Cell Wall Composition as a Marker of the Reprogramming of the Cell Fate on the Example of a Daucus carota (L.) Hypocotyl in Which Somatic Embryogenesis Was Induced
Abstract
:1. Introduction
2. Results
2.1. Explant Histology
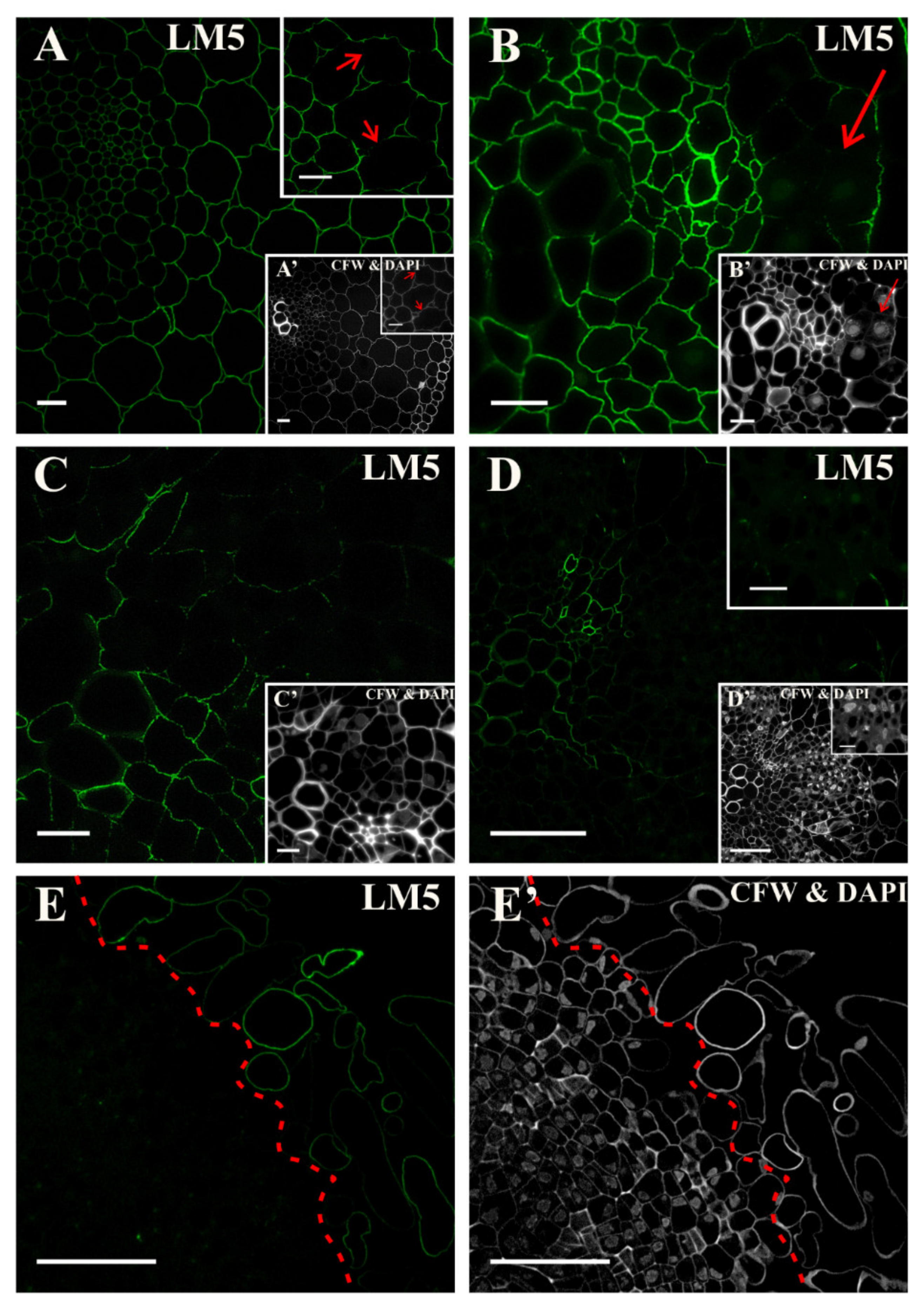
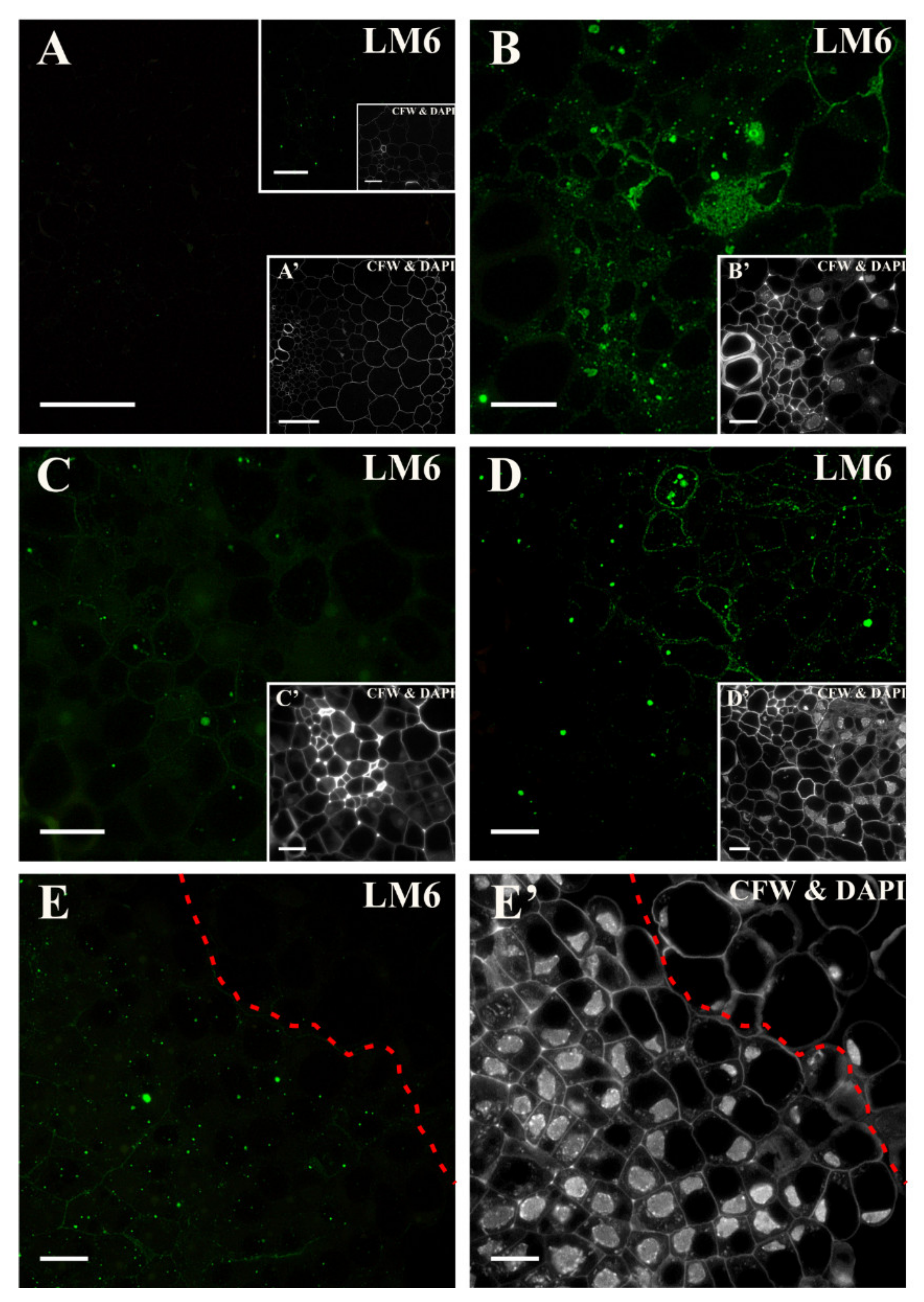
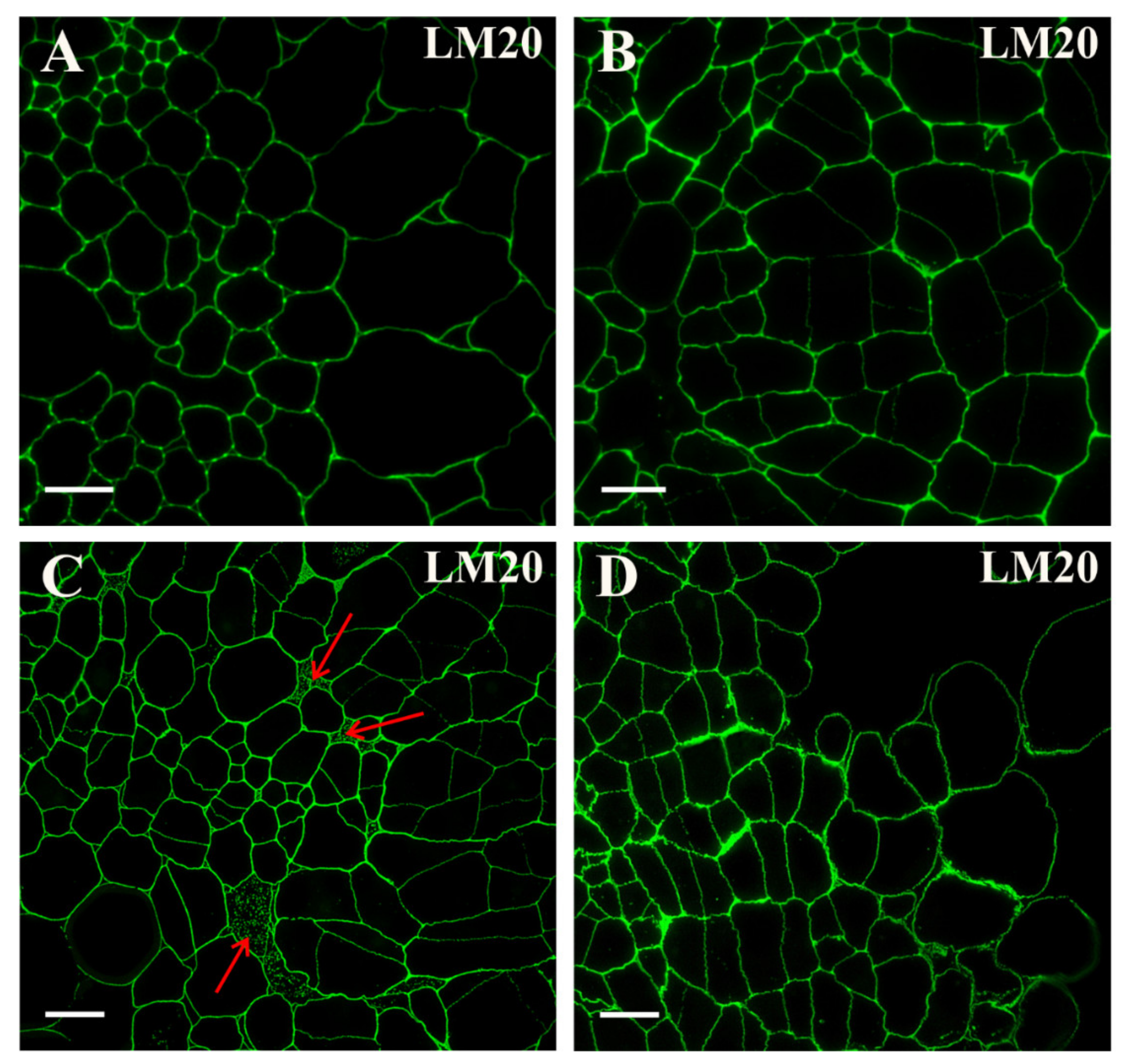
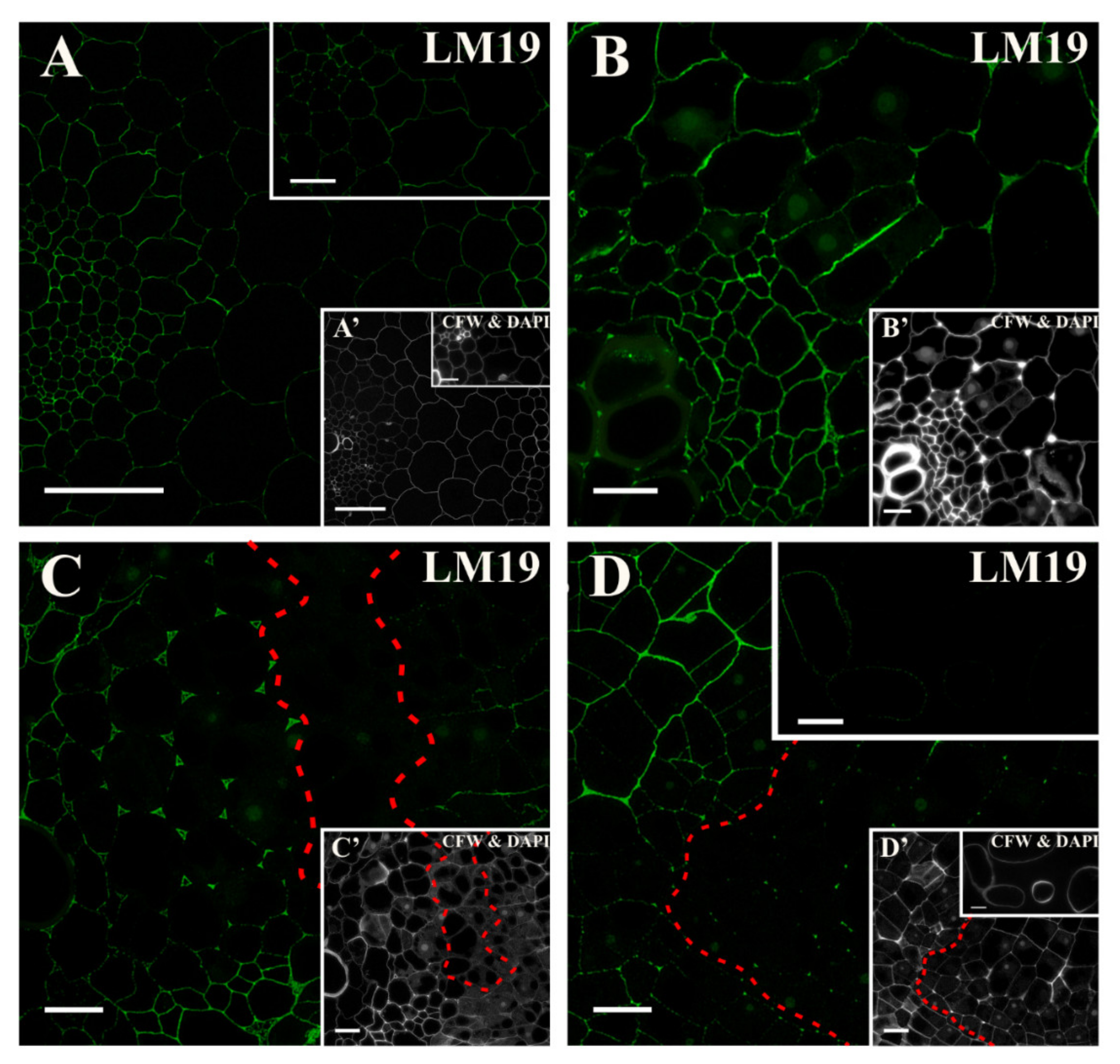
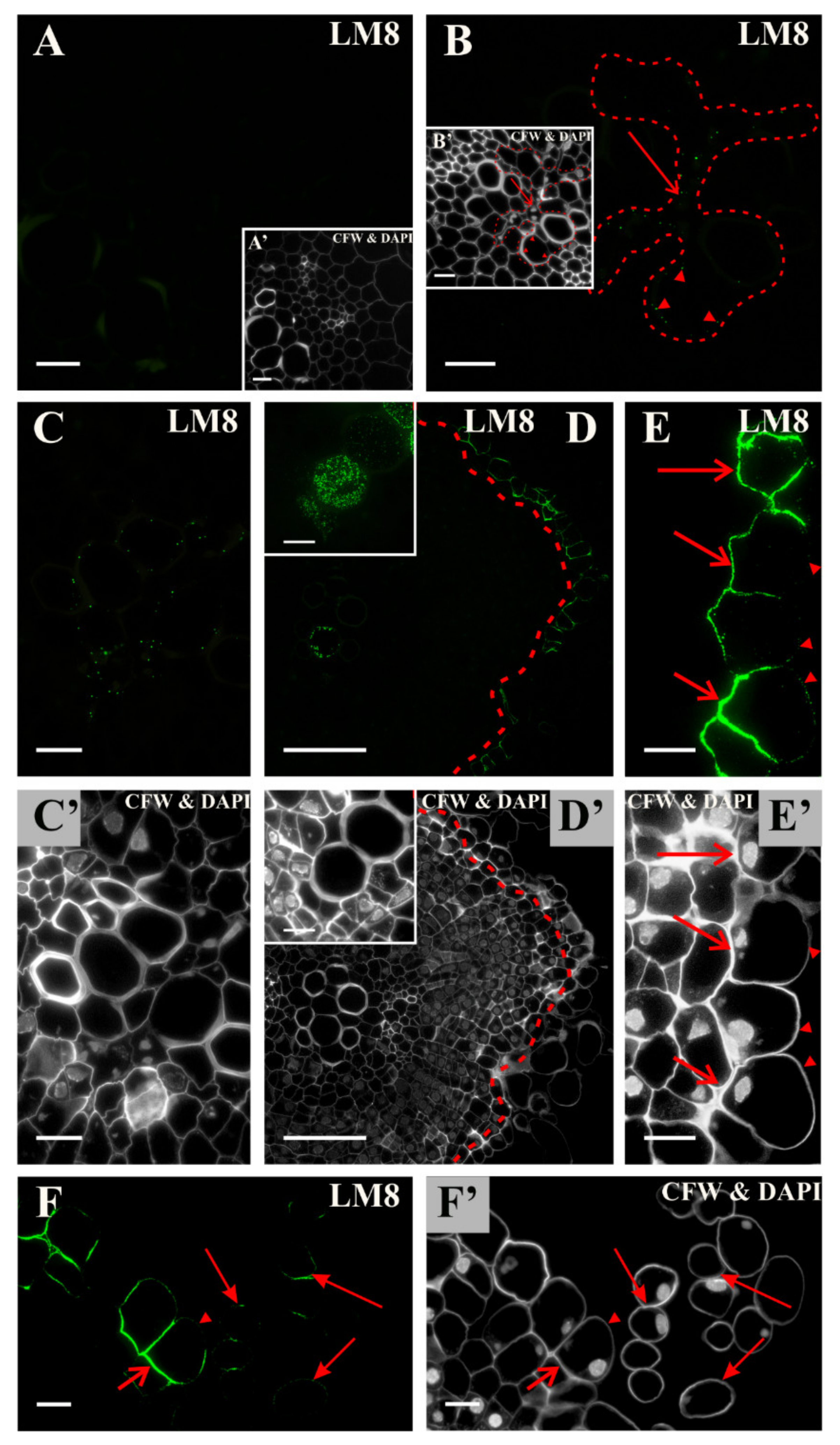
2.2. Distribution of the Pectic Epitopes in the Explant
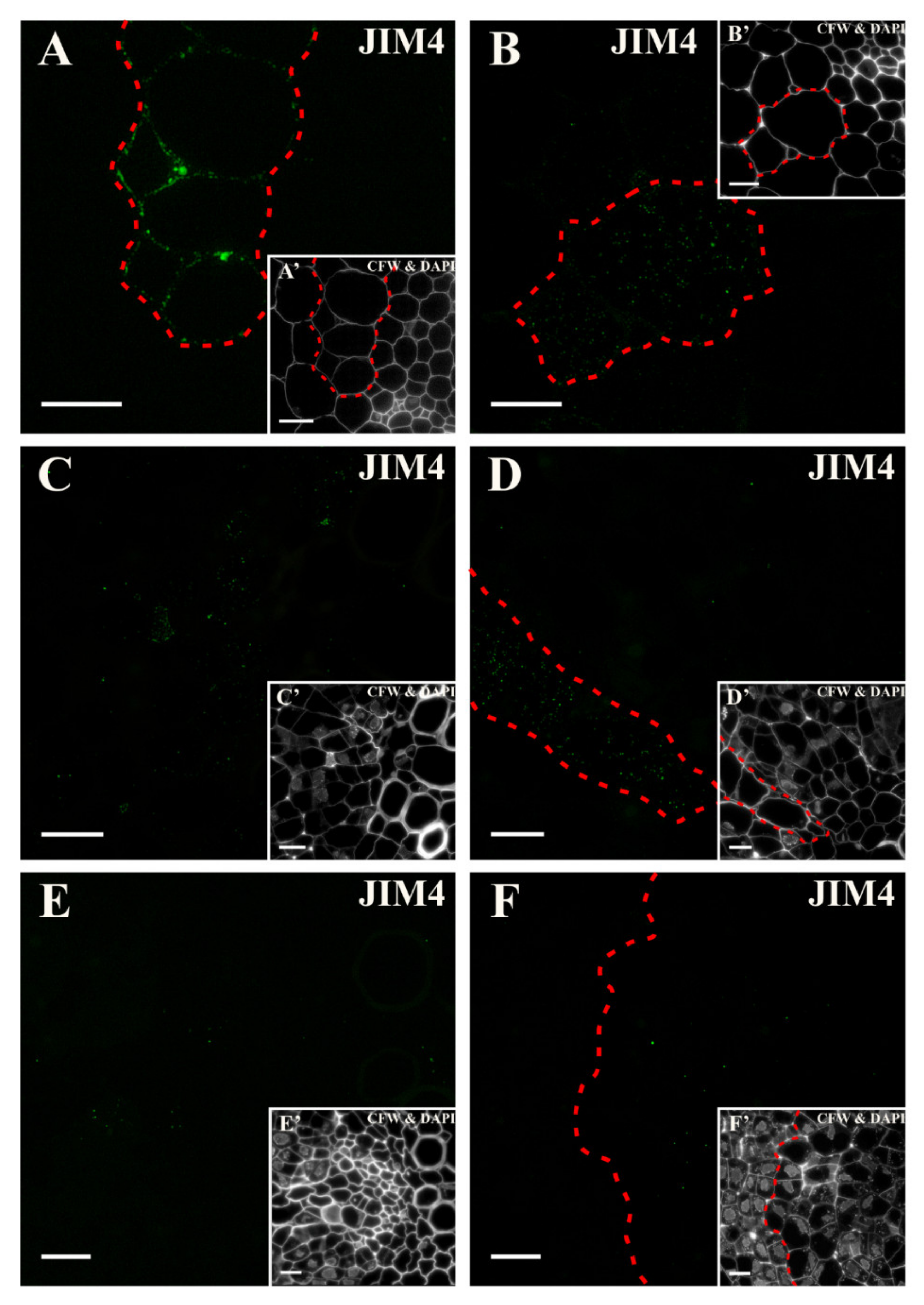
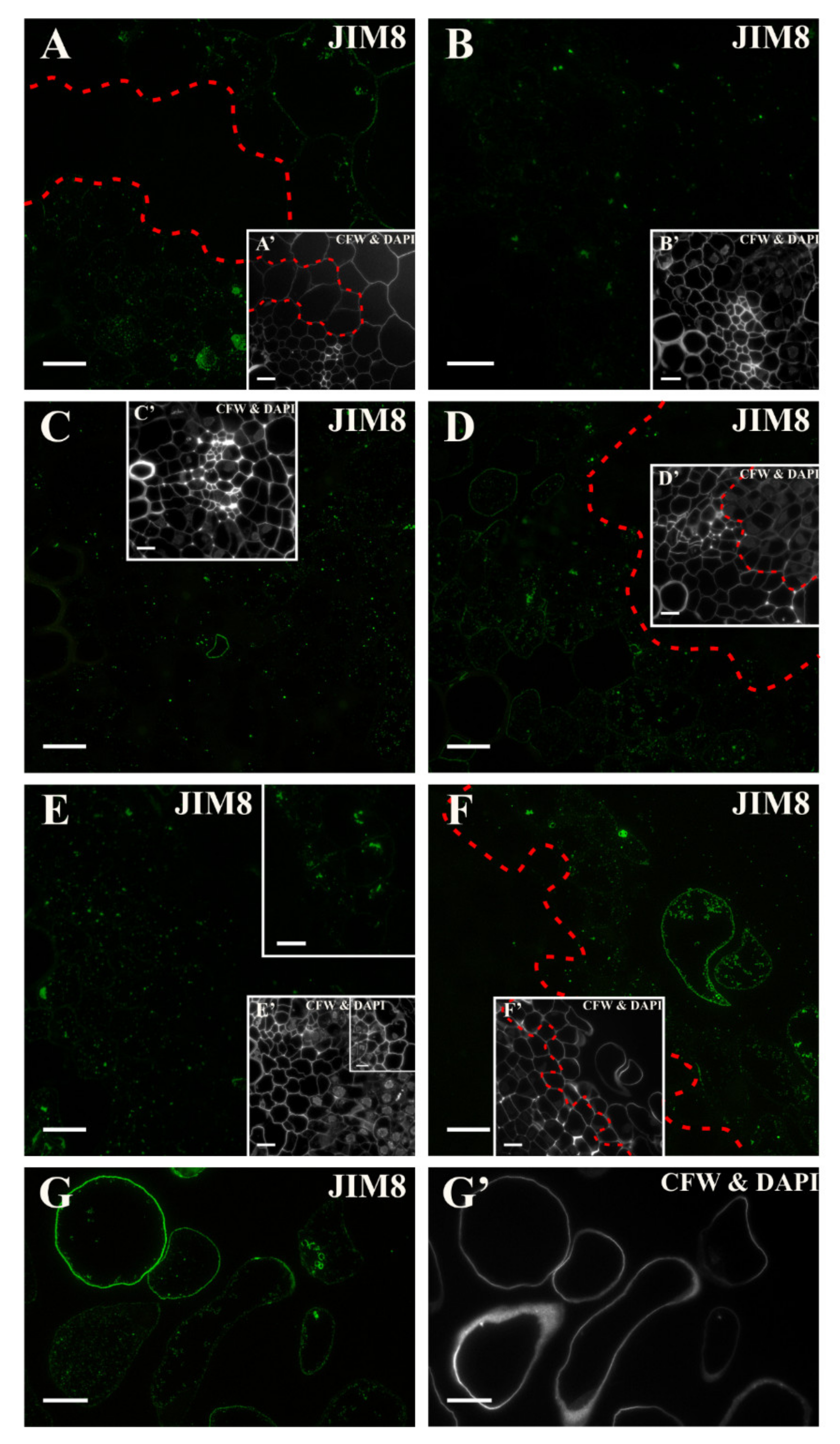
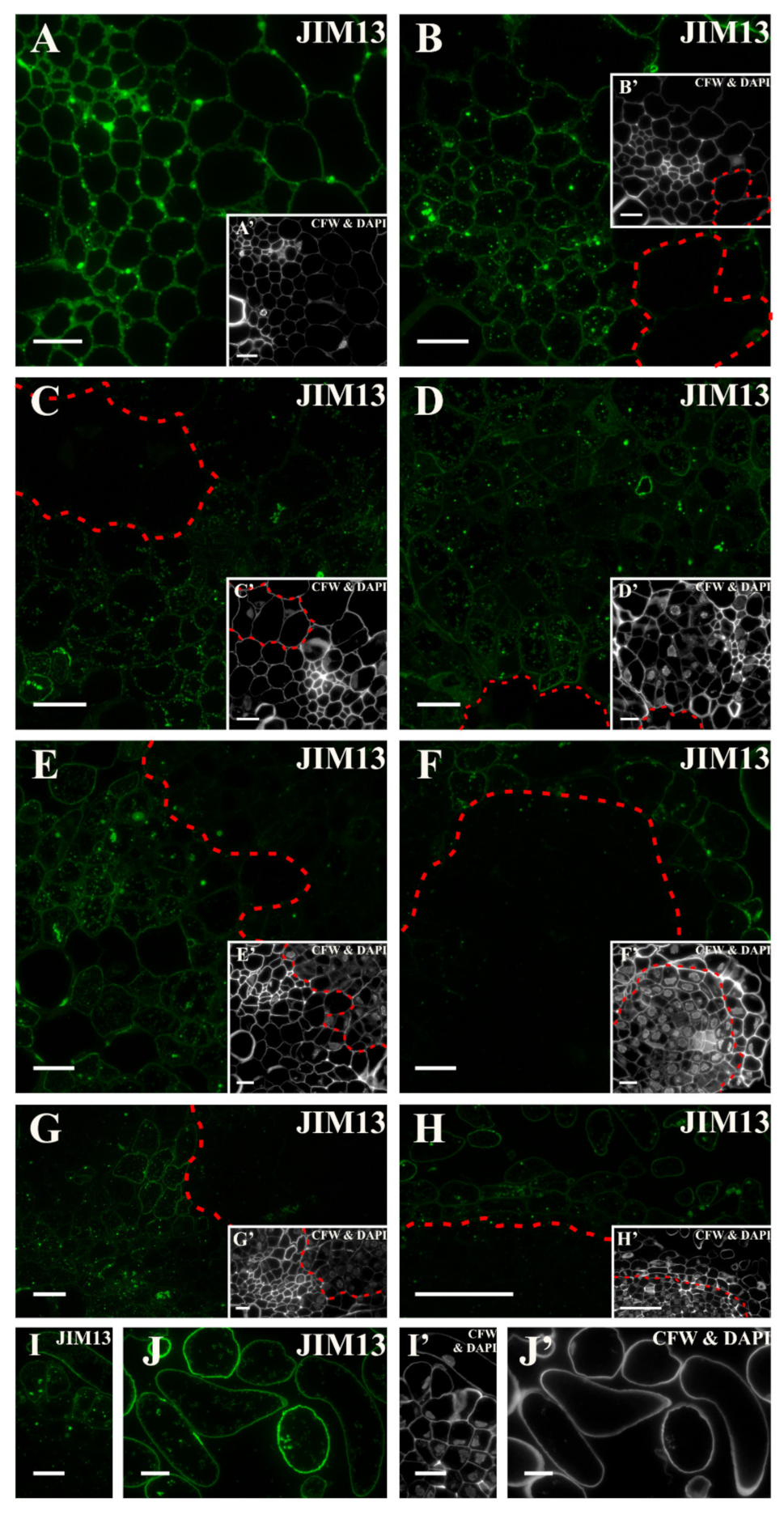
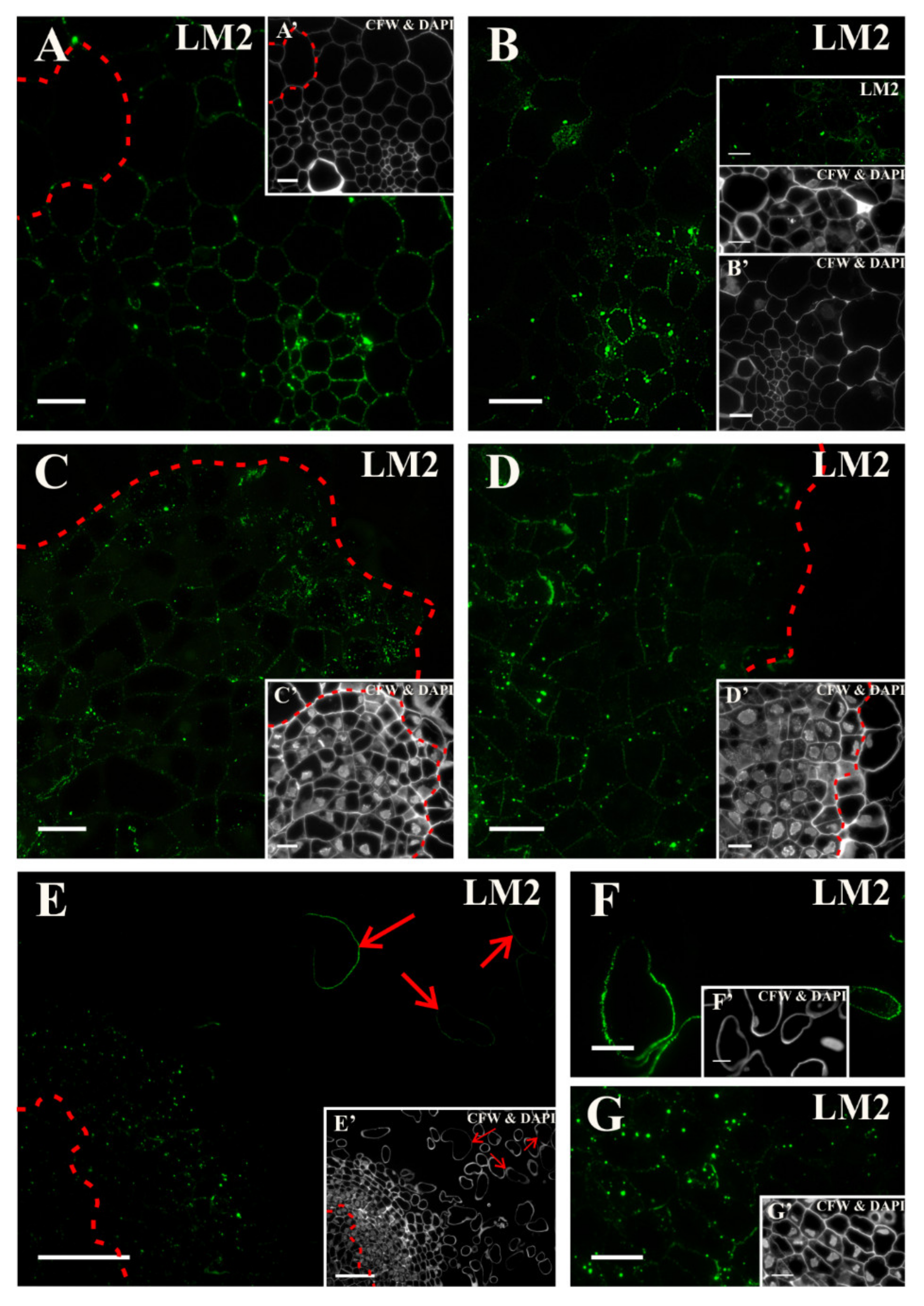
2.3. Distribution of the AGP Epitopes
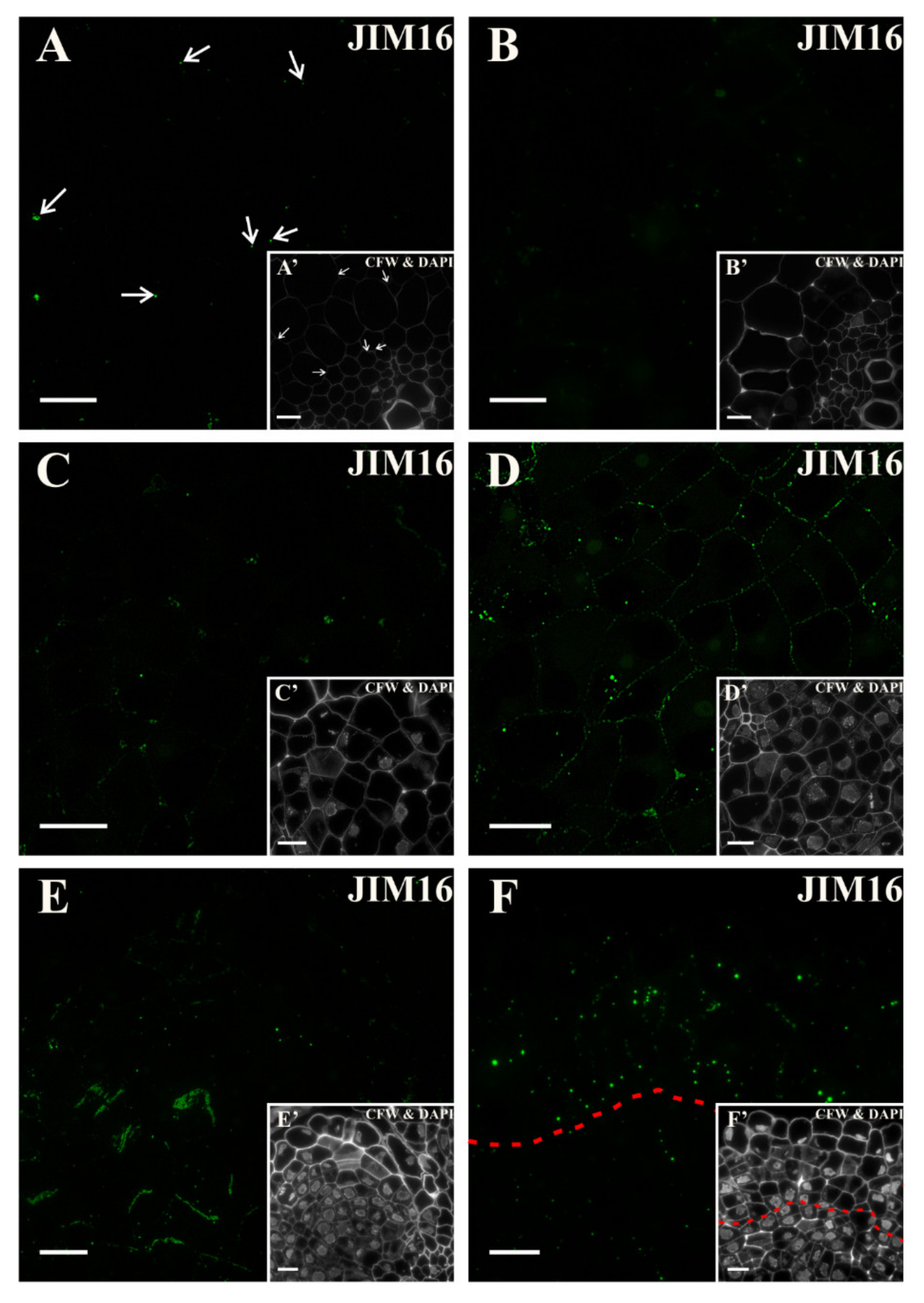
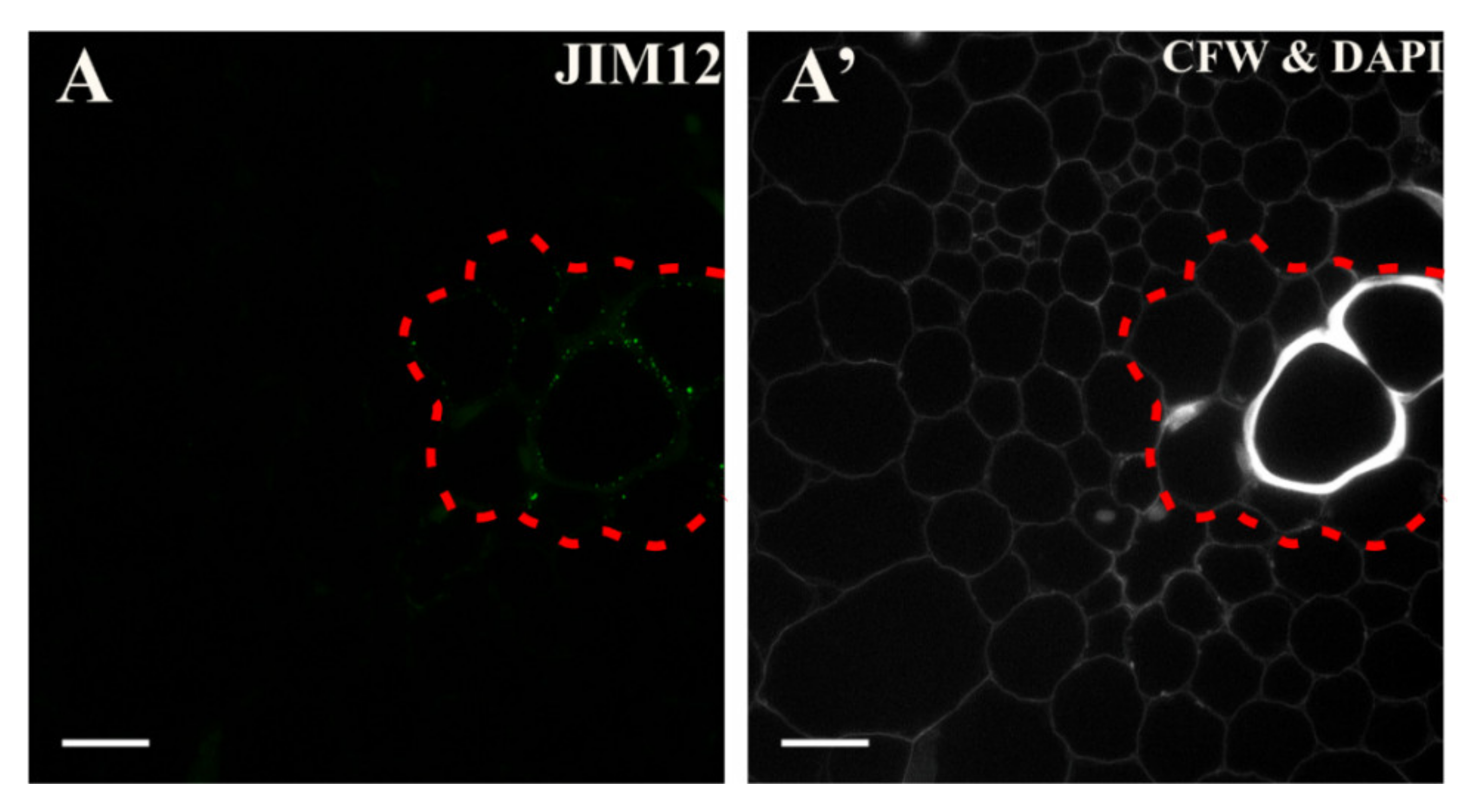
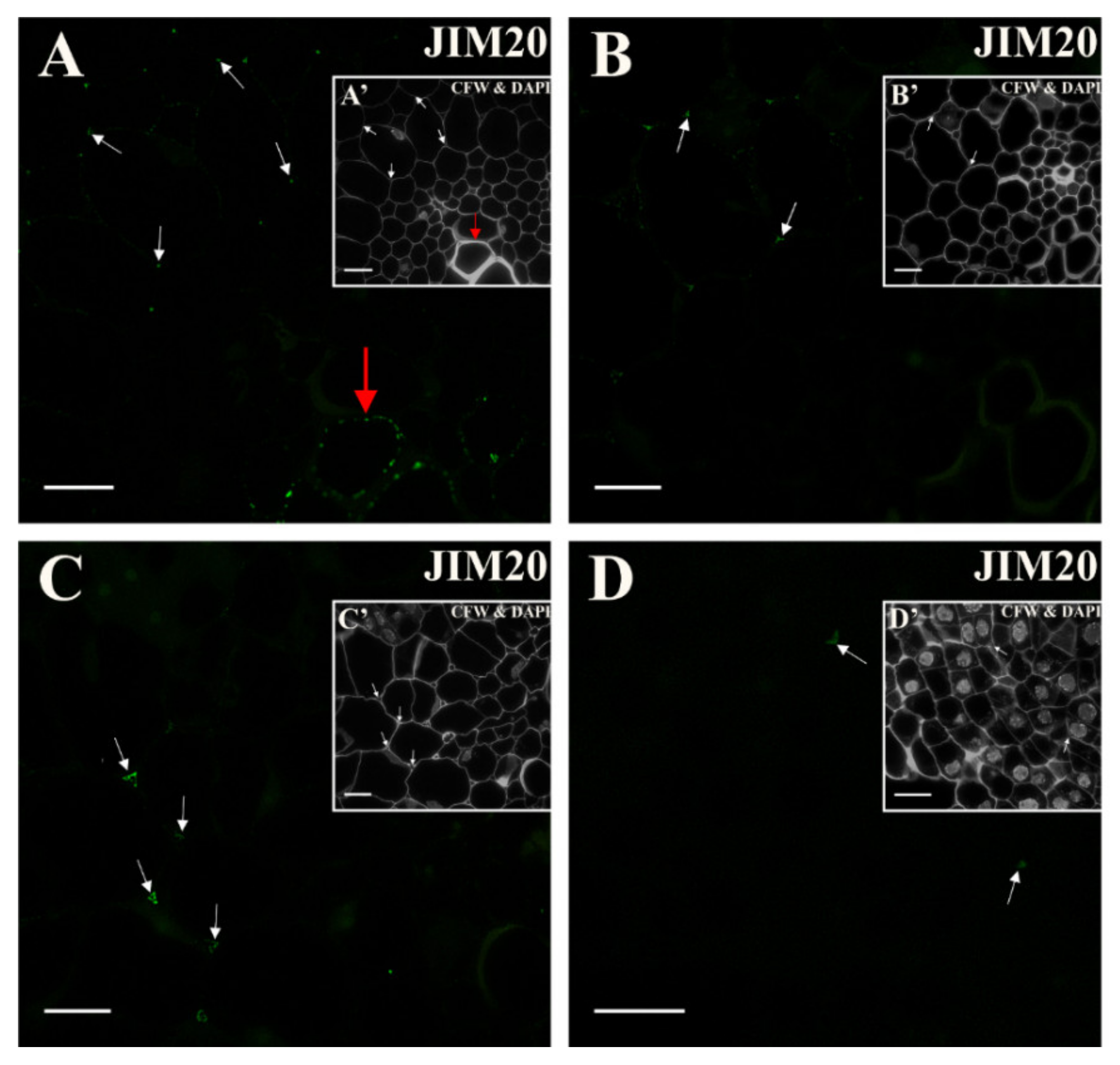
2.4. Extensins
2.5. Results Summary
3. Discussion
3.1. Morpho-Histological Changes in an Explant that Had Been Subjected to SE Induction
3.2. Cytological Identification of the Explant Cells That are Undergoing Reprogramming
3.3. Spatio-Temporal Changes in Pectins as Markers of the State of Cell Differentiation
3.4. Spatio-Temporal Changes of the AGPs as Markers of the State of Cell Differentiation
3.5. Spatio-Temporal Changes of the Extensins as Markers of the State of Cell Differentiation
4. Materials and Methods
4.1. Plant material and Culture Condition
4.2. In Vitro Culture
4.3. Histochemistry and Immunohistochemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGP | Arabinogalactan protein |
| DAPI | 4′,6-diamidino-2-phenylindole |
| HG | Homogalacturonan |
| RG-I | Ramnogalacturonan I |
| HRGP | Hydroxyproline-rich glycoproteins |
| SE | Somatic embryogenesis |
| XGA | Xylogalacturonan |
References
- Wang, S.; Schiefelbein, J. Regulation of cell fate determination in plants. Front. Plant Sci. 2014, 5, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romberger, J.A.; Hejnowicz, Z.; Hill, J.F. Plant Structure: Function and Development. A Treatise on Anatomy and Vegetative Development, with Special Reference to Woody Plants; Springer: Berlin/Heidelberg, Germany, 1993; pp. 167–199. [Google Scholar]
- Quiroz-Figueroa, F.R.; Rojas-Herrera, R.; Galaz-Avalos, R.M.; Loyola-Vargas, V.M. Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell. Tissue Organ Cult. 2006, 86, 285–301. [Google Scholar] [CrossRef]
- Gaj, M.D.; Zhang, S.; Harada, J.J.; Lemaux, P.G. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 2005, 222, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Kumar, P. Regulation of somatic embryogenesis in crops: A review. Agric. Rev. 2013, 34, 1–20. [Google Scholar]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, X. Regulation of somatic embryogenesis in higher plants. CRC. Crit. Rev. Plant Sci. 2010, 29, 36–57. [Google Scholar] [CrossRef]
- Winkelmann, T. Somatic versus zygotic embryogenesis: Learning from seeds. In Methods in Molecular Biology; Humana Press Inc.: Clifton, NJ, USA, 2016; Volume 1359, pp. 25–46. [Google Scholar]
- Rocha, D.I.; Kurczyńska, E.; Potocka, I.; Steinmacher, D.A.; Otoni, W.C. Histology and histochemistry of somatic embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 471–494. [Google Scholar]
- Knox, J.P. Revealing the structural and functional diversity of plant cell walls. Curr. Opin. Plant Biol. 2008, 11, 308–313. [Google Scholar] [CrossRef]
- Tchorbadjieva, M.I. Advances in proteomics of somatic embryogenesis. In Somatic Embryogenesis in Ornamentals and Its Applications; Springer: New Dehli, India, 2015; pp. 67–90. [Google Scholar]
- O’Neill, M.A.; York, W.S. The Composition and Structure of Plant Primary Cell Walls. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 1–54. [Google Scholar]
- Seifert, G.J.; Blaukopf, C. Irritable walls: The plant extracellular matrix and signaling. Plant Physiol. 2010, 153, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Keegstra, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Underwood, W. The plant cell wall: A dynamic barrier against pathogen invasion. Front. Plant Sci. 2012, 3, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 2013, 25, 270–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosgrove, D.J.; Jarvis, M.C. Comparative structure and biomechanics of plant primary and secondary cell walls. Front. Plant Sci. 2012, 3, 204. [Google Scholar] [CrossRef] [Green Version]
- Knox, J.P. The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int. Rev. Cytol. 1997, 171, 79–120. [Google Scholar]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Coelho, L.C.B.B.; Teixeira, J.A.; Carneiro-Da-Cunha, M.G. Approaches in biotechnological applications of natural polymers. AIMS Mol. Sci. 2016, 3, 386–425. [Google Scholar] [CrossRef] [Green Version]
- Palin, R.; Geitmann, A. The role of pectin in plant morphogenesis. BioSystems 2012, 109, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan methyl-esterification and plant development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Villarreal, M.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Martínez-Téllez, M.A. Plant Cell Wall Polymers: Function, Structure and Biological Activity of Their Derivatives. In Polymerization; InTech: Vienna, Austria, 2012; pp. 63–86. [Google Scholar]
- Bárány, I.; Fadón, B.; Risueño, M.C.; Testillano, P.S. Cell wall components and pectin esterification levels as markers of proliferation and differentiation events during pollen development and pollen embryogenesis in Capsicum annuum L. J. Exp. Bot. 2010, 61, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.; Blervacq, A.-S.; Hendriks, T.; Slomianny, C.; Vasseur, J.; Hilbert, J.-L. Cell wall differentiation during early somatic embryogenesis in plants. II. Ultrastructural study and pectin immunolocalization on chicory embryos. Can. J. Bot. 2000, 78, 824–831. [Google Scholar]
- Xu, C.; Zhao, L.; Pan, X.; Šamaj, J. Developmental Localization and Methylesterification of Pectin Epitopes during Somatic Embryogenesis of Banana (Musa spp. AAA). PLoS ONE 2011, 6, e22992. [Google Scholar] [CrossRef]
- Sala, K.; Potocka, I.; Kurczynska, E. Spatio-temporal distribution and methyl-esterification of pectic epitopes provide evidence of developmental regulation of pectins during somatic embryogenesis in Arabidopsis thaliana. Biol. Plant. 2013, 57, 410–416. [Google Scholar] [CrossRef]
- Verdeil, J.L.; Hocher, V.; Huet, C.; Grosdemange, F.; Escoute, J.; Ferrière, N.; Nicole, M. Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann. Bot. 2001, 88, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Betekhtin, A.; Rojek, M.; Milewska-Hendel, A.; Gawecki, R.; Karcz, J.; Kurczynska, E.; Hasterok, R. Spatial distribution of selected chemical cell wall components in the embryogenic callus of Brachypodium distachyon. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, M.; Knox, J.P.; Konieczny, R. Arabinogalactan-protein and pectin epitopes in relation to an extracellular matrix surface network and somatic embryogenesis and callogenesis in Trifolium nigrescens Viv. Plant Cell. Tissue Organ Cult. 2013, 115, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Sala, K.; Malarz, K.; Barlow, P.W.; Kurczyńska, E.U. Distribution of some pectic and arabinogalactan protein epitopes during Solanum lycopersicum (L.) adventitious root development. BMC Plant Biol. 2017, 17, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Rumyantseva, N.I. Arabinogalactan proteins: Involvement in plant growth and morphogenesis. Biokhimiya 2005, 70, 1301–1317. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.A.; Li, T.; Held, M.; Kieliszewski, M.J. The role of the primary cell wall in plant morphogenesis. Int. J. Mol. Sci. 2018, 19, 2674. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.M.; Pereira, L.G.; Coimbra, S. Arabinogalactan proteins: Rising attention from plant biologists. Plant Reprod. 2015, 28, 1–15. [Google Scholar] [CrossRef]
- Steinmacher, D.A.; Saare-Surminski, K.; Lieberei, R. Arabinogalactan proteins and the extracellular matrix surface network during peach palm somatic embryogenesis. Physiol. Plant. 2012, 146, 336–349. [Google Scholar] [CrossRef]
- Saare-Surminski, K.; Preil, W.; Knox, J.P.; Lieberei, R. Arabinogalactan proteins in embryogenic and non-embryogenic callus cultures of Euphorbia pulcherrima. Physiol. Plant. 2000, 108, 180–187. [Google Scholar] [CrossRef]
- Pan, X.; Yang, X.; Lin, G.; Zou, R.; Chen, H.; Šamaj, J.; Xu, C. Ultrastructural changes and the distribution of arabinogalactan proteins during somatic embryogenesis of banana (Musa spp. AAA cv. ’Yueyoukang 1′). Physiol. Plant. 2011, 142, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Stacey, N.J.; Roberts, K.; Knox, J.P. Patterns of expression of the JIM4 arabinogalactan-protein epitope in cell cultures and during somatic embryogenesis in Daucus carota L. Planta 1990, 180, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.; Blervacq, A.S.; Vasseur, J.; Hilbert, J.L. Arabinogalactan-proteins in Cichorium somatic embryogenesis: Effect of β-glucosyl Yariv reagent and epitope localisation during embryo development. Planta 2000, 211, 305–314. [Google Scholar] [CrossRef]
- Konieczny, R.; Świerczyńska, J.; Czaplicki, A.Z.; Bohdanowicz, J. Distribution of pectin and arabinogalactan protein epitopes during organogenesis from androgenic callus of wheat. Plant Cell Rep. 2007, 26, 355–363. [Google Scholar] [CrossRef]
- Trifunović, M.; Subotić, A.; Petrić, M.; Jevremović, S. The role of arabinogalactan proteins in morphogenesis of centaurium erythraea rafn in vitro. In The Gentianaceae-Volume 2: Biotechnology and Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 113–138. [Google Scholar]
- Filonova, L.H.; Bozhkov, P.V.; Von Arnold, S. Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J. Exp. Bot. 2000, 51, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Dolan, L.; Linstead, P.; Roberts, K. An AGP epitope distinguishes a central metaxylem initial from other vascular initials in the Arabidopsis root. Protoplasma 1995, 189, 149–155. [Google Scholar] [CrossRef]
- Casero, P.J.; Casimiro, I.; Knox, J.P. Occurrence of cell surface arabinogalactan-protein and extensin epitopes in relation to pericycle and vascular tissue development in the root apex of four species. Planta 1998, 204, 252–259. [Google Scholar] [CrossRef]
- Zhang, X.L.; Ren, Y.J.; Zhao, J. Roles of extensins in cotyledon primordium formation and shoot apical meristem activity in Nicotiana tabacum. J. Exp. Bot. 2008, 59, 4045–4058. [Google Scholar] [CrossRef] [Green Version]
- Smallwood, M.; Beven, A.; Donovan, N.; Neill, S.J.; Peart, J.; Roberts, K.; Knox, J.P. Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant J. 1994, 5, 237–246. [Google Scholar] [CrossRef]
- Xu, C.; Takáč, T.; Burbach, C.; Menzel, D.; Šamaj, J. Developmental localization and the role of hydroxyproline rich glycoproteins during somatic embryogenesis of banana (Musa spp. AAA). BMC Plant Biol. 2011, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Zagorchev, L.; Odjakova, M. Hydroxyproline rich proteins in salt adapted embryogenic suspension cultures of Dactylis glomerata L. Biotechnol. Biotechnol. Equip. 2011, 25, 2321–2328. [Google Scholar] [CrossRef] [Green Version]
- Pennell, R.I.; Janniche, L.; Scofield, G.N.; Booij, H.; De Vries, S.C.; Roberts, K. Identification of a transitional cell state in the developmental pathway to carrot somatic embryogenesis. J. Cell Biol. 1992, 119, 1371–1380. [Google Scholar] [CrossRef] [Green Version]
- Toonen, M.A.J.; Schmidt, E.D.L.; Hendriks, T.; Verhoeven, H.A.; Van Kammen, A.; De Vries, S.C. Expression of the JIM8 cell wall epitope in carrot somatic embryogenesis. Planta 1996, 200, 167–173. [Google Scholar] [CrossRef]
- McCabe, P.F.; Valentine, T.A.; Forsberg, L.S.; Pennell, R.I. Soluble signals from cells identified at the cell wall establish a developmental pathway in carrot. Plant Cell 1997, 9, 2225–2241. [Google Scholar] [CrossRef] [PubMed]
- Betekhtin, A.; Pinski, A.; Milewska-Hendel, A.; Kurczynska, E.; Hasterok, R. Stability and instability processes in the calli of Fagopyrum tataricum that have different morphogenic potentials. Plant Cell. Tissue Organ Cult. 2019, 137, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Potocka, I.; Godel, K.; Dobrowolska, I.; Kurczyńska, E.U. Spatio-temporal localization of selected pectic and arabinogalactan protein epitopes and the ultrastructural characteristics of explant cells that accompany the changes in the cell fate during somatic embryogenesis in Arabidopsis thaliana. Plant Physiol. Biochem. 2018, 127, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Popielarska-Konieczna, M.; Sala, K.; Abdullah, M.; Tuleja, M.; Kurczyńska, E. Extracellular matrix and wall composition are diverse in the organogenic and non-organogenic calli of Actinidia arguta. Plant Cell Rep. 2020, 39, 779–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdeil, J.L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: Dependence versus autonomy? Trends Plant Sci. 2007, 12, 245–252. [Google Scholar] [CrossRef]
- Namasivayam, P. Acquisition of embryogenic competence during somatic embryogenesis. Plant Cell. Tissue Organ Cult. 2007, 90, 1–8. [Google Scholar] [CrossRef]
- She, W.; Grimanelli, D.; Rutowicz, K.; Whitehead, M.W.J.; Puzio, M.; Kotliński, M.; Jerzmanowski, A.; Baroux, C. Chromatin reprogramming during the somatic-to-reproductive cell fate transition in plants. Development 2013, 140, 4008–4019. [Google Scholar] [CrossRef] [Green Version]
- Wójcikowska, B.; Wójcik, A.M.; Gaj, M.D. Epigenetic regulation of auxin-induced somatic embryogenesis in plants. Int. J. Mol. Sci. 2020, 21, 2307. [Google Scholar] [CrossRef] [Green Version]
- Horstman, A.; Bemer, M.; Boutilier, K. A transcriptional view on somatic embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Yuan, D.; Jin, F.; Zhang, Y.; Xu, J. Transcript profiling reveals complex auxin signalling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton. BMC Plant Biol. 2012, 12, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Márquez-López, R.E.; Pérez-Hernández, C.; Ku-González, Á.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Localization and transport of indole-3-acetic acid during somatic embryogenesis in Coffea canephora. Protoplasma 2018, 255, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Godel-Jedrychowska, K.; Kulinska-Lukaszek, K.; Horstman, A.; Soriano, M.; Li, M.; Malota, K.; Boutilier, K.; Kurczynska, E.U. Symplasmic isolation marks cell fate changes during somatic embryogenesis. J. Exp. Bot. 2020, 71, 2612–2628. [Google Scholar] [CrossRef]
- Fehér, A.; Bernula, D.; Gémes, K. The many ways of somatic embryo initiation. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 23–37. [Google Scholar]
- Guzzo, F.; Baldan, B.; Levi, M.; Sparvoli, E.; Lo Schiavo, F.; Terzi, M.; Mariani, P. Early cellular events during induction of carrot explants with 2,4-D. Protoplasma 1995, 185, 28–36. [Google Scholar] [CrossRef]
- Toma, I.; Toma, C.; Ghiorghita, G. Histo-anatomy and in vitro morphogenesis in Hyssopus officinalis L. (Lamiaceae). Acta Bot. Croat 2004, 63, 59–68. [Google Scholar]
- Boerjan, W.; Cervera, M.T.; Delarue, M.; Beeckman, T.; Dewitte, W.; Bellini, C.; Caboche, M.; Van Onckelen, H.; Van Montagu, M.; Inze, D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 1995, 7, 1405–1419. [Google Scholar]
- Dubrovsky, J.G.; Doerner, P.W.; Colón-Carmona, A.; Rost, T.L. Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol. 2000, 124, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Parizot, B.; Laplaze, L.; Ricaud, L.; Boucheron-Dubuisson, E.; Bayle, V.; Bonke, M.; De Smet, I.; Poethig, S.R.; Helariutta, Y.; Haseloff, J.; et al. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol. 2008, 146, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casero, P.J.; García-Sánchez, C.; Lloret, P.G.; Navascués, J. Morphological features of pericycle cells in relation to their topographical location in onion adventitious roots. New Phytol. 1989, 112, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, J.G.; Rost, T.L. Pericycle. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2005; pp. 1–6. [Google Scholar]
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’H, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009, 57, 626–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Seong, E.S.; Kim, M.J.; Ghimire, B.K.; Kang, W.H.; Yu, C.Y.; Li, C.H. Direct somatic embryogenesis from pericycle cells of broccoli (Brassica oleracea L. var. italica) root explants. Plant Cell. Tissue Organ Cult. 2010, 100, 49–58. [Google Scholar] [CrossRef]
- Michalczuk, L.; Ribnicky, D.M.; Cooke, T.J.; Cohen, J.D. Regulation of indole-3-acetic acid biosynthetic pathways in carrot cell cultures. Plant Physiol. 1992, 100, 1346–1353. [Google Scholar] [CrossRef]
- Ayil-Gutiérrez, B.; Galaz-Ávalos, R.M.; Peña-Cabrera, E.; Loyola-Vargas, V.M. Dynamics of the concentration of IAA and some of its conjugates during the induction of somatic embryogenesis in Coffea canephora. Plant Signal. Behav. 2013, 8, e26998-1. [Google Scholar] [CrossRef] [Green Version]
- Pescador, R.; Kerbauy, G.B.; de Ferreira, W.M.; Purgatto, E.; Suzuki, R.M.; Guerra, M.P. A hormonal misunderstanding in Acca sellowiana embryogenesis: Levels of zygotic embryogenesis do not match those of somatic embryogenesis. Plant Growth Regul. 2012, 68, 67–76. [Google Scholar] [CrossRef]
- Rocha, D.I.; Vieira, L.M.; Tanaka, F.A.O.; da Silva, L.C.; Otoni, W.C. Somatic embryogenesis of a wild passion fruit species Passiflora cincinnata Masters: Histocytological and histochemical evidences. Protoplasma 2012, 249, 747–758. [Google Scholar] [CrossRef]
- McCartney, L.; Steele-King, C.G.; Jordan, E.; Knox, J.P. Cell wall pectic (1→4)-β-D-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 2003, 33, 447–454. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Mccartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Steele-King, C.G.; Marcus, S.E.; Knox, J.P. Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. Plant J. 1999, 20, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 1997, 390, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Satoh, S.; Nakamura, N.; Fujii, T. Differences in pectic polysaccharides between carrot embryogenic and non-embryogenic calli. Plant Cell Rep. 1995, 14, 279–284. [Google Scholar] [CrossRef]
- Majewska-Sawka, A.; Münster, A. Cell-wall antigens in mesophyll cells and mesophyll-derived protoplasts of sugar beet: Possible implication in protoplast recalcitrance? Plant Cell Rep. 2003, 21, 946–954. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [Green Version]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef]
- Pérez-Pérez, Y.; Carneros, E.; Berenguer, E.; Solís, M.T.; Bárány, I.; Pintos, B.; Gómez-Garay, A.; Risueño, M.C.; Testillano, P.S. Pectin de-methylesterification and AGP increase promote cell wall remodeling and are required during somatic embryogenesis of Quercus suber. Front. Plant Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Briggs, S.P.H.; Knox, J.P. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003, 26, 977–989. [Google Scholar] [CrossRef]
- Willats, W.G.T.; McCartney, L.; Steele-King, C.G.; Marcus, S.E.; Mort, A.; Huisman, M.; Van Alebeek, G.J.; Schols, H.A.; Voragen, A.G.J.; Le Goff, A.; et al. A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta 2004, 218, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, M.; Czaplicki, A.Z.; Konieczny, R. Patterns of pectin epitope expression during shoot and root regeneration in androgenic cultures of two wheat cultivars. Acta Biol Cracov. Bot. 2007, 49, 69–72. [Google Scholar]
- Coimbra, S.; Almeida, J.; Jungueira, V.; Costa, M.L.; Pereira, L.G. Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. J. Exp. Bot. 2007, 58, 4027–4035. [Google Scholar] [CrossRef] [Green Version]
- Lamport, D.T.A.; Várnai, P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 2013, 197, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-proteins: Key regulators at the cell surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 47–56. [Google Scholar]
- Portillo, L.; Olmedilla, A.; Santacruz-Ruvalcaba, F. Cellular and molecular changes associated with somatic embryogenesis induction in Agave tequilana. Protoplasma 2012, 249, 1101–1107. [Google Scholar] [CrossRef]
- Namasivayam, P.; Skepper, J.N.; Hanke, D. Distribution of arabinogalactan protein (AGP) epitopes on the anther-derived embryoid cultures of Brassica napus. Pertanika J. Trop. Agric. Sci. 2010, 33, 303–313. [Google Scholar]
- Yates, E.A.; Valdor, J.F.; Haslam, S.M.; Morris, H.R.; Dell, A.; Mackie, W.; Knox, J.P. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 1996, 6, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Lamport, D.T.A.; Kieliszewski, M.J.; Chen, Y.; Cannon, M.C. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011, 156, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Herger, A.; Dünser, K.; Kleine-Vehn, J.; Ringli, C. Leucine-rich repeat extensin proteins and their role in cell wall sensing. Curr. Biol. 2019, 29, R851–R858. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Sala, K.; Karcz, J.; Rypień, A.; Kurczyńska, E.U. Unmethyl-esterified homogalacturonan and extensins seal Arabidopsis graft union. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Jones, L.; Seymour, G.B.; Knox, J.P. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-D-galactan. Plant Physiol. 1997, 113, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Willats, W.G.T.; Marcus, S.E.; Knox, J.P. Generation of a monoclonal antibody specific to (1 → 5)-α-L-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef]
- Smallwood, M.; Yates, E.A.; Willats, W.G.T.; Martin, H.; Knox, J.P. Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 1996, 198, 452–459. [Google Scholar] [CrossRef]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 1991, 3, 1317–1326. [Google Scholar] [CrossRef]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]

| Cells Reprogramming to the Meristematic/Pluripotent State | Cells Undergoing Separation | Separated Cells | Formative Divisions | Constitutive Wall Component | Cell Position Marker | |
|---|---|---|---|---|---|---|
| Positive Marker | LM6, LM2 | LM8, JIM8, JIM13, LM2, JIM11 | LM5, JIM8, JIM13, LM2 | LM6, JIM16, LM2, JIM13 | LM19, LM5, LM20, JIM13, LM2 | JIM4, for pericycle cells on xylem pole |
| Negative Marker | LM5, JIM8, JIM13 | LM6 | LM6 | LM5 | LM6, JIM4, JIM8, JIM16, JIM12, JIM20 | LM5, JIM8; JIM13, LM2 for pericycle cells on xylem pole |
| Antibody | Epitope | References |
|---|---|---|
| Pectins | ||
| LM5 | linear tetrasaccharide in (1–4)-β-D-galactans (RG I side chain) | [101] |
| LM6 | linear pentasaccharide in (1–5)-α-l-arabinans (RG I side chain) | [102] |
| LM8 | xylogalacturonan domain in HG | [88] |
| LM19 | non-methyl-esterified, partially methyl-esterified HG | [85] |
| LM20 | methyl-esterified HG | [85] |
| Extensins | ||
| JIM11 | Extensin/ HRGP glycoprotein | [47] |
| JIM12 | Extensin/ HRGP glycoprotein | [47] |
| JIM20 | Extensin/ HRGP glycoprotein | [47] |
| AGPs | ||
| LM2 | Arabinogalactan protein, carbohydrate epitope containing β -linked GlcA | [103] |
| JIM4 | Arabinogalactan glycoprotein, βGlcA-(1,3)- α GalA-(1,2)-Rha | [93,96] |
| JIM8 | Arabinogalactan | [104] |
| JIM13 | Arabinogalactan/Arabinogalactan protein, carbohydrate epitope (β)GlcA1->3(α)GalA1->2Rha | [105] |
| JIM16 | Arabinogalactan/Arabinogalactan protein | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuczak, M.; Kurczyńska, E. Cell Wall Composition as a Marker of the Reprogramming of the Cell Fate on the Example of a Daucus carota (L.) Hypocotyl in Which Somatic Embryogenesis Was Induced. Int. J. Mol. Sci. 2020, 21, 8126. https://doi.org/10.3390/ijms21218126
Kuczak M, Kurczyńska E. Cell Wall Composition as a Marker of the Reprogramming of the Cell Fate on the Example of a Daucus carota (L.) Hypocotyl in Which Somatic Embryogenesis Was Induced. International Journal of Molecular Sciences. 2020; 21(21):8126. https://doi.org/10.3390/ijms21218126
Chicago/Turabian StyleKuczak, Michał, and Ewa Kurczyńska. 2020. "Cell Wall Composition as a Marker of the Reprogramming of the Cell Fate on the Example of a Daucus carota (L.) Hypocotyl in Which Somatic Embryogenesis Was Induced" International Journal of Molecular Sciences 21, no. 21: 8126. https://doi.org/10.3390/ijms21218126
APA StyleKuczak, M., & Kurczyńska, E. (2020). Cell Wall Composition as a Marker of the Reprogramming of the Cell Fate on the Example of a Daucus carota (L.) Hypocotyl in Which Somatic Embryogenesis Was Induced. International Journal of Molecular Sciences, 21(21), 8126. https://doi.org/10.3390/ijms21218126






