in vitro activated toward the M2a subtype relevant in allergy. Dev. Comp. Immunol. 2018, 82, 118–127. [Google Scholar] [CrossRef]
- Heinrich, F.; Contioso, V.B.; Stein, V.M.; Carlson, R.; Tipold, A.; Ulrich, R.; Puff, C.; Baumgärtner, W.; Spitzbarth, I. Passage-dependent morphological and phenotypical changes of a canine histiocytic sarcoma cell line (DH82 cells). Vet. Immunol. Immunopathol. 2015, 163, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.; Bee, A.; Bell, S.; Gilmore, W.; Mee, A.; Morris, R.; Carter, S.D. Immunological and inflammatory characterisation of three canine cell lines: K1, K6 and DH82. Vet. Immunol Immunopathol. 2000, 75, 9–25. [Google Scholar] [CrossRef]
- Mendonça, P.H.B.; da Rocha, R.F.D.B.; Moraes, J.B.d.B.; LaRocque-de-Freitas, I.F.; Logullo, J.; Morrot, A.; Nunes, M.P.; Freire-de-Lima, C.G.; Decote-Ricardo, D. Canine Macrophage DH82 Cell Line As a Model to Study Susceptibility to Trypanosoma cruzi Infection. Front. Immunol. 2017, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Armando, F.; Gambini, M.; Corradi, A.; Giudice, C.; Pfankuche, V.M.; Brogden, G.; Attig, F.; von Kockritz-Blickwede, M.; Baumgartner, W.; Puff, C. Oxidative Stress in Canine Histiocytic Sarcoma Cells Induced by an Infection with Canine Distemper Virus Led to a Dysregulation of HIF-1alpha Downstream Pathway Resulting in a Reduced Expression of VEGF-B in vitro. Viruses 2020, 12, 200. [Google Scholar] [CrossRef]
- Nadaes, N.R.; Silva da Costa, L.; Santana, R.C.; LaRocque-de-Freitas, I.F.; Vivarini, Á.C.; Soares, D.C.; Wardini, A.B.; Gazos Lopes, U.; Saraiva, E.M.; Freire-de-Lima, C.G.; et al. DH82 Canine and RAW264.7 Murine Macrophage Cell Lines Display Distinct Activation Profiles Upon Interaction with Leishmania infantum and Leishmania amazonensis. Front. Cell Infect. Microbiol. 2020, 10, 247. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Nakatani, N.; Kubo, T.; Semi, Y.; Yoshida, N.; Nakajima, H.; Iseri, T.; Azuma, Y.T.; Takeuchi, T. Adenosine and ATP affect LPS-induced cytokine production in canine macrophage cell line DH82 cells. J. Vet. Med. Sci. 2012, 74, 27–34. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Y.; Zhao, Z.; Yan, L.; Xu, T.; Wang, X.; He, H.; Xia, X.; Zheng, W.; Xue, X. RNA sequencing analyses of gene expressions in a canine macrophages cell line DH82 infected with canine distemper virus. Infect. Genet. Evol. 2020, 80, 104206. [Google Scholar] [CrossRef]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar]
- Stevenson, R.O.; Taylor, R.M.; Wiley, J.S.; Sluyter, R. The P2X7 receptor mediates the uptake of organic cations in canine erythrocytes and mononuclear leukocytes: Comparison to equivalent human cell types. Purinergic Signal. 2009, 5, 385–394. [Google Scholar] [CrossRef]
- Jalilian, I.; Peranec, M.; Curtis, B.L.; Seavers, A.; Spildrejorde, M.; Sluyter, V.; Sluyter, R. Activation of the damage-associated molecular pattern receptor P2X7 induces interleukin-1 beta release from canine monocytes. Vet. Immunol. Immunopathol. 2012, 149, 86–91. [Google Scholar] [CrossRef]
- Ase, A.R.; Honson, N.S.; Zaghdane, H.; Pfeifer, T.A.; Seguela, P. Identification and characterization of a selective allosteric antagonist of human P2X4 receptor channels. Mol. Pharmacol. 2015, 87, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.; Donnelly-Roberts, D.; Namovic, M.; Zhong, C.M.; Wade, C.; Chandran, P.; Zhu, C.; Carroll, W.; Perez-Medrano, A.; Iwakura, Y.; et al. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1 alpha beta knockout mice. Behav. Brain Res. 2009, 204, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Virginio, C.; Surprenant, A.; Rice, J.; Dubyak, G.R. Isoquinolines as antagonists of the P2X7 nucleotide receptor: High selectivity for the human versus rat receptor homologues. Mol. Pharmacol. 1998, 54, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Layhadi, J.A.; Fountain, S.J. P2X4 Receptor-Dependent Ca2+ Influx in Model Human Monocytes and Macrophages. Int. J. Mol. Sci. 2017, 18, 2261. [Google Scholar] [CrossRef] [PubMed]
- Micklewright, J.J.; Layhadi, J.A.; Fountain, S.J. P2Y12 receptor modulation of ADP-evoked intracellular Ca2+ signalling in THP-1 human monocytic cells. Br. J. Pharmacol. 2018, 175, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hao, K.; Li, H.; Lu, R.; Liu, C.; Zhou, M.; Li, B.; Meng, Z.; Hu, Q.; Jiang, C. Design, synthesis and anti-inflammatory evaluation of 3-amide benzoic acid derivatives as novel P2Y14 receptor antagonists. Eur. J. Med. Chem. 2019, 181, 111564. [Google Scholar] [CrossRef]
- Tu, Y.M.; Gong, C.X.; Ding, L.; Liu, X.Z.; Li, T.; Hu, F.F.; Wang, S.; Xiong, C.P.; Liang, S.D.; Xu, H. A high concentration of fatty acids induces TNF-alpha as well as NO release mediated by the P2X4 receptor, and the protective effects of puerarin in RAW264.7 cells. Food Funct. 2017, 8, 4336–4346. [Google Scholar] [CrossRef]
- Gadeock, S.; Tran, J.; Georgiou, J.G.; Jalilian, I.; Taylor, R.M.; Wiley, J.S.; Sluyter, R. TGF-beta 1 prevents up-regulation of the P2X7 receptor by IFN-gamma and LPS in leukemic THP-1 monocytes. Biochim. Biophys. Acta 2010, 1798, 2058–2066. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y Receptor Pharmacology: IUPHAR Review:27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Mathie, A.; Peters, J.A.; Veale, E.L.; Striessnig, J.; Kelly, E.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; et al. The Concise Guide to Pharmacology 2019/20: Ion channels. Br. J. Pharmacol 2019, 176 (Suppl. 1), S142–S228. [Google Scholar] [CrossRef]
- Sophocleous, R.A.; Berg, T.; Finol-Urdaneta, R.K.; Sluyter, V.; Keshiya, S.; Bell, L.; Curtis, S.J.; Curtis, B.L.; Seavers, A.; Bartlett, R.; et al. Pharmacological and genetic characterisation of the canine P2X4 receptor. Br. J. Pharmacol. 2020, 177, 2812–2829. [Google Scholar] [CrossRef] [PubMed]
- Virginio, C.; Robertson, G.; Surprenant, A.; North, R.A. Trinitrophenyl-Substituted Nucleotides Are Potent Antagonists Selective for P2X1, P2X3, and Heteromeric P2X2/3 Receptors. Mol. Pharmacol. 1998, 53, 969. [Google Scholar] [PubMed]
- Nagata, K.; Imai, T.; Yamashita, T.; Tsuda, M.; Tozaki-Saitoh, H.; Inoue, K. Antidepressants inhibit P2X4 Receptor function: A possible involvement in neuropathic pain relief. Mol. Pain 2009, 5, 20. [Google Scholar] [CrossRef]
- Abdelrahman, A.; Namasivayam, V.; Hinz, S.; Schiedel, A.C.; Kose, M.; Burton, M.; El-Tayeb, A.; Gillard, M.; Bajorath, J.; de Ryck, M.; et al. Characterization of P2X4 receptor agonists and antagonists by calcium influx and radioligand binding studies. Biochem. Pharmacol. 2017, 125, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiang, Z.-H.; Jiang, C.-L.; Liu, W.-Z.; Shang, Z.-L. Effects of antidepressants on P2X7 receptors. Psychiatry Res. 2016, 242, 281–287. [Google Scholar] [CrossRef]
- Dao-Ung, P.; Skarratt, K.K.; Fuller, S.J.; Stokes, L. Paroxetine suppresses recombinant human P2X7 responses. Purinergic Signal. 2015, 11, 481–490. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef]
- Thastrup, O.; Cullen, P.J.; Drøbak, B.K.; Hanley, M.R.; Dawson, A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. USA 1990, 87, 2466–2470. [Google Scholar] [CrossRef]
- Zambon, A.C.; Hughes, R.J.; Meszaros, J.G.; Wu, J.J.; Torres, B.; Brunton, L.L.; Insel, P.A. P2Y2 receptor of MDCK cells: Cloning, expression, and cell-specific signaling. Am. J. Physiol. Renal Physiol. 2000, 279, F1045–F1052. [Google Scholar] [CrossRef]
- Kempson, S.A.; Edwards, J.M.; Osborn, A.; Sturek, M. Acute inhibition of the betaine transporter by ATP and adenosine in renal MDCK cells. Am. J. Physiol. Renal Physiol. 2008, 295, F108–F117. [Google Scholar] [CrossRef][Green Version]
- Charlton, S.J.; Brown, C.A.; Weisman, G.A.; Turner, J.T.; Erb, L.; Boarder, M.R. Cloned and transfected P2Y4 receptors: Characterization of a suramin and PPADS-insensitive response to UTP. Br. J. Pharmacol. 1996, 119, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, M.; Burbiel, J.C.; Attah, I.Y.; Abdelrahman, A.; Müller, C.E. Synthesis, characterization, and in vitro evaluation of the selective P2Y2 receptor antagonist AR-C118925. Purinergic Signal. 2017, 13, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.C.; Qi, A.D.; Harden, T.K.; Nicholas, R.A. Polarized expression of human P2Y receptors in epithelial cells from kidney, lung, and colon. Am. J. Physiol. Cell Physiol. 2005, 288, C624–C632. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.D.; Wolff, S.C.; Nicholas, R.A. The apical targeting signal of the P2Y2 receptor is located in its first extracellular loop. J. Biol. Chem. 2005, 280, 29169–29175. [Google Scholar] [CrossRef]
- Xue, X.; Zhu, Y.; Yan, L.; Wong, G.; Sun, P.; Zheng, X.; Xia, X. Antiviral efficacy of favipiravir against canine distemper virus infection in vitro. BMC Vet. Res. 2019, 15, 316. [Google Scholar] [CrossRef]
- Pfankuche, V.M.; Sayed-Ahmed, M.; Contioso, V.B.; Spitzbarth, I.; Rohn, K.; Ulrich, R.; Deschl, U.; Kalkuhl, A.; Baumgärtner, W.; Puff, C. Persistent Morbillivirus Infection Leads to Altered Cortactin Distribution in Histiocytic Sarcoma Cells with Decreased Cellular Migration Capacity. PLoS ONE 2016, 11, e0167517. [Google Scholar] [CrossRef]
- Armando, F.; Gambini, M.; Corradi, A.; Becker, K.; Marek, K.; Pfankuche, V.M.; Mergani, A.E.; Brogden, G.; de Buhr, N.; von Köckritz-Blickwede, M.; et al. Mesenchymal to epithelial transition driven by canine distemper virus infection of canine histiocytic sarcoma cells contributes to a reduced cell motility in vitro. J. Cell Mol. Med. 2020, 24, 9332–9348. [Google Scholar] [CrossRef]
- Balazs, B.; Danko, T.; Kovacs, G.; Koles, L.; Hediger, M.A.; Zsembery, A. Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptors by two complementary methods. Cell Physiol. Biochem. 2013, 32, 11–24. [Google Scholar] [CrossRef]
- Stokes, L.; Bidula, S.; Bibič, L.; Allum, E. To Inhibit or Enhance? Is There a Benefit to Positive Allosteric Modulation of P2X Receptors? Front. Pharmacol. 2020, 11, 627. [Google Scholar] [CrossRef]
- von Kugelgen, I. Pharmacology of P2Y receptors. Brain Res. Bull. 2019. [Google Scholar] [CrossRef]
- Jan, C.R.; Ho, C.M.; Wu, S.N.; Tseng, C.J. Mechanisms of rise and decay of ADP-evoked calcium signal in MDCK cells. Chin. J. Physiol. 1998, 41, 67–73. [Google Scholar] [PubMed]
- Katzur, A.C.; Koshimizu, T.-A.; Tomic, M.; Schultze-Mosgau, A.; Ortmann, O.; Stojilkovic, S.S. Expression and Responsiveness of P2Y2 Receptors in Human Endometrial Cancer Cell Lines. J. Clin. Endocrinol. Metab. 1999, 84, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Wildman, S.S.; Unwin, R.J.; King, B.F. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br. J. Pharmacol. 2003, 140, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Post, S.R.; Rump, L.C.; Zambon, A.; Hughes, R.J.; Buda, M.D.; Jacobson, J.P.; Kao, C.C.; Insel, P.A. ATP activates cAMP production via multiple purinergic receptors in MDCK-D1 epithelial cells. Blockade of an autocrine/paracrine pathway to define receptor preference of an agonist. J. Biol. Chem. 1998, 273, 23093–23097. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qi, A.D.; Zambon, A.C.; Insel, P.A.; Nicholas, R.A. An arginine/glutamine difference at the juxtaposition of transmembrane domain 6 and the third extracellular loop contributes to the markedly different nucleotide selectivities of human and canine P2Y11 receptors. Mol. Pharmacol. 2001, 60, 1375–1382. [Google Scholar] [CrossRef]
- Communi, D.; Robaye, B.; Boeynaems, J.M. Pharmacological characterization of the human P2Y11 receptor. Br. J. Pharmacol 1999, 128, 1199–1206. [Google Scholar] [CrossRef]
- Hughes, R.J.; Torres, B.; Zambon, A.; Arthur, D.; Bohmann, C.; Rump, L.C.; Insel, P.A. Expression of multiple P2Y receptors by MDCK-D1 cells: P2Y1 receptor cloning and signaling. Drug Dev. Res. 2003, 59, 1–7. [Google Scholar] [CrossRef]
- Nicholas, R.A.; Watt, W.C.; Lazarowski, E.R.; Li, Q.; Harden, K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996, 50, 224–229. [Google Scholar]
- Communi, D.; Parmentier, M.; Boeynaems, J.M. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem. Biophys. Res. Commun. 1996, 222, 303–308. [Google Scholar] [CrossRef]
- Insel, P.A.; Ostrom, R.S.; Zambon, A.C.; Hughes, R.J.; Balboa, M.A.; Shehnaz, D.; Gregorian, C.; Torres, B.; Firestein, B.L.; Xing, M.; et al. P2Y receptors of MDCK cells: Epithelial cell regulation by extracellular nucleotides. Clin. Exp. Pharmacol. Physiol. 2001, 28, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cao, W.; Li, J.; Liu, J. Lysosomal exocytosis of ATP is coupled to P2Y2 receptor in marginal cells in the stria vascular in neonatal rats. Cell Calcium 2018, 76, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Chessell, I.P.; Simon, J.; Barnard, E.A.; Miller, K.J.; Michel, A.D.; Humphrey, P.P.A. Functional characterization of the P2X4 receptor orthologues. Br. J. Pharmacol. 2000, 129, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Muzzachi, S.; Blasi, A.; Ciani, E.; Favia, M.; Cardone, R.A.; Marzulli, D.; Reshkin, S.J.; Merizzi, G.; Casavola, V.; Soleti, A.; et al. MED1101: A new dialdehydic compound regulating P2x7 receptor cell surface expression in U937 cells. Biol. Cell 2013, 105, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; Ase, A.R.; Seguela, P. P2X receptor channels in chronic pain pathways. Br. J. Pharmacol. 2018, 175, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Role of the P2X4 receptor in neuropathic pain. Curr. Opin. Pharmacol. 2019, 47, 33–39. [Google Scholar] [CrossRef]
- Domercq, M.; Matute, C. Targeting P2X4 and P2X7 receptors in multiple sclerosis. Curr. Opin. Pharmacol. 2019, 47, 119–125. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Paramasivam, A.; Yu, J.C.; Murrell-Lagnado, R.D. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 2007, 120, 3838–3849. [Google Scholar] [CrossRef]
- Sivaramakrishnan, V.; Bidula, S.; Campwala, H.; Katikaneni, D.; Fountain, S.J. Constitutive lysosome exocytosis releases ATP and engages P2Y receptors in human monocytes. J. Cell Sci. 2012, 125, 4567–4575. [Google Scholar] [CrossRef]
- Toyomitsu, E.; Tsuda, M.; Yamashita, T.; Tozaki-Saitoh, H.; Tanaka, Y.; Inoue, K. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 2012, 8, 301–310. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; Zarruk, J.G.; Healy, L.M.; Baskar Jesudasan, S.J.; Jhelum, P.; Salmon, C.K.; Formanek, A.; Russo, M.V.; Antel, J.P.; McGavern, D.B.; et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 2018, 16, e2005264. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, V.; Drogemuller, C.; Leeb, T. A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and eight wolves. Anim. Genet. 2019, 50, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Büscher, R.; Hoerning, A.; Patel, H.H.; Zhang, S.; Arthur, D.B.; Grasemann, H.; Ratjen, F.; Insel, P.A. P2Y2 receptor polymorphisms and haplotypes in cystic fibrosis and their impact on Ca2+ influx. Pharmacogenet. Genom. 2006, 16, 199–205. [Google Scholar] [CrossRef]
- Stokes, L.; Scurrah, K.; Ellis, J.A.; Cromer, B.A.; Skarratt, K.K.; Gu, B.J.; Harrap, S.B.; Wiley, J.S. A loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Hypertension 2011, 58, 1086–1092. [Google Scholar] [CrossRef]
- Sophocleous, R.A.; Sluyter, V.; Curtis, B.L.; Curtis, S.J.; Jurak, L.M.; Faulks, M.; Spildrejorde, M.; Gates, S.; Proctor, E.J.; Seavers, A.; et al. Association of a P2RX7 gene missense variant with brachycephalic dog breeds. Anim. Genet. 2020, 51, 127–131. [Google Scholar] [CrossRef]
- Spildrejorde, M.; Bartlett, R.; Stokes, L.; Jalilian, I.; Peranec, M.; Sluyter, V.; Curtis, B.L.; Skarratt, K.K.; Skora, A.; Bakhsh, T.; et al. R270C polymorphism leads to loss of function of the canine P2X7 receptor. Physiol. Genom. 2014, 46, 512–522. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Paredes, R.M.; Etzler, J.C.; Watts, L.T.; Lechleiter, J.D. Chemical Calcium Indicators. Methods (San Diego, Calif.) 2008, 46, 143–151. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The Concise Guide to Pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176, S21–S141. [Google Scholar] [CrossRef]
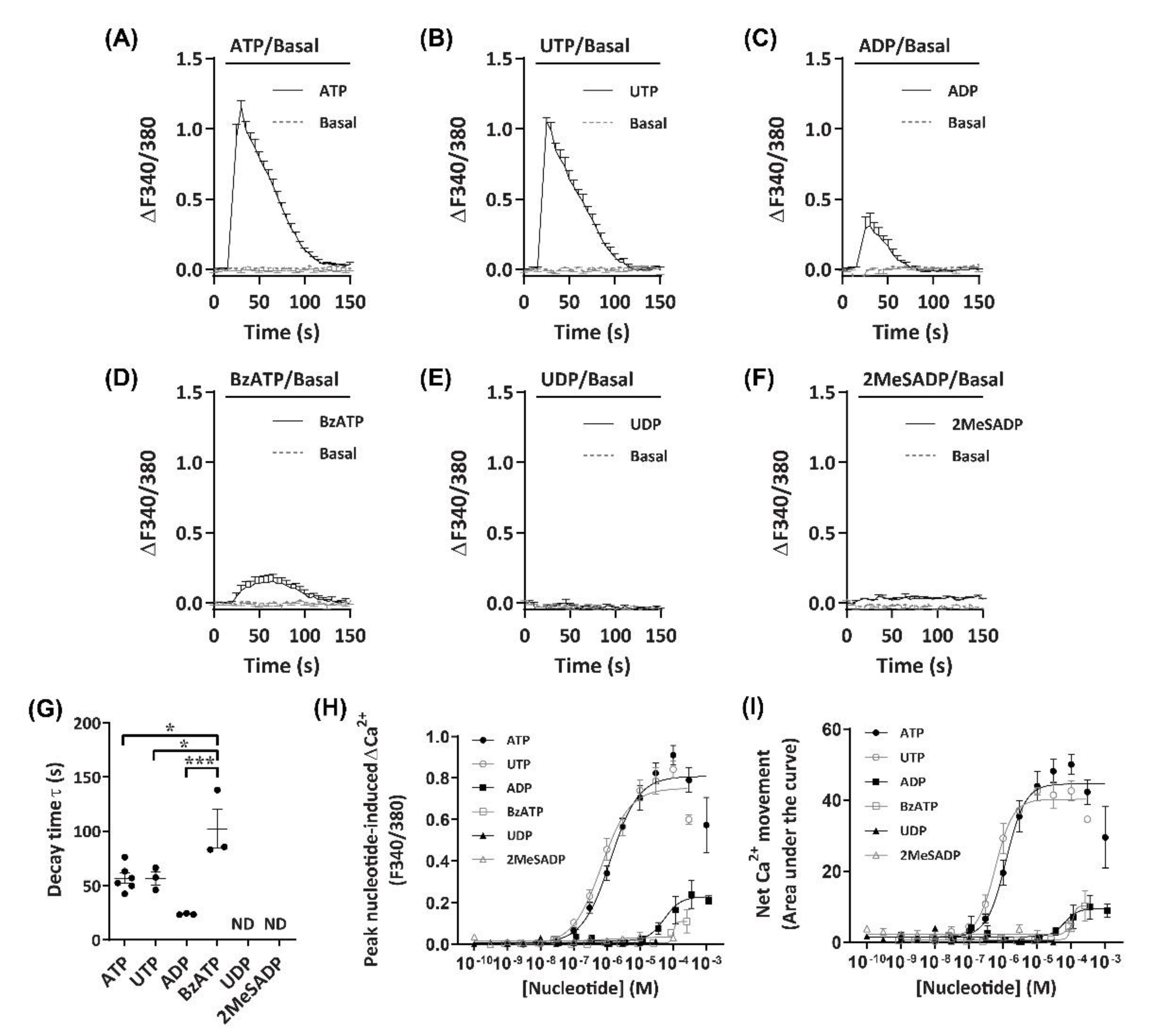
| Nucleotide | Peak Ca2+ Response | Net Ca2+ Response | ||
|---|---|---|---|---|
| pEC50 | Hill Coefficient | pEC50 | Hill Coefficient | |
| ATP | 5.88 ± 0.05 (100%) | 0.99 | 5.92 ± 0.09 (100%) | 1.43 |
| UTP | 6.16 ± 0.09 (65.9%) | 1.02 | 6.26 ± 0.12 (69.1%) | 1.71 |
| ADP 1 | 4.03 ± 0.30 (26.4%) 2 | 1.47 | 4.07 ± 0.21 (19.9%) 2 | 1.00 |
| BzATP | <4.00 (12.1%) 3 | 2.26 | <4.00 (20.5%) 3 | 2.66 |
| UDP | ND (<10%) | - | ND (<10%) | - |
| 2MeSADP | ND (<10%) | - | ND (<10%) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sophocleous, R.A.; Miles, N.A.; Ooi, L.; Sluyter, R. P2Y2 and P2X4 Receptors Mediate Ca2+ Mobilization in DH82 Canine Macrophage Cells. Int. J. Mol. Sci. 2020, 21, 8572. https://doi.org/10.3390/ijms21228572
Sophocleous RA, Miles NA, Ooi L, Sluyter R. P2Y2 and P2X4 Receptors Mediate Ca2+ Mobilization in DH82 Canine Macrophage Cells. International Journal of Molecular Sciences. 2020; 21(22):8572. https://doi.org/10.3390/ijms21228572
Chicago/Turabian StyleSophocleous, Reece Andrew, Nicole Ashleigh Miles, Lezanne Ooi, and Ronald Sluyter. 2020. "P2Y2 and P2X4 Receptors Mediate Ca2+ Mobilization in DH82 Canine Macrophage Cells" International Journal of Molecular Sciences 21, no. 22: 8572. https://doi.org/10.3390/ijms21228572
APA StyleSophocleous, R. A., Miles, N. A., Ooi, L., & Sluyter, R. (2020). P2Y2 and P2X4 Receptors Mediate Ca2+ Mobilization in DH82 Canine Macrophage Cells. International Journal of Molecular Sciences, 21(22), 8572. https://doi.org/10.3390/ijms21228572






