Fibromyalgia: Recent Advances in Diagnosis, Classification, Pharmacotherapy and Alternative Remedies
Abstract
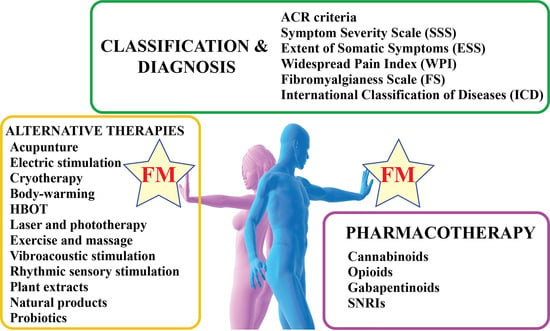
:1. Introduction
2. Diagnosis
3. Therapy
3.1. Pharmacotherapy of FM
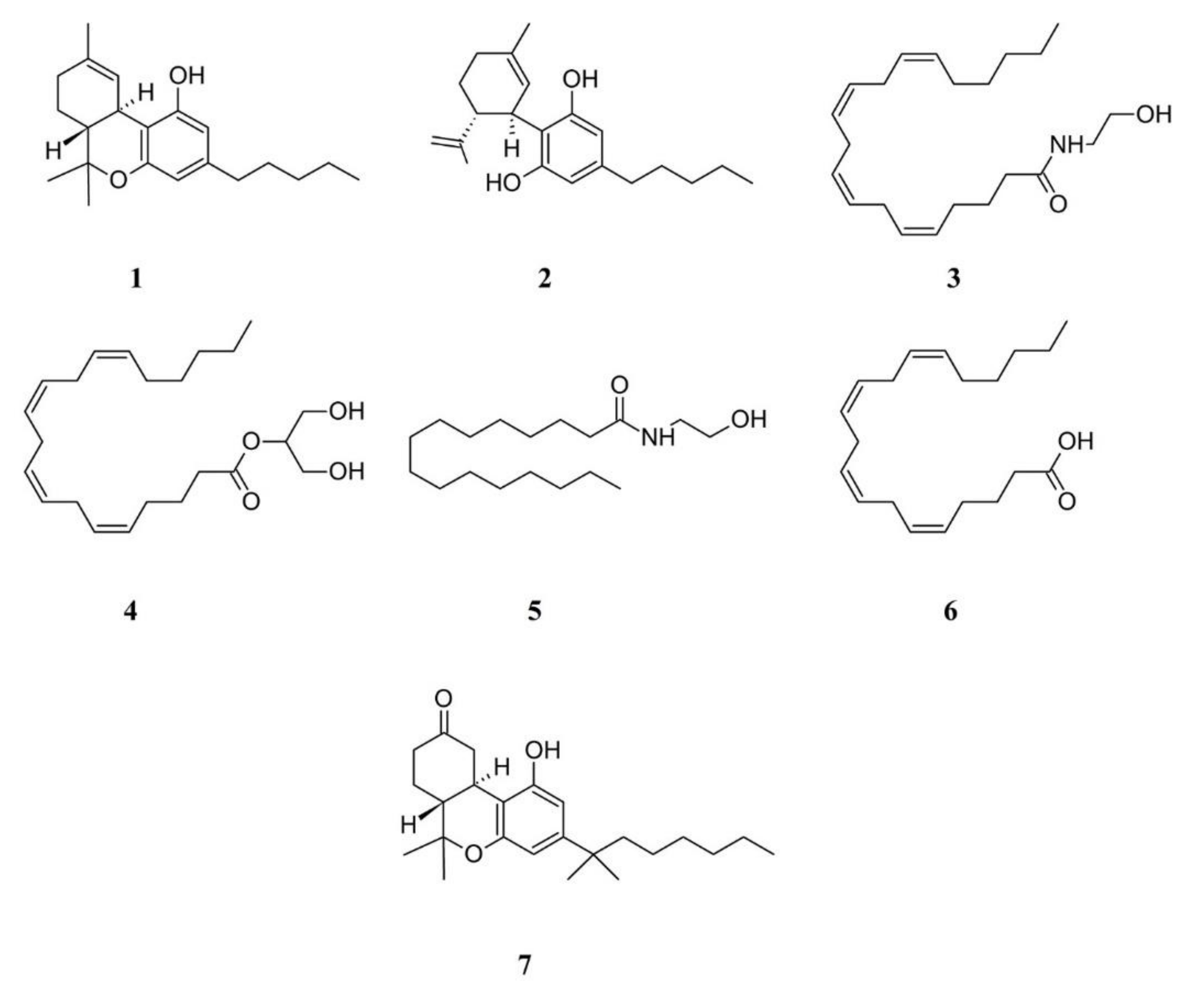
3.1.1. Cannabinoids in FM Therapy
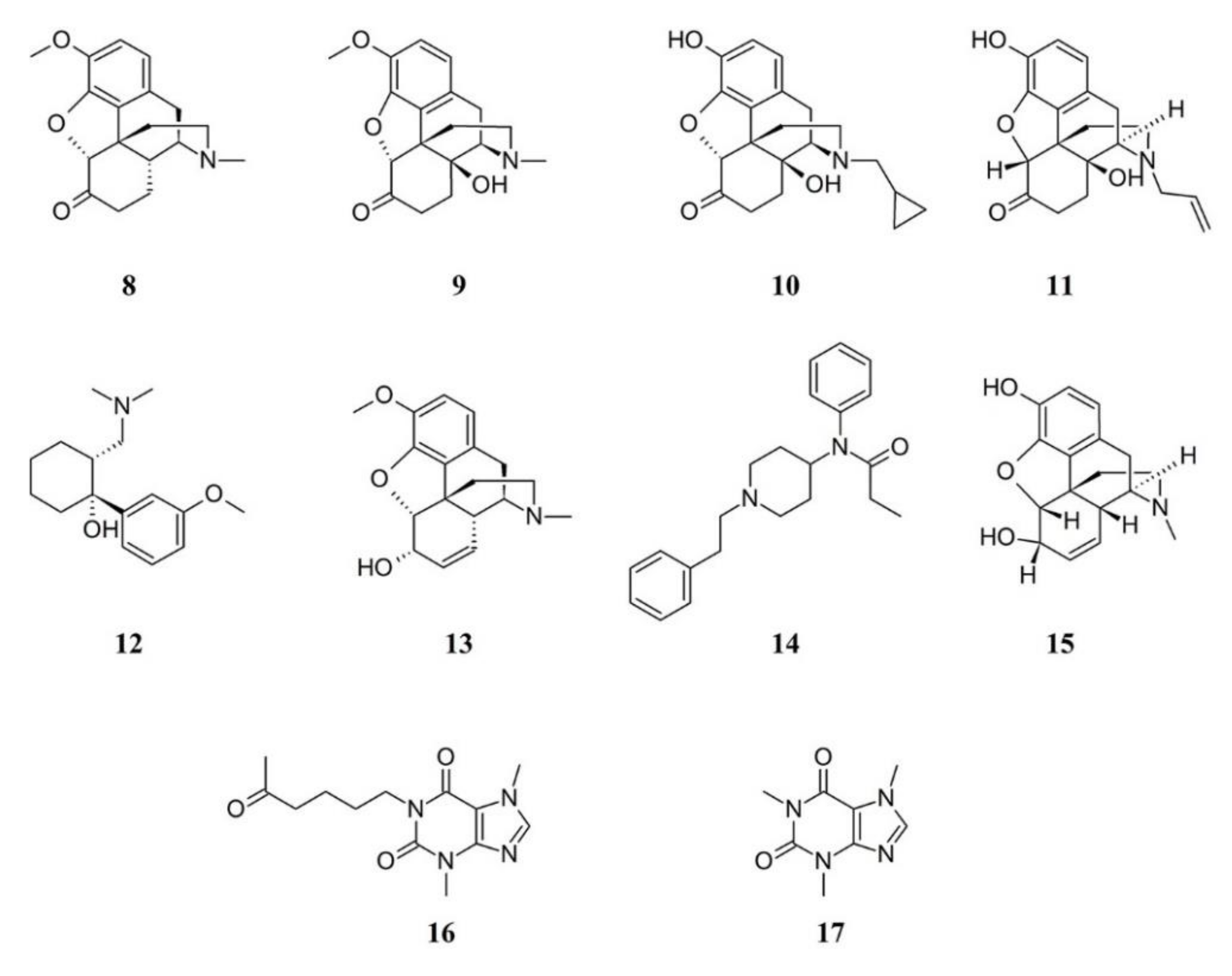
3.1.2. Opioids in FM Therapy
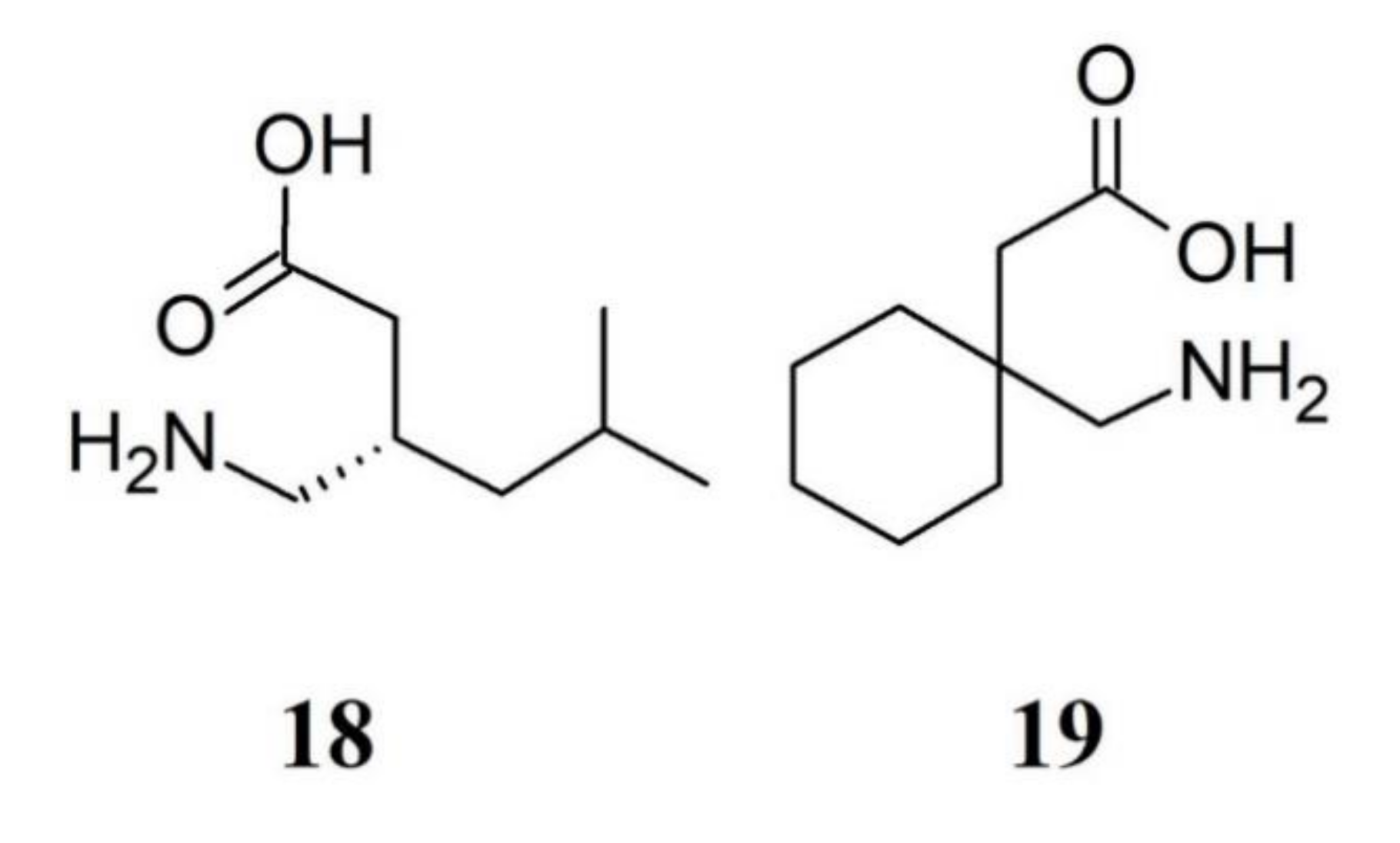
3.1.3. Gabapentinoids in FM Therapy
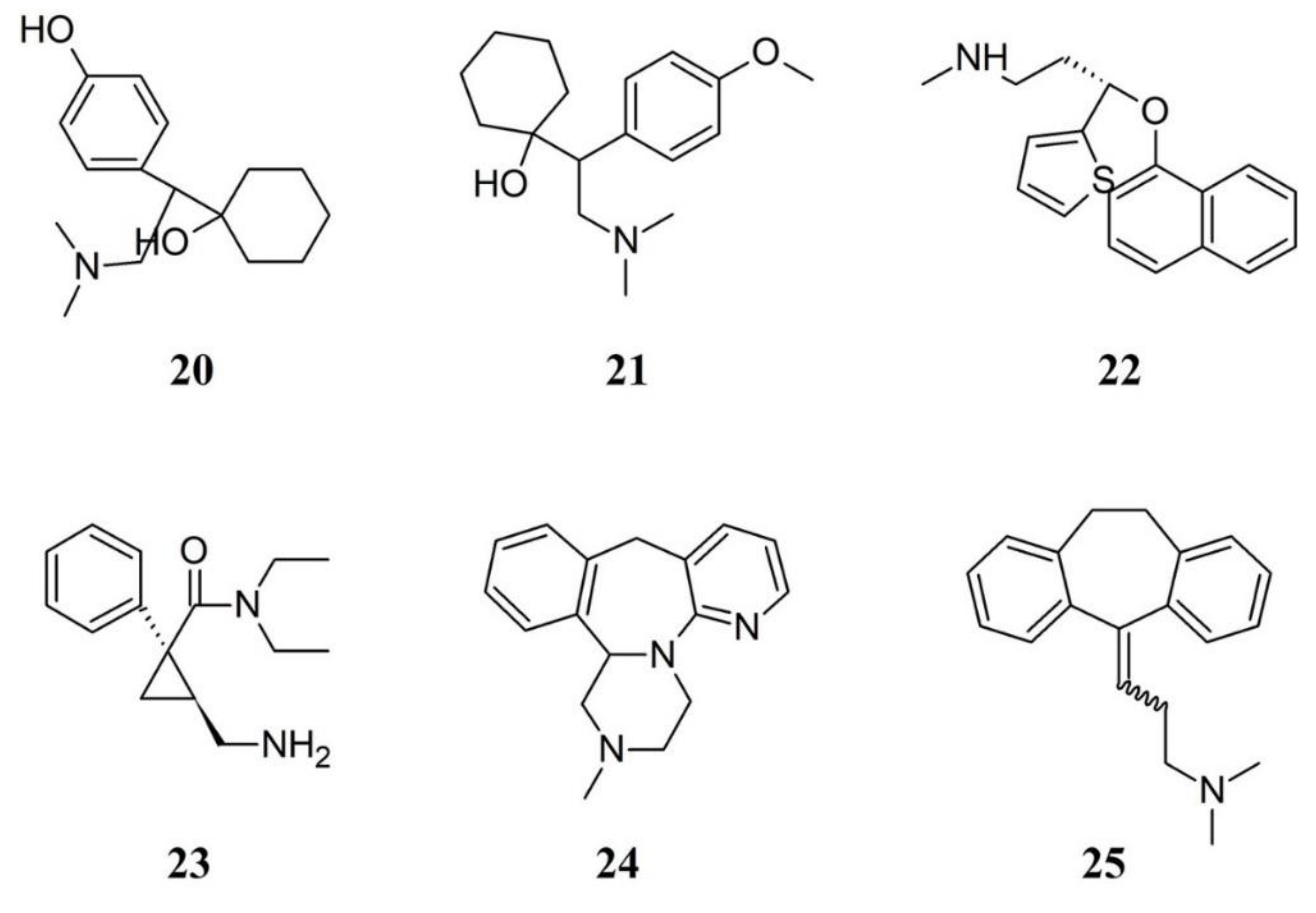
3.1.4. Serotonin–Norepinephrine Reuptake Inhibitors in FM Therapy
3.2. Alternative Therapies for FM
3.2.1. Acupunture
3.2.2. Electric Stimulation
3.2.3. Vibroacoustic and Rhythmic Sensory Stimulation
3.2.4. Thermal Therapies
3.2.5. Hyperbaric Treatment
3.2.6. Laser Therapy and Phototherapy
3.2.7. Exercise and Massage
3.2.8. Probiotics and FM Therapy
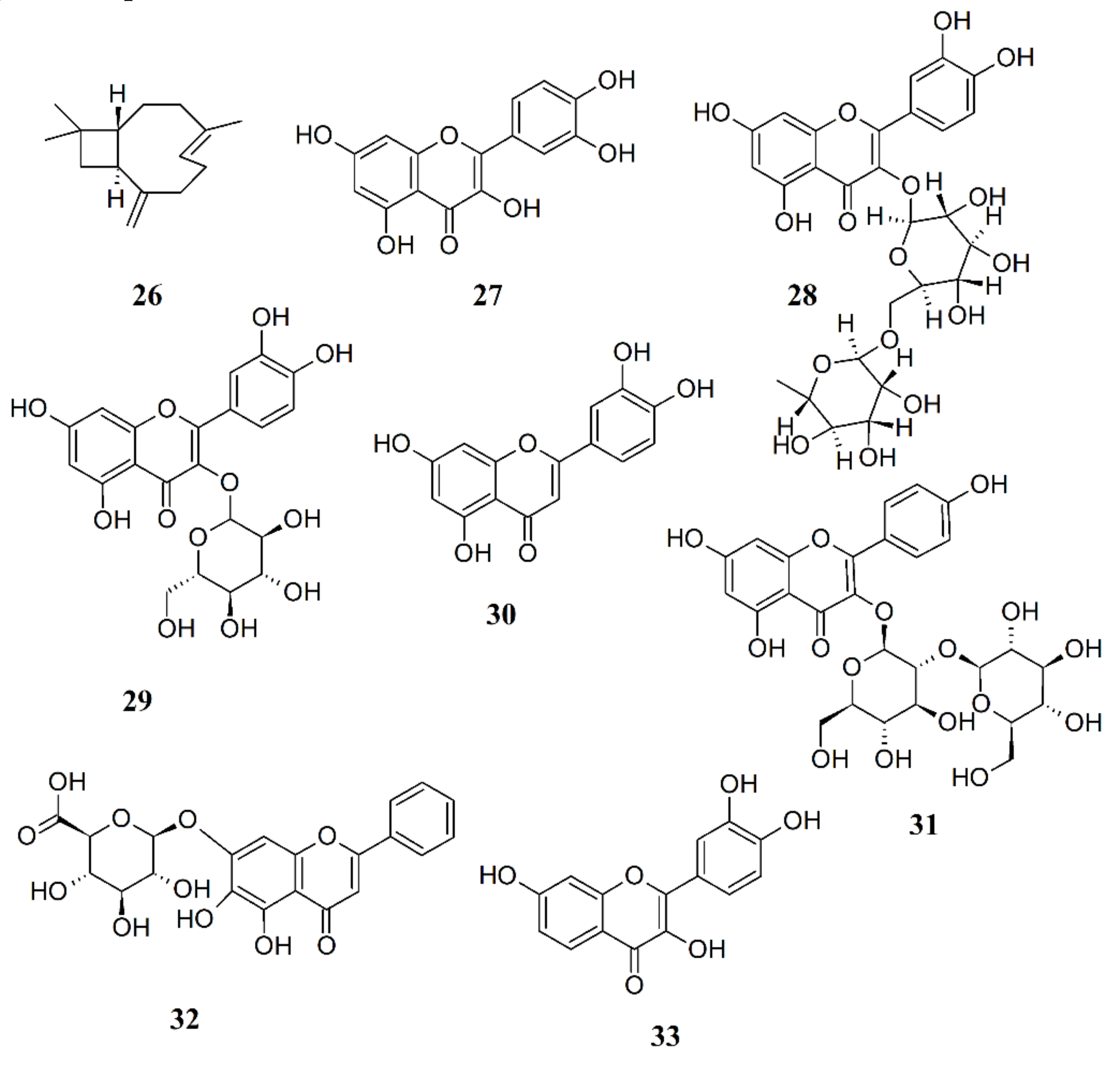
3.2.9. Use of Plant Extracts and Natural Products for FM Treatment
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 2-AG | 2-ArachidonoylGlycerol |
| AA | Arachidonic Acid |
| ACR | American College of Rheumatology |
| ACTH | Adrenocorticotropic hormone |
| AEA | N-arachidonoylethanolamine |
| BDNF | Brain-Derived Neurotrophic Factors |
| CB1 | Cannabinoid Receptor 1 |
| CB2 | Cannabinoid Receptor 2 |
| CBD | Cannabidiol |
| CNS | Central Nervous System |
| EA | Electro-Acupuncture |
| ESS | Extent of Somatic Symptoms |
| FIQ | FM Impact Questionnaire |
| FIQR | FM Impact Questionnaire Revised version |
| FM | Fibromyalgia |
| FS | Fibromyalgianess Scale |
| GABA | Gamma-Aminobutyric Acid |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
| HBOT | Hyperbaric Oxygen Therapy |
| ICD-11 | International Classification of Diseases |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| MA | Manual Acupuncture |
| PEA | Palmitoylethanolamide |
| PFM | Primary FM |
| ROS | Reactive Oxygen Species |
| SIQ | Symptom Impact Questionnaire |
| SFM | Secondary FM |
| SNRIs | Serotonin and Norepinephrine Reuptake Inhibitors |
| SSRIs | Serotonin Selective Reuptake Inhibitors |
| SSS | Symptom Severity Scale |
| TCAs | Tricyclic Antidepressant |
| TNFα | Tumor necrosis factor alpha |
| VAS | Visual Analog Scale |
| WPI | Widespread Pain Index |
| Δ9-THC | Delta 9-tetrahydrocannabinol |
References
- Wang, S.M.; Han, C.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Fibromyalgia diagnosis: A review of the past, present and future. Expert Rev. Neurother. 2015, 15, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia pathogenesis and treatment options update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Beritze, N.; Arguelles, M.; Carcaba, V.; Fernandez, F.; Janciauskiene, S.; Oikonomopoulou, K.; de Serres, F.J.; Fernandez-Bustillo, E.; Hollenberg, M.D. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin. Rheumatol. 2010, 29, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Cabo-Meseguer, A.; Cerda-Olmedo, G.; Trillo-Mata, J.L. Fibromyalgia: Prevalence, epidemiologic profiles and economic costs. Med. Clin. 2017, 149, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A.; Schilling, S. Advances in the assessment of fibromyalgia. Rheum. Dis. Clin. N. Am. 2009, 35, 339–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Underwood, M.; Carnes, D. Fibromyalgia. BMJ Br. Med. J. 2014, 348. [Google Scholar] [CrossRef] [PubMed]
- McBeth, J.; Mulvey, M.R. Fibromyalgia: Mechanisms and potential impact of the acr 2010 classification criteria. Nat. Rev. Rheumatol. 2012, 8, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Clauw, D.J.; McCarberg, B.H.; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin. Proc. 2011, 86, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The american-college-of-rheumatology 1990 criteria for the classification of fibromyalgia—Report of the multicenter criteria committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; McDermott, M.P.; Peirce-Sandner, S.; Burke, L.B.; Cowan, P.; Farrar, J.T.; Hertz, S.; Raja, S.N.; Rappaport, B.A.; et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: Immpact recommendations. Pain 2009, 146, 238–244. [Google Scholar] [CrossRef]
- Arnold, L.M.; Crofford, L.J.; Mease, P.J.; Burgess, S.M.; Palmer, S.C.; Abetz, L.; Martin, S.A. Patient perspectives on the impact of fibromyalgia. Patient Educ. Couns. 2008, 73, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, F.; Hauser, W. Fibromyalgia diagnosis and diagnostic criteria. Ann. Med. 2011, 43, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F. New american college of rheumatology criteria for fibromyalgia: A twenty-year journey. Arthritis Care Res. 2010, 62, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the acr preliminary diagnostic criteria for fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef]
- Oncu, J.; Iliser, R.; Kuran, B. Do new diagnostic criteria for fibromyalgia provide treatment opportunity to those previously untreated? J. Back Musculoskelet. Rehabil. 2013, 26, 437–443. [Google Scholar] [CrossRef]
- Wolfe, F.; Walitt, B.; Rasker, J.J.; Hauser, W. Primary and secondary fibromyalgia are the same: The universality of polysymptomatic distress. J. Rheumatol. 2019, 46, 204–212. [Google Scholar] [CrossRef]
- Bellato, E.; Marini, E.; Castoldi, F.; Barbasetti, N.; Mattei, L.; Bonasia, D.E.; Blonna, D. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat. 2012, 2012, 426130. [Google Scholar] [CrossRef] [Green Version]
- Bennett, R.M.; Friend, R.; Marcus, D.; Bernstein, C.; Han, B.K.; Yachoui, R.; Deodhar, A.; Kaell, A.; Bonafede, P.; Chino, A.; et al. Criteria for the diagnosis of fibromyalgia: Validation of the modified 2010 preliminary american college of rheumatology criteria and the development of alternative criteria. Arthritis Care Res. 2014, 66, 1364–1373. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ringold, S.; Khanna, D.; Neogi, T.; Johnson, S.R.; Miller, A.; Brunner, H.I.; Ogawa, R.; Felson, D.; Ogdie, A.; et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res. 2015, 67, 891–897. [Google Scholar] [CrossRef]
- Taylor, W.J.; Fransen, J. Distinctions between diagnostic and classification criteria: Comment on the article by Aggarwal et al. Arthritis Care Res. 2016, 68, 149–150. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bidari, A.; Parsa, B.G.; Ghalehbaghi, B. Challenges in fibromyalgia diagnosis: From meaning of symptoms to fibromyalgia labeling. Korean J. Pain 2018, 31, 147–154. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Schmukler, J.; Jamal, S.; Castrejon, I.; Gibson, K.A.; Srinivasan, S.; Hauser, W.; Pincus, T. Diagnosis of fibromyalgia: Disagreement between fibromyalgia criteria and clinician-based fibromyalgia diagnosis in a university clinic. Arthritis Care Res. 2019, 71, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Eich, W.; Bar, K.J.; Bernateck, M.; Burgmer, M.; Dexl, C.; Petzke, F.; Sommer, C.; Winkelmann, A.; Hauser, W. Definition, classification, clinical diagnosis and prognosis of fibromyalgia syndrome: Updated guidelines 2017 and overview of systematic review articles. Schmerz 2017, 31, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffaeli, W.; Malafoglia, V.; Bonci, A.; Tenti, M.; Ilari, S.; Gremigni, P.; Iannuccelli, C.; Gioia, C.; Di Franco, M.; Mollace, V.; et al. Identification of mor-positive b cell as possible innovative biomarker (mu lympho-marker) for chronic pain diagnosis in patients with fibromyalgia and osteoarthritis diseases. Int. J. Mol. Sci. 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackshaw, K.V.; Aykas, D.P.; Sigurdson, G.T.; Plans, M.; Madiai, F.; Yu, L.B.; Buffington, C.A.T.; Giusti, M.M.; Rodriguez-Saona, L. Metabolic fingerprinting for diagnosis of fibromyalgia and other rheumatologic disorders. J. Biol. Chem. 2019, 294, 2555–2568. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F. Criteria for fibromyalgia? What is fibromyalgia? Limitations to current concepts of fibromyalgia and fibromyalgia criteria. Clin. Exp. Rheumatol. 2017, 35, S3–S5. [Google Scholar]
- Walitt, B.; Nahin, R.L.; Katz, R.S.; Bergman, M.J.; Wolfe, F. The prevalence and characteristics of fibromyalgia in the 2012 national health interview survey. PLoS ONE 2015, 10, e0138024. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.A.; Straube, S.; Aldington, D. Pain measures and cut-offs—No worse than mild pain as a simple, universal outcome. Anaesthesia 2013, 68, 400–412. [Google Scholar] [CrossRef]
- Espejo, J.A.; Garcia-Escudero, M.; Oltra, E. Unraveling the molecular determinants of manual therapy: An approach to integrative therapeutics for the treatment of fibromyalgia and chronic fatigue syndrome/myalgic encephalomyelitis. Int. J. Mol. Sci. 2018, 19, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin. Pharmacother. 2015, 16, 1347–1368. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.; Shum, B.; Moore, R.A.; Wiffen, P.J.; Gilron, I. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst. Rev. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Seymour, K. Fibromyalgia: Should the treatment paradigm be monotherapy or combination pharmacotherapy? Curr. Pain Headache Rep. 2008, 12, 399–405. [Google Scholar] [CrossRef]
- Kwiatek, R. Treatment of fibromyalgia. Aust. Prescr. 2017, 40, 179–183. [Google Scholar] [CrossRef]
- Wright, C.L.; Mist, S.D.; Ross, R.L.; Jones, K.D. Duloxetine for the treatment of fibromyalgia. Expert Rev. Clin. Immunol. 2010, 6, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [Green Version]
- De Vries, M.; van Rijckevorsel, D.C.M.; Wilder-Smith, O.H.G.; van Goor, H. Dronabinol and chronic pain: Importance of mechanistic considerations. Expert Opin. Pharmacother. 2014, 15, 1525–1534. [Google Scholar] [CrossRef]
- Russo, E.B. Clinical endocannabinoid deficiency (CECD)—Can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuroendocr. Lett. 2004, 25. (Reprinted from Neuroendocrinilogy, 2004, 25, 31–39). [Google Scholar]
- Smith, S.C.; Wagner, M.S. Clinical endocannabinoid deficiency (CECD) revisited: Can this concept explain the therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuroendocr. Lett. 2014, 35, 198–201. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abushaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Skrabek, R.Q.; Gallmova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Klose, P.; Fitzcharles, M.A.; Phillips, T.; Hauser, W. Cannabinoids for fibromyalgia. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of cb1 and cb2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, D.; Eich, W.; Lerner, R.; Lutz, B.; Bindila, L.; Tesarz, J. Plasma parameters of the endocannabinoid system are unaltered in fibromyalgia. Psychother. Psychosom. 2018, 87, 377–379. [Google Scholar] [CrossRef]
- Kaufmann, I.; Schelling, G.; Eisner, C.; Richter, H.P.; Krauseneck, T.; Vogeser, M.; Hauer, D.; Campolongo, P.; Chouker, A.; Beyer, A.; et al. Anandamide and neutrophil function in patients with fibromyalgia. Psychoneuroendocrinology 2008, 33, 676–685. [Google Scholar] [CrossRef]
- Agarwal, N.; Pacher, P.; Tegeder, I.; Amaya, F.; Constantin, C.E.; Brenner, G.J.; Rubino, T.; Michalski, C.W.; Marsicano, G.; Monory, K.; et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007, 10, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Schley, M.; Legler, A.; Skopp, G.; Schmelz, M.; Konrad, C.; Rukwied, R. Delta-9-thc based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr. Med. Res. Opin. 2006, 22, 1269–1276. [Google Scholar] [CrossRef]
- Fiz, J.; Duran, M.; Capella, D.; Carbonell, J.; Farre, M. Cannabis use in patients with fibromyalgia: Effect on symptoms relief and health-related quality of life. PLoS ONE 2011, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth. Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Ste-Marie, P.A.; Goldenberg, D.L.; Pereira, J.X.; Abbey, S.; Choiniere, M.; Ko, G.; Moulin, D.E.; Panopalis, P.; Proulx, J.; et al. 2012 Canadian guidelines for the diagnosis and management of fibromyalgia syndrome: Executive summary. Pain Res. Manag. 2013, 18, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Ste-Marie, P.A.; Fitzcharles, M.A.; Gamsa, A.; Ware, M.A.; Shir, Y. Association of herbal cannabis use with negative psychosocial parameters in patients with fibromyalgia. Arthritis Care Res. 2012, 64, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.T.; Crofford, L.J. Chronic opioid use in fibromyalgia syndrome a clinical review. JCR J. Clin. Rheumatol. 2013, 19, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.L.; Clauw, D.J.; Palmer, R.E.; Clair, A.G. Opioid use in fibromyalgia: A cautionary tale. Mayo Clin. Proc. 2016, 91, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraniuk, J.N.; Whalen, G.; Cunningham, J.; Clauw, D.J. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet. Disord. 2004, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Fitzcharles, M.-A.; Faregh, N.; Ste-Marie, P.A.; Shir, Y. Opioid use in fibromyalgia is associated with negative health related measures in a prospective cohort study. Pain Res. Treat. 2013, 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.M.; Robinson, R.L.; Mease, P.; Kroenke, K.; Williams, D.A.; Chen, Y.; Faries, D.; Wohlreich, M.; McCarberg, B.; Hann, D. Long-term evaluation of opioid treatment in fibromyalgia. Clin. J. Pain 2015, 31, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.M.; Lee, B.J.; Oh, T.H.; Park, D.; Kim, C.H. Association between initial opioid use and response to a brief interdisciplinary treatment program in fibromyalgia. Medicine 2019, 98, 8. [Google Scholar] [CrossRef]
- Harris, R.E.; Clauw, D.J.; Scott, D.J.; McLean, S.A.; Gracely, R.H.; Zubieta, J.K. Decreased central mu-opioid receptor availability in fibromyalgia. J. Neurosci. 2007, 27, 10000–10006. [Google Scholar] [CrossRef] [Green Version]
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An internet survey of 2596 people with fibromyalgia. BMC Musculoskelet. Disord. 2007, 8, 27. [Google Scholar]
- Hilliard, P.E.; Waljee, J.; Moser, S.; Metz, L.; Mathis, M.; Goesling, J.; Cron, D.; Clauw, D.J.; Englesbe, M.; Abecasis, G.; et al. Prevalence of preoperative opioid use and characteristics associated with opioid use among patients presenting for surgeryprevalence of preoperative opioid use and associated patient characteristicsprevalence of preoperative opioid use and associated patient characteristics. JAMA Surg. 2018, 153, 929–937. [Google Scholar] [PubMed] [Green Version]
- Gaskell, H.; Moore, R.A.; Derry, S.; Stannard, C. Oxycodone for pain in fibromyalgia in adults. Cochrane Database Syst. Rev. 2016, 23. [Google Scholar] [CrossRef]
- Ruette, P.; Stuyck, J.; Debeer, P. Neuropathic arthropathy of the shoulder and elbow associated with syringomyelia: A report of 3 cases. Acta Orthop. Belg. 2007, 73, 525–529. [Google Scholar] [PubMed]
- Williams, E.R.; Ford, C.M.; Simonds, J.G.; Leal, A.K. Blocking peripheral opioid receptors with naloxone methiodide prevents acute and chronic training-induced analgesia in a rat model of fibromyalgia. FASEB J. 2017, 31, 1. [Google Scholar]
- Hermans, L.; Nijs, J.; Calders, P.; De Clerck, L.; Moorkens, G.; Hans, G.; Grosemans, S.; De Mettelinge, T.R.; Tuynman, J.; Meeus, M. Influence of morphine and naloxone on pain modulation in rheumatoid arthritis, chronic fatigue syndrome/fibromyalgia, and controls: A double-blind, randomized, placebo-controlled, cross-over study. Pain Pract. 2018, 18, 418–430. [Google Scholar] [CrossRef]
- MacLean, A.J.B.; Schwartz, T.L. Tramadol for the treatment of fibromyalgia. Expert Rev. Neurother. 2015, 15, 469–475. [Google Scholar] [CrossRef]
- Gur, A.; Calgan, N.; Nas, K.; Cevik, R.; Sarac, A.J. Low dose of tramadol in the treatment of fibromyalgia syndrome: A controlled clinical trial versus placebo. Ann. Rheum. Dis. 2006, 65, 556. [Google Scholar]
- Mullican, W.S.; Lacy, J.R.; TRAMAP-ANAG-006 Study Group. Tramadol/acetaminophen combination tablets and codeine/acetaminophen combination capsules for the management of chronic pain: A comparative trial. Clin. Ther. 2001, 23, 1429–1445. [Google Scholar] [CrossRef]
- Price, D.D.; Staud, R.; Robinson, M.E.; Mauderli, A.P.; Cannon, R.; Vierck, C.J. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002, 99, 49–59. [Google Scholar] [CrossRef]
- Larabi, I.A.; Martin, M.; Fabresse, N.; Etting, I.; Edel, Y.; Pfau, G.; Alvarez, J.C. Hair testing for 3-fluorofentanyl, furanylfentanyl, methoxyacetylfentanyl, carfentanil, acetylfentanyl and fentanyl by lc-ms/ms after unintentional overdose. Forensic Toxicol. 2020, 38, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Comer, S.D.; Cahill, C.M. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci. Biobehav. Rev. 2019, 106, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Abeles, A.M.; Pillinger, M.H.; Solitar, B.M.; Abeles, M. Narrative review: The pathophysiology of fibromyalgia. Ann. Intern. Med. 2007, 146, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Watkins, L.R.; Maier, S.F. Immune regulation of central nervous system functions: From sickness responses to pathological pain. J. Intern. Med. 2005, 257, 139–155. [Google Scholar] [CrossRef]
- Khalil, R.B. Pentoxifylline’s theoretical efficacy in the treatment of fibromyalgia syndrome. Pain Med. 2013, 14, 549–550. [Google Scholar] [CrossRef] [Green Version]
- Polli, A.; Ghosh, M.; Bakusic, J.; Ickmans, K.; Monteyne, D.; Velkeniers, B.; Bekaert, B.; Godderis, L.; Nijs, J. DNA methylation and brain-derived neurotrophic factor expression account for symptoms and widespread hyperalgesia in patients with chronic fatigue syndrome and comorbid fibromyalgia. Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef]
- Scott, J.R.; Hassett, A.L.; Brummett, C.M.; Harris, R.E.; Clauw, D.J.; Harte, S.E. Caffeine as an opioid analgesic adjuvant in fibromyalgia. J. Pain Res. 2017, 10, 1801–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, C.W.; Brett, A.S. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern. Med. 2019, 179, 695–701. [Google Scholar] [CrossRef]
- Micheva, K.D.; Buchanan, J.; Holz, R.W.; Smith, S.J. Retrograde regulation of synaptic vesicle endocytosis and recycling. Nat. Neurosci. 2003, 6, 925–932. [Google Scholar] [CrossRef]
- Deitos, A.; Soldatelli, M.D.; Dussan-Sarria, J.A.; Souza, A.; Torres, I.L.D.; Fregni, F.; Caumo, W. Novel insights of effects of pregabalin on neural mechanisms of intracortical disinhibition in physiopathology of fibromyalgia: An explanatory, randomized, double-blind crossover study. Front. Hum. Neurosci. 2018, 12, 14. [Google Scholar] [CrossRef]
- Kiso, T.; Moriyama, A.; Furutani, M.; Matsuda, R.; Funatsu, Y. Effects of pregabalin and duloxetine on neurotransmitters in the dorsal horn of the spinal cord in a rat model of fibromyalgia. Eur. J. Pharmacol. 2018, 827, 117–124. [Google Scholar] [CrossRef]
- Gerardi, M.C.; Atzeni, F.; Batticciotto, A.; Di Franco, M.; Rizzi, M.; Sarzi-Puttini, P. The safety of pregabalin in the treatment of fibromyalgia. Expert Opin. Drug Saf. 2016, 15, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Hirakata, M.; Yoshida, S.; Tanaka-Mizuno, S.; Kuwauchi, A.; Kawakami, K. Pregabalin prescription for neuropathic pain and fibromyalgia: A descriptive study using administrative database in Japan. Pain Res. Manag. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asomaning, K.; Abramsky, S.; Liu, Q.; Zhou, X.; Sobel, R.E.; Watt, S. Pregabalin prescriptions in the United Kingdom: A drug utilisation study of the health improvement network (thin) primary care database. Int J. Clin. Pr. 2016, 70, 380–388. [Google Scholar] [CrossRef]
- Ferreira-Dos-Santos, G.; Sousa, D.C.; Costa, J.; Vaz-Carneiro, A. Analysis of the cochrane review: Pregabalin for pain in fibromyalgia in adults. Cochrane database syst rev. 2016; 9: Cd011790 and 2016; 4: Cd009002. Acta Med. Port. 2018, 31, 376–381. [Google Scholar] [CrossRef]
- Bhusal, S.; Diomampo, S.; Magrey, M.N. Clinical utility, safety, and efficacy of pregabalin in the treatment of fibromyalgia. Drug Healthc. Patient Saf. 2016, 8, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Arnold, L.M.; Choy, E.; Clauw, D.J.; Oka, H.; Whalen, E.; Semel, D.; Pauer, L.; Knapp, L. An evidence-based review of pregabalin for the treatment of fibromyalgia. Curr. Med. Res. Opin. 2018, 34, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.E.; Derry, S.; Wiffen, P.J.; Moore, R.A. Gabapentin for fibromyalgia pain in adults. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Urrutia, G.; Nishishinya, M.B.; Cantrell, S.E.; Hauser, W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst. Rev. 2015, 66. [Google Scholar] [CrossRef]
- Welsch, P.; Uceyler, N.; Klose, P.; Walitt, B.; Hauser, W. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst. Rev. 2018, 111. [Google Scholar] [CrossRef] [Green Version]
- Grubisic, F. Are serotonin and noradrenaline reuptake inhibitors effective, tolerable, and safe for adults with fibromyalgia? A cochrane review summary with commentary. J. Musculoskelet. Neuronal. Interact. 2018, 18, 404–406. [Google Scholar]
- VanderWeide, L.A.; Smith, S.M.; Trinkley, K.E. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J. Clin. Pharm. Ther. 2015, 40, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Murakami, M.; Oka, H.; Onozawa, K.; Yoshida, S.; Osada, K. Efficacy of mirtazapine for the treatment of fibromyalgia without concomitant depression: A randomized, double-blind, placebo-controlled phase IIa study in Japan. Pain 2016, 157, 2089–2096. [Google Scholar] [CrossRef] [Green Version]
- Deboer, T. The pharmacologic profile of mirtazapine. J. Clin. Psychiatry 1996, 57, 19–25. [Google Scholar]
- Ottman, A.A.; Warner, C.B.; Brown, J.N. The role of mirtazapine in patients with fibromyalgia: A systematic review. Rheumatol. Int. 2018, 38, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Rico-Villademoros, F.; Slim, M.; Calandre, E.P. Amitriptyline for the treatment of fibromyalgia: A comprehensive review. Expert Rev. Neurother. 2015, 15, 1123–1150. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for fibromyalgia in adults. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- De Tommaso, M.; Delussi, M.; Ricci, K.; D’Angelo, G. Abdominal acupuncture changes cortical responses to nociceptive stimuli in fibromyalgia patients. CNS Neurosci. Ther. 2014, 20, 565–567. [Google Scholar] [CrossRef]
- Karatay, S.; Okur, S.C.; Uzkeser, H.; Yildirim, K.; Akcay, F. Effects of acupuncture treatment on fibromyalgia symptoms, serotonin, and substance p levels: A randomized sham and placebo-controlled clinical trial. Pain Med. 2018, 19, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Deare, J.C.; Zheng, Z.; Xue, C.C.L.; Liu, J.P.; Shang, J.S.; Scott, S.W.; Littlejohn, G. Acupuncture for treating fibromyalgia. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.J.; Li, X.; Han, M.; Liu, J.P. Acupoint stimulation for fibromyalgia: A systematic review of randomized controlled trials. Evid. Based Complementary Altern. Med. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Zhang, X.C.; Chen, H.; Xu, W.T.; Song, Y.Y.; Gu, Y.H.; Ni, G.X. Acupuncture therapy for fibromyalgia: A systematic review and meta-analysis of randomized controlled trials. J. Pain Res. 2019, 12, 527–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesio, V.; Torta, D.M.E.; Colonna, F.; Leombruni, P.; Ghiggia, A.; Fusaro, E.; Geminiani, G.C.; Torta, R.; Castelli, L. Are fibromyalgia patients cognitively impaired? Objective and subjective neuropsychological evidence. Arthritis Care Res. 2015, 67, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gelonch, O.; Garolera, M.; Valls, J.; Rossello, L.; Pifarre, J. Executive function in fibromyalgia: Comparing subjective and objective measures. Compr. Psychiatry 2016, 66, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.E.; Yu, B.; Zhang, W.; Chen, W.H.; Qi, Q.; Miao, Y. Effectiveness and safety of transcranial direct current stimulation in fibromyalgia: A systematic review and meta-analysis. J. Rehabil. Med. 2017, 49, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Brighina, F.; Curatolo, M.; Cosentino, G.; De Tommaso, M.; Battaglia, G.; Sarzi-Puttini, P.C.; Guggino, G.; Fierro, B. Brain modulation by electric currents in fibromyalgia: A structured review on non-invasive approach with transcranial electrical stimulation. Front. Hum. Neurosci. 2019, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, V.S.; Zortea, M.; Alves, R.L.; Naziazeno, C.C.D.; Saldanha, J.S.; de Carvalho, S.D.R.; Leite, A.J.D.; Torres, I.L.D.; de Souza, A.; Calvetti, P.U.; et al. Cognitive effects of transcranial direct current stimulation combined with working memory training in fibromyalgia: A randomized clinical trial. Sci Rep. 2018, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Eken, A.; Kara, M.; Baskak, B.; Baltaci, A.; Gokcay, D. Differential efficiency of transcutaneous electrical nerve stimulation in dominant versus nondominant hands in fibromyalgia: Placebo-controlled functional near-infrared spectroscopy study. Neurophotonics 2018, 5, 15. [Google Scholar] [CrossRef]
- Yuksel, M.; Ayas, S.; Cabioglu, M.T.; Yilmaz, D.; Cabioglu, C. Quantitative data for transcutaneous electrical nerve stimulation and acupuncture effectiveness in treatment of fibromyalgia syndrome. Evid. Based Complementary Altern. Med. 2019, 12, 362831. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Plazier, M.; Ost, J.; Stassijns, G.; Deleye, S.; Ceyssens, S.; Dupont, P.; Stroobants, S.; Staelens, S.; De Ridder, D.; et al. The effect of occipital nerve field stimulation on the descending pain pathway in patients with fibromyalgia: A water pet and EEG imaging study. BMC Neurol. 2018, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Sutbeyaz, S.T.; Sezer, N.; Koseoglu, F.; Kibar, S. Low-frequency pulsed electromagnetic field therapy in fibromyalgia a randomized, double-blind, sham-controlled clinical study. Clin. J. Pain 2009, 25, 722–728. [Google Scholar] [CrossRef]
- Multanen, J.; Hakkinen, A.; Heikkinen, P.; Kautiainen, H.; Mustalampi, S.; Ylinen, J. Pulsed electromagnetic field therapy in the treatment of pain and other symptoms in fibromyalgia: A randomized controlled study. Bioelectromagnetics 2018, 39, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Cruccu, G.; Garcia-Larrea, L.; Hansson, P.; Keindl, M.; Lefaucheur, J.P.; Paulus, W.; Taylor, R.; Tronnier, V.; Truini, A.; Attal, N. Ean guidelines on central neurostimulation therapy in chronic pain conditions. Eur. J. Neurol. 2016, 23, 1489–1499. [Google Scholar] [CrossRef]
- Knijnik, L.M.; Dussan-Sarria, J.A.; Rozisky, J.R.; Torres, I.L.S.; Brunoni, A.R.; Fregni, F.; Caumo, W. Repetitive transcranial magnetic stimulation for fibromyalgia: Systematic review and meta-analysis. Pain Pract. 2016, 16, 294–304. [Google Scholar] [CrossRef]
- Thut, G.; Schyns, P.G.; Gross, J. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front. Psychol. 2011, 2, 170. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.; Werneck, L.; Paiva, E.; Gans, P. Effects of music in combination with vibration in acupuncture points on the treatment of fibromyalgia. J. Altern. Complement. Med. 2015, 21, 77–82. [Google Scholar] [CrossRef]
- Chesky, K.S.; Russell, I.J.; Lopez, Y.; Kondraske, G.V. Fibromyalgia tender point pain: A double-blind, placebo-controlled pilot study of music vibration using the music vibration table. J. Musculoskelet. Pain 1997, 5, 33–52. [Google Scholar] [CrossRef]
- Naghdi, L.; Ahonen, H.; Macario, P.; Bartel, L. The effect of low-frequency sound stimulation on patients with fibromyalgia: A clinical study. Pain Res. Manag. 2015, 20, E21–E27. [Google Scholar] [CrossRef] [PubMed]
- Janzen, T.B.; Paneduro, D.; Picard, L.; Gordon, A.; Bartel, L.R. A parallel randomized controlled trial examining the effects of rhythmic sensory stimulation on fibromyalgia symptoms. PLoS ONE 2019, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Ablin, J.N.; Hauser, W.; Buskila, D. Spa Treatment (Balneotherapy) for Fibromyalgia—A Qualitative-Narrative Review and a Historical Perspective. Evid. Based Complementary Altern. Med. 2013, 2013, 638050. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L.; Sukenik, S.; Bolotin, A.; Abu-Shakra, M.; Amir, A.; Flusser, D.; Buskila, D. The effect of balneotherapy at the dead sea on the quality of life of patients with fibromyalgia syndrome. Clin. Rheumatol. 2001, 20, 15–19. [Google Scholar] [CrossRef]
- Mist, S.D.; Firestone, K.A.; Jones, K.D. Complementary and alternative exercise for fibromyalgia: A meta-analysis. J. Pain Res. 2013, 6, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Fioravanti, A.; Perpignano, G.; Tirri, G.; Cardinale, G.; Gianniti, C.; Lanza, C.E.; Loi, A.; Tirri, E.; Sfriso, P.; Cozzi, F. Effects of mud-bath treatment on fibromyalgia patients: A randomized clinical trial. Rheumatol. Int. 2007, 27, 1157–1161. [Google Scholar] [CrossRef]
- Maeda, T.; Kudo, Y.; Horiuchi, T.; Makino, N. Clinical and anti-aging effect of mud-bathing therapy for patients with fibromyalgia. Mol. Cell. Biochem. 2018, 444, 87–92. [Google Scholar] [CrossRef]
- Guidelli, G.M.; Tenti, S.; De Nobili, E.; Fioravanti, A. Fibromyalgia syndrome and spa therapy: Myth or reality? Clin. Med. Insights Arthritis Musculoskelet. Disord. 2012, 5, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Ernst, E.; Fialka, V. Ice freezes pain—A review of the clinical effectiveness of analgesic cold therapy. J. Pain Symptom Manag. 1994, 9, 56–59. [Google Scholar] [CrossRef]
- Rivera, J.; Tercero, M.J.; Salas, J.S.; Gimeno, J.H.; Alejo, J.S. The effect of cryotherapy on fibromyalgia: A randomised clinical trial carried out in a cryosauna cabin. Rheumatol. Int. 2018, 38, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Bettoni, L.; Bonomi, F.G.; Zani, V.; Manisco, L.; Indelicato, A.; Lanteri, P.; Banfi, G.; Lombardi, G. Effects of 15 consecutive cryotherapy sessions on the clinical output of fibromyalgic patients. Clin. Rheumatol. 2013, 32, 1337–1345. [Google Scholar] [CrossRef]
- Sutherland, A.M.; Clarke, H.A.; Katz, J.; Katznelson, R. Hyperbaric oxygen therapy: A new treatment for chronic pain? Pain Pract. 2016, 16, 620–628. [Google Scholar] [CrossRef]
- Bennett, M.H.; French, C.; Schnabel, A.; Wasiak, J.; Kranke, P.; Weibel, S. Normobaric and hyperbaric oxygen therapy for the treatment and prevention of migraine and cluster headache. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Yildiz, S.; Kiralp, M.Z.; Akin, A.; Keskin, I.; Ay, H.; Dursun, H.; Cimsit, M. A new treatment modality for fibromyalgia syndrome: Hyperbaric oxygen therapy. J. Int. Med Res. 2004, 32, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussi-Gross, R.; Golan, H.; Fishlev, G.; Bechor, Y.; Volkov, O.; Bergan, J.; Friedman, M.; Hoofien, D.; Shlamkovitch, N.; Ben-Jacob, E.; et al. Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury—Randomized prospective trial. PLoS ONE 2013, 8, e79995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efrati, S.; Golan, H.; Bechor, Y.; Faran, Y.; Daphna-Tekoah, S.; Sekler, G.; Fishlev, G.; Ablin, J.N.; Bergan, J.; Volkov, O.; et al. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome—Prospective clinical trial. PLoS ONE 2015, 10, e0127012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shewy, K.M.; Kunbaz, A.; Gad, M.M.; Al-Husseini, M.J.; Saad, A.M.; Sammour, Y.M.; Abdel-Daim, M.M. Hyperbaric oxygen and aerobic exercise in the long-term treatment of fibromyalgia: A narrative review. Biomed. Pharmacother. 2019, 109, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kisselev, S.B.; Moskvin, S.V. The use of laser therapy for patients with fibromyalgia: A critical literary review. J. Lasers Med. Sci. 2019, 10, 12–20. [Google Scholar] [CrossRef] [Green Version]
- White, P.F.; Zafereo, J.; Elvir-Lazo, O.L.; Hernandez, H. Treatment of drug-resistant fibromyalgia symptoms using high-intensity laser therapy: A case-based review. Rheumatol. Int. 2018, 38, 517–523. [Google Scholar] [CrossRef]
- Gur, A.; Karakoc, M.; Nas, K.; Cevik, R.; Sarac, A.J.; Ataoglu, S. Effects of low power laser and low dose amitriptyline therapy on clinical symptoms and quality of life in fibromyalgia: A single-blind, placebo-controlled trial. Rheumatol. Int. 2002, 22, 188–193. [Google Scholar]
- Panton, L.; Simonavice, E.; Williams, K.; Mojock, C.; Kim, J.S.; Kingsley, J.D.; McMillan, V.; Mathis, R. Effects of class IV laser therapy on fibromyalgia impact and function in women with fibromyalgia. J. Altern. Complement. Med. 2013, 19, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Ruaro, J.A.; Frez, A.R.; Ruaro, M.B.; Nicolau, R.A. Low-level laser therapy to treat fibromyalgia. Lasers Med. Sci. 2014, 29, 1815–1819. [Google Scholar] [CrossRef]
- Da Silva, M.M.; Albertini, R.; Leal, E.C.P.; de Carvalho, P.D.C.; Silva, J.A.; Bussadori, S.K.; de Oliveira, L.V.F.; Casarin, C.A.S.; Andrade, E.L.; Bocalini, D.S.; et al. Effects of exercise training and photobiomodulation therapy (extraphoto) on pain in women with fibromyalgia and temporomandibular disorder: Study protocol for a randomized controlled trial. Trials 2015, 16, 8. [Google Scholar] [CrossRef] [Green Version]
- Busch, A.J.; Webber, S.C.; Brachaniec, M.; Bidonde, J.; Dal Bello-Haas, V.; Danyliw, A.D.; Overend, T.J.; Richards, R.S.; Sawant, A.; Schachter, C.L. Exercise therapy for fibromyalgia. Curr. Pain Headache Rep. 2011, 15, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.D.; Adams, D.; Winters-Stone, K.; Burckhardt, C.S. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988–2005). Health Qual. Life Outcomes 2006, 4, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidonde, J.; Busch, A.J.; Schachter, C.L.; Webber, S.C.; Musselman, K.E.; Overend, T.J.; Goes, S.M.; Dal Bello-Haas, V.; Boden, C. Mixed exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2019, 208. [Google Scholar] [CrossRef]
- Assumpção, A.; Matsutani, L.A.; Yuan, S.L.; Santo, A.S.; Sauer, J.; Mango, P.; Marques, A.P. Muscle stretching exercises and resistance training in fibromyalgia: Which is better? A three-arm randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 663–670. [Google Scholar] [CrossRef]
- Nelson, N.L. Muscle strengthening activities and fibromyalgia: A review of pain and strength outcomes. J. Bodyw. Mov. Ther. 2015, 19, 370–376. [Google Scholar] [CrossRef]
- Sanudo, B.; Galiano, D.; Carrasco, L.; Blagojevic, M.; de Hoyo, M.; Saxton, J. Aerobic exercise versus combined exercise therapy in women with fibromyalgia syndrome: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2010, 91, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Corbin, L.W.; Maluf, K.S. Pain mediates the association between physical activity and the impact of fibromyalgia on daily function. Clin. Rheumatol. 2015, 34, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Bement, M.K.H.; Weyer, A.; Hartley, S.; Drewek, B.; Harkins, A.L.; Hunter, S.K. Pain perception after isometric exercise in women with fibromyalgia. Arch. Phys. Med. Rehabil. 2011, 92, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Carson, J.W.; Carson, K.M.; Jones, K.D.; Bennett, R.M.; Wright, C.L.; Mist, S.D. A pilot randomized controlled trial of the yoga of awareness program in the management of fibromyalgia. Pain 2010, 151, 530–539. [Google Scholar] [CrossRef]
- De Oliveira, F.R.; Goncalves, L.C.V.; Borghi, F.; da Silva, L.; Gomes, A.E.; Trevisan, G.; de Souza, A.L.; Grassi-Kassisse, D.M.; Crege, D. Massage therapy in cortisol circadian rhythm, pain intensity, perceived stress index and quality of life of fibromyalgia syndrome patients. Complement. Ther. Clin. Pract. 2018, 30, 85–90. [Google Scholar] [CrossRef]
- Tomas-Carus, P.; Hakkinen, A.; Gusi, N.; Leal, A.; Hakkinen, K.; Ortega-Alonso, A. Aquatic training and detraining on fitness and quality of life in fibromyalgia. Med. Sci. Sports Exerc. 2007, 39, 1044–1050. [Google Scholar] [CrossRef]
- Gusi, N.; Tomas-Carus, P. Cost-utility of an 8-month aquatic training for women with fibromyalgia: A randomized controlled trial. Arthritis Res. Ther. 2008, 10, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, C.P.; Zamuner, A.R.; Forti, M.; Franca, T.F.; Tamburus, N.Y.; Silva, E. Oxygen uptake and body composition after aquatic physical training in women with fibromyalgia: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 751–758. [Google Scholar]
- Bidonde, J.; Busch, A.J.; Schachter, C.L.; Overend, T.J.; Kim, S.Y.; Goes, S.; Boden, C.; Foulds, H.J.A. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Bidonde, J.; Busch, A.J.; Webber, S.C.; Schachter, C.L.; Danyliw, A.; Overend, T.J.; Richards, R.S.; Rader, T. Aquatic exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2014, 177. [Google Scholar] [CrossRef] [PubMed]
- Marske, C.; Bernard, N.; Palacios, A.; Wheeler, C.; Preiss, B.; Brown, M.; Bhattacharya, S.; Klapstein, G. Fibromyalgia with gabapentin and osteopathic manipulative medicine: A pilot study. J. Altern. Complement. Med. 2018, 24, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.S.; Villela, A.L.; Jones, A.; Natour, J. Effectiveness of dance in patients with fibromyalgia: A randomised, single-blind, controlled study. Clin. Exp. Rheumatol. 2012, 30, S18–S23. [Google Scholar]
- Assunção, J.C.; Silva, H.J.D.; da Silva, J.F.C.; Cruz, R.D.; Lins, C.A.D.; de Souza, M.C. Zumba dancing can improve the pain and functional capacity in women with fibromyalgia. J. Bodyw. Mov. Ther. 2018, 22, 455–459. [Google Scholar] [CrossRef]
- Wang, C.C.; Schmid, C.H.; Fielding, R.A.; Harvey, W.F.; Reid, K.F.; Price, L.L.; Driban, J.B.; Kalish, R.; Rones, R.; McAlindon, T. Effect of tai chi versus aerobic exercise for fibromyalgia: Comparative effectiveness randomized controlled trial. BMJ Br. Med J. 2018, 360, 14. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Galland, L. The gut microbiome and the brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Goebel, A.; Buhner, S.; Schedel, R.; Lochs, H.; Sprotte, G. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology 2008, 47, 1223–1227. [Google Scholar] [CrossRef] [Green Version]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Roman, P.; Estevez, A.F.; Mires, A.; Sanchez-Labraca, N.; Canadas, F.; Vivas, A.B.; Cardona, D. A pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Sci Rep. 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Butler, D. Translational research: Crossing the valley of death. Nature 2008, 453, 840–842. [Google Scholar] [CrossRef]
- Nascimento, S.D.; DeSantana, J.M.; Nampo, F.K.; Ribeiro, E.A.N.; da Silva, D.L.; Araujo, J.X.; Almeida, J.; Bonjardim, L.R.; Araujo, A.A.D.; Quintans, L.J. Efficacy and safety of medicinal plants or related natural products for fibromyalgia: A systematic review. Evid. Based Complementary Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, M.J. A brief-history of opiates, opioid-peptides, and opioid receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 5391–5393. [Google Scholar] [CrossRef] [Green Version]
- Benyhe, S. Morphine—New aspects in the study of an ancient compound. Life Sci. 1994, 55, 969–979. [Google Scholar] [CrossRef]
- Meng, I.D.; Manning, B.H.; Martin, W.J.; Fields, H.L. An analgesia circuit activated by cannabinoids. Nature 1998, 395, 381–383. [Google Scholar] [CrossRef]
- Sagy, I.; Schleider, L.B.L.; Abu-Shakra, M.; Novack, V. Safety and efficacy of medical cannabis in fibromyalgia. J. Clin. Med. 2019, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Van de Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; van Velzen, M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain 2019, 160, 860–869. [Google Scholar] [CrossRef]
- Habib, G.; Artul, S. Medical cannabis for the treatment of fibromyalgia. JCR J. Clin. Rheumatol. 2018, 24, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Avisar, I. The consumption of cannabis by fibromyalgia patients in Israel. Pain Res. Treat. 2018, 5, 7829427. [Google Scholar] [CrossRef] [Green Version]
- Maffei, M.E. Plant natural sources of the endocannabinoid (e)-β-caryophyllene: A systematic quantitative analysis of published literature. Int. J. Mol. Sci. 2020, 21, 6540. [Google Scholar] [CrossRef] [PubMed]
- Paula-Freire, L.I.G.; Andersen, M.L.; Gama, V.S.; Molska, G.R.; Carlini, E.L.A. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine 2014, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, G.L.D.; Machado, K.C.; Machado, K.C.; da Silva, A.; Feitosa, C.M.; Almeida, F.R.D. Non-clinical toxicity of beta-caryophyllene, a dietary cannabinoid: Absence of adverse effects in female swiss mice. Regul. Toxicol. Pharmacol. 2018, 92, 338–346. [Google Scholar] [CrossRef]
- Quintans, L.J.; Araujo, A.A.S.; Brito, R.G.; Santos, P.L.; Quintans, J.S.S.; Menezes, P.P.; Serafini, M.R.; Silva, G.F.; Carvalho, F.M.S.; Brogden, N.K.; et al. Beta-caryophyllene, a dietary cannabinoid, complexed with beta-cyclodextrin produced anti-hyperalgesic effect involving the inhibition of Fos expression in superficial dorsal horn. Life Sci. 2016, 149, 34–41. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Porreca, F.; Lai, J.; Albrecht, P.J.; Rice, F.L.; Khodorova, A.; Davar, G.; Makriyannis, A.; Vanderah, T.W.; Mata, H.P.; et al. Cb2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. USA 2005, 102, 3093–3098. [Google Scholar] [CrossRef] [Green Version]
- Fidyt, K.; Fiedorowicz, A.; Strzadala, L.; Szumny, A. Beta-caryophyllene and beta-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid cb2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Melo, A.J.D.; Heimarth, L.; Carvalho, A.M.D.; Quintans, J.D.S.; Serafini, M.R.; Araujo, A.A.D.; Alves, P.B.; Ribeiro, A.M.; Shanmugam, S.; Quintans, L.J.; et al. Eplingiella fruticosa (Lamiaceae) Essential Oil Complexed with Beta-Cyclodextrin Improves Its Anti-Hyperalgesic Effect in a Chronic Widespread Non-Inflammatory Muscle Pain Animal Model. Food Chem. Toxicol. 2020, 135, 7. [Google Scholar]
- Dolara, P.; Luceri, C.; Ghelardini, C.; Monserrat, C.; Aiolli, S.; Luceri, F.; Lodovici, M.; Menichetti, S.; Romanelli, M.N. Analgesic effects of myrrh. Nature 1996, 379, 29. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, J.K. Myrrh: An analgesic with a 4000-year history. Drug News Perspect. 1996, 9, 554–557. [Google Scholar]
- Su, S.L.; Hua, Y.Q.; Wang, Y.Y.; Gu, W.; Zhou, W.; Duan, J.A.; Jiang, H.F.; Chen, T.; Tang, Y.P. Evaluation of the anti-inflammatory and analgesic properties of individual and combined extracts from Commiphora myrrha, and Boswellia carterii. J. Ethnopharmacol. 2012, 139, 649–656. [Google Scholar] [CrossRef]
- Lee, D.; Ju, M.K.; Kim, H. Commiphora extract mixture ameliorates monosodium iodoacetate-induced osteoarthritis. Nutrients 2020, 12, 17. [Google Scholar] [CrossRef]
- Germano, A.; Occhipinti, A.; Barbero, F.; Maffei, M.E. A pilot study on bioactive constituents and analgesic effects of myrliq®, a Commiphora myrrha extract with a high furanodiene content. Biomed. Res. Int 2017, 2017, 3804356. [Google Scholar] [CrossRef] [Green Version]
- Galeotti, N. Hypericum perforatum (St John’s Wort) beyond Depression: A Therapeutic Perspective for Pain Conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef]
- Khan, H.; Pervaiz, A.; Intagliata, S.; Das, N.; Venkata, K.C.N.; Atanasov, A.G.; Najda, A.; Nabavi, S.M.; Wang, D.D.; Pittala, V.; et al. The analgesic potential of glycosides derived from medicinal plants. DARU 2020, 28, 387–401. [Google Scholar] [CrossRef]
- Wang, R.Y.; Qiu, Z.; Wang, G.Z.; Hu, Q.; Shi, N.H.; Zhang, Z.Q.; Wu, Y.Q.; Zhou, C.H. Quercetin attenuates diabetic neuropathic pain by inhibiting mtor/p70s6k pathway-mediated changes of synaptic morphology and synaptic protein levels in spinal dorsal horn of db/db mice. Eur. J. Pharmacol. 2020, 882, 7. [Google Scholar] [CrossRef]
- Carvalho, T.T.; Mizokami, S.S.; Ferraz, C.R.; Manchope, M.F.; Borghi, S.M.; Fattori, V.; Calixto-Campos, C.; Camilios-Neto, D.; Casagrande, R.; Verri, W.A. The granulopoietic cytokine granulocyte colony-stimulating factor (G-CSF) induces pain: Analgesia by rutin. Inflammopharmacology 2019, 27, 1285–1296. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.Y.; Gui, X.; Chen, L.; Huang, B.K. Natural flavonoids as promising analgesic candidates: A systematic review. Chem. Biodivers. 2016, 13, 1427–1440. [Google Scholar] [CrossRef]
- Yao, X.L.; Li, L.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Attenuation of reserpine-induced fibromyalgia via ros and serotonergic pathway modulation by fisetin, a plant flavonoid polyphenol. Exp. Ther. Med. 2020, 19, 1343–1355. [Google Scholar] [CrossRef] [Green Version]
- Shakiba, M.; Moazen-Zadeh, E.; Noorbala, A.A.; Jafarinia, M.; Divsalar, P.; Kashani, L.; Shahmansouri, N.; Tafakhori, A.; Bayat, H.; Akhondzadeh, S. Saffron (Crocus sativus) versus duloxetine for treatment of patients with fibromyalgia: A randomized double-blind clinical trial. Avicenna J. Phytomedicine 2018, 8, 513–523. [Google Scholar]
- McCarberg, B.H. Clinical overview of fibromyalgia. Am. J. Ther. 2012, 19, 357–368. [Google Scholar] [CrossRef] [PubMed]
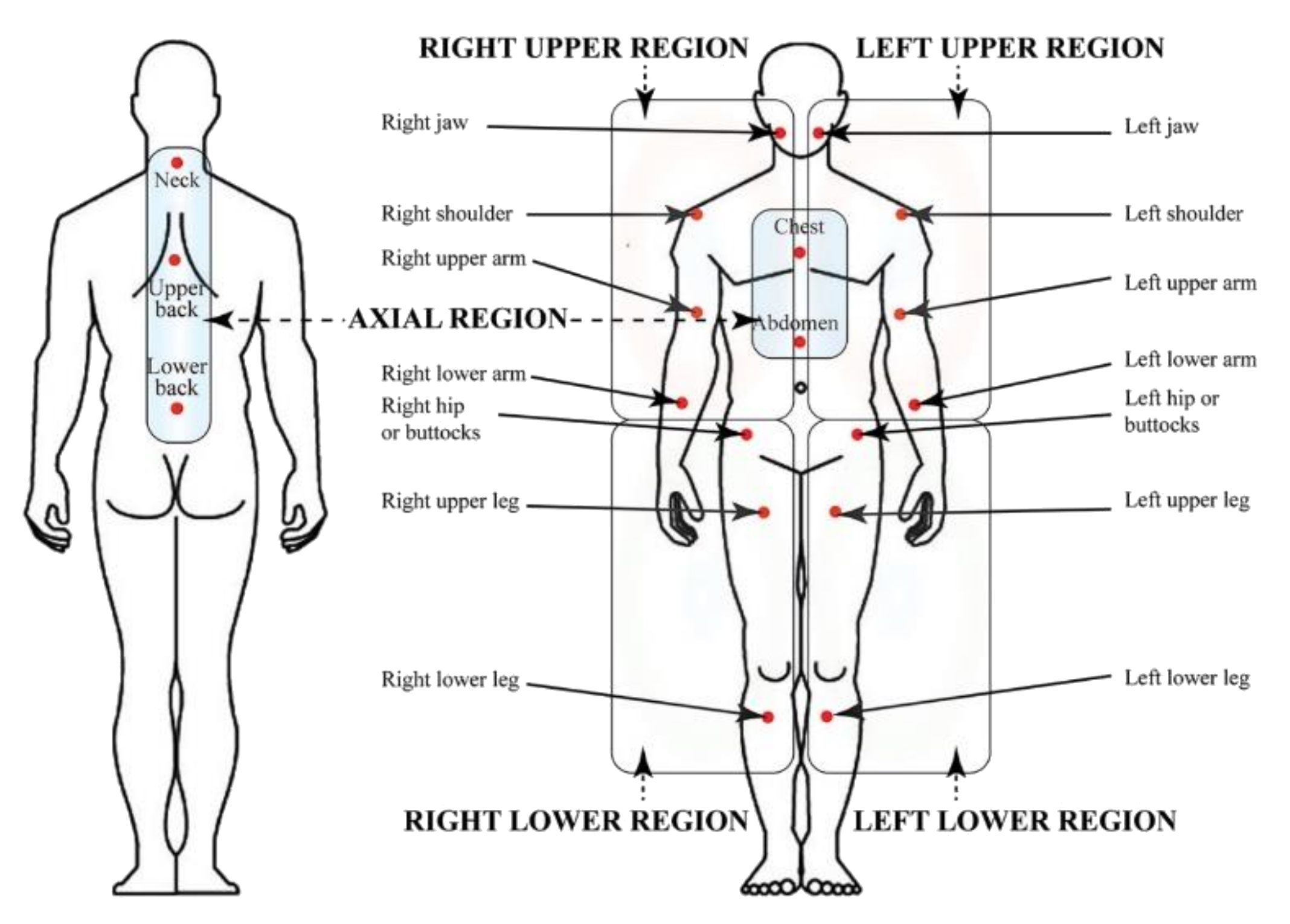
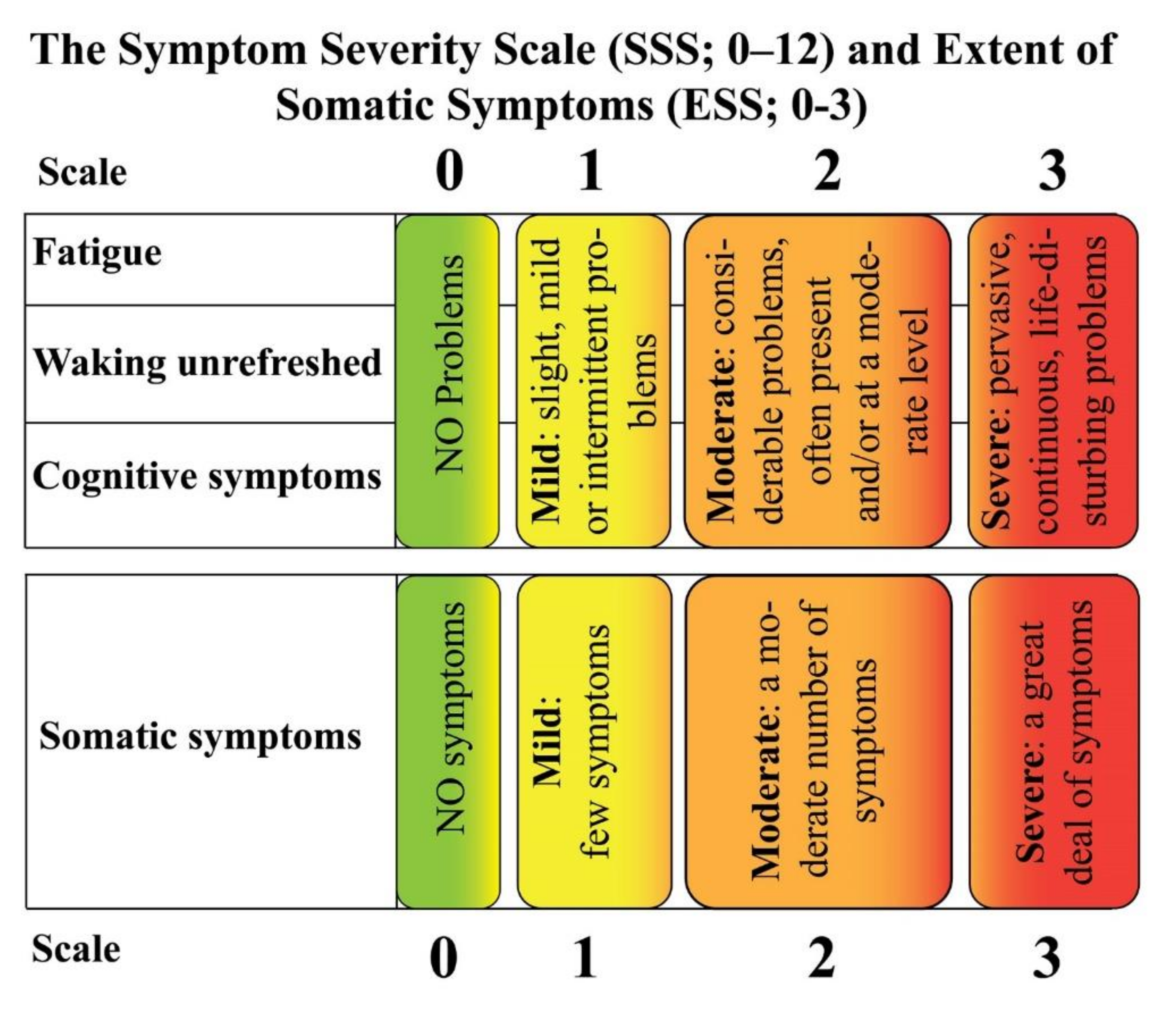
| Widespread pain index (WPI) | |||
| Areas | specification | ||
| Number of areas in which the patient has had pain over the past week | 0–19 points | ||
| Areas to be considered | shoulder girdle, hip (buttock, trochanter), jaw, upper back, lower back, upper arm, upper leg, chest, neck, abdomen, lower arm, and lower leg (all these areas should be considered bilaterally) | ||
| Symptom Severity Scale (SSS) score | |||
| Symptom | Level of severity | Symptom level | Score |
| Fatigue Waking unrefreshed Cognitive symptoms (e.g., working memory capacity, recognition memory, verbal knowledge, anxiety, and depression) | For each of these 3 symptoms, indicate the level of severity over the past week using the following scale: 0 = no problem 1 = slight or mild problems, generally mild or intermittent 2 = moderate; considerable problems, often present and/or at a moderate level 3 = severe; pervasive, continuous, life-disturbing problems | Considering somatic symptoms in general, indicate whether the patient has the following: 0 = no symptoms 1 = few symptoms 2 = a moderate number of symptoms 3 = a great deal of symptoms | Final score between 0 and 12 |
| Criteria | |||
| Specification | Conditions | ||
| A patient satisfies diagnostic criteria for fibromyalgia if the following 3 conditions are met | (a)WPI ≥ 7/19 and SS scale score ≥ 5 or WPI 3–6 and SS scale score ≥ 9 (b) symptoms have been present as a similar level for at least 3 months (c) the patient does not have a disorder that would otherwise explain the pain | ||
| Modified criteria | |||
| Specification | Conditions | Final Score | |
| A patient satisfies diagnostic criteria for fibromyalgia if the following 3 conditions are met | (a)WPI (as above) (b) SS scale score (as above, but without extent of somatic symptoms) (c) presence of abdominal pain, depression, headaches (yes = 1, no = 0) | The number of pain sites (WPI), the SS scale score, and the presence of associated symptoms are summed to give a final score between 0 and 31 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maffei, M.E. Fibromyalgia: Recent Advances in Diagnosis, Classification, Pharmacotherapy and Alternative Remedies. Int. J. Mol. Sci. 2020, 21, 7877. https://doi.org/10.3390/ijms21217877
Maffei ME. Fibromyalgia: Recent Advances in Diagnosis, Classification, Pharmacotherapy and Alternative Remedies. International Journal of Molecular Sciences. 2020; 21(21):7877. https://doi.org/10.3390/ijms21217877
Chicago/Turabian StyleMaffei, Massimo E. 2020. "Fibromyalgia: Recent Advances in Diagnosis, Classification, Pharmacotherapy and Alternative Remedies" International Journal of Molecular Sciences 21, no. 21: 7877. https://doi.org/10.3390/ijms21217877
APA StyleMaffei, M. E. (2020). Fibromyalgia: Recent Advances in Diagnosis, Classification, Pharmacotherapy and Alternative Remedies. International Journal of Molecular Sciences, 21(21), 7877. https://doi.org/10.3390/ijms21217877