Identifying Cancer-Relevant Mutations in the DLC START Domain Using Evolutionary and Structure-Function Analyses
Abstract
:1. Introduction
2. Results and Discussion
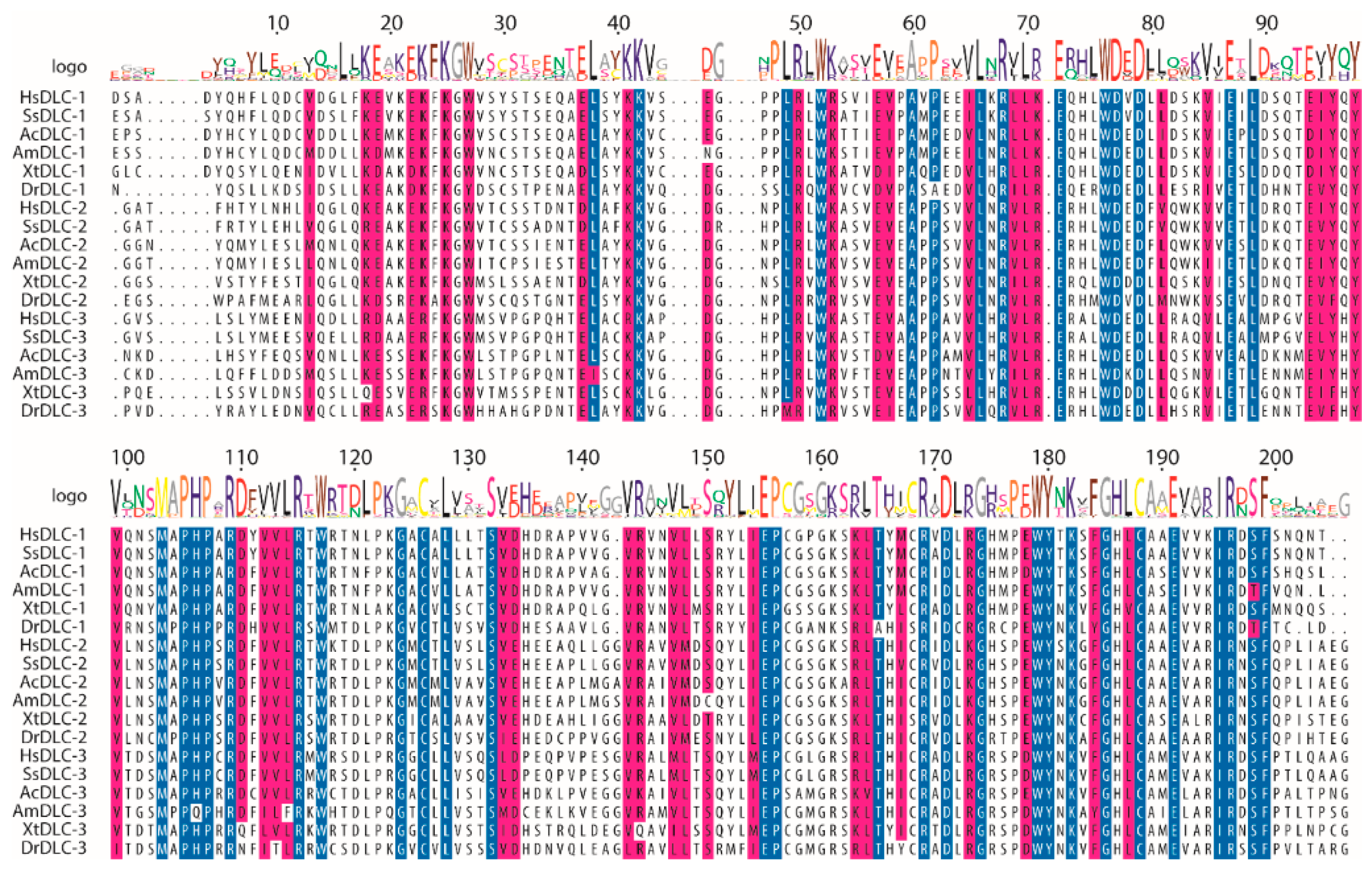
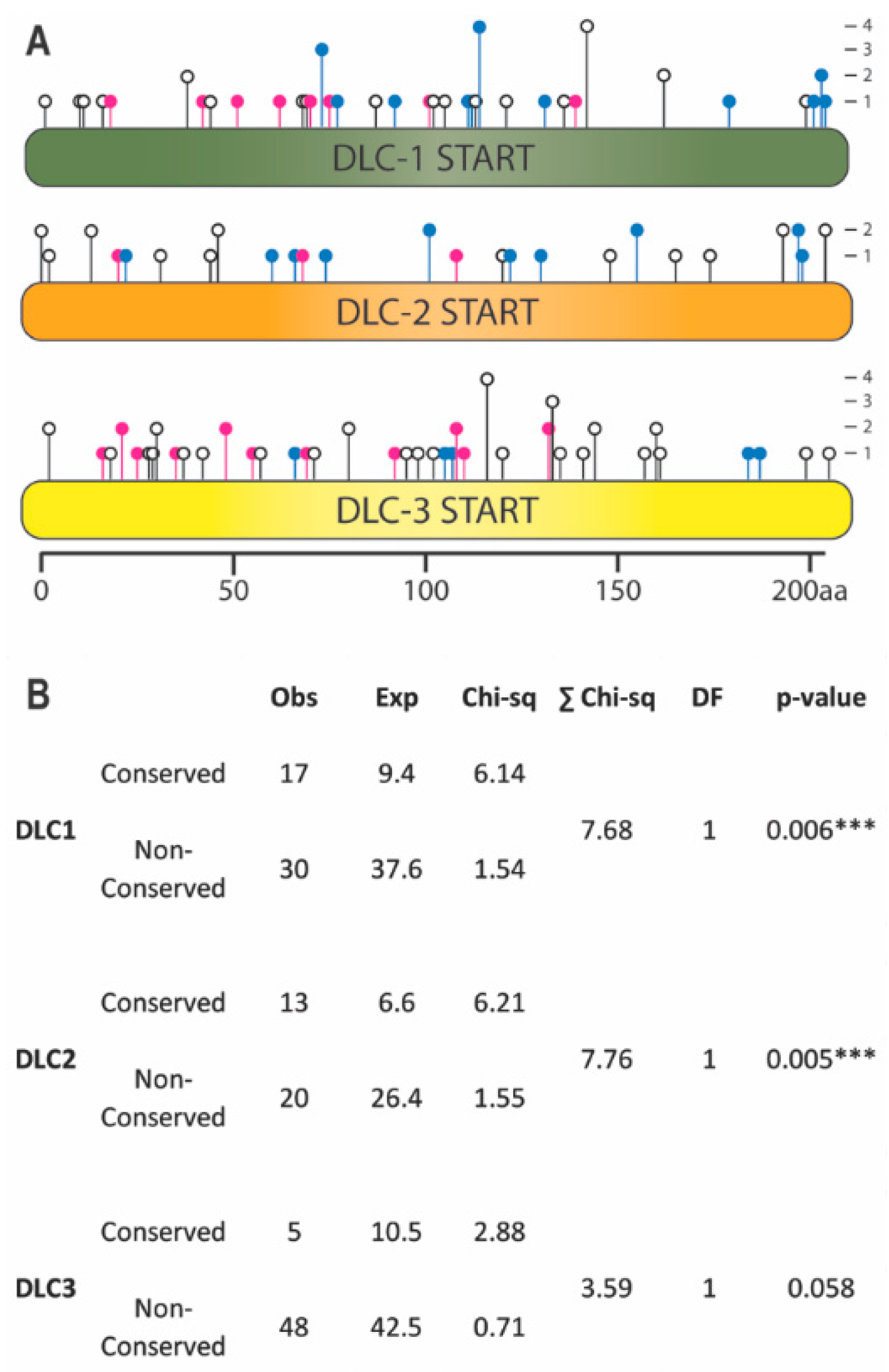
2.1. Mutations in Conserved Residues of DLC-1 and DLC-2 START Domains Are Overrepresented in Tumors
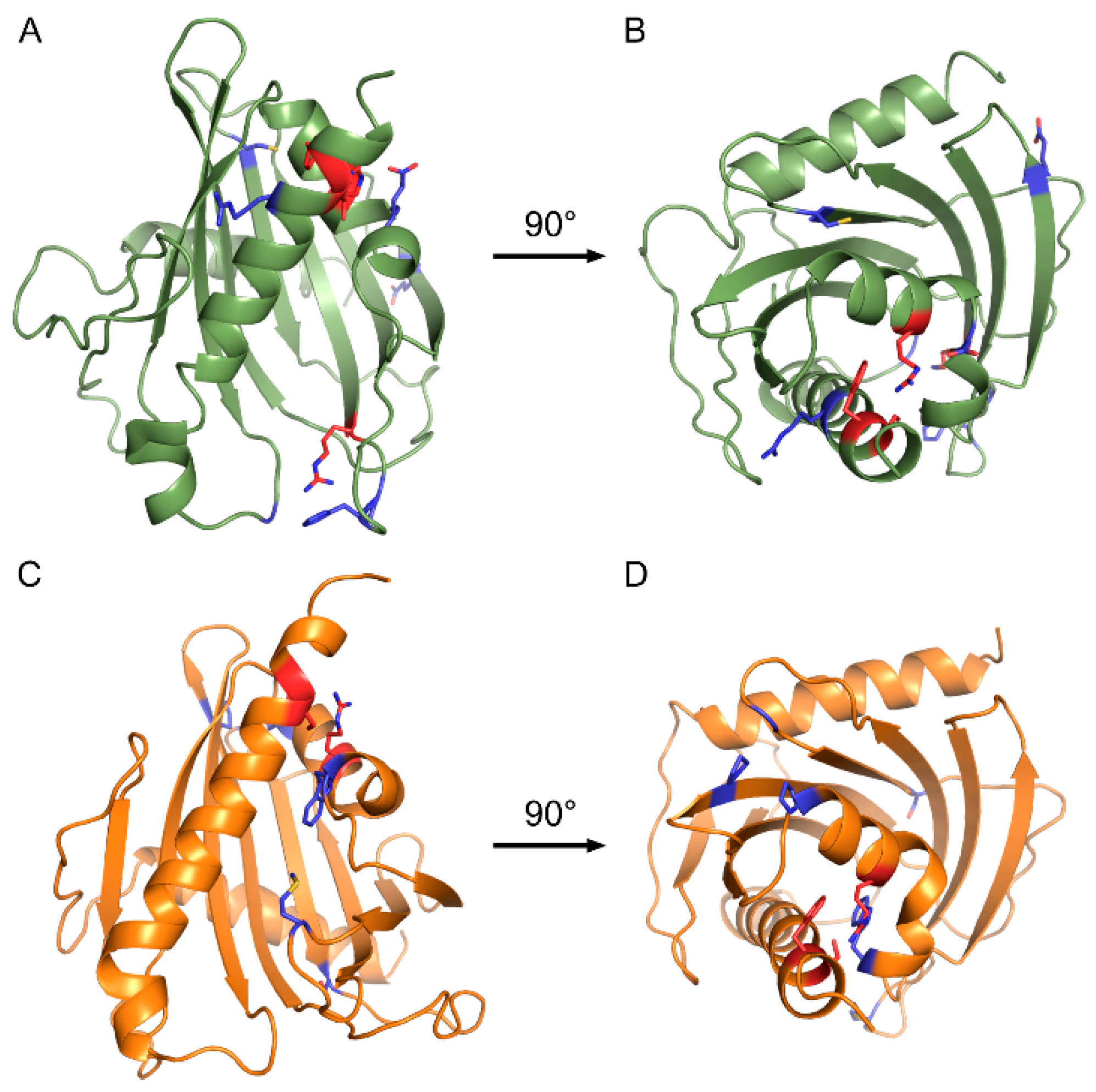
2.2. Conserved Residues form Multiple Bonds within the START Tertiary Structure
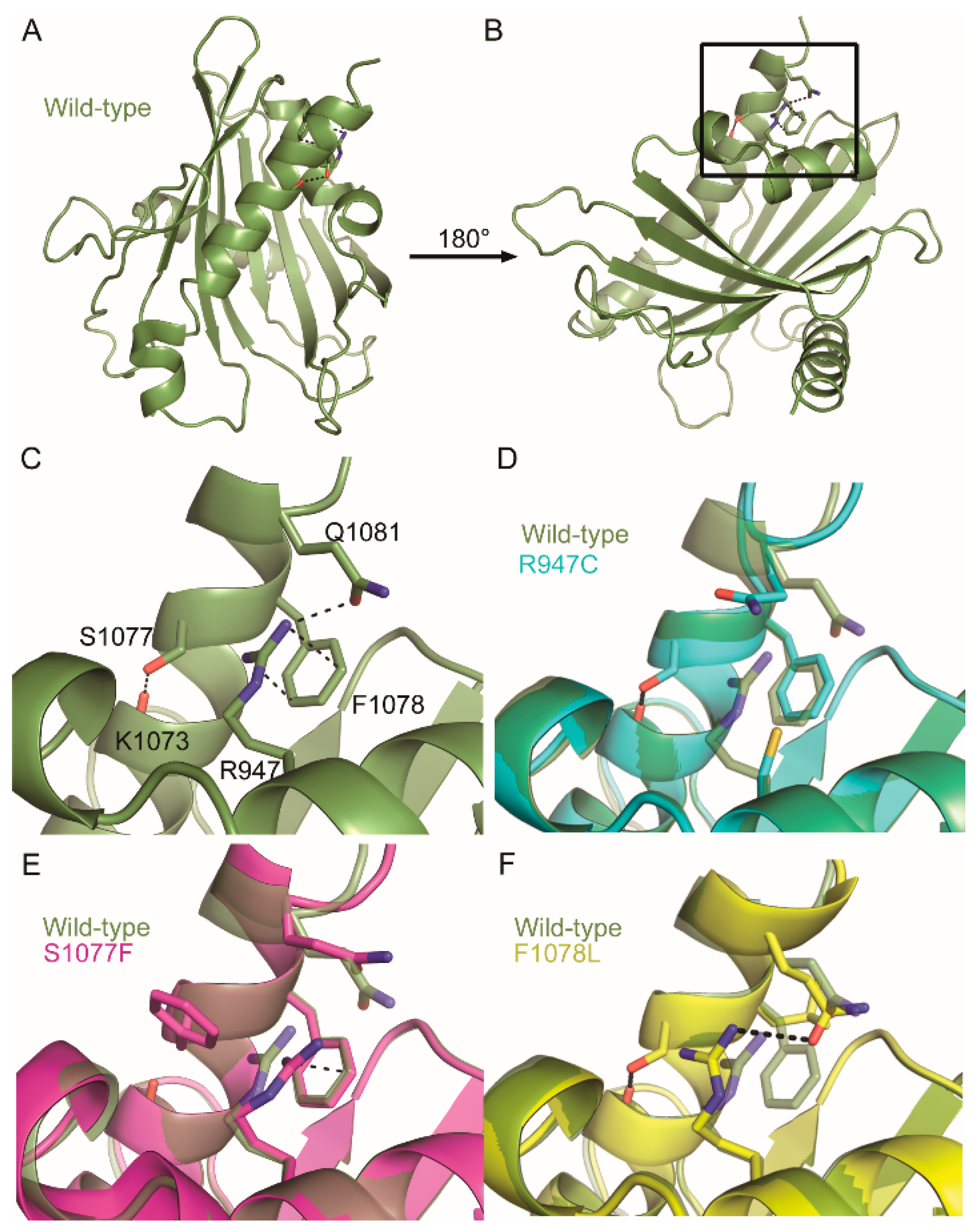
2.3. COSMIC Missense Mutations Disrupt Tertiary-Level Interactions in DLC-1 and DLC-2 START Domains
3. Conclusions
4. Materials and Methods
4.1. Mutation Identification and Kolmogorov-Smirnov Test of Uniformity
4.2. Multiple Sequence Alignment (MSA)
4.3. Mutation Counts and Chi-Square Analysis
4.4. Homology Modeling and Identification of Tertiary-Level Interactions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.M.; Ridley, A.J. Rho GTPases in cancer cell biology. FEBS Lett. 2008, 582, 2093–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haga, R.B.; Ridley, A.J. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases 2016, 7, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.; Yoon, S.R.; Lim, J.; Cho, H.J.; Lee, H.G. Dysregulation of Rho GTPases in Human Cancers. Cancers 2020, 12, 1179. [Google Scholar] [CrossRef]
- Maldonado, M.D.M.; Dharmawardhane, S. Targeting Rac and Cdc42 GTPases in Cancer. Cancer Res. 2018, 78, 3101–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, X.; Marchioni, F.; Sipes, N.; Evelyn, C.R.; Jerabek-Willemsen, M.; Duhr, S.; Seibel, W.; Wortman, M.; Zheng, Y. Rational Design of Small Molecule Inhibitors Targeting RhoA Subfamily Rho GTPases. Chem. Biol. 2012, 19, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Zheng, Y. Approaches of targeting Rho GTPases in cancer drug discovery. Expert Opin. Drug Discov. 2015, 10, 991–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto-Dominguez, N.; Parnell, C.; Teng, Y. Drugging the Small GTPase Pathways in Cancer Treatment: Promises and Challenges. Cells 2019, 8, 255. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mata, R.; Boulter, E.; Burridge, K. The invisible hand: Regulation of RHO GTPases by RHOGDIs. Nat. Rev. Mol. Cell Biol. 2011, 12, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, H.; Li, J.; Liu, Y.; Ding, Y. Overexpression of Rho GDP-Dissociation Inhibitor Alpha Is Associated with Tumor Progression and Poor Prognosis of Colorectal Cancer. J. Proteome Res. 2008, 7, 3994–4003. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.B.; Krutzsch, H.; Shu, H.; Zhao, Y.; Liotta, L.A.; Kohn, E.C.; Petricoin, E.F. Proteomic analysis and identification of new biomarkers and therapeutic targets for invasive ovarian cancer. Proteomics 2002, 2, 76–84. [Google Scholar] [CrossRef]
- Wang, D.; Qian, X.; Rajaram, M.; Durkin, M.E.; Lowy, D.R. DLC1 is the principal biologically-relevant down-regulated DLC family member in several cancers. Oncotarget 2016, 7, 45144–45157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, J.; Douglas, G.; Wu, C.H.; Burbelo, P.D. Human RhoGAP domain-containing proteins: Structure, function and evolutionary relationships. FEBS Lett. 2002, 528, 27–34. [Google Scholar] [CrossRef]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Wang, D.; Qian, X.; Sanchez-Solana, B.; Tripathi, B.K.; Durkin, M.E.; Lowy, D. Cancer-Associated Point Mutations in the DLC1 Tumor Suppressor and Other Rho-GAPs Occur Frequently and Are Associated with Decreased Function. Cancer Res. 2020, 80, 3568–3579. [Google Scholar] [CrossRef]
- Durkin, M.E.; Avner, M.R.; Huh, C.-G.; Yuan, B.-Z.; Thorgeirsson, S.S.; Popescu, N.C. DLC-1, a Rho GTPase-activating protein with tumor suppressor function, is essential for embryonic development. FEBS Lett. 2005, 579, 1191–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, T.O.; Leung, T.H.Y.; Lam, S.; Cheung, O.F.; Tung, E.K.K.; Khong, P.L.; Lam, A.; Chung, S.; Ng, I.O.-L. Deleted in Liver Cancer 2 (DLC2) Was Dispensable for Development and Its Deficiency Did Not Aggravate Hepatocarcinogenesis. PLoS ONE 2009, 4, e6566. [Google Scholar] [CrossRef] [Green Version]
- Durkin, M.E.; Ullmannova, V.; Guan, M.; Popescu, N.C. Deleted in liver cancer 3 (DLC-3), a novel Rho GTPase-activating protein, is downregulated in cancer and inhibits tumor cell growth. Oncogene 2007, 26, 4580–4589. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Sun, J.; Song, J. High expression of DLC family proteins predicts better prognosis and inhibits tumor progression in NSCLC. Mol. Med. Rep. 2019, 19, 4881–4889. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Qian, X.; Papageorge, A.; Schetter, A.J.; Vass, W.C.; Liu, X.; Braverman, R.; Robles, A.I.; Lowy, D. Functional interaction of tumor suppressor DLC1 and caveolin-1 in cancer cells. Cancer Res. 2012, 72, 4405–4416. [Google Scholar] [CrossRef] [Green Version]
- Healy, K.D.; Hodgson, L.; Kim, T.-Y.; Shutes, A.; Maddileti, S.; Juliano, R.L.; Hahn, K.M.; Harden, T.K.; Bang, Y.-J.; Der, C.J. DLC-1 suppresses non-small cell lung cancer growth and invasion by RhoGAP-dependent and independent mechanisms. Mol. Carcinog. 2008, 47, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Chen, N.-T.; Shih, Y.-P.; Liao, Y.-C.; Xue, L.; Lo, S.H. DLC2 modulates angiogenic responses in vascular endothelial cells by regulating cell attachment and migration. Oncogene 2010, 29, 3010–3016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, T.H.-Y.; Ching, Y.P.; Yam, J.W.P.; Wong, C.; Yau, T.-O.; Jin, N.-Y.; Ng, I.O.-L. Deleted in liver cancer 2 (DLC2) suppresses cell transformation by means of inhibition of RhoA activity. Proc. Natl. Acad. Sci. USA 2005, 102, 15207–15212. [Google Scholar] [CrossRef] [Green Version]
- Ching, Y.-P.; Wong, C.-M.; Chan, S.-F.; Leung, T.H.-Y.; Ng, D.C.-H.; Jin, D.-Y.; Ng, I.O.-L. Deleted in Liver Cancer (DLC) 2 Encodes a RhoGAP Protein with Growth Suppressor Function and Is Underexpressed in Hepatocellular Carcinoma. J. Biol. Chem. 2003, 278, 10824–10830. [Google Scholar] [CrossRef] [Green Version]
- Iyer, L.; Koonin, E.; Aravind, L. Adaption of the helix-grip fold for ligand binding and catalysis in the START domain superfamily. Proteins Struct. Funct. Genetics 2001, 43, 134–144. [Google Scholar] [CrossRef]
- Roderick, S.L.; Chan, W.W.; Agate, D.S.; Olsen, L.R.; Vetting, M.W.; Rajashankar, K.; Cohen, D.E. Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nat. Genet. 2002, 9, 507–511. [Google Scholar] [CrossRef]
- Murcia, M.; Faráldo-Gómez, J.D.; Maxfield, F.R.; Roux, B. Modeling the structure of the StART domains of MLN64 and StAR proteins in complex with cholesterol. J. Lipid Res. 2006, 47, 2614–2630. [Google Scholar] [CrossRef] [Green Version]
- Soccio, R.E.; Breslow, J.L. StAR-related Lipid Transfer (START) Proteins: Mediators of Intracellular Lipid Metabolism. J. Biol. Chem. 2003, 278, 22183–22186. [Google Scholar] [CrossRef] [Green Version]
- Alpy, F. Give lipids a START: The StAR-related lipid transfer (START) domain in mammals. J. Cell Sci. 2005, 118, 2791–2801. [Google Scholar] [CrossRef] [Green Version]
- Raya, A.; Revert, F.; Navarro, S.; Saus, J. Characterization of a Novel Type of Serine/Threonine Kinase That Specifically Phosphorylates the Human Goodpasture Antigen. J. Biol. Chem. 1999, 274, 12642–12649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrick, K.; Nguyen, D.; Karlowski, W.M.; Mayer, K.F. START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 2004, 5, R41. [Google Scholar] [CrossRef] [Green Version]
- Tillman, M.C.; Imai, N.; Li, Y.; Khadka, M.; Okafor, C.D.; Juneja, P.; Adhiyaman, A.; Hagen, S.J.; Cohen, D.E.; Ortlund, E.A. Allosteric regulation of thioesterase superfamily member 1 by lipid sensor domain binding fatty acids and lysophosphatidylcholine. Proc. Natl. Acad. Sci. USA 2020, 117, 22080–22089. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.-P.; Wang, J.; Yan, Y.; Zhang, G.-L.; Zhang, H.; Wu, J.; Chen, F.; Wang, X.; Kang, Z.; et al. YR36/WKS1-Mediated Phosphorylation of PsbO, an Extrinsic Member of Photosystem II, Inhibits Photosynthesis and Confers Stripe Rust Resistance in Wheat. Mol. Plant 2019, 12, 1639–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafif, M.; Balagué, C.; Huard-Chauveau, C.; Roby, D. An essential role for the VASt domain of the Arabidopsis VAD1 protein in the regulation of defense and cell death in response to pathogens. PLoS ONE 2017, 12, e0179782. [Google Scholar] [CrossRef]
- Kanno, K.; Wu, M.K.; Agate, D.S.; Fanelli, B.J.; Wagle, N.; Scapa, E.F.; Ukomadu, C.; Cohen, D.E. Interacting Proteins Dictate Function of the Minimal START Domain Phosphatidylcholine Transfer Protein/StarD2. J. Biol. Chem. 2007, 282, 30728–30736. [Google Scholar] [CrossRef] [Green Version]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2018, 47, D941–D947. [Google Scholar] [CrossRef] [Green Version]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, Y.; Morales, D.; Pardo, M.C. A comparison of uniformity tests. Statistics 2005, 39, 315–327. [Google Scholar] [CrossRef]
- Capra, J.A.; Singh, M. Predicting functionally important residues from sequence conservation. Bioinformatics 2007, 23, 1875–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, B.Y.; Epand, R.F.; Epand, R.M.; Miller, W.L. Cholesterol Binding Does Not Predict Activity of the Steroidogenic Acute Regulatory Protein, StAR. J. Biol. Chem. 2007, 282, 10223–10232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Chavez, F. The rule of variation: Amio acid exchange according to the rotating circular genetic code. J. Theor. Biol. 2010, 264, 711–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsell, A.-G.; Lee, W.H.; Persson, C.; Siponen, M.I.; Nilsson, M.; Busam, R.D.; Kotenyova, T.; Schüler, H.; Lehtiö, L. Comparative Structural Analysis of Lipid Binding START Domains. PLoS ONE 2011, 6, e19521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, D. Cation-π Interactions in Chemistry and Biology: A New View of Benen, Phe, Tyr, and Trp. Science 1996, 271, 163–168. [Google Scholar] [CrossRef]
- Gallivan, J.P.; Dougherty, D.A. Cation-pi interactions in structural biology. Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464. [Google Scholar] [CrossRef] [Green Version]
- Anoosha, P.; Sakthivel, R.; Gromiha, M.M. Exploring preferred amino acid mutations in cancer genes: Applications to identify potential drug targets. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2016, 1862, 155–165. [Google Scholar] [CrossRef]
- Popescu, N.C.; Goodison, S. Deleted in liver cancer-1 (DLC1): An emerging metastasis suppressor gene. Mol. Diagn. Ther. 2014, 18, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Miyakawa, T.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Tanokura, M. Structure and function of abscisic acid receptors. Trends Plant Sci. 2013, 18, 259–266. [Google Scholar] [CrossRef]
- Vaidya, A.S.; Helander, J.D.M.; Peterson, F.C.; Elzinga, D.; Dejonghe, W.; Kaundal, A.; Park, S.-Y.; Xing, Z.; Mega, R.; Takeuchi, J.; et al. Dynamic control of plant water use using designed ABA receptor agonists. Science 2019, 366, eaaw8848. [Google Scholar] [CrossRef]
- Zimmermann, L.; Stephens, A.; Nam, S.-Z.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at its Core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef]
- Yates, A.D.; Achuthan, P.; Akanni, W.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl. Nucleic Acids Res. 2019, 48, D682–D688. [Google Scholar] [CrossRef]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bodenhofer, U.; Bonatesta, E.; Horejš-Kainrath, C.; Hochreiter, S. Msa: An R package for multiple sequence alignment. Bioinformatics 2015, 31, 3997–3999. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, J.T.; Carroll, S.M.; Thornton, J.W. Evolution of Hormone-Receptor Complexity by Molecular Exploitation. Science 2006, 312, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Šali, A.; Potterton, L.; Yuan, F.; Van Vlijmen, H.; Karplus, M. Evaluation of comparative protein modeling by MODELLER. Proteins Struct. Funct. Bioinform. 1995, 23, 318–326. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holub, A.S.; Bouley, R.A.; Petreaca, R.C.; Husbands, A.Y. Identifying Cancer-Relevant Mutations in the DLC START Domain Using Evolutionary and Structure-Function Analyses. Int. J. Mol. Sci. 2020, 21, 8175. https://doi.org/10.3390/ijms21218175
Holub AS, Bouley RA, Petreaca RC, Husbands AY. Identifying Cancer-Relevant Mutations in the DLC START Domain Using Evolutionary and Structure-Function Analyses. International Journal of Molecular Sciences. 2020; 21(21):8175. https://doi.org/10.3390/ijms21218175
Chicago/Turabian StyleHolub, Ashton S., Renee A. Bouley, Ruben C. Petreaca, and Aman Y. Husbands. 2020. "Identifying Cancer-Relevant Mutations in the DLC START Domain Using Evolutionary and Structure-Function Analyses" International Journal of Molecular Sciences 21, no. 21: 8175. https://doi.org/10.3390/ijms21218175
APA StyleHolub, A. S., Bouley, R. A., Petreaca, R. C., & Husbands, A. Y. (2020). Identifying Cancer-Relevant Mutations in the DLC START Domain Using Evolutionary and Structure-Function Analyses. International Journal of Molecular Sciences, 21(21), 8175. https://doi.org/10.3390/ijms21218175




