Meiotic Nuclear Architecture in Distinct Mole Vole Hybrids with Robertsonian Translocations: Chromosome Chains, Stretched Centromeres, and Distorted Recombination
Abstract
1. Introduction
2. Results
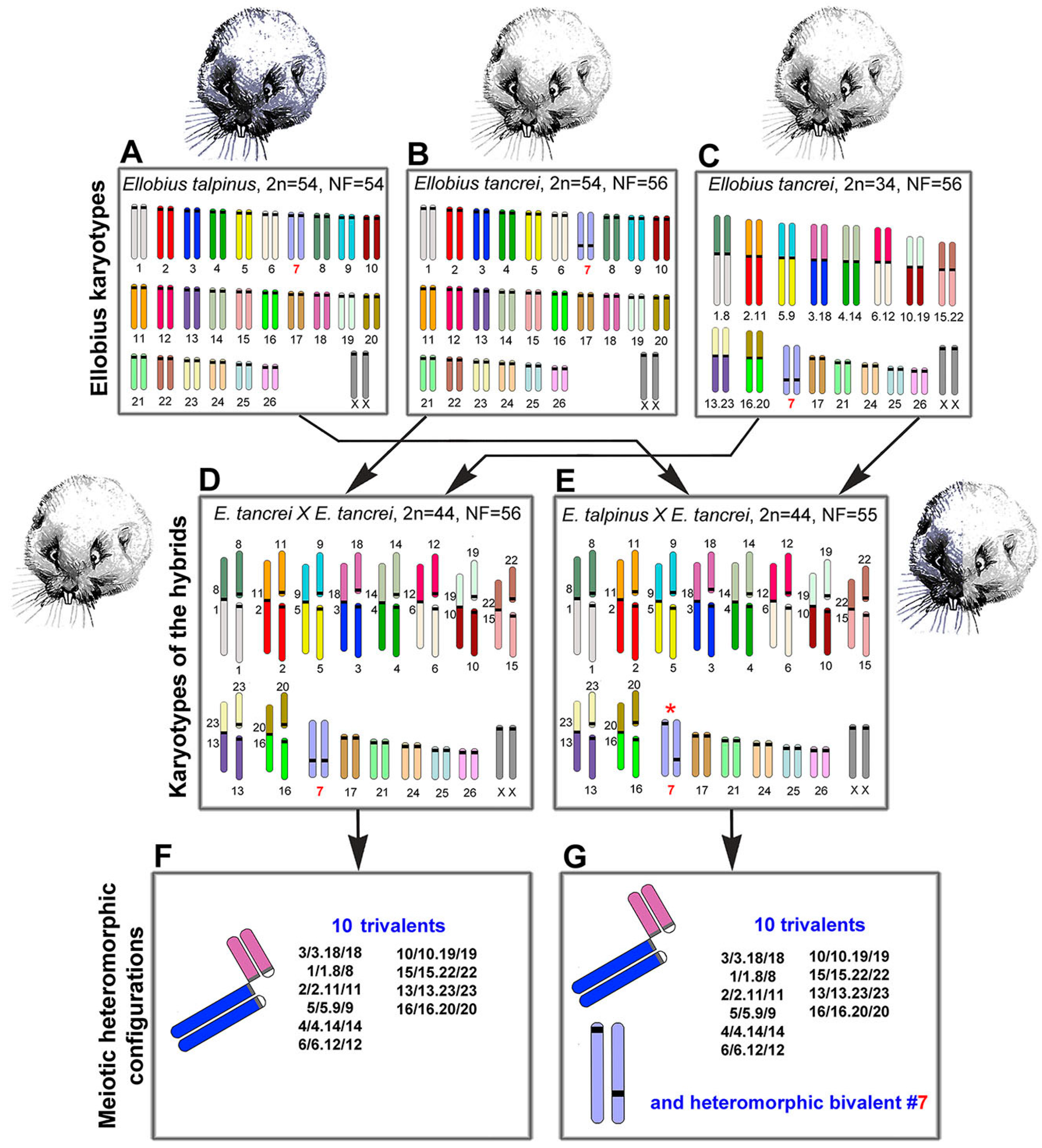
2.1. Experimental Hybridization
2.2. Synaptic Behavior of Chromosomes
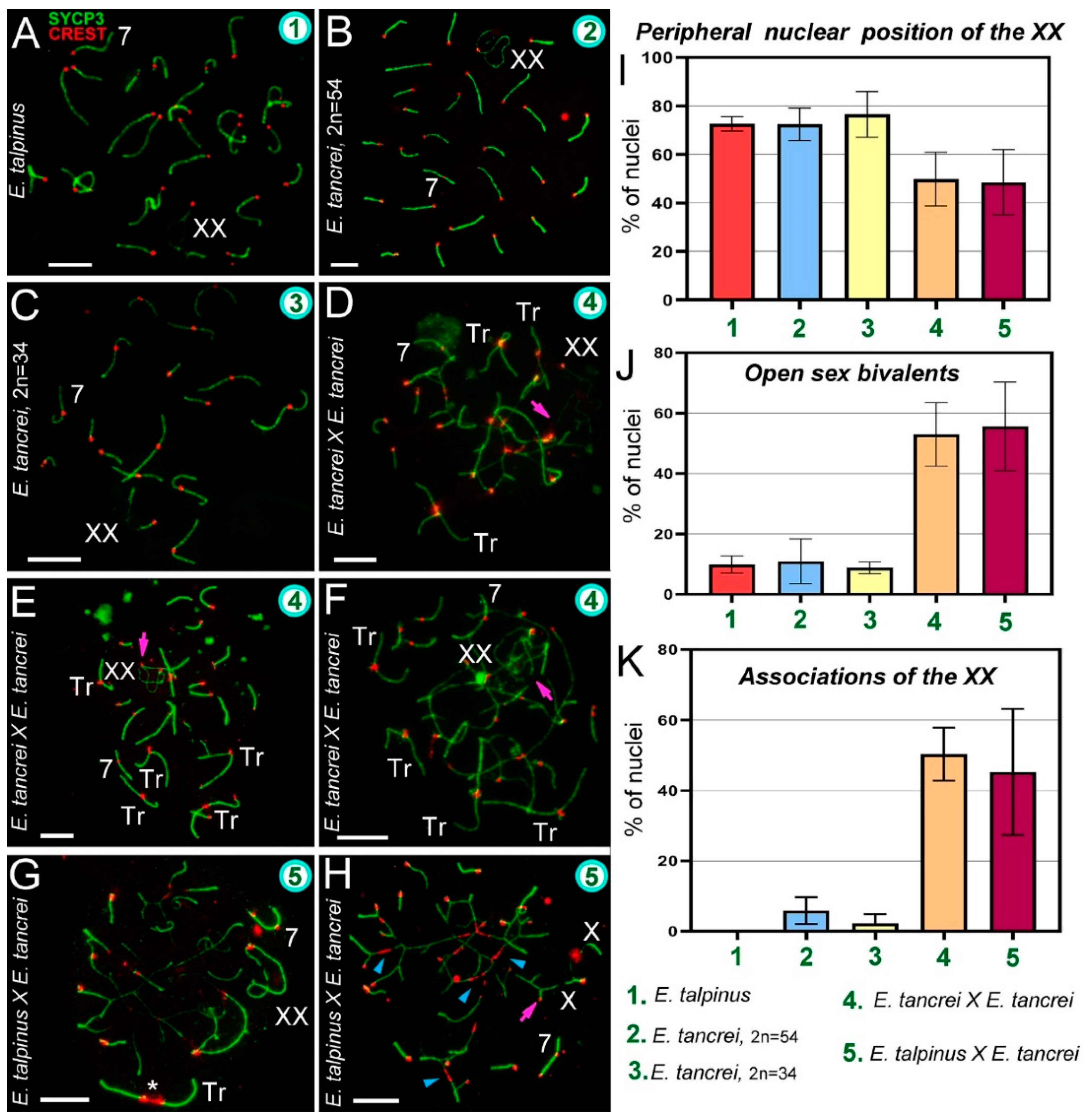
2.3. Synapsis Defects and Associations of the XX and SC Trivalents in Hybrids
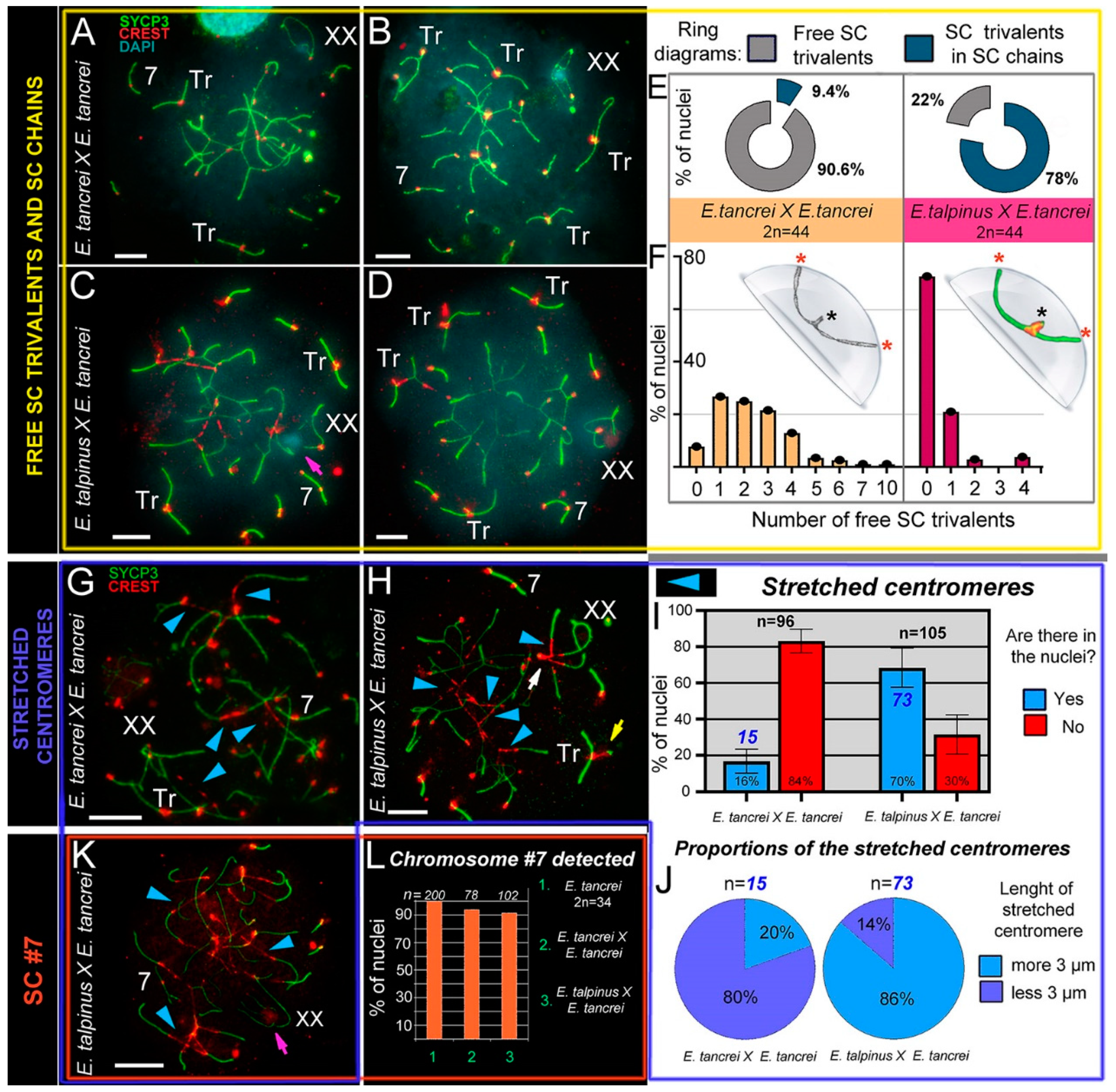
2.4. Free Closed SC Trivalents and Open SC Trivalents in Chains
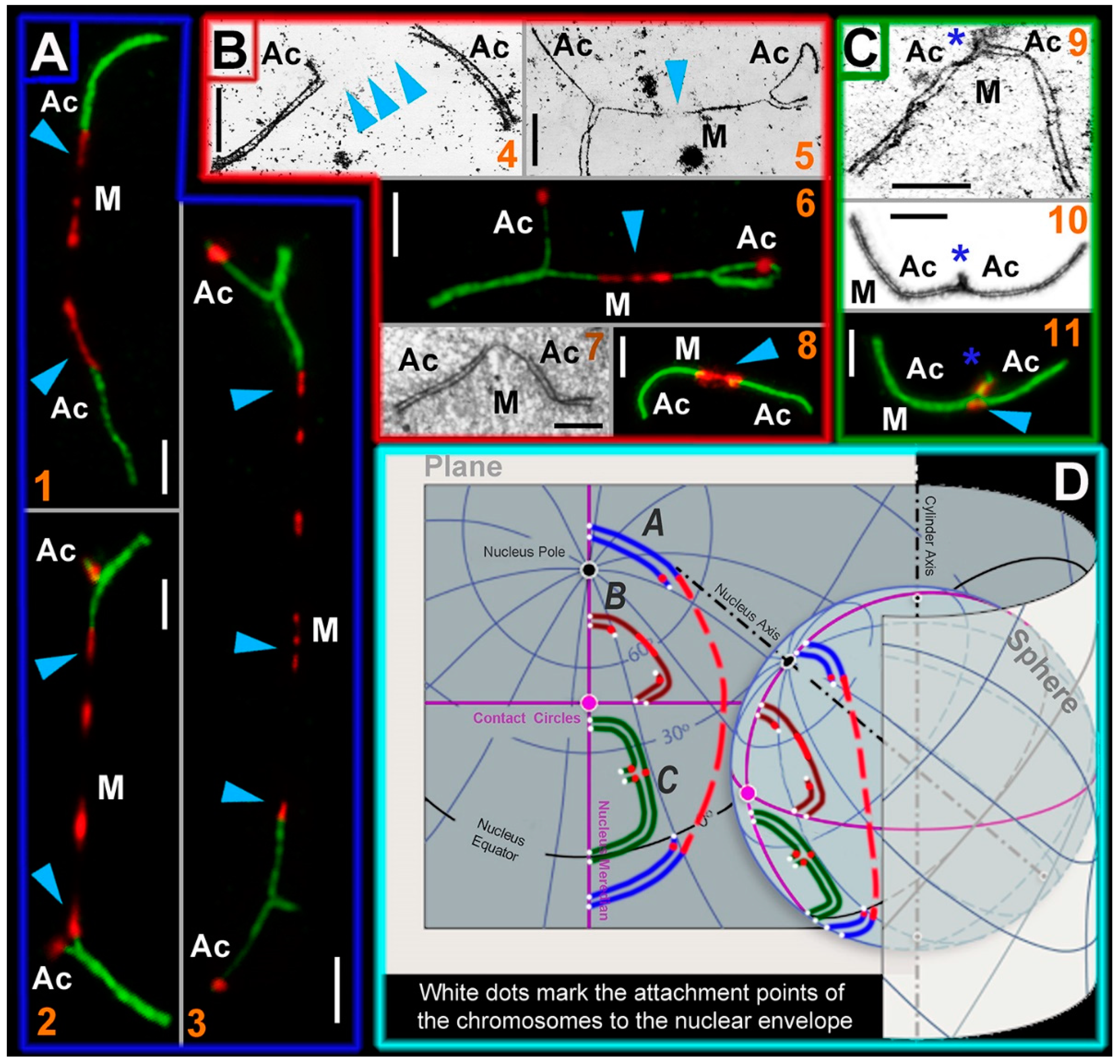
2.5. Stretched Centromeres in SC Trivalents and Their Participation in SC Trivalents Chains
2.6. Bivalent #7 Behavior
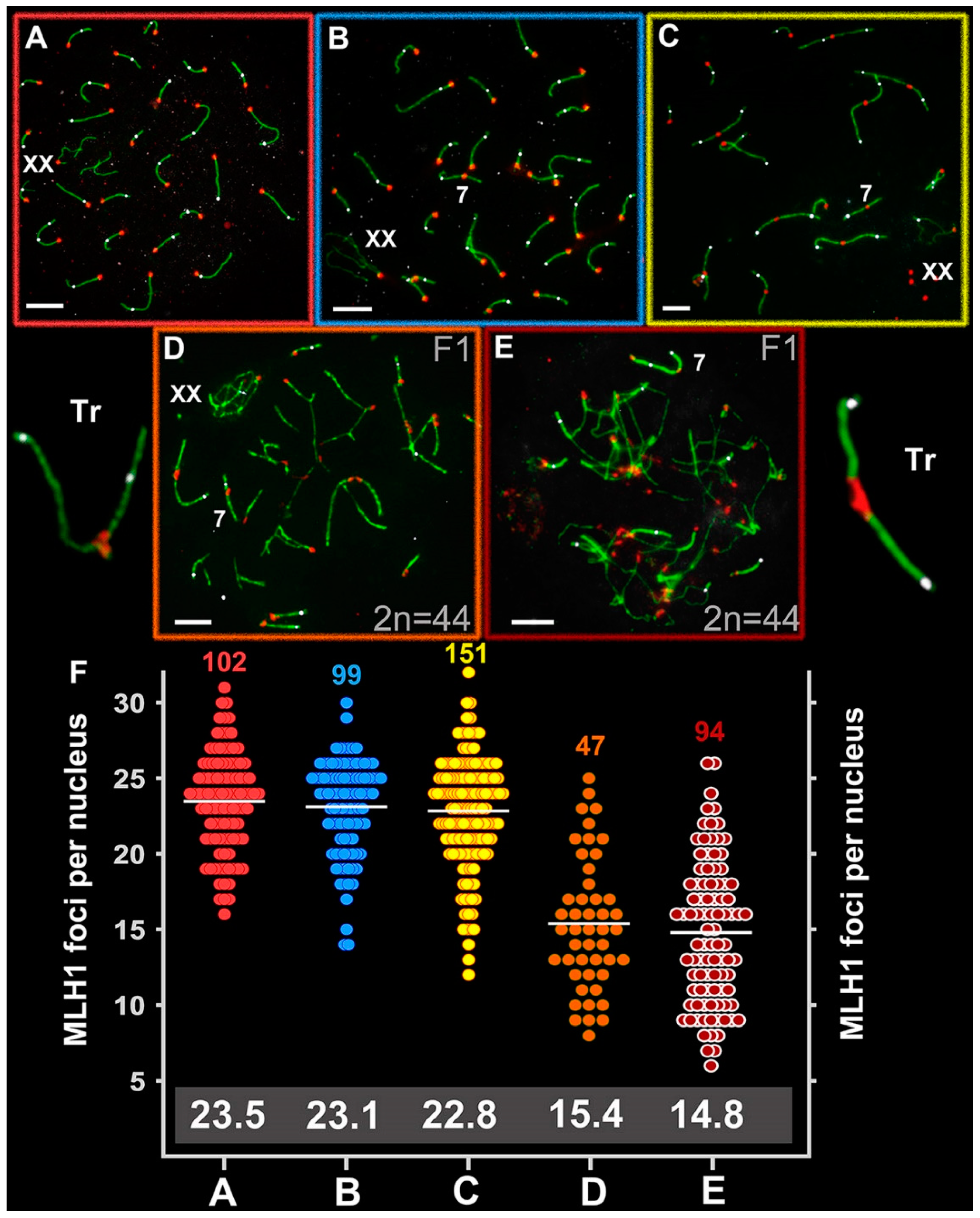
2.7. Chromosome Recombination
3. Discussion
3.1. Chromosome Synapsis Instability and Reduced Recombination in Hybrids
3.2. Centromere Identity and Stretched Centromeres of the SC Trivalents
3.3. Chromosome #7: Implications from the Hybrid Meiotic Nuclei Studies
3.4. Nuclear Architecture: Simulated Chromosome Configurations in Mole Vole Pachytene Spermatocytes
4. Materials and Methods
4.1. Animals
4.2. Ethics Statement
4.3. Spermatocytes Suspensions
4.4. Spermatocytes Spreads
4.5. Spermatocytes Squashes
4.6. Antibodies and Immunocytochemistry
4.7. AgNO3-Staining and Electron Microscopy
4.8. Histological Analysis of Testis Sections
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACA | Anticentromere antibody |
| CENPA | Centromere protein A |
| ChB | Chromatin-dense body, chromatin body |
| CREST | Calcinosis Raynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasia |
| GSI | Gonadosomatic index |
| MLH1 | MutL homolog 1 |
| PBS | Phosphate buffered saline |
| SC | Synaptonemal complex |
| SYCP3 | Synaptonemal complex protein 3 |
| γH2AFX | Phosphorylated H2A histone family, member X |
References
- Dobzhansky, T. Genetics and the Origin of Species; Columbia University Press: New York, NY, USA, 1937; p. 364. [Google Scholar]
- Benirschke, K. Sterility and fertility of interspecific mammalian hybrids. In Comparative Aspects of Reproductive Failure; Benirschke, K., Ed.; Springer: New York, NY, USA, 1967; pp. 218–234. [Google Scholar]
- Basrur, P.K. Hybrid sterility. In Comparative Mammalian Cytogenetics; Benirschke, K., Ed.; Springer: New York, NY, USA, 1969; pp. 107–131. [Google Scholar]
- White, M.J.D. Animal Cytology and Evolution; Cambridge University Press: London, UK; New York, NY, USA; Melbourne, Australia, 1977; p. 696. [Google Scholar]
- Pavlova, S.V.; Searle, J.B. Chromosomes and speciation in mammals. In Mammalian Evolution, Diversity and Systematics; Zahos, F.E., Asher, A.J., Eds.; De Gruyter: Berlin, Germany; Boston, MA, UAS, 2018; pp. 17–38. [Google Scholar] [CrossRef]
- Runemark, A.; Vallejo-Marin, M.; Meier, J.I. Eukaryote hybrid genomes. PLoS Genet. 2019, 15, e1008404. [Google Scholar] [CrossRef]
- Johnson, N.A. Hybrid incompatibility genes: Remnants of a genomic battlefield? Trends Genet. 2010, 26, 317–325. [Google Scholar] [CrossRef]
- Crespi, B.; Nosil, P. Conflictual speciation: Species formation via genomic conflict. Trend Ecol. Evol. 2013, 28, 48–57. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. The significance of responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef]
- Ha, M.; Lu, J.; Tian, L.; Ramachandran, V.; Kasschau, K.D.; Chapman, E.J.; Chen, X.; Wang, X.J.; Chen, Z.J. Small RNAs serve as a genetic buffer against genomic shock in Arabidopsis interspecific hybrids and allopolyploids. Proc. Natl. Acad. Sci. USA 2009, 106, 17835–17840. [Google Scholar] [CrossRef] [PubMed]
- Petrov, D.A.; Schutzman, J.L.; Hartl, D.L.; Lozovskaya, E.R. Diverse transposable elements are mobilized in hybrid dysgenesis in Drosophila virilis. Proc. Natl. Acad. Sci. USA 1995, 92, 8050–8054. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Langdon, T. The plant nucleus at war and peace: Genome organization in the interphase nucleus. In Plant Genome Diversity; Leitch, I.J., Greilhuber, J., Dolezel, J., Wendel, J.F., Eds.; Springer: Vienna, Austria, 2013; Volume 2, pp. 13–31. [Google Scholar]
- Watkins, A.E. Hybrid sterility and incompatibility. J. Genet. 1932, 25, 125–162. [Google Scholar] [CrossRef]
- Muller, H.J. Isolating mechanisms, evolution, and temperature. Biol. Symp. 1942, 6, 71–125. [Google Scholar]
- Hale, D.W.; Washburn, L.; Eicher, E.M. Meiotic abnormalities in hybrid mice of the C57BL/6J × Mus spretus cross suggest a cytogenetic basis for Haldane’s rule of hybrid sterility. Cytogenet. Genome Res. 1993, 63, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Forejt, J. Hybrid sterility in the mouse. Trends Genet. 1996, 12, 412–417. [Google Scholar] [CrossRef]
- Ishishita, S.; Tsuboi, K.; Ohishi, N.; Tsuchiya, K.; Matsuda, Y. Abnormal pairing of X and Y sex chromosomes during meiosis I in interspecific hybrids of Phodopus campbelli and P. sungorus. Sci. Rep. 2015, 5, 9435. [Google Scholar] [CrossRef] [PubMed]
- Haerty, W.; Singh, R.S. Gene regulation divergence is a major contributor to the evolution of Dobzhansky–Muller incompatibilities between species of Drosophila. Mol. Biol. Evol. 2006, 23, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Landry, C.R.; Hartl, D.L.; Ranz, J.M. Genome clashes in hybrids: Insights from gene expression. Heredity 2007, 99, 483–493. [Google Scholar] [CrossRef]
- Brown, J.D.; O’Neill, R.J. Chromosomes, conflict, and epigenetics: Chromosomal speciation revisited. Annu. Rev. Genom. Hum. Genet. 2010, 11, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Johannisson, R.; Winking, H. Synaptonemal complexes of chains and rings in mice heterozygous for multiple Robertsonian translocations. Chromosome Res. 1994, 2, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Johannisson, R.; Winking, H. Pachytene chromosomes in trisomy 19 male mice with Robertsonian translocations. Chromosome Res. 1998, 6, 285–294. [Google Scholar] [CrossRef]
- Sharma, T.; Bardhan, A.; Bahadur, M. Reduced meiotic fitness in hybrids with heterozygosity for heterochromatin in the speciating Mus terricolor complex. J. Biosci. 2003, 28, 189–198. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Gregorova, S.; Mihola, O.; Anger, M.; Sebestova, J.; Denny, P.; Simecek, P.; Forejt, J. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc. Natl. Acad. Sci. USA 2013, 110, E468–E477. [Google Scholar] [CrossRef]
- Forejt, J.; Čapková, J.; Gregorová, S. T (16:17) 43H translocation as a tool in analysis of the proximal part of chromosome 17 (including Tt gene complex) of the mouse. Genet. Res. 1980, 35, 165–177. [Google Scholar] [CrossRef]
- Gill, A.E. Partial reproductive isolation of subspecies of the California vole, Microtus californicus. Genetica 1980, 52, 105–117. [Google Scholar] [CrossRef]
- Tambovtseva, V.G.; Matveevsky, S.N.; Kashintsova, A.A.; Tretiakov, A.V.; Kolomiets, O.L.; Bakloushinskaya, I.Y. A meiotic mystery in experimental hybrids of the eastern mole vole (Ellobius tancrei, Mammalia, Rodentia). Vavilov J. Genet. Breed. 2019, 23, 239–243. [Google Scholar] [CrossRef]
- Matsuda, Y.; Moens, P.B.; Chapman, V.M. Deficiency of X and Y chromosomal pairing at meiotic prophase in spermatocytes of sterile interspecific hybrids between laboratory mice (Mus domesticus) and Mus spretus. Chromosoma 1992, 101, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Matveevsky, S.N.; Malygin, V.M.; Lebedev, V.S.; Poplavskaya, N.S.; Surov, A.V.; Kolomiets, O.L. Sporadic disorders in the meiotic prophase I in Cricetulus barabensis hybrids (Cricetidae, Rodentia) do not lead to reproductive isolation between karyomorphs. Caryologia 2014, 67, 149–154. [Google Scholar] [CrossRef][Green Version]
- Gureeva, A.V.; Feoktistova, N.Y.; Matveevsky, S.N.; Kolomiets, O.L.; Surov, A.V. Speciation of Eversman and Mongolian hamsters (Allocricetulus, Cricetinae): Experimental hybridization. Biol. Bull. 2016, 43, 736–742. [Google Scholar] [CrossRef]
- Ivanitskaya, E.; Rashkovetsky, L.; Nevo, E. Chromosomes in a hybrid zone of Israeli mole rats (Spalax, Rodentia). Russ. J. Genet. 2010, 46, 1149–1151. [Google Scholar] [CrossRef]
- Matveevsky, S.N.; Pavlova, S.V.; Acaeva, M.M.; Kolomiets, O.L. Synaptonemal complex analysis of interracial hybrids between the Moscow and Neroosa chromosomal races of the common shrew Sorex araneus showing regular formation of a complex meiotic configuration (ring-of-four). Comp. Cytogenet. 2012, 6, 301–314. [Google Scholar] [CrossRef]
- Lin, D.; Bi, K.; Conroy, C.J.; Lacey, E.A.; Schraiber, J.G.; Bowie, R.C. Mito-nuclear discordance across a recent contact zone for California voles. Ecol. Evol. 2018, 8, 6226–6241. [Google Scholar] [CrossRef]
- Chang, M.C.; Pickworth, S.; McGaughey, R.W. Experimental hybridization and chromosomes of hybrids. In Comparative Mammalian Cytogenetics; Benirschke, K., Ed.; Springer: New York, NY, USA, 1969; pp. 132–145. [Google Scholar]
- King, M. Species Evolution: The Role of Chromosome Change; Cambridge University Press: New York, NY, USA, 1993; p. 336. [Google Scholar]
- Lebedev, V.; Bogdanov, A.; Brandler, O.; Melnikova, M.; Enkhbat, U.; Tukhbatullin, A.; Abramov, A.; Surov, A.; Bakloushinskaya, I.; Bannikova, A. Cryptic variation in mole voles Ellobius (Arvicolinae, Rodentia) of Mongolia. Zool. Scripta 2020, 49, 535–548. [Google Scholar] [CrossRef]
- Vorontsov, N.N.; Lyapunova, E.A.; Zakarjan, G.G.; Ivanov, V.G. The karyology and taxonomy of the genus Ellobius (Microtinae, Rodentia). In The Mammals: Evolution, Karyology, Faunistics, Systematics; Vorontsov, N.N., Ed.; Nauka: Novosibirsk, Russia, 1969; pp. 127–129. (In Russian) [Google Scholar]
- Lyapunova, E.A.; Ivnitskii, S.B.; Korablev, V.P.; Yanina, I.Y. Complete Robertsonian fan of the chromosomal forms in the mole-vole superspecies Ellobius talpinus. Proc. USSR Acad. Sci. 1984, 274, 1209–1213. (In Russian) [Google Scholar]
- Lyapunova, E.A.; Bakloushinskaya, I.Y.; Saidov, A.S.; Saidov, K.K. Dynamics of chromosome variation in mole voles Ellobius tancrei (Mammalia, Rodentia) in Pamiro-Alay in the period from 1982 to 2008. Russ. J. Genet. 2010, 46, 566–571. [Google Scholar] [CrossRef]
- Bakloushinskaya, I.; Romanenko, S.A.; Serdukova, N.A.; Graphodatsky, A.S.; Lyapunova, E.A. A new form of the mole vole Ellobius tancrei Blasius, 1884 (Mammalia, Rodentia) with the lowest chromosome number. Comp. Cytogenet. 2013, 7, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bakloushinskaya, I.; Lyapunova, E.A.; Saidov, A.S.; Romanenko, S.A.; O’Brien, P.C.; Serdyukova, N.A.; Ferguson-Smith, M.; Matveevsky, S.N.; Bogdanov, A. Rapid chromosomal evolution in enigmatic mammal with XX in both sexes, the Alay mole vole Ellobius alaicus Vorontsov et al., 1969 (Mammalia, Rodentia). Comp. Cytogenet. 2019, 13, 147–177. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, S.A.; Lyapunova, E.A.; Saidov, A.S.; O’Brien, P.; Serdyukova, N.A.; Ferguson-Smith, M.A.; Graphodatsky, A.S.; Bakloushinskaya, I. Chromosome translocations as a driver of diversification in Mole Voles Ellobius (Rodentia, Mammalia). Int. J. Mol. Sci. 2019, 20, 4466. [Google Scholar] [CrossRef]
- Romanenko, S.A.; Sitnikova, N.A.; Serdukova, N.A.; Perelman, P.L.; Rubtsova, N.V.; Bakloushinskaya, I.Y.; Lyapunova, E.A.; Just, W.; Ferguson-Smith, M.A.; Yang, F.; et al. Chromosomal evolution of Arvicolinae (Cricetidae, Rodentia). II. The genome homology of two mole voles (genus Ellobius), the field vole and golden hamster revealed by comparative chromosome painting. Chromosome Res. 2007, 15, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Kolomiets, O.L.; Lyapunova, E.A.; Kalikinskaya, E.I.; Vorontsov, N.N.; Bogdanov, Y.F. Karyotyping of synaptonemal complexes of wild and hybrid forms of mole voles Ellobius, carrying Robertsonian rearrangements. In Proceedings of the Materials of the 5th All-Union Symposium “Molecular mechanisms of genetic processes”, Moscow, Russia, 26–29 December 1983; Nauka Press: Moscow, Russia, 1983; p. 31. (In Russian). [Google Scholar]
- Kolomiets, O.L.; Lyapunova, E.A.; Mazurova, T.F.; Yanina, I.Y.; Bogdanov, Y.F. Varying ways of formation of synaptonemal complex trivalent in heterozygote hybrids by Robertson’s translocation. In Molecular Mechanisms of Genetic Processes. Molecular Genetics, Evolution and Molecular-Genetic Bases of Selection; Nauka Press: Moscow, Russia, 1985; pp. 72–84. (In Russian) [Google Scholar]
- Kolomiets, O.L.; Lyapunova, E.A.; Mazurova, T.F.; Yanina, I.Y.; Bogdanov, Y.F. Participation of heterochromatin in formation of synaptonemal complex chains in animals heterozygous for multiple Robertsonian translocation. Russ. J. Genet. 1986, 22, 273–283. (In Russian) [Google Scholar]
- Lyapunova, E.A.; Yakimenko, L.V. Genetics of Ellobius (Ellobius Rodentia). IV. Decrease in the fertility of hybrids between the forms of Ellobius talpinus subspecies with different chromosome numbers. Genetika 1985, 21, 1960–1968. (In Russian) [Google Scholar]
- Lyapunova, E.A.; Baklushinskaya, I.Y.; Kolomiets, O.L.; Mazurova, T.F. Analysis of fertility of hybrids of multi-chromosomal forms in mole-voles of the super-species Ellobius tancrei differing in a single pair of Robertsonian metacentrics. Proc. USSR Acad. Sci. 1990, 310, 26–29. (In Russian) [Google Scholar]
- Bakloushinskaya, I.Y.; Romanenko, S.A.; Graphodatsky, A.S.; Matveevsky, S.N.; Lyapunova, E.A.; Kolomiets, O.L. The role of chromosome rearrangements in the evolution of mole voles of the genus Ellobius (Rodentia, Mammalia). Russ. J. Genet. 2010, 46, 1143–1145. [Google Scholar] [CrossRef]
- Bakloushinskaya, I.Y.; Matveevsky, S.N.; Romanenko, S.A.; Serdukova, N.A.; Kolomiets, O.L.; Spangenberg, V.E.; Lyapunova, E.A.; Graphodatsky, A.S. A comparative analysis of the mole vole sibling species Ellobius tancrei and E. talpinus (Cricetidae, Rodentia) through chromosome painting and examination of synaptonemal complex structures in hybrids. Cytogenet. Genome Res. 2012, 136, 199–207. [Google Scholar] [CrossRef]
- Matveevsky, S.; Bakloushinskaya, I.; Tambovtseva, V.; Romanenko, S.; Kolomiets, O. Analysis of meiotic chromosome structure and behavior in Robertsonian heterozygotes of Ellobius tancrei (Rodentia, Cricetidae): A case of monobrachial homology. Comp. Cytogenet. 2015, 9, 691. [Google Scholar] [CrossRef]
- Matveevsky, S.; Kolomiets, O.; Bogdanov, A.; Hakhverdyan, M.; Bakloushinskaya, I. Chromosomal evolution in mole voles Ellobius (Cricetidae, Rodentia): Bizarre sex chromosomes, variable autosomes and meiosis. Genes 2017, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, Y.F.; Kolomiets, O.L.; Lyapunova, E.A.; Yanina, I.Y.; Mazurova, T.F. Synaptonemal complexes and chromosome chains in the rodent Ellobius talpinus heterozygous for ten Robertsonian translocations. Chromosoma 1986, 94, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, A.O.; Oke, B.O.; Akinloye, A.K. Characterizing the gonadosomatic index and its relationship with age in greater cane rat (Thryonomys swinderianus, Temminck). J. Vet. Anat. 2009, 2, 53–59. [Google Scholar] [CrossRef]
- Pochron, S.T.; Wright, P.C.; Schaentzler, E.; Ippolito, M.; Rakotonirina, G.; Ratsimbazafy, R.; Rakotosoa, R. Effect of season and age on the gonadosomatic index of Milne-Edwards’ sifakas (Propithecus diadema edwardsi) in Ranomafana National Park, Madagascar. Int. J. Primatol. 2002, 23, 355–364. [Google Scholar] [CrossRef]
- Gomendio, M.; Martin-Coello, J.; Crespo, C.; Magaña, C.; Roldan, E.R. Sperm competition enhances functional capacity of mammalian spermatozoa. Proc. Natl. Acad. Sci. USA 2006, 103, 15113–15117. [Google Scholar] [CrossRef] [PubMed]
- De Boer, P.D.; de Jong, J.H. Chromosome pairing and fertility in mice. In Fertility and Chromosome Pairing: Recent Studies in Plants and Animals; Gillies, C.B., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1989; pp. 37–76. [Google Scholar]
- Kolomiets, O.L.; Vorontsov, N.N.; Lyapunova, E.A.; Mazurova, T.F. Ultrastructure, meiotic behavior, and evolution of sex chromosomes of the genus Ellobius. Genetica 1991, 84, 179–189. [Google Scholar] [CrossRef]
- Matveevsky, S.; Bakloushinskaya, I.; Kolomiets, O. Unique sex chromosome systems in Ellobius: How do male XX chromosomes recombine and undergo pachytene chromatin inactivation? Sci. Rep. 2016, 6, 29949. [Google Scholar] [CrossRef] [PubMed]
- Matveevsky, S.N. Neocentromeres in the structure of the non-Robertsonian submetacentric chromosome pairs of Ellobius tancrei. In Proceedings of the Materials of the XVIII International Conference “Lomonosov-2011” for Students, Graduate Students and Young Scientists (Genetics Subsection), Moscow, Russia, 11–15 April 2011; Andreev, A.I., Andriyanov, A.V., Antipov, E.A., Chistyakova, M.V., Eds.; p. 94. (In Russian). [Google Scholar]
- Matveevsky, S.N. Signs of Sexual Dimorphism in Meiosis and Karyotype Variability of Mole Vole Ellobius (Rodentia, Mammalia). Ph.D. Thesis, NI Vavilov Institute of General Genetics of Russian Academy of Science, Moscow, Russia, 2011. Supervisor Dr. O.L. Kolomiets. pp. 1–172. (In Russian). [Google Scholar]
- Segura, J.; Ferretti, L.; Ramos-Onsins, S.; Capilla, L.; Farré, M.; Reis, F.; Oliver-Bonet, M.; Fernández-Bellón, H.; Garcia, F.; Garcia-Caldés, M.; et al. Evolution of recombination in eutherian mammals: Insights into mechanisms that affect recombination rates and crossover interference. Proc. Biol. Sci. 2013, 280, 20131945. [Google Scholar] [CrossRef]
- Arnold, M.L. Natural Hybridization and Evolution; Oxford University Press: New York, NY, USA, 1993; p. 214. [Google Scholar]
- Abbott, R.J.; Barton, N.H.; Good, J.M. Genomics of hybridization and its evolutionary consequences. Mol. Ecol. 2016, 25, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.J.; Karatsis, P.A.; Hamilton, A.E. Synaptonemal complex analysis of heteromorphic trivalents in lemur hybrids. Chromosoma 1979, 70, 141–160. [Google Scholar] [CrossRef]
- Ratomponirina, C.; Brun, B.; Rumpler, Y. Synaptonemal complexes in Robertsonian translocation heterozygous in lemurs. In Kew Chromosome Conference 3; Brandham, P.E., Ed.; HMSO: London, UK, 1988; pp. 65–73. [Google Scholar]
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World: A Taxonomic and Geographic Reference; JHU Press: Baltimore, MA, USA, 2005; Volume 1. [Google Scholar]
- Schwitzer, C.; Mittermeier, R.A.; Louis, E.E., Jr.; Richardson, M. Family Lemuridae (Bamboo, True and Ruffed Lemurs). In Handbook of the Mammals of the World. Volume 3: Primates; Mittermeier, R.A., Wilson, D.E., Eds.; Lynx Edicions: Barcelona, Spain, 2013; pp. 90–141. [Google Scholar]
- Hauffe, H.C.; Searle, J.B. Chromosomal heterozygosity and fertility in house mice (Mus musculus domesticus) from Northern Italy. Genetics 1998, 150, 1143–1154. [Google Scholar]
- Grao, P.; Coll, M.D.; Ponsa, M.; Egozcue, J. Trivalent behavior during prophase I in male mice heterozygous for three Robertsonian translocations: An electron-microscopic study. Cytogenet. Genome Res. 1989, 52, 105–110. [Google Scholar] [CrossRef]
- Wallace, B.M.N.; Searle, J.B.; Everett, C.A. The effect of multiple simple Robertsonian heterozygosity on chromosome pairing and fertility of wild-stock house mice (Mus musculus domesticus). Cytogenet. Cell Genet. 2002, 96, 276–286. [Google Scholar] [CrossRef]
- Manterola, M.; Page, J.; Vasco, C.; Berríos, S.; Parra, M.T.; Viera, A.; Rufas, J.S.; Zuccotti, M.; Garagna, S.; Fernández-Donoso, R. A high incidence of meiotic silencing of unsynapsed chromatin is not associated with substantial pachytene loss in heterozygous male mice carrying multiple simple Robertsonian translocations. PLoS Genet. 2009, 5, e1000625. [Google Scholar] [CrossRef]
- Borodin, P.M.; Rogatcheva, M.B.; Zhelezova, A.I.; Oda, S.I. Chromosome pairing in inter-racial hybrids of the house musk shrew (Suncus murinus, Insectivora, Soricidae). Genome 1998, 41, 79–90. [Google Scholar] [CrossRef]
- Rogatcheva, M.B.; Aulchenko, Y.S.; Oda, S.I.; Zhelezova, A.I.; Serova, I.A.; Axenovich, T.I.; Borodin, P.M. Chromosomal and genic mechanisms of reproductive isolation: The case of Suncus murinus. Acta Theriol. 2000, 45, 147–160. [Google Scholar] [CrossRef]
- Rieseberg, L.H. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 2001, 16, 351–358. [Google Scholar] [CrossRef]
- Livingstone, K.; Rieseberg, L. Chromosomal evolution and speciation: A recombination-based approach. New Phytol. 2004, 161, 107–112. [Google Scholar] [CrossRef]
- Cattanach, B.M.; Moseley, H. Nondisjunction and reduced fertility caused by the tobacco mouse metacentric chromosomes. Cytogenet. Genome Res. 1973, 12, 264–287. [Google Scholar] [CrossRef]
- Davisson, M.T.; Akeson, E.C. Recombination suppression by heterozygous Robertsonian chromosomes in the mouse. Genetics 1993, 133, 649–667. [Google Scholar]
- Baker, S.M.; Plug, A.W.; Prolla, T.A.; Bronner, C.E.; Harris, A.C.; Yao, X.; Christie, D.M.; Monell, C.; Arnheim, N.; Bradley, N.; et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 1996, 13, 336–342. [Google Scholar] [CrossRef]
- Eaker, S.; Pyle, A.; Cobb, J.; Handel, M.A. Evidence for meiotic spindle checkpoint from analysis of spermatocytes from Robertsonian-chromosome heterozygous mice. J. Cell Sci. 2001, 114, 2953–2965. [Google Scholar] [PubMed]
- Eaker, S.; Cobb, J.; Pyle, A.; Handel, M.A. Meiotic prophase abnormalities and metaphase cell death in MLH1-deficient mouse spermatocytes: Insights into regulation of spermatogenic progress. Dev. Biol. 2002, 249, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.A.; Cohen, P.E. Not all germ cells are created equal: Aspects of sexual dimorphism in mammalian meiosis. Reproduction 2005, 130, 761–781. [Google Scholar] [CrossRef]
- Cheeseman, I.M. The kinetochore. Cold Spring Harb. Perspect. Biol. 2014, 6, a015826. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Henikoff, S. What makes a centromere? Exp. Cell Res. 2020, 389, 111895. [Google Scholar] [CrossRef]
- Henikoff, S.; Ahmad, K.; Malik, H.S. The centromere paradox: Stable inheritance with rapidly evolving DNA. Science 2001, 293, 1098–1102. [Google Scholar] [CrossRef]
- Garagna, S.; Zuccotti, M.; Capanna, E.; Redi, C.A. High-resolution organization of mouse telomeric and pericentromeric DNA. Cytogenet. Genome Res. 2002, 96, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Plohl, M.; Luchetti, A.; Meštrović, N.; Mantovani, B. Satellite DNAs between selfishness and functionality: Structure, genomics and evolution of tandem repeats in centromeric (hetero) chromatin. Gene 2008, 409, 72–82. [Google Scholar] [CrossRef]
- Ferreri, G.C.; Brown, J.D.; Obergfell, C.; Jue, N.; Finn, C.E.; O’Neill, M.J.; O’Neill, R.J. Recent amplification of the kangaroo endogenous retrovirus, KERV, limited to the centromere. J. Virol. 2011, 85, 4761–4771. [Google Scholar] [CrossRef]
- Chang, C.H.; Chavan, A.; Palladino, J.; Wei, X.; Martins, N.M.; Santinello, B. Islands of retroelements are major components of Drosophila centromeres. PLoS Biol. 2019, 17, e3000241. [Google Scholar] [CrossRef] [PubMed]
- Amor, D.J.; Choo, K.A. Neocentromeres: Role in human disease, evolution, and centromere study. Am. J. Hum. Genet. 2002, 71, 695–714. [Google Scholar] [CrossRef]
- Dalal, Y. Epigenetic specification of centromeres. Biochem. Cell Biol. 2009, 87, 273–282. [Google Scholar] [CrossRef]
- Bernad, R.; Sánchez, P.; Losada, A. Epigenetic specification of centromeres by CENP-A. Exp. Cell Res. 2009, 315, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, A.; Desai, A. A molecular view of kinetochore assembly and function. Biology 2017, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Berríos, S.; Manieu, C.; López-Fenner, J.; Ayarza, E.; Page, J.; González, M.; Manterola, M.; Fernández-Donoso, R. Robertsonian chromosomes and the nuclear architecture of mouse meiotic prophase spermatocytes. Biol. Res. 2014, 47, 16. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.J.; Elgin, S.C. Epigenetic codes for heterochromatin formation and silencing: Rounding up the usual suspects. Cell 2002, 108, 489–500. [Google Scholar] [CrossRef]
- Nishibuchi, G.; Déjardin, J. The molecular basis of the organization of repetitive DNA-containing constitutive heterochromatin in mammals. Chromosome Res. 2017, 25, 77–87. [Google Scholar] [CrossRef]
- Ribeiro, S.A.; Vagnarelli, P.; Dong, Y.; Hori, T.; McEwen, B.F.; Fukagawa, T.; Flors, C.; Earnshaw, W.C. A super-resolution map of the vertebrate kinetochore. Proc. Natl. Acad. Sci. USA 2010, 107, 10484–10489. [Google Scholar] [CrossRef]
- Gil-Fernández, A.; Saunders, P.; Martín-Ruiz, M.; Ribagorda, M.; López-Jiménez, P.; Jeffries, D.L.; Parra, M.T.; Viera, A.; Rufas, J.S.; Perrin, N.; et al. Meiosis reveals the early steps in the evolution of a neo-XY sex chromosome pair in the African pygmy mouse Mus minutoides. bioRxiv 2020, 177329. [Google Scholar] [CrossRef]
- Mestrović, N.; Plohl, M.; Mravinac, B.; Ugarković, D. Evolution of satellite DNAs from the genus Palorus-experimental evidence for the” library” hypothesis. Mol. Biol. Evol. 1998, 15, 1062–1068. [Google Scholar] [CrossRef]
- Lee, H.R.; Zhang, W.; Langdon, T.; Jin, W.; Yan, H.; Cheng, Z.; Jiang, J. Chromatin immunoprecipitation cloning reveals rapid evolutionary patterns of centromeric DNA in Oryza species. Proc. Natl. Acad. Sci. USA 2005, 102, 11793–11798. [Google Scholar] [CrossRef]
- Talbert, P.B.; Kasinathan, S.; Henikoff, S. Simple and complex centromeric satellites in Drosophila sibling species. Genetics 2018, 208, 977–990. [Google Scholar] [CrossRef]
- Salser, W.; Bowen, S.; Browne, D.; El-Adli, F.; Fedoroff, N.; Fry, K. Investigation of the organization of mammalian chromosomes at the DNA sequence level. Fed. Proc. 1976, 35, 23–35. [Google Scholar]
- Miklos, G.L.G. Localized highly repetitive DNA sequences in vertebrate and invertebrate genomes. In Molecular Evolutionary Genetics; MacIntyre, R.J., Ed.; Plenum Press: New York, NY, USA, 1985; pp. 241–321. [Google Scholar]
- Smalec, B.M.; Heider, T.N.; Flynn, B.L.; O’Neill, R.J. A centromere satellite concomitant with extensive karyotypic diversity across the Peromyscus genus defies predictions of molecular drive. Chromosome Res. 2019, 27, 237–252. [Google Scholar] [CrossRef]
- O’Neill, R.J.W.; O’Neill, M.J.; Graves, J.A.M. Undermethylation associated with retroelement activation and chromosome remodelling in an interspecific mammalian hybrid. Nature 1998, 393, 68–72. [Google Scholar] [CrossRef]
- Metcalfe, C.J.; Bulazel, K.V.; Ferreri, G.C.; Schroeder-Reiter, E.; Wanner, G.; Rens, W.; Obergfell, C.; Eldridge, M.D.B.; O’Neill, R.J. Genomic instability within centromeres of interspecific marsupial hybrids. Genetics 2007, 177, 2507–2517. [Google Scholar] [CrossRef]
- Brown, J.D.; Mitchell, S.E.; O’Neill, R.J. Making a long story short: Noncoding RNAs and chromosome change. Heredity 2012, 108, 42–49. [Google Scholar] [CrossRef]
- Lyapunova, E.A.; Vorontsov, N.N. Genetics of Ellobius (Rodentia). I. Karyological characteristics of four Ellobius species. Russ. J. Genet. 1978, 14, 2012–2024. (In Russian) [Google Scholar]
- Matveevsky, S.; Kolomiets, O.; Bogdanov, A.; Alpeeva, E.; Bakloushinskaya, I. Meiotic chromosome contacts as a plausible prelude for Robertsonian translocations. Genes 2020, 11, 386. [Google Scholar] [CrossRef]
- Cremer, T.; Kurz, A.; Zirbel, R.; Dietzel, S.; Rinke, B.; Schröck, E.; Speicher, M.R.; Mathieu, U.; Jauch, A.; Emmerich, P.; et al. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb. Symp. Quant. Biol. 1993, 58, 777–792. [Google Scholar] [CrossRef]
- Parada, L.A.; Misteli, T. Chromosome positioning in the interphase nucleus. Trend Cell Biol. 2002, 12, 425–432. [Google Scholar] [CrossRef]
- Foster, H.A.; Abeydeera, L.R.; Griffin, D.K.; Bridger, J.M. Non-random chromosome positioning in mammalian sperm nuclei, with migration of the sex chromosomes during late spermatogenesis. J. Cell Sci. 2005, 118, 1811–1820. [Google Scholar] [CrossRef]
- Cremer, T.; Cremer, M. Chromosome territories. Cold Spring Harb. Perspect. Biol. 2010, 2, a003889. [Google Scholar] [CrossRef]
- Cremer, T.; Cremer, M.; Dietzel, S.; Müller, S.; Solovei, I.; Fakan, S. Chromosome territories–a functional nuclear landscape. Curr. Opin. Cell Biol. 2006, 18, 307–316. [Google Scholar] [CrossRef]
- Cremer, T.; Cremer, C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2001, 2, 292–301. [Google Scholar] [CrossRef]
- Misteli, T. Chromosome territories: The arrangement of chromosomes in the nucleus. Nat. Educ. 2008, 1, 167. [Google Scholar]
- Tanabe, H.; Habermann, F.A.; Solovei, I.; Cremer, M.; Cremer, T. Non-random radial arrangements of interphase chromosome territories: Evolutionary considerations and functional implications. Mutat. Res. 2002, 504, 37–45. [Google Scholar] [CrossRef]
- Mora, L.; Ponsà, M.; Garcia, M. Chromosomal territories in evolutionary rearranged primate Chromosomes. In Primate Cytogenetics and Comparative Genomics; Sineo, L., Stanyon, R., Eds.; Firenze University Press: Firenze, Italy, 2006; pp. 77–85. [Google Scholar]
- Politz, J.C.R.; Scalzo, D.; Groudine, M. The redundancy of the mammalian heterochromatic compartment. Curr. Opin. Genet. Dev. 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Garagna, S.; Zuccotti, M.; Thornhill, A.; Fernandez-Donoso, R.; Berrios, S.; Capanna, E.; Redi, C.A. Alteration of nuclear architecture in male germ cells of chromosomally derived subfertile mice. J. Cell Sci. 2001, 114, 4429–4434. [Google Scholar]
- Oberdoerffer, P.; Sinclair, D.A. The role of nuclear architecture in genomic instability and ageing. Nat. Rev. Mol. Cell Biol. 2007, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Lammerding, J. Mechanics of the nucleus. Compr. Physiol. 2011, 1, 783–807. [Google Scholar] [CrossRef]
- Fournier, A.; McLeer-Florin, A.; Lefebvre, C.; Duley, S.; Barki, L.; Ribeyron, J.; Alboukadel, K.; Hamaidia, S.; Granjon, A.; Gressin, R.; et al. 1q12 chromosome translocations form aberrant heterochromatic foci associated with changes in nuclear architecture and gene expression in B cell lymphoma. EMBO Mol. Med. 2010, 2, 159–171. [Google Scholar] [CrossRef]
- Berríos, S.; Fernández-Donoso, R.; Page, J.; Ayarza, E.; Capanna, E.; Solano, E.; Castiglia, R. Hexavalents in spermatocytes of Robertsonian heterozygotes between Mus m. domesticus 2n = 26 from the Vulcano and Lipari Islands (Aeolian Archipelago, Italy). Eur. J. Histochem. 2018, 62. [Google Scholar] [CrossRef]
- Abdelhedi, F.; Chalas, C.; Petit, J.M.; Abid, N.; Mokadem, E.; Hizem, S.; Kamoun, H.; Keskes, L.; Dupont, J. Altered three-dimensional organization of sperm genome in DPY19L2-deficient globozoospermic patients. J. Assist. Reprod. Genet. 2019, 36, 69–77. [Google Scholar] [CrossRef]
- Rosin, L.F.; Crocker, O.; Isenhart, R.L.; Nguyen, S.C.; Xu, Z.; Joyce, E.F. Chromosome territory formation attenuates the translocation potential of cells. eLife 2019, 8, e49553. [Google Scholar] [CrossRef]
- Alsheimer, M.; von Glasenapp, E.; Hock, R.; Benavente, R. Architecture of the nuclear periphery of rat pachytene spermatocytes: Distribution of nuclear envelope proteins in relation to synaptonemal complex attachment sites. Mol. Biol. Cell 1999, 10, 1235–1245. [Google Scholar] [CrossRef]
- Hernández-Hernández, A.; Vázquez-Nin, G.H.; Echeverría, O.M.; Recillas-Targa, F. Chromatin structure contribution to the synaptonemal complex formation. Cell. Mol. Life Sci. 2009, 66, 1198–1208. [Google Scholar] [CrossRef]
- Alsheimer, M. The dance floor of meiosis: Evolutionary conservation of nuclear envelope attachment and dynamics of meiotic telomeres. In Meiosis. Genome Dynamics; Benavente, R., Wolf, J.N., Eds.; Karger Publishers: Basel, Switzerland, 2009; pp. 81–93. [Google Scholar] [CrossRef]
- Schimenti, J. Synapsis or silence. Nat. Genet. 2005, 37, 11–13. [Google Scholar] [CrossRef]
- Turner, J.M.; Mahadevaiah, S.K.; Fernandez-Capetillo, O.; Nussenzweig, A.; Xu, X.; Deng, C.X.; Burgoyne, P.S. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 2005, 37, 41–47. [Google Scholar] [CrossRef]
- Berrios, S.; Ayarza, E.; Moreno, M.; Paulos, A.; Fernández-Donoso, R. Non-random distribution of the pericentromeric heterochromatin in meiotic prophase nuclei of mammalian spermatocytes. Genetica 1999, 106, 187–195. [Google Scholar] [CrossRef]
- Berríos, S.; Fernández-Donoso, R.; Pincheira, J.; Page, J.; Manterola, M.; Cerda, M.C. Number and nuclear localisation of nucleoli in mammalian spermatocytes. Genetica 2004, 121, 219–228. [Google Scholar] [CrossRef]
- Berríos, S. Nuclear architecture of mouse spermatocytes: Chromosome topology, heterochromatin, and nucleolus. Cytogenet. Genome Res. 2017, 151, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ribagorda, M.; Berríos, S.; Solano, E.; Ayarza, E.; Martín-Ruiz, M.; Gil-Fernández, A.; Parra, M.T.; Viera, A.; Rufas, J.S.; Capanna, E.; et al. Meiotic behavior of a complex hexavalent in heterozygous mice for Robertsonian translocations: Insights for synapsis dynamics. Chromosoma 2019, 128, 149–163. [Google Scholar] [CrossRef]
- Matveevsky, S.N.; Kolomiets, O.L. Types of synaptonemal complex chains in heterozygotes for multiple Robertsonian translocations. Factors Exp. Evol. Org. 2011, 10, 42–47. (In Russian) [Google Scholar]
- Matveevsky, S.N.; Kolomiets, O.L. Synaptonemal complex configurations in Robertsonian heterozygotes. Tsitologia 2016, 58, 309–314. [Google Scholar]
- Turner, J.M. Meiotic sex chromosome inactivation. Development 2007, 134, 1823–1831. [Google Scholar] [CrossRef]
- Burgoyne, P.S.; Mahadevaiah, S.K.; Turner, J.M. The consequences of asynapsis for mammalian meiosis. Nat. Rev. Genet. 2009, 10, 207–216. [Google Scholar] [CrossRef]
- Homolka, D.; Ivanek, R.; Capkova, J.; Jansa, P.; Forejt, J. Chromosomal rearrangement interferes with meiotic X chromosome inactivation. Genome Res. 2007, 17, 1431–1437. [Google Scholar] [CrossRef]
- Berríos, S.; Manterola, M.; Prieto, Z.; López-Fenner, J.; Page, J.; Fernández-Donoso, R. Model of chromosome associations in Mus domesticus spermatocytes. Biol. Res. 2010, 43, 275–285. [Google Scholar] [CrossRef]
- Kolomiets, O.; Matveevsky, S.; Bakloushinskaya, I. Sexual dimorphism in prophase I of meiosis in the Northern mole vole (Ellobius talpinus Pallas, 1770) with isomorphic (XX) chromosomes in males and females. Comp. Cytogenet. 2010, 455. [Google Scholar] [CrossRef]
- Page, J.; Suja, J.A.; Santos, J.L.; Rufas, J.S. Squash procedure for protein immunolocalization in meiotic cells. Chromosome Res. 1998, 6, 639–642. [Google Scholar] [CrossRef]
- De Boer, P. Chromosomal causes for fertility reduction in mammals. In Chemical Mutagens; De Serres, F.G., Ed.; Springer: Boston, MA, USA, 1986; pp. 427–467. [Google Scholar]
- Miklos, G.L.G. Sex-chromosome pairing and male fertility. Cytogenet. Cell Genet. 1974, 13, 558–577. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, X.; Wang, K.; Gent, J.I.; Zhang, W.; Dawe, R.K.; Jiang, J. Gene expression and chromatin modifications associated with maize centromeres. G3 Genes Genomes Genet. 2016, 6, 183–192. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveevsky, S.; Tretiakov, A.; Kashintsova, A.; Bakloushinskaya, I.; Kolomiets, O. Meiotic Nuclear Architecture in Distinct Mole Vole Hybrids with Robertsonian Translocations: Chromosome Chains, Stretched Centromeres, and Distorted Recombination. Int. J. Mol. Sci. 2020, 21, 7630. https://doi.org/10.3390/ijms21207630
Matveevsky S, Tretiakov A, Kashintsova A, Bakloushinskaya I, Kolomiets O. Meiotic Nuclear Architecture in Distinct Mole Vole Hybrids with Robertsonian Translocations: Chromosome Chains, Stretched Centromeres, and Distorted Recombination. International Journal of Molecular Sciences. 2020; 21(20):7630. https://doi.org/10.3390/ijms21207630
Chicago/Turabian StyleMatveevsky, Sergey, Artemii Tretiakov, Anna Kashintsova, Irina Bakloushinskaya, and Oxana Kolomiets. 2020. "Meiotic Nuclear Architecture in Distinct Mole Vole Hybrids with Robertsonian Translocations: Chromosome Chains, Stretched Centromeres, and Distorted Recombination" International Journal of Molecular Sciences 21, no. 20: 7630. https://doi.org/10.3390/ijms21207630
APA StyleMatveevsky, S., Tretiakov, A., Kashintsova, A., Bakloushinskaya, I., & Kolomiets, O. (2020). Meiotic Nuclear Architecture in Distinct Mole Vole Hybrids with Robertsonian Translocations: Chromosome Chains, Stretched Centromeres, and Distorted Recombination. International Journal of Molecular Sciences, 21(20), 7630. https://doi.org/10.3390/ijms21207630




