Mass Spectrometry-Based Solid Phase Peptide Reaction Assay for Detecting Allergenicity Using an Immobilized Peptide-Conjugating Photo-Cleavable Linker
Abstract
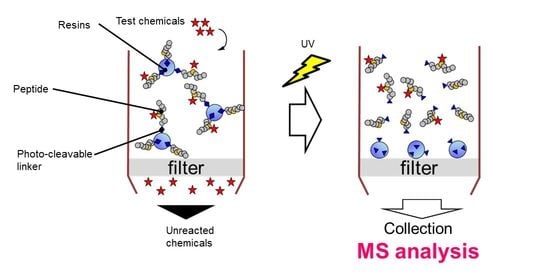
:1. Introduction
2. Results and Discussion
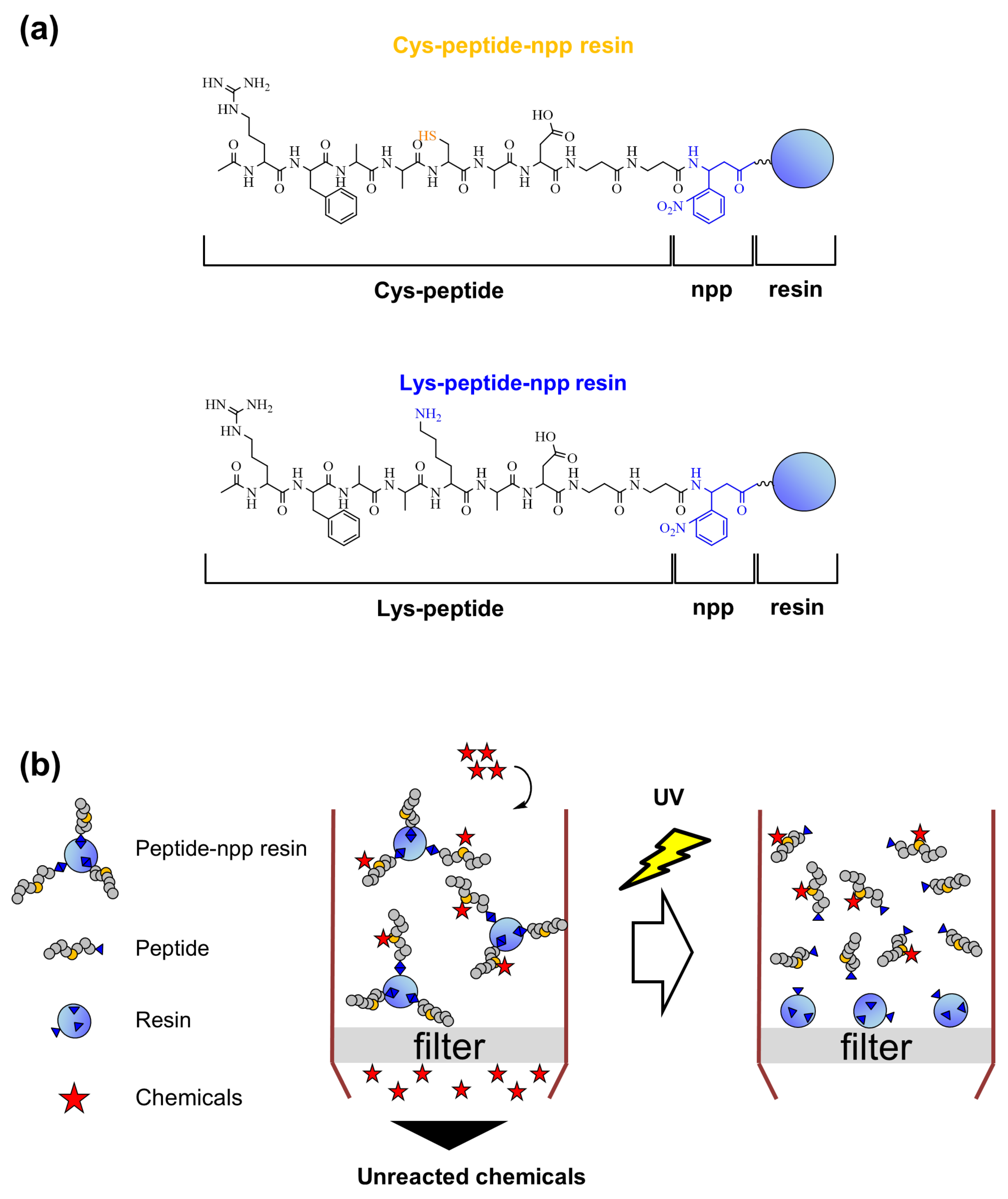
2.1. Design and Synthesis of Cys- and Lys-Peptides via a Photo-Cleavable Linker on the Resin
2.2. Optimization of MALDI-TOF MS Analysis Using FITC-Modified Peptides
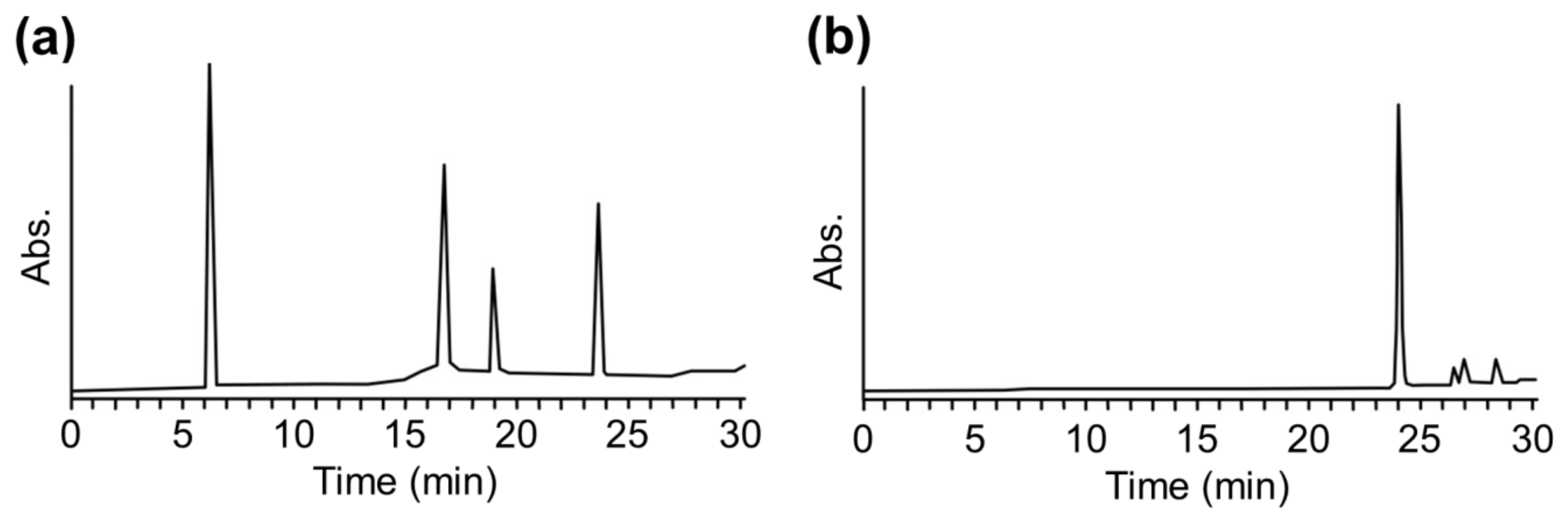
2.3. MALDI-TOF MS Analysis of Sensitizer-Modified Peptides
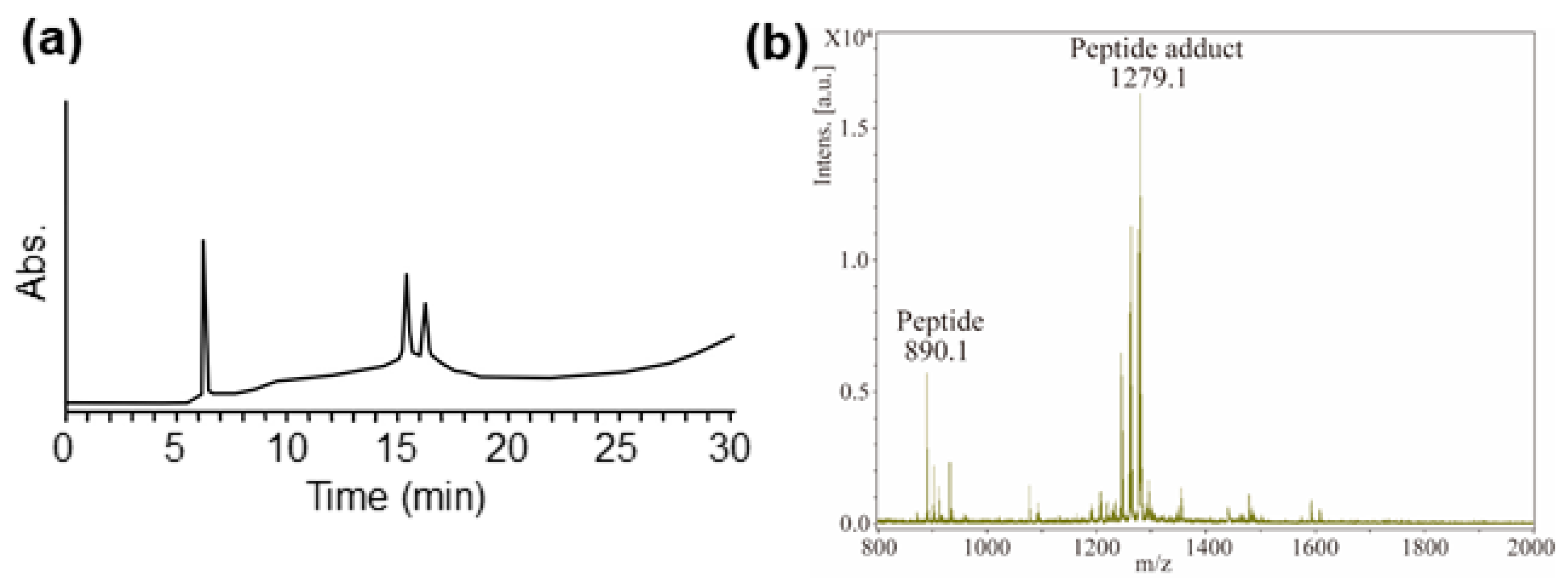
2.4. Confirmation of Reactivity with Adduct Analysis
2.5. Reaction of Mixture with the Peptide-npp Resin
3. Materials and Methods
3.1. Test Chemicals and Materials
3.2. Synthesis of Cys-Peptide-npp and Lys-Peptide-npp Resins
3.3. Procedure Using Cys-Peptide-npp Resins
3.4. Procedure Using Lys-Peptide-npp Resins
3.5. Analysis Using HPLC
3.6. Analysis Using MALDI-TOF MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACA | a-Amyl cinnamic aldehyde |
| BA | Benzylidene acetone |
| BC | Benzyl cinnamate |
| BQ | p-Benzoquinone |
| DMF | Dimethyl formamide |
| DP | Dibutyl phthalate |
| DPRA | Direct peptide reactivity assay |
| FITC | Fluorescein-5-isothiocyanate |
| HCA | a-Hexyl cinnamic aldehyde |
| HPLC | High-performance liquid chromatography |
| IPA | Isopropanol |
| MS | Mass spectrometry |
| MPH | 5-Methyl-2-phenyl-2-hexenal |
| M-SPRA | Mass spectrometry-based solid-phase peptide reaction assay |
| UE | Undec-10-enal |
References
- Kimner, I.; Mitchell, J.; Griffin, A.C. Development of a murine local lymph node assay for the determination of sensitizing potential. Food Chem. Toxicol. 1986, 24, 585–586. [Google Scholar]
- EU. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (7th Amendment to the European Cosmetics Directive). Off. J. Eur. Communities Legis 2003, 66, 26–35. [Google Scholar]
- Kaplan, D.H.; Igyarto, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Gerberick, G.F.; Vassallo, J.D.; Bailey, R.E.; Chaney, J.G.; Morrall, S.W.; Lepoittevin, J.P. Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci. 2004, 81, 332–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerberick, G.F.; Vassallo, J.D.; Foertsch, L.M.; Price, B.B.; Chaney, J.G.; Lepoittevin, J.P. Quantification of chemical peptide reactivity for screening contact allergens: A classification tree model approach. Toxicol. Sci. 2007, 97, 417–427. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidelines for testing of chemicals No. 442C. In Key-Event-Based Test Guideline for in Chemico Skin Sensitisation Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Natsch, A.; Gfeller, H. LC-MS-based characterization of the peptide reactivity of chemicals to improve the in vitro prediction of the skin sensitization potential. Toxicol. Sci. 2008, 106, 464–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalko, J.F.; Kimber, I.; Dearman, R.J.; Gerberick, G.F.; Sarlo, K.; Api, A.M. Chemical reactivity measurements: Potential for characterization of respiratory chemical allergens. Toxicol. Vitro 2011, 25, 433–445. [Google Scholar] [CrossRef]
- EURL-ECVAM. EURL ECVAM Recommendation on the Direct Peptide Reactivity Assay (Dpra) for Skin Sensitisation Test; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Miyazaki, H.; Hamada, Y.; Takaishi, H.; Minamino, Y.; Ikeda, H.; Mekata, H.; Takaishi, M.; Yamashita, K.; Usui, K. Development of a chromophore-solid phase peptide reaction assay (C-SPRA) for assessing skin sensitization in vitro. Analyst 2020, 145, 3211–3216. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.J.; Greenberg, M.M. Synthesis of oligonucleotides containing 3′-alkyl carboxylic acids using universal, photolabile solid phase synthesis supports. J. Org. Chem. 1995, 60, 3358–3364. [Google Scholar] [CrossRef]
- Holmes, C.P. Model studies for new o-nitrobenzyl photolabile linkers: Substituent effects on the rates of photochemical cleavage. J.Org. Chem. 1997, 62, 2370–2380. [Google Scholar] [CrossRef]
- Ariyasu, S.; Hanaya, K.; Watanabe, E.; Suzuki, T.; Horie, K.; Hayase, M.; Abe, R.; Aoki, S. Selective capture and collection of live target cells using a photoreactive silicon wafer device modified with antibodies via a photocleavable linker. Langmuir 2012, 28, 13118–13126. [Google Scholar] [CrossRef]
- Usui, K.; Tomizaki, K.-y.; Mihara, H. A cell microarray format: A peptide release system using a photo-cleavable linker for cell toxicity and cell uptake analysis. Methods. Mol. Biol. 2016, 1352, 199–210. [Google Scholar]
- Kakiyama, T.; Usui, K.; Tomizaki, K.-y.; Mie, M.; Kobatake, E.; Mihara, H. A peptide release system using a photo-cleavable linker in a cell array format for cell-toxicity analysis. Polym. J. 2013, 45, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Usui, K.; Kikuchi, T.; Tomizaki, K.-y.; Kakiyama, T.; Mihara, H. A novel array format for monitoring cellular uptake using a photo-cleavable linker for peptide release. Chem. Commun. 2013, 49, 6394–6396. [Google Scholar] [CrossRef]
- Mabuchi, M.; Shimizu, T.; Haramura, M.; Tanaka, A. Identification and purification of target protein using affinity resin bearing a photo-labile linker. Bioorg. Med. Chem. Lett. 2015, 25, 3373–3377. [Google Scholar] [CrossRef]
- Usui, K.; Yokota, S.; Iwata, K.; Hamada, Y. Novel purification process for amyloid beta peptide(1-40). Processes 2020, 8, 464. [Google Scholar] [CrossRef]
- Otsubo, Y.; Nishijo, T.; Miyazawa, M.; Saito, K.; Mizumachi, H.; Sakaguchi, H. Binary test battery with KeratinoSensTM and h-CLAT as part of a bottom-up approach for skin sensitization hazard prediction. Regul. Toxicol. Pharmacol. 2017, 88, 118–124. [Google Scholar] [CrossRef]
- Aleksic, M.; Thain, E.; Roger, D.; Saib, O.; Davies, M.; Li, J.; Aptula, A.; Zazzeroni, R. Reactivity profiling: Covalent modification of single nucleophile peptides for skin sensitization risk assessment. Toxicol. Sci. 2009, 108, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.W.; Aptula, A.O.; Patlewicz, G. Electrophilic chemistry related to skin sensitization. Reaction mechanistic applicability domain classification for a published data set of 106 chemicals tested in the mouse local lymph node assay. Chem. Res. Toxicol. 2007, 20, 44–60. [Google Scholar] [CrossRef]
- Roberts, D.W.; Patlewicz, G.; Kern, P.S.; Gerberick, F.; Kimber, I.; Dearman, R.J.; Ryan, C.A.; Basketter, D.A.; Aptula, A.O. Mechanistic applicability domain classification of a local lymph node assay dataset for skin sensitization. Chem. Res. Toxicol. 2007, 20, 1019–1030. [Google Scholar] [CrossRef]
- Urbisch, D.; Mehling, A.; Guth, K.; Ramirez, T.; Honarvar, N.; Kolle, S.; Landsiedel, R.; Jaworska, J.; Kern, P.S.; Gerberick, F.; et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul. Toxicol. Pharm. 2015, 71, 337–351. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.K.; Hotchkiss, S.A.M. Allergic Contact Dermatitis—Chemical and Metabolic Mechanisms; Taylor & Francis: London, UK, 2001. [Google Scholar]
- Lepoittevin, J.P. Metabolism versus chemical transformation or proversus prehaptens? Contact Derm. 2006, 54, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; White, P.D. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
| Test Chemicals | LLNA a | DPRA b | Adduct Formation in this Study c | |
|---|---|---|---|---|
| Potency Category | Results d | Cys-Peptide | Lys-Peptide | |
| p-Benzoquinone (BQ) | Extreme | P | + | + |
| Fluorescein-5-isothiocyanate (FITC) | Strong | P | + | + |
| Benzylidene acetone (BA) | Moderate | P | + | + |
| 5-Methyl-2-phenyl-2-hexenal (MPH) | Moderate | - | + | + |
| Undec-10-enal (UE) | Moderate | N | − | + |
| α-Hexyl cinnamic aldehyde (HCA) | Weak | N | + | + |
| α-Amyl cinnamic aldehyde (ACA) | Weak | N | + | + |
| Benzyl cinnamate (BC) | Weak | N | + | − |
| Dibutyl phthalate (DP) | Non-sensitizer | N | − | − |
| Isopropanol (IP) | Non-sensitizer | N | − | − |
| Test Chemicals | MS Signal (m/z) | Adduct Interpretation | |
|---|---|---|---|
| Cys-Peptide | Lys-Peptide | ||
| BQ | Cys1: 973 [M+H]+ Cys2: 1113 [M+H]+ | Lys1: 1101 [M+H]+ | Cys1: Michael adduct, Cys2: bimolecular addition by Michael adduct and oxidation to sulfone, Lys1: bimolecular addition by Michael adduct |
| FITC | Cys1: 1254 [M+H]+ | Lys1: 1279 [M+H]+ | Cys1: Acylation, Lys1: Acylation |
| BA | Cys1: 1011 [M+H]+ | Lys1: 1164 [M+H]+ | Cys1: Michael adduct, Lys1: bimolecular addition by Michael adduct and Schiff base formation |
| MPH | Cys1: 1053 [M+H]+ | Lys1: 1074 [M+H]+ | Cys1: Michael adduct, Lys1: unknown |
| UE | No signal | Lys1: 1042 [M+H]+ | Lys1: Schiff base formation |
| HCA | Cys1: 1081 [M+H]+ | Lys1: 1120 [M+H]+ | Cys1: Michael adduct, Lys1: Michael adduct |
| ACA | Cys1: 1067 [M+H]+ | Lys1: 1074 [M+H]+ | Cys1: Michael adduct, Lys1: Schiff base formation |
| BC | Cys1: 1085 [M+H]+ Cys2: 1131 [M+H]+ | No signal | Cys1: unknown, Cys2: unknown, |
| DP | No signal | No signal | Not adduct |
| IP | No signal | No signal | Not adduct |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, H.; Samejima, Y.; Iwata, K.; Minamino, Y.; Hikida, S.; Ariumi, H.; Ikeda, H.; Hamada, Y.; Yamashita, K.; Usui, K. Mass Spectrometry-Based Solid Phase Peptide Reaction Assay for Detecting Allergenicity Using an Immobilized Peptide-Conjugating Photo-Cleavable Linker. Int. J. Mol. Sci. 2020, 21, 8332. https://doi.org/10.3390/ijms21218332
Miyazaki H, Samejima Y, Iwata K, Minamino Y, Hikida S, Ariumi H, Ikeda H, Hamada Y, Yamashita K, Usui K. Mass Spectrometry-Based Solid Phase Peptide Reaction Assay for Detecting Allergenicity Using an Immobilized Peptide-Conjugating Photo-Cleavable Linker. International Journal of Molecular Sciences. 2020; 21(21):8332. https://doi.org/10.3390/ijms21218332
Chicago/Turabian StyleMiyazaki, Hiroshi, Yasutaka Samejima, Kazuya Iwata, Yuuki Minamino, Shinya Hikida, Hideto Ariumi, Hidefumi Ikeda, Yoshio Hamada, Kunihiko Yamashita, and Kenji Usui. 2020. "Mass Spectrometry-Based Solid Phase Peptide Reaction Assay for Detecting Allergenicity Using an Immobilized Peptide-Conjugating Photo-Cleavable Linker" International Journal of Molecular Sciences 21, no. 21: 8332. https://doi.org/10.3390/ijms21218332
APA StyleMiyazaki, H., Samejima, Y., Iwata, K., Minamino, Y., Hikida, S., Ariumi, H., Ikeda, H., Hamada, Y., Yamashita, K., & Usui, K. (2020). Mass Spectrometry-Based Solid Phase Peptide Reaction Assay for Detecting Allergenicity Using an Immobilized Peptide-Conjugating Photo-Cleavable Linker. International Journal of Molecular Sciences, 21(21), 8332. https://doi.org/10.3390/ijms21218332