Varying Atmospheric CO2 Mediates the Cold-Induced CBF-Dependent Signaling Pathway and Freezing Tolerance in Arabidopsis
Abstract
1. Introduction
2. Results
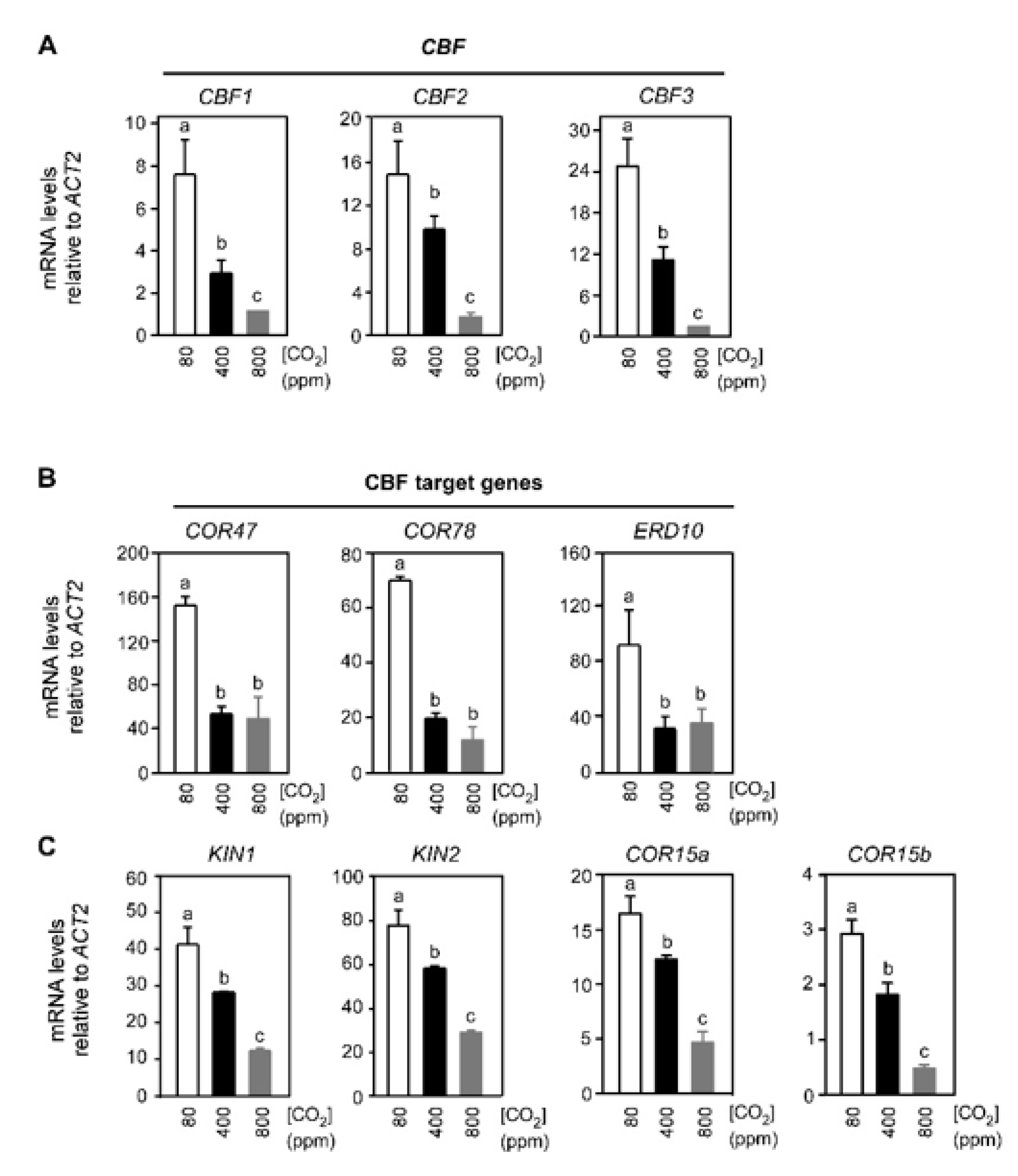
2.1. Varying Atmospheric CO2 Modulates the CBF-Mediated Cold Signaling Pathway
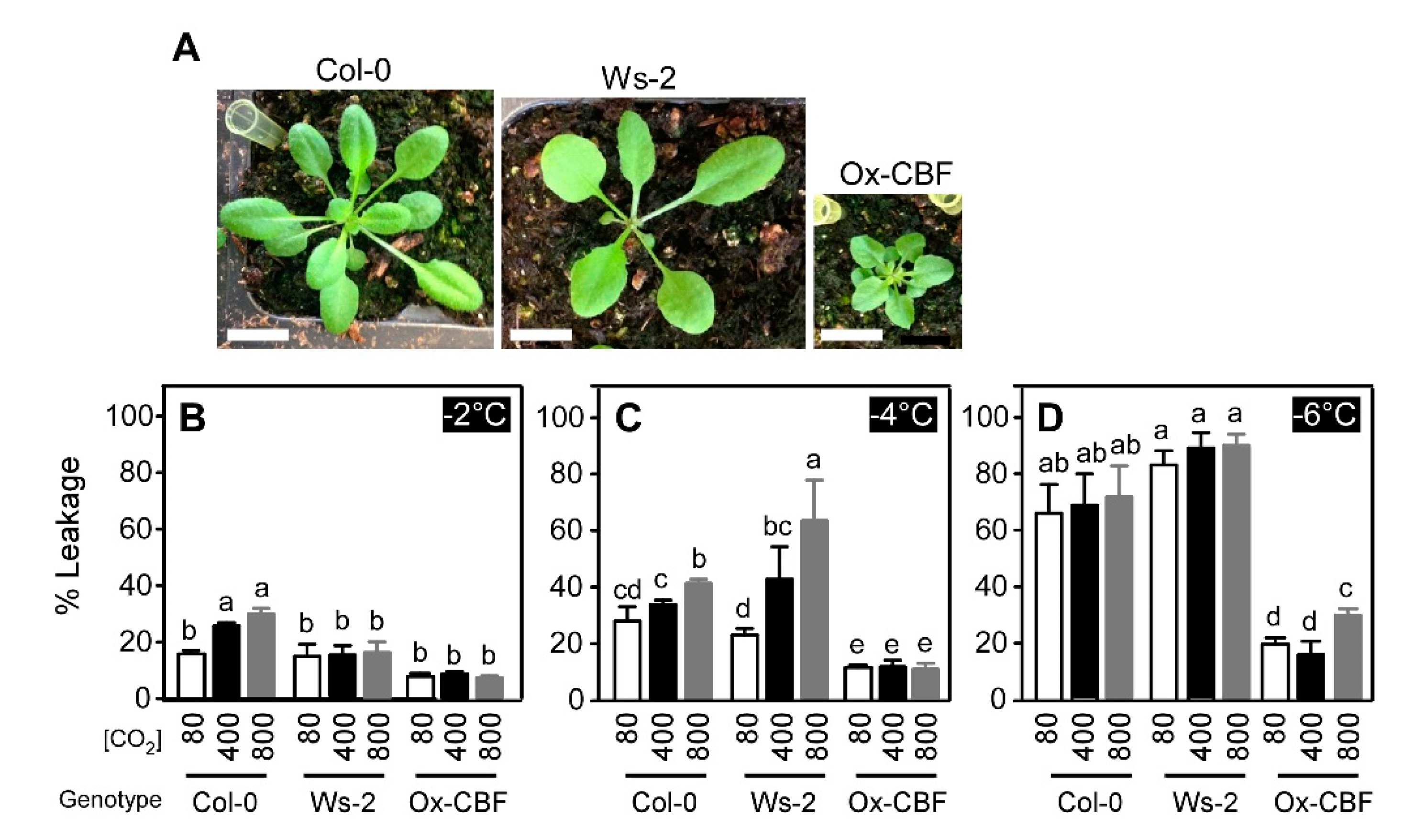
2.2. Varying Atmospheric CO2 Modifies the Freezing Tolerance of Arabidopsis
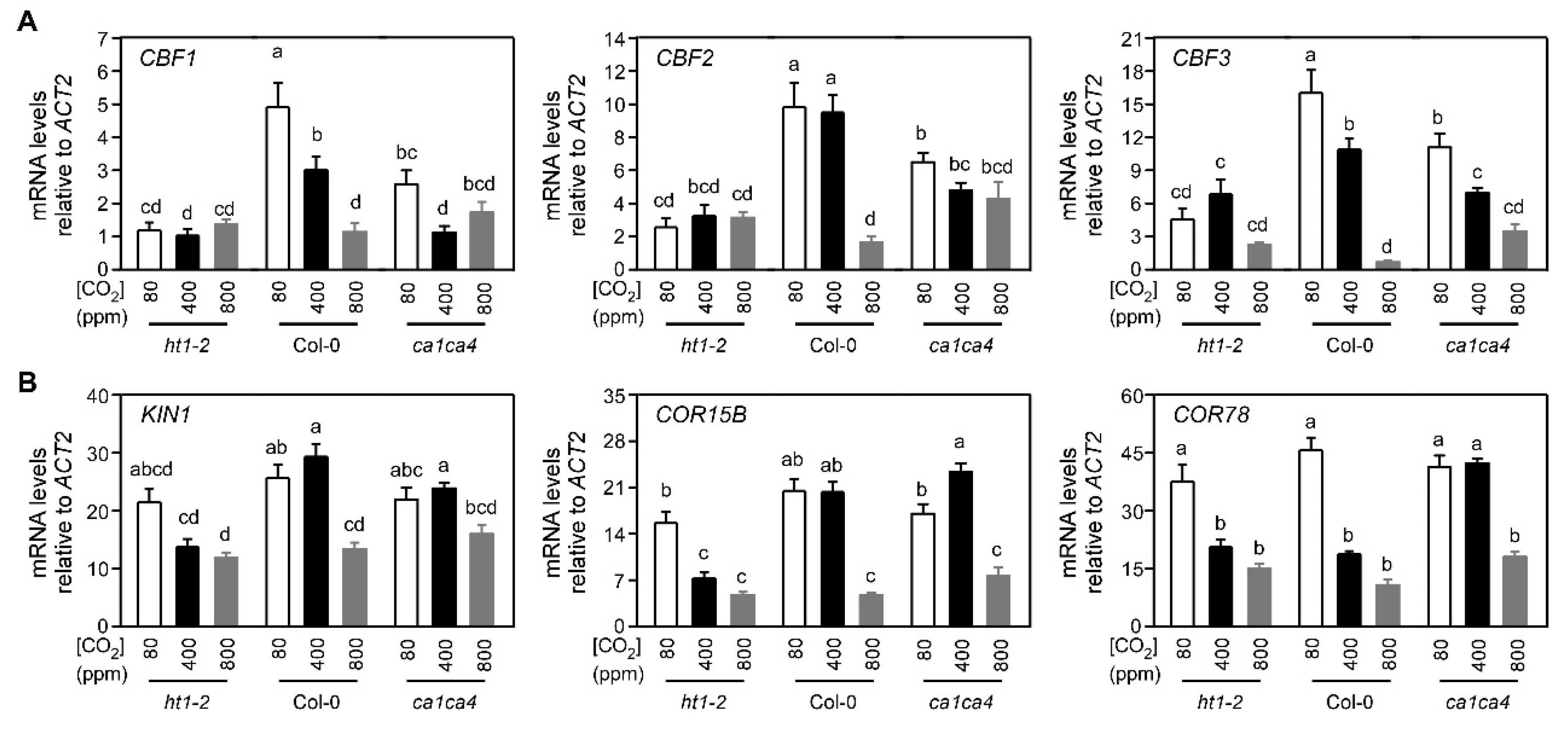
2.3. Arabidopsis Mutants Defective in Stomatal Responses to CO2 Exhibit Altered CBF-Mediated Cold Signaling
2.4. Defects in Stomatal Responses to CO2 Alter Freezing Tolerance in Arabidopsis
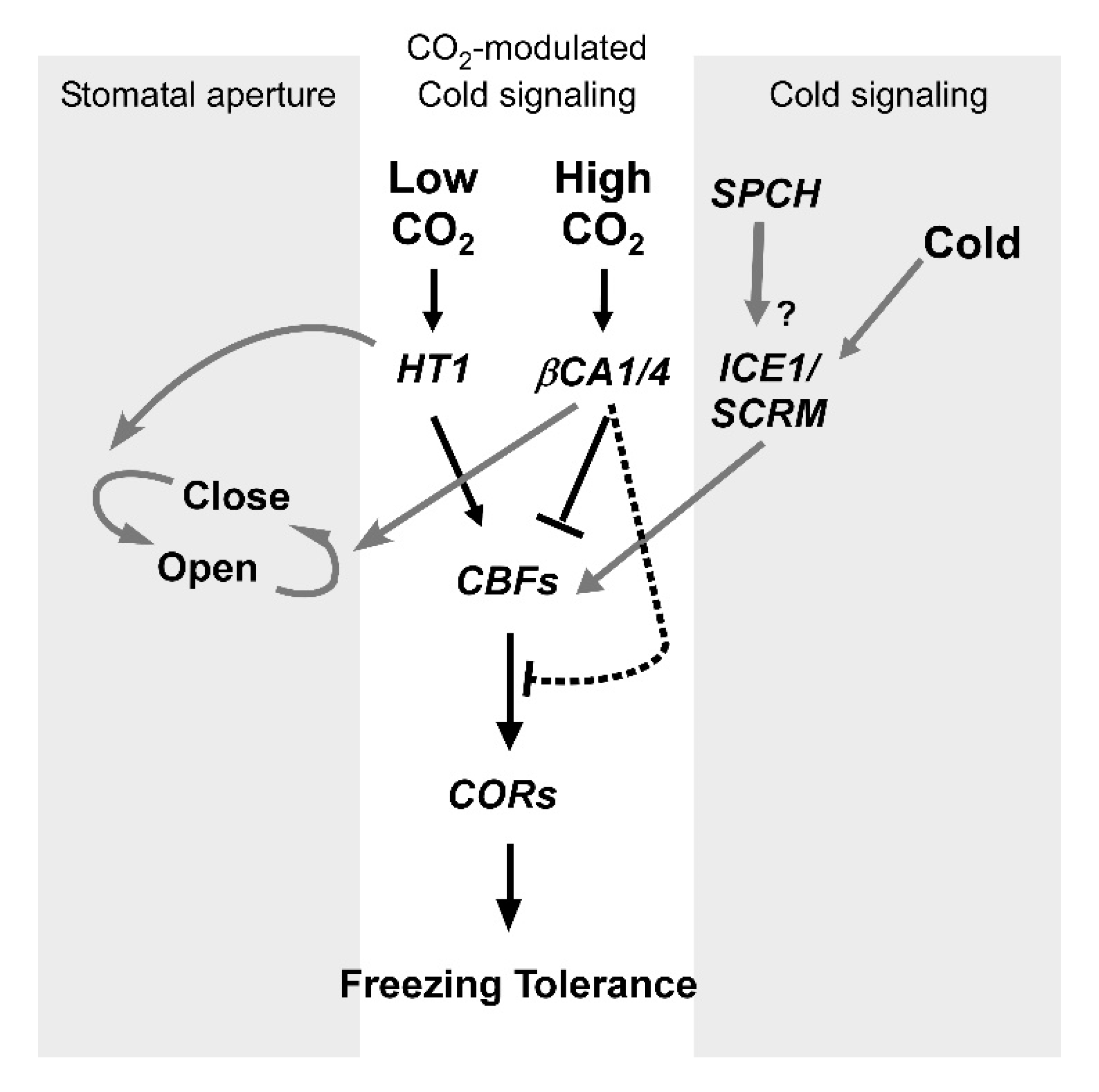
2.5. Relationship between CO2 and Cold Signaling in Stomatal Movement
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Growth Conditions
5.2. Low Temperature and CO2 Treatments
5.3. Transcript Analysis
5.4. Electrolyte Leakage Freeze Test
5.5. Stomatal Conductance Determinations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ht1-2 | high leaf temperature 1-2 |
| ca1ca4 | β-carbonic anhydrase 1 and 4 |
| CRT/DRE | C-repeat/dehydration response element |
| COR | Cold-responsive |
| ERD | Early dehydration inducible |
| RD | Responsive to dehydration |
| KIN | Cold-inducible |
| ICE1 | Inducer of CBF Expression 1 |
| SCRM | SCREAM |
| SPCH | SPEECHLESS |
| ACT2 | Actin 2 |
| gs | Stomatal conductance |
| ATHB2 | Arabidopsis thaliana homeobox 2 |
| CHS | Chalcone synthase |
| LEA | late embryogenesis abundant |
References
- Körner, C. Plant adaptation to cold climates. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF–COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.J.; Zarka, D.G.; Stockinger, E.J.; Salazar, M.P.; Houghton, J.M.; Thomashow, M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998, 16, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Bargues, M.; Terol, J.; Perez-Alonso, M.; Salinas, J. The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol. 1999, 119, 463–470. [Google Scholar] [CrossRef]
- Baker, S.S.; Wilhelm, K.S.; Thomashow, M.F. The 5’-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol. Biol. 1994, 24, 701–713. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994, 6, 251–264. [Google Scholar] [CrossRef]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef]
- Thomashow, M.F. So what’s new in the field of plant cold acclimation? Lots! Plant Physiol. 2001, 125, 89–93. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant cold acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.K. Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, J.; Sun, T.; Wang, X.; Zhu, C.; Ai, Y.; Gu, H. The precise regulation of different COR genes by individual CBF transcription factors in Arabidopsis thaliana. J. Integr. Plant Biol. 2017, 59, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.; Thomashow, M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell. 2002, 14, 1675–1690. [Google Scholar] [CrossRef]
- Leuendorf, J.E.; Frank, M.; Schmülling, T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Cold Signal Transduction and its Interplay with Phytohormones During Cold Acclimation. Plant Cell Physiol. 2014, 56, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Leyva, A.; Jarillo, J.A.; Salinas, J.; Martinez-Zapater, J.M. Low Temperature Induces the Accumulation of Phenylalanine Ammonia-Lyase and Chalcone Synthase mRNAs of Arabidopsis thaliana in a Light-Dependent Manner. Plant Physiol. 1995, 108, 39–46. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef]
- Thomashow, M.F. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998, 118, 1–7. [Google Scholar] [CrossRef]
- Xiong, L.M.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002, 14, S165–S183. [Google Scholar] [CrossRef]
- Qi, X.; Torii, K.U. Hormonal and environmental signals guiding stomatal development. BMC Biol. 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 Response of Stomata and Its Dependence on Environmental Factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.T.; Pallardy, S.G. Effects of Low-Temperature on Leaf Diffusion Resistance of Ulmus-Americana and Fraxinus-Pennsylvanica Seedlings. Can. J. Bot. 1979, 57, 2466–2470. [Google Scholar] [CrossRef]
- Wilson, J.M. Mechanism of Chill-Hardening and Drought-Hardening of Phaseolus-Vulgaris Leaves. New Phytol. 1976, 76, 257–270. [Google Scholar] [CrossRef]
- Wilkinson, S.; Clephan, A.L.; Davies, W.J. Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol. 2001, 126, 1566–1578. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.H.; Hong, X.; Agarwal, M.; Zhu, J.K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhao, L.; Ren, Y.; Yang, S.; Zhu, J.K.; Zhao, C. The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J. Integr. Plant Biol. 2020, 62, 258–263. [Google Scholar] [CrossRef]
- Kanaoka, M.M.; Pillitteri, L.J.; Fujii, H.; Yoshida, Y.; Bogenschutz, N.L.; Takabayashi, J.; Zhu, J.K.; Torii, K.U. SCREAM/ICE1 and SCREAM2 Specify Three Cell-State Transitional Steps Leading to Arabidopsis Stomatal Differentiation. Plant Cell 2008, 20, 1775–1785. [Google Scholar] [CrossRef]
- Lau, O.S.; Davies, K.A.; Chang, J.; Adrian, J.; Rowe, M.H.; Ballenger, C.E.; Bergmann, D.C. Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells. Science 2014. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Dong, J. Stomatal development in Arabidopsis. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0162. [Google Scholar] [CrossRef]
- Lau, O.S.; Bergmann, D.C. Stomatal development: A plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development 2012, 139, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Putarjunan, A.; Ruble, J.; Srivastava, A.; Zhao, C.; Rychel, A.L.; Hofstetter, A.K.; Tang, X.; Zhu, J.K.; Tama, F.; Zheng, N.; et al. Bipartite anchoring of SCREAM enforces stomatal initiation by coupling MAP kinases to SPEECHLESS. Nat. Plants 2019, 5, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F.; Torii, K.U. SCREAMing Twist on the Role of ICE1 in Freezing Tolerance. Plant Cell 2020, 32, 816–819. [Google Scholar] [CrossRef]
- Engineer, C.B.; Ghassemian, M.; Anderson, J.C.; Peck, S.C.; Hu, H.; Schroeder, J.I. Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 2014, 513, 246–250. [Google Scholar] [CrossRef]
- Hu, H.H.; Boisson-Dernier, A.; Israelsson-Nordstrom, M.; Bohmer, M.; Xue, S.W.; Ries, A.; Godoski, J.; Kuhn, J.M.; Schroeder, J.I. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 2010, 12, 87–93. [Google Scholar] [CrossRef]
- Hashimoto, M.; Negi, J.; Young, J.; Israelsson, M.; Schroeder, J.I.; Iba, K. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol. 2006, 8, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.J.; Sebolt, A.M.; Salazar, M.P.; Everard, J.D.; Thomashow, M.F. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2000, 124, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Steponkus, P.L.; Uemura, M.; Webb, M.S. Advances in Low Temperature Biology; JAI: London, UK, 1993; Volume 2, pp. 211–312. [Google Scholar]
- Guy, C.; Haskell, D.; Neven, L.; Klein, P.; Smelser, C. Hydration-state-responsive proteins link cold and drought stress in spinach. Planta 1992, 188, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Cox, W. Interrelations between Environmental Factors and Freezing Resistance of Cabbage Leaves. Plant Physiol. 1976, 57, 553–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siminovitch, D.; Cloutier, Y. Drought and freezing tolerance and adaptation in plants: Some evidence of near equivalences. Cryobiology 1983, 20, 487–503. [Google Scholar] [CrossRef]
- Dure, L., 3rd; Crouch, M.; Harada, J.; Ho, T.H.; Mundy, J.; Quatrano, R.; Thomas, T.; Sung, Z.R. Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol. Biol. 1989, 12, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol. Gen. Genet. 1993, 236, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.S.; Wolfraim, L.; Poole, R.J.; Dhindsa, R.S. Molecular cloning and relationship to freezing tolerance of cold-acclimation-specific genes of alfalfa. Plant Physiol. 1989, 89, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.S.; Poole, R.J.; Dhindsa, R.S. Changes in Protein Patterns and Translatable Messenger RNA Populations during Cold Acclimation of Alfalfa. Plant Physiol. 1987, 84, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.W.; Ward, R.W.; Thomashow, M.F. Characterization of a Cold-Regulated Wheat Gene Related to Arabidopsis Cor47. Plant Physiol. 1992, 100, 915–922. [Google Scholar] [CrossRef]
- Artus, N.N.; Uemura, M.; Steponkus, P.L.; Gilmour, S.J.; Lin, C.; Thomashow, M.F. Constitutive expression of the cold-regulated Arabidopsis thaliana COR15a gene affects both chloroplast and protoplast freezing tolerance. Proc. Natl. Acad. Sci. USA 1996, 93, 13404–13409. [Google Scholar] [CrossRef]
- Wang, H.; Datla, R.; Georges, F.; Loewen, M.; Cutler, A.J. Promoters from kin1 and cor6.6, two homologous Arabidopsis thaliana genes: Transcriptional regulation and gene expression induced by low temperature, ABA, osmoticum and dehydration. Plant Mol. Biol. 1995, 28, 605–617. [Google Scholar] [CrossRef]
- Kreps, J.A.; Wu, Y.; Chang, H.-S.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002, 130, 2129–2141. [Google Scholar] [CrossRef]
- Harvaux, M.; Kloppstech, K. The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta 2001, 213, 953–966. [Google Scholar] [CrossRef]
- Catala, R.; Medina, J.; Salinas, J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16475–16480. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, Y.R.; Hossain, M.A.; Hong, S.W.; Lee, B.H.; Lee, H. A mutation in ELA1, an age-dependent negative regulator of PAP1/MYB75, causes UV- and cold stress-tolerance in Arabidopsis thaliana seedlings. Plant Sci. 2009, 176, 678–686. [Google Scholar] [CrossRef]
- Sicher, R.C.; Barnaby, J.Y. Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiol. Plant. 2012, 144, 238–253. [Google Scholar] [CrossRef]
- Sicher, R. Carbon partitioning and the impact of starch deficiency on the initial response of Arabidopsis to chilling temperatures. Plant Sci. 2011, 181, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Brock, A.K.; Willmann, R.; Kolb, D.; Grefen, L.; Lajunen, H.M.; Bethke, G.; Lee, J.; Nurnberger, T.; Gust, A.A. The Arabidopsis Mitogen-Activated Protein Kinase Phosphatase PP2C5 Affects Seed Germination, Stomatal Aperture, and Abscisic Acid-Inducible Gene Expression. Plant Physiol. 2010, 153, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Joseph, R.A.; Steponkus, P.L. Cold-Acclimation of Arabidopsis thaliana—Effect on Plasma-Membrane Lipid-Composition and Freeze-Induced Lesions. Plant Physiol. 1995, 109, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.J.; Hajela, R.K.; Thomashow, M.F. Cold-Acclimation in Arabidopsis thaliana. Plant Physiol. 1988, 87, 745–750. [Google Scholar] [CrossRef]
- Sukumara, N.P.; Weiser, C.J. A Possible Standard Freezing Test for Evaluating Frost Tolerance in Potato Varieties. Am. Potato J. 1970, 47, 360. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnaby, J.Y.; Kim, J.; Devi, M.J.; Fleisher, D.H.; Tucker, M.L.; Reddy, V.R.; Sicher, R.C. Varying Atmospheric CO2 Mediates the Cold-Induced CBF-Dependent Signaling Pathway and Freezing Tolerance in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 7616. https://doi.org/10.3390/ijms21207616
Barnaby JY, Kim J, Devi MJ, Fleisher DH, Tucker ML, Reddy VR, Sicher RC. Varying Atmospheric CO2 Mediates the Cold-Induced CBF-Dependent Signaling Pathway and Freezing Tolerance in Arabidopsis. International Journal of Molecular Sciences. 2020; 21(20):7616. https://doi.org/10.3390/ijms21207616
Chicago/Turabian StyleBarnaby, Jinyoung Y., Joonyup Kim, Mura Jyostna Devi, David H. Fleisher, Mark L. Tucker, Vangimalla R. Reddy, and Richard C. Sicher. 2020. "Varying Atmospheric CO2 Mediates the Cold-Induced CBF-Dependent Signaling Pathway and Freezing Tolerance in Arabidopsis" International Journal of Molecular Sciences 21, no. 20: 7616. https://doi.org/10.3390/ijms21207616
APA StyleBarnaby, J. Y., Kim, J., Devi, M. J., Fleisher, D. H., Tucker, M. L., Reddy, V. R., & Sicher, R. C. (2020). Varying Atmospheric CO2 Mediates the Cold-Induced CBF-Dependent Signaling Pathway and Freezing Tolerance in Arabidopsis. International Journal of Molecular Sciences, 21(20), 7616. https://doi.org/10.3390/ijms21207616





