Analysis of Spatio-Temporal Transcriptome Profiles of Soybean (Glycine max) Tissues during Early Seed Development
Abstract
1. Introduction
2. Results and Discussion
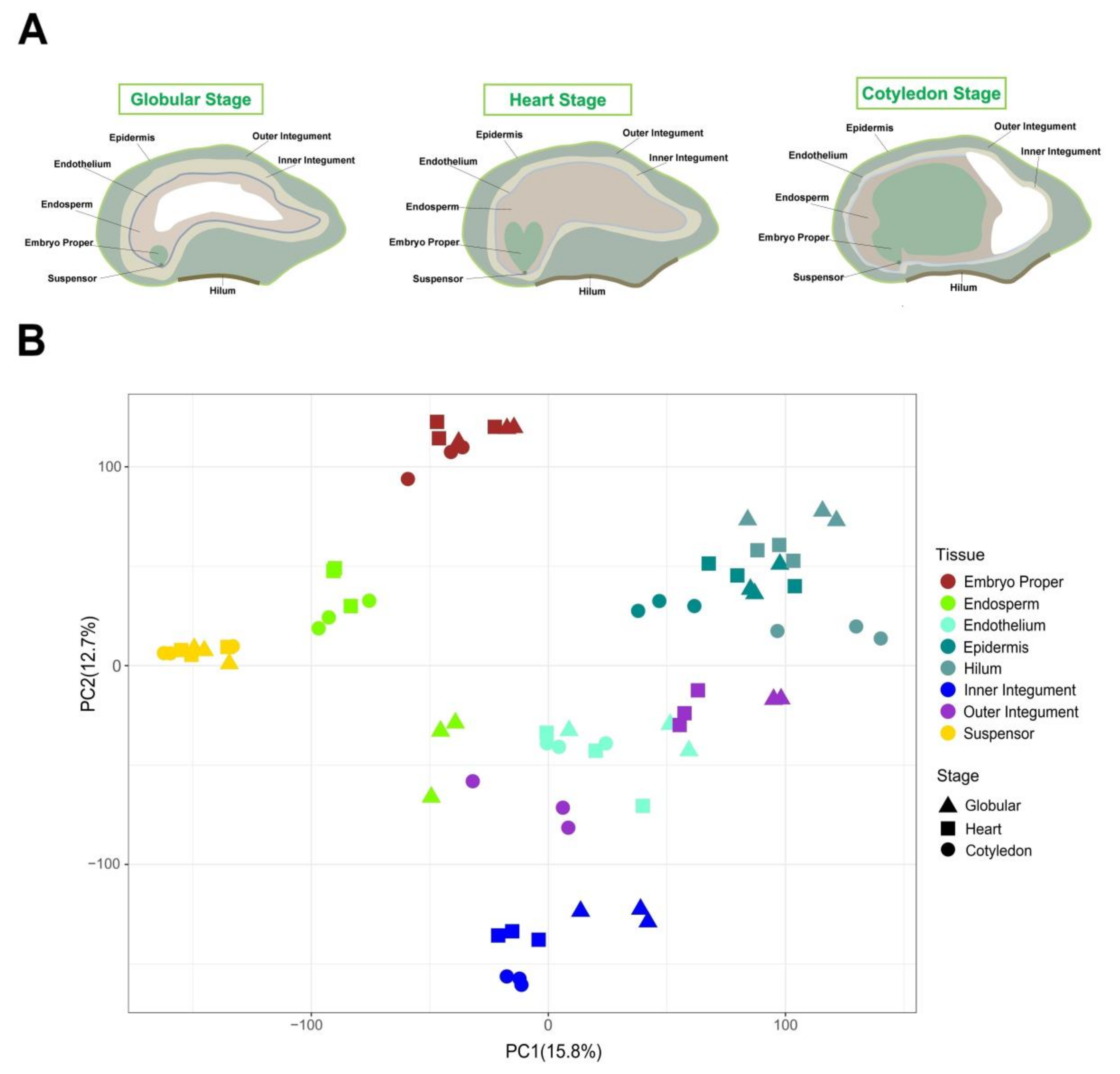
2.1. Source of Spatial and Temporal Transcriptome Data during Soybean Seed Early Development
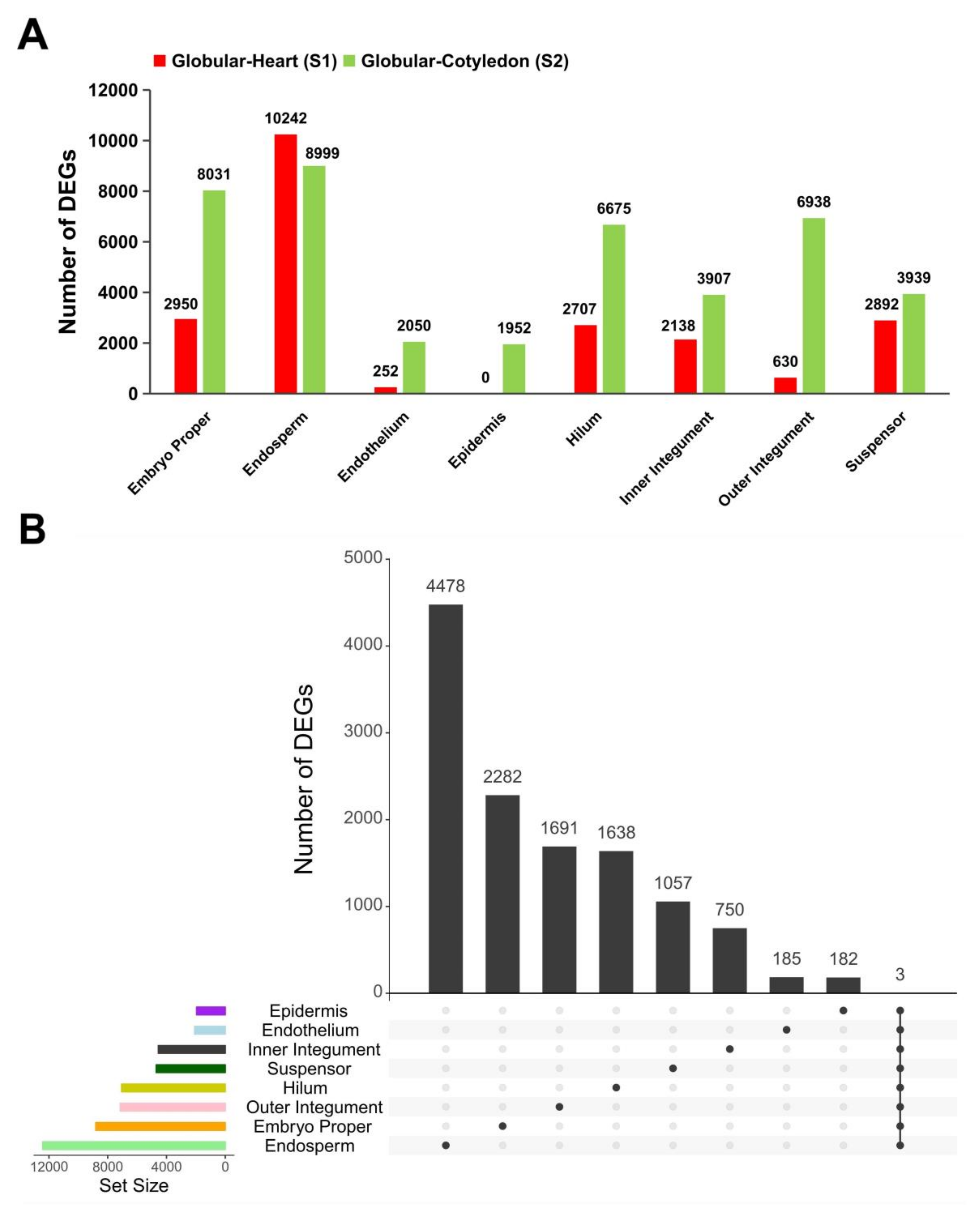
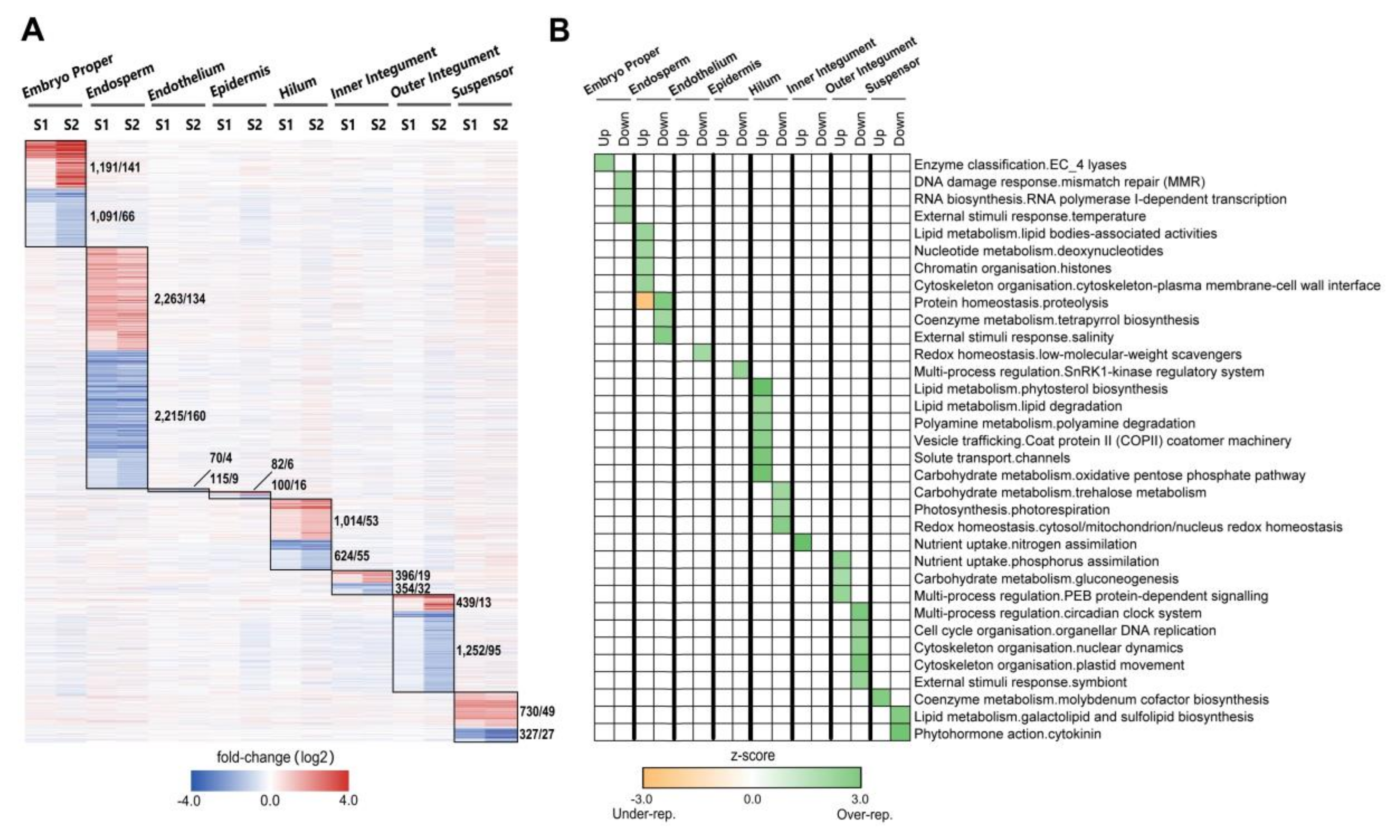
2.2. Tissue-Specific Regulation of Gene Expression Occurs over Developmental Stages
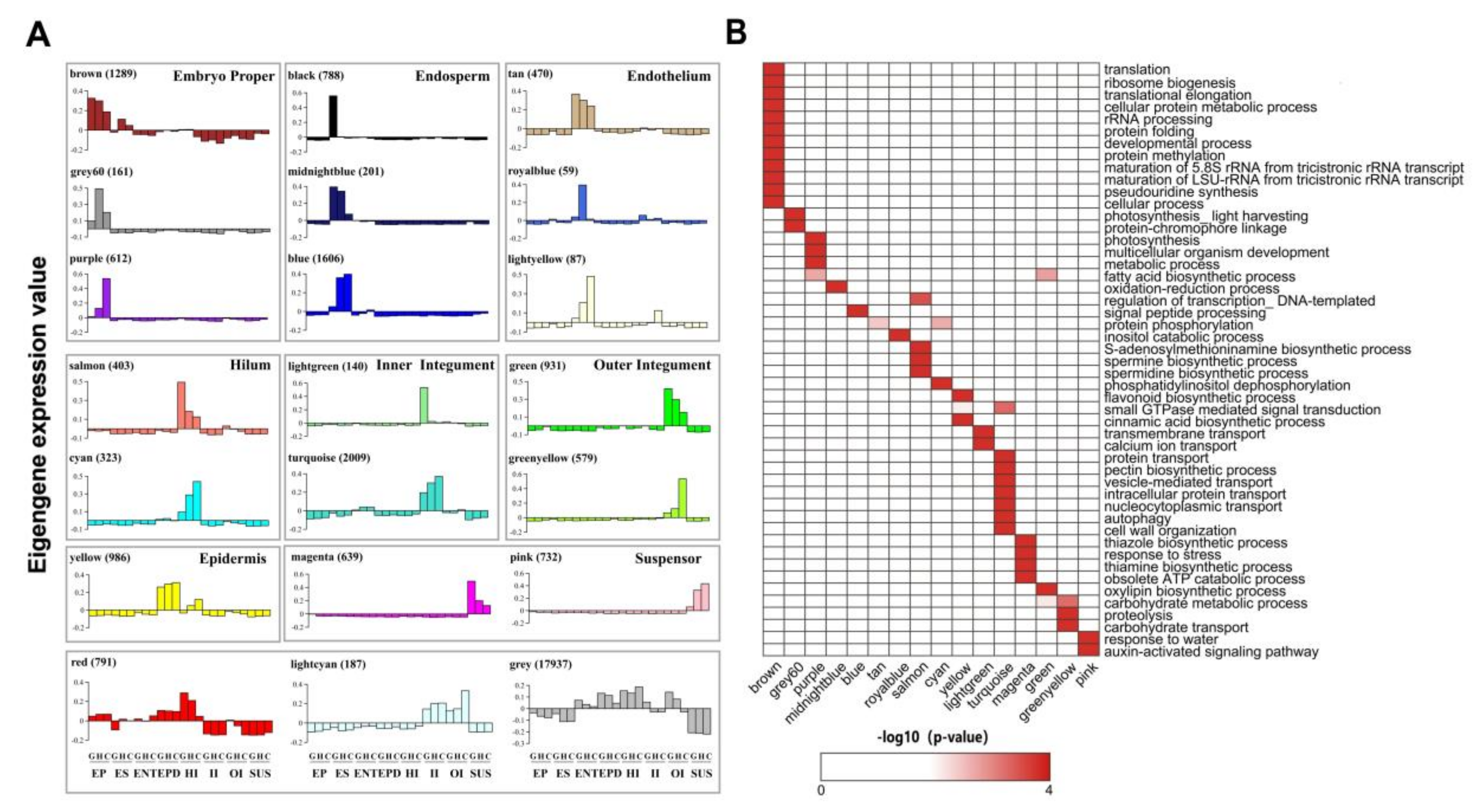
2.3. Predicting Candidate Transcriptional Regulators Using Weighted Gene Correlation Network Analysis
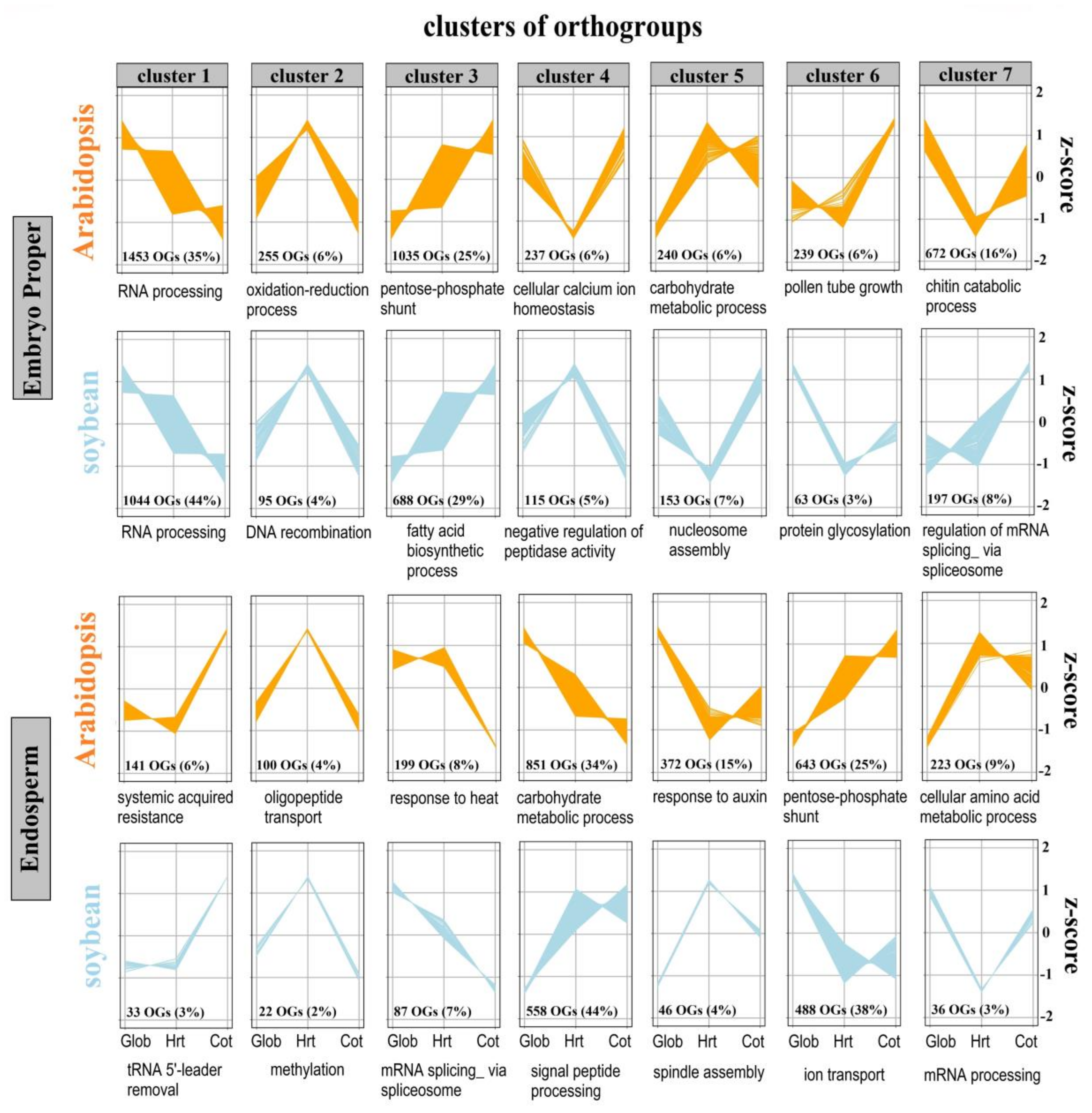
2.4. Integration of Orthology and Expression Information Identifies Conserved Orthologues between Soybean and Arabidopsis
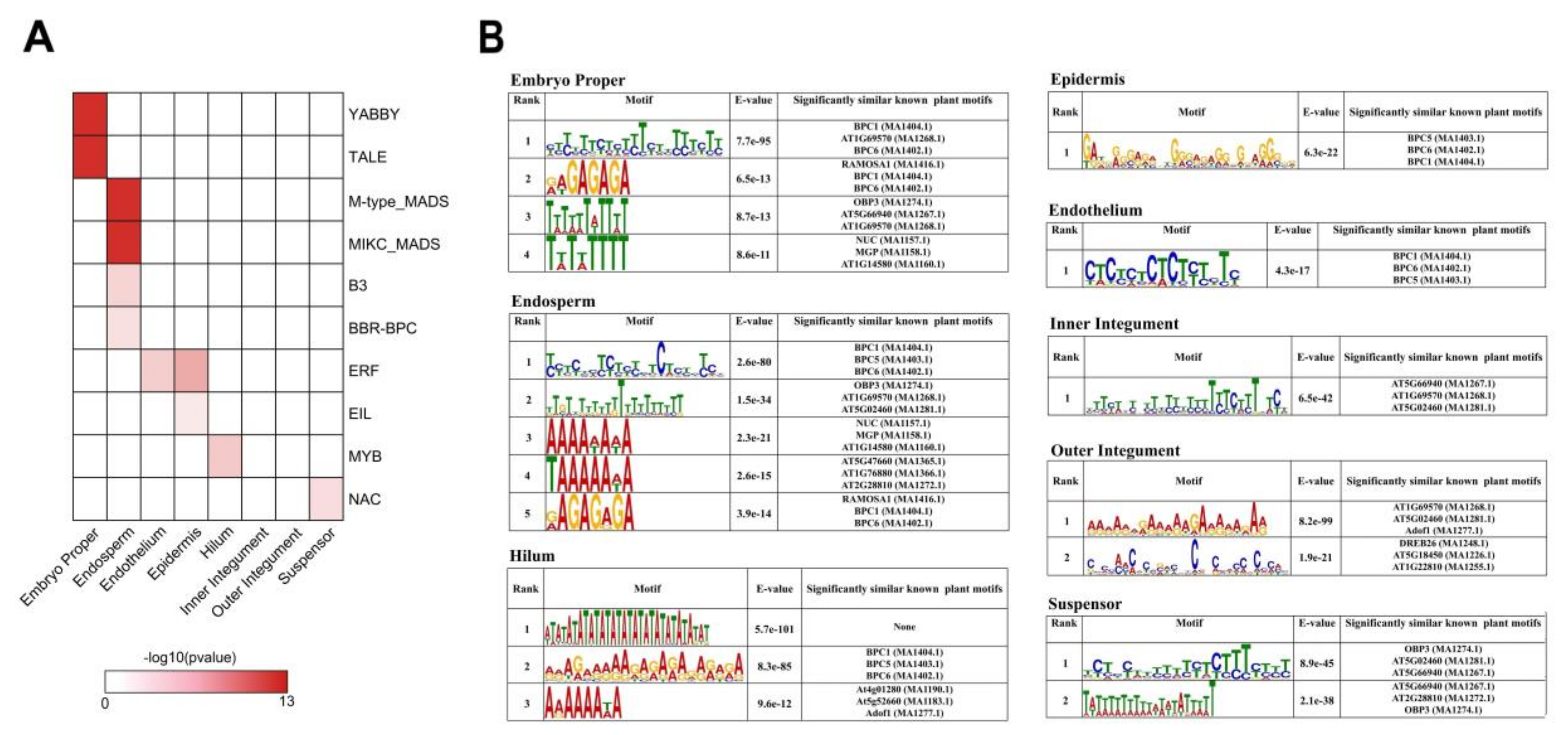
2.5. Tissue-Specific Expression of Transcriptional Regulators during Early Seed Development
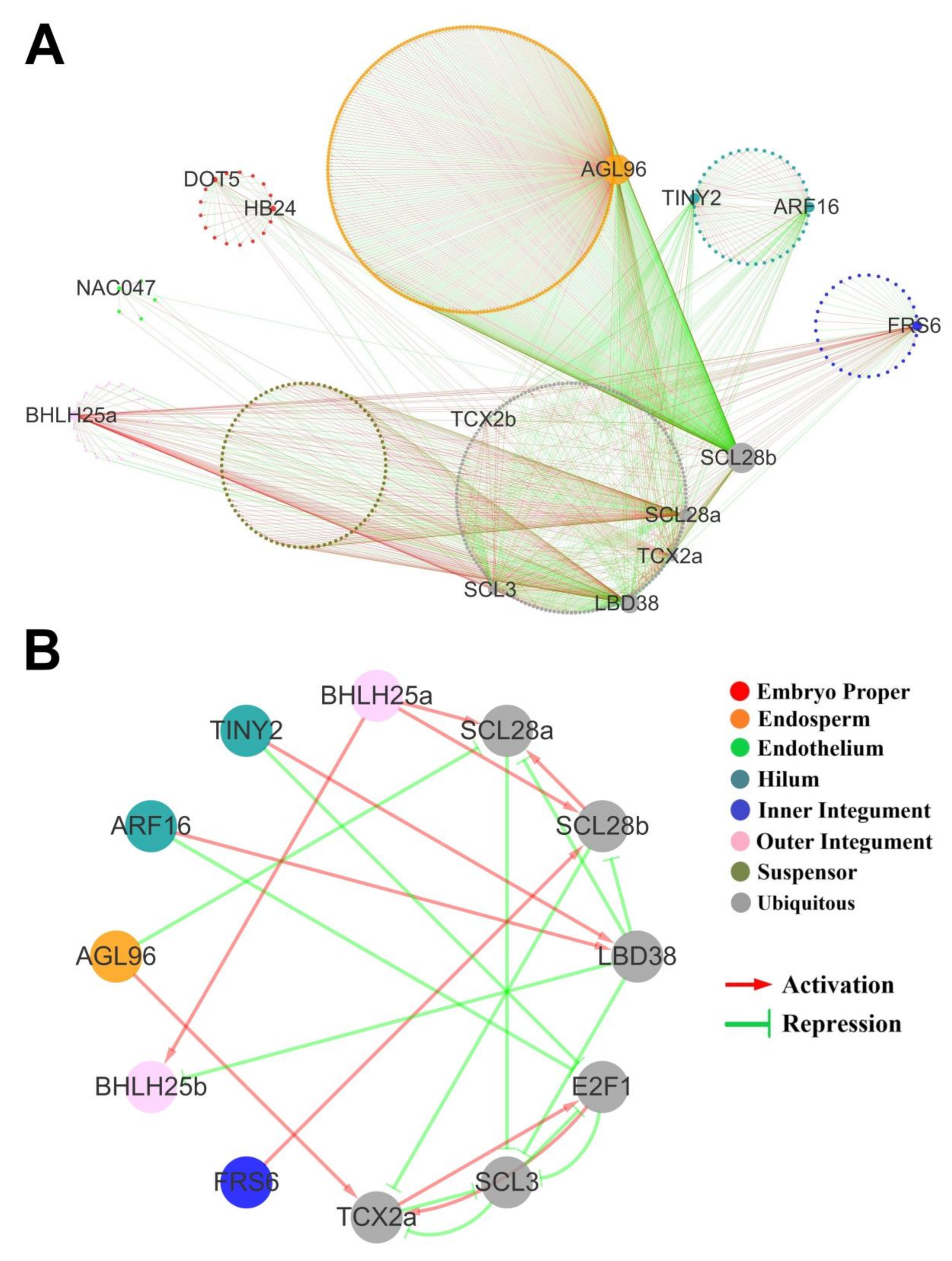
2.6. Gene Regulatory Network (GRN) of the Seed Tissue-Specific Genes
3. Summary
4. Materials and Methods
4.1. RNA-Seq Data Quality Control Analysis
4.2. PageMan Analysis of DEGs
4.3. Identification of Co-Expression Modules and Hub Genes
4.4. Orthologues Identification between Soybean and Arabidopsis
4.5. Transcription Factor and Promoter Motif Enrichment
4.6. Gene Regulatory Network Inference
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGL | AGAMOUS-like |
| ARF | AUXIN RESPONSE FACTOR |
| CV | Coefficient of variation |
| DAP | Days after pollination |
| DE | Differentially expressed |
| DEG | Differentially expressed gene |
| ERF | ETHYLENE RESPONSE FACTOR |
| GEO | Gene Expression Omnibus |
| GIF | Growth regulating factor-interacting factor |
| GO | Gene Ontology |
| GRN | Gene regulatory network |
| LCM | Laser capture microdissection |
| PCA | Principle component analysis |
| SCL | SCARECROW-LIKE |
| TF | Transcription factor |
| TPM | Transcripts per million |
| WGCNA | Weighted gene co-expression network analysis |
References
- Goldberg, R.B.; Barker, S.J.; Perez-Grau, L. Regulation of gene expression during plant embryogenesis. Cell 1989, 56, 149–160. [Google Scholar] [CrossRef]
- Johnson, S.; Liu, C.M.; Hedley, C.L.; Wang, T.L. An analysis of seed development in pisum-sativum. The isolation of mutants defective in embryo development. J. Exp. Bot. 1994, 45, 1503–1511. [Google Scholar] [CrossRef]
- Coste, F.; Ney, B.; Crozat, Y. Seed development and seed physiological quality of field grown beans (Phaseolus vulgaris L.). Seed Sci. Technol. 2001, 29, 121–136. [Google Scholar]
- Weterings, K.; Apuya, N.R.; Bi, Y.P.; Fischer, R.L.; Harada, J.J.; Goldberg, R.B. Regional localization of suspensor mRNAs during early embryo development. Plant Cell 2001, 13, 2409–2425. [Google Scholar] [CrossRef]
- Weber, H.; Borisjuk, L.; Wobus, U. Molecular physiology of legume seed development. Annu. Rev. Plant Biol. 2005, 56, 253–279. [Google Scholar] [CrossRef] [PubMed]
- Le, B.H.; Wagmaister, J.A.; Kawashima, T.; Bui, A.Q.; Harada, J.J.; Goldberg, R.B. Using genomics to study legume seed development. Plant Physiol. 2007, 144, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; Depaiva, G.; Yadegari, R. Plant embryogenesis—Zygote to seed. Science 1994, 266, 605–614. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.X.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.J.; Thelen, J.J.; Cheng, J.L.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Severin, A.J.; Woody, J.L.; Bolon, Y.T.; Joseph, B.; Diers, B.W.; Farmer, A.D.; Muehlbauer, G.J.; Nelson, R.T.; Grant, D.; Specht, J.E.; et al. RNA-Seq Atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol. 2010, 10, 16. [Google Scholar] [CrossRef]
- Jones, S.I.; Gonzalez, D.O.; Vodkin, L.O. Flux of transcript patterns during soybean seed development. BMC Genom. 2010, 11, 15. [Google Scholar] [CrossRef]
- Jones, S.I.; Vodkin, L.O. Using RNA-Seq to Profile Soybean Seed Development from Fertilization to Maturity. PLoS ONE 2013, 8, e59270. [Google Scholar] [CrossRef]
- Jang, Y.E.; Kim, M.Y.; Shim, S.; Lee, J.; Lee, S.-H. Gene expression profiling for seed protein and oil synthesis during early seed development in soybean. Genes Genom. 2015, 37, 409–418. [Google Scholar] [CrossRef]
- Lu, X.; Li, Q.-T.; Xiong, Q.; Li, W.; Bi, Y.-D.; Lai, Y.-C.; Liu, X.-L.; Man, W.-Q.; Zhang, W.-K.; Ma, B.; et al. The transcriptomic signature of developing soybean seeds reveals the genetic basis of seed trait adaptation during domestication. Plant J. 2016, 86, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, S.; He, C.; Zhou, B.; Ruan, Y.L.; Shou, H. Identification of regulatory networks and hub genes controlling soybean seed set and size using RNA sequencing analysis. J. Exp. Bot. 2017, 68, 1955–1972. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, Z.; Wang, Z.; Yu, J.; Qin, H.; Mao, X.; Jiang, H.; Xin, D.; Yin, Z.; Zhu, R.; et al. Meta-analysis and transcriptome profiling reveal hub genes for soybean seed storage composition during seed development. Plant Cell Environ. 2018, 41, 2109–2127. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.M.; Kwong, R.W.; Park, S.; Le, B.H.; Baden, R.; Cagliari, A.; Hashimoto, M.; Munoz, M.D.; Fischer, R.L.; Goldberg, R.B.; et al. LEC1 sequentially regulates the transcription of genes involved in diverse developmental processes during seed development. Proc. Natl. Acad. Sci. USA 2017, 114, E6710–E6719. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, H.; Shen, Y.; Wang, J. Transcriptomic analysis of rice (Oryza sativa) endosperm using the RNA-Seq technique. Plant Mol. Biol. 2013, 81, 363–378. [Google Scholar] [CrossRef]
- Xu, H.; Gao, Y.; Wang, J. Transcriptomic analysis of rice (Oryza sativa) developing embryos using the RNA-Seq technique. PLoS ONE 2012, 7, e30646. [Google Scholar] [CrossRef]
- Belmonte, M.F.; Kirkbride, R.C.; Stone, S.L.; Pelletier, J.M.; Bui, A.Q.; Yeung, E.C.; Hashimoto, M.; Fei, J.; Harada, C.M.; Munoz, M.D.; et al. Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proc. Natl. Acad. Sci. USA 2013, 110, E435–E444. [Google Scholar] [CrossRef]
- Ziegler, D.J.; Khan, D.; Kalichuk, J.L.; Becker, M.G.; Belmonte, M.F. Transcriptome landscape of the early Brassica napus seed. J. Integr. Plant Biol. 2019, 61, 639–650. [Google Scholar] [CrossRef]
- Liew, L.C.; Narsai, R.; Wang, Y.; Berkowitz, O.; Whelan, J.; Lewsey, M.G. Temporal tissue-specific regulation of transcriptomes during barley (Hordeum vulgare) seed germination. Plant J. 2020, 101, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Doll, N.M.; Just, J.; Brunaud, V.; Caius, J.; Grimault, A.; Depege-Fargeix, N.; Esteban, E.; Pasha, A.; Provart, N.J.; Ingram, G.C.; et al. Transcriptomics at Maize Embryo/Endosperm Interfaces Identifies a Transcriptionally Distinct Endosperm Subdomain Adjacent to the Embryo Scutellum (OPEN). Plant Cell 2020, 32, 833–852. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, W.Y.; Kwak, K.J.; Oh, S.H.; Han, Y.S.; Kang, H. Glycine-rich RNA-binding proteins are functionally conserved in Arabidopsis thaliana and Oryza sativa during cold adaptation process. J. Exp. Bot. 2010, 61, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, U.; Horiguchi, G.; Ponce, M.R.; Micol, J.L.; Tsukaya, H. Coordination of cell proliferation and cell expansion mediated by ribosome-related processes in the leaves of Arabidopsis thaliana. Plant J. 2009, 59, 499–508. [Google Scholar] [CrossRef]
- Nelissen, H.; Eeckhout, D.; Demuynck, K.; Persiau, G.; Walton, A.; van Bel, M.; Vervoort, M.; Candaele, J.; De Block, J.; Aesaert, S.; et al. Dynamic Changes in ANGUSTIFOLIA3 Complex Composition Reveal a Growth Regulatory Mechanism in the Maize Leaf. Plant Cell 2015, 27, 1605–1619. [Google Scholar] [CrossRef]
- Schrick, K.; Bruno, M.; Khosla, A.; Cox, P.N.; Marlatt, S.A.; Roque, R.A.; Nguyen, H.C.; He, C.W.; Snyder, M.P.; Singh, D.; et al. Shared functions of plant and mammalian StAR-related lipid transfer (START) domains in modulating transcription factor activity. BMC Biol. 2014, 12, 20. [Google Scholar] [CrossRef]
- Chye, M.L.; Huang, B.Q.; Zee, S.Y. Isolation of a gene encoding Arabidopsis membrane-associated acyl-CoA binding protein and immunolocalization of its gene product. Plant J. 1999, 18, 205–214. [Google Scholar] [CrossRef]
- Lung, S.C.; Liao, P.; Yeung, E.C.; Hsiao, A.S.; Xue, Y.; Chye, M.L. Acyl-CoA-Binding Protein ACBP1 Modulates Sterol Synthesis during Embryogenesis. Plant Physiol. 2017, 174, 1420–1435. [Google Scholar] [CrossRef]
- Lung, S.C.; Liao, P.; Yeung, E.C.; Hsiao, A.S.; Xue, Y.; Chye, M.L. Arabidopsis ACYL-COA-Binding Protein1 interacts with Sterol C4-Methyl Oxidase1-2 to modulate gene expression of homeodomain-leucine zipper IV transcription factors. New Phytol. 2018, 218, 183–200. [Google Scholar] [CrossRef]
- Chen, Q.F.; Xiao, S.; Qi, W.Q.; Mishra, G.; Ma, J.Y.; Wang, M.F.; Chye, M.L. The Arabidopsis acbp1acbp2 double mutant lacking acyl-CoA-binding proteins ACBP1 and ACBP2 is embryo lethal. New Phytol. 2010, 186, 843–855. [Google Scholar] [CrossRef]
- Pignocchi, C.; Minns, G.E.; Nesi, N.; Koumproglou, R.; Kitsios, G.; Benning, C.; Lloyd, C.W.; Doonan, J.H.; Hills, M.J. Endosperm Defective1 Is a Novel Microtubule-Associated Protein Essential for Seed Development in Arabidopsis. Plant Cell 2009, 21, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Hehenberger, E.; Kradolfer, D.; Kohler, C. Endosperm cellularization defines an important developmental transition for embryo development. Development 2012, 139, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Komis, G.; Luptovciak, I.; Doskocilova, A.; Samaj, J. Biotechnological aspects of cytoskeletal regulation in plants. Biotechnol. Adv. 2015, 33, 1043–1062. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Venglat, P.; Tibiche, C.; Yang, H.; Risseeuw, E.; Cao, Y.; Babic, V.; Cloutier, M.; Keller, W.; Wang, E.; et al. Genome-wide analysis reveals gene expression and metabolic network dynamics during embryo development in Arabidopsis. Plant Physiol. 2011, 156, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Schultz, G.; Katchalski, E. Early ribosomal rna transcription and appearance of cytoplasmic ribosomes during germination of wheat embryo. Nat.-New Biol. 1971, 231, 69. [Google Scholar] [CrossRef]
- Sela, A.; Piskurewicz, U.; Megies, C.; Mene-Saffrane, L.; Finazzi, G.; Lopez-Molina, L. Embryonic Photosynthesis Affects Post-Germination Plant Growth. Plant Physiol. 2020, 182, 2166–2181. [Google Scholar] [CrossRef]
- Tejos, R.I.; Mercado, A.V.; Meisel, L.A. Analysis of chlorophyll fluorescence reveals stage specific patterns of chloroplast-containing cells during Arabidopsis embryogenesis. Biol. Res. 2010, 43, 99–111. [Google Scholar] [CrossRef]
- Singh, D. Histopathology of some seed-borne infections—A review of recent investigations. Seed Sci. Technol. 1983, 11, 651–663. [Google Scholar]
- Radchuk, V.; Borisjuk, L. Physical, metabolic and developmental functions of the seed coat. Front. Plant Sci. 2014, 5, 510. [Google Scholar] [CrossRef]
- Babu, Y.; Musielak, T.; Henschen, A.; Bayer, M. Suspensor Length Determines Developmental Progression of the Embryo in Arabidopsis. Plant Physiol. 2013, 162, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Goldberg, R.B. The suspensor: Not just suspending the embryo. Trends Plant Sci. 2010, 15, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Sarojam, R.; Sappl, P.G.; Goldshmidt, A.; Efroni, I.; Floyd, S.K.; Eshed, Y.; Bowman, J.L. Differentiating Arabidopsis Shoots from Leaves by Combined YABBY Activities. Plant Cell 2010, 22, 2113–2130. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, M.K.; Bowman, J.L.; Sundaresan, V. YABBY polarity genes mediate the repression of KNOX homeobox genes in Arabidopsis. Plant Cell 2002, 14, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Villanneva, J.M.; Broadhvest, J.; Hauser, B.A.; Meister, R.J.; Schneitz, K.; Gasser, C.S. Inner noouter regulates abaxial-adaxial patterning in Arabidopsis ovules. Genes Dev. 1999, 13, 3160–3169. [Google Scholar] [CrossRef]
- Finet, C.; Floyd, S.K.; Conway, S.J.; Zhong, B.J.; Scutt, C.P.; Bowmanb, J.L. Evolution of the YABBY gene family in seed plants. Evol. Dev. 2016, 18, 116–126. [Google Scholar] [CrossRef]
- Yoong, F.Y.; O’Brien, L.K.; Truco, M.J.; Huo, H.Q.; Sideman, R.; Hayes, R.; Michelmore, R.W.; Bradford, K.J. Genetic Variation for Thermotolerance in Lettuce Seed Germination Is Associated with Temperature-Sensitive Regulation of Ethylene Response Factor1 (ERF1). Plant Physiol. 2016, 170, 472–488. [Google Scholar] [CrossRef]
- Fitzgerald, M.S.; McKnight, T.D.; Shippen, D.E. Characterization and developmental patterns of telomerase expression in plants. Proc. Natl. Acad. Sci. USA 1996, 93, 14422–14427. [Google Scholar] [CrossRef] [PubMed]
- Heller, K.; Kilian, A.; Piatyszek, M.A.; Kleinhofs, A. Telomerase activity in plant extracts. Mol. Gen. Genet. 1996, 252, 342–345. [Google Scholar] [CrossRef]
- Sabelli, P.A.; Larkins, B.A. The Development of Endosperm in Grasses. Plant Physiol. 2009, 149, 14–26. [Google Scholar] [CrossRef]
- Deshpande, P.K. Development of embryo and endosperm in eragrostis-unioloides (poaceae). Plant Syst. Evol. 1976, 125, 253–259. [Google Scholar] [CrossRef]
- Huysmans, M.; Buono, R.A.; Skorzinski, N.; Radio, M.C.; De Winter, F.; Parizot, B.; Mertens, J.; Karimi, M.; Fendrych, M.; Nowack, M.K. NAC Transcription Factors ANAC087 and ANAC046 Control Distinct Aspects of Programmed Cell Death in the Arabidopsis Columella and Lateral Root Cap. Plant Cell 2018, 30, 2197–2213. [Google Scholar] [CrossRef]
- Yanofsky, M.F.; Ma, H.; Bowman, J.L.; Drews, G.N.; Feldmann, K.A.; Meyerowitz, E.M. The protein encoded by the arabidopsis homeotic gene agamous resembles transcription factors. Nature 1990, 346, 35–39. [Google Scholar] [CrossRef]
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genetic interactions among floral homeotic genes of arabidopsis. Development 1991, 112, 1–20. [Google Scholar] [PubMed]
- Shahan, R.; Zawora, C.; Wight, H.; Sittmann, J.; Wang, W.P.; Mount, S.M.; Liu, Z.C. Consensus Coexpression Network Analysis Identifies Key Regulators of Flower and Fruit Development in Wild Strawberry. Plant Physiol. 2018, 178, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Le, B.H.; Cheng, C.; Bui, A.Q.; Wagmaister, J.A.; Henry, K.F.; Pelletier, J.; Kwong, L.; Belmonte, M.; Kirkbride, R.; Horvath, S.; et al. Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 8063–8070. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 14. [Google Scholar] [CrossRef]
- Abu-Jamous, B.; Kelly, S. Clust: Automatic extraction of optimal co-expressed gene clusters from gene expression data. Genome Biol. 2018, 19, 172. [Google Scholar] [CrossRef]
- Monfared, M.M.; Simon, M.K.; Meister, R.J.; Roig-Villanova, I.; Kooiker, M.; Colombo, L.; Fletcher, J.C.; Gasser, C.S. Overlapping and antagonistic activities of BASIC PENTACYSTEINE genes affect a range of developmental processes in Arabidopsis. Plant J. 2011, 66, 1020–1031. [Google Scholar] [CrossRef]
- Kang, H.G.; Singh, K.B. Characterization of salicylic acid-responsive, Arabidopsis Dof domain proteins: Overexpression of OBP3 leads to growth defects. Plant J. 2000, 21, 329–339. [Google Scholar] [CrossRef]
- Spurney, R.J.; Van den Broeck, L.; Clark, N.M.; Fisher, A.P.; Balaguer, M.A.D.; Sozzani, R. Tuxnet: A simple interface to process RNA sequencing data and infer gene regulatory networks. Plant J. 2020, 101, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Walia, H.; Josefsson, C.; Dilkes, B.; Kirkbride, R.; Harada, J.; Comai, L. Dosage-Dependent Deregulation of an AGAMOUS-LIKE Gene Cluster Contributes to Interspecific Incompatibility. Curr. Biol. 2009, 19, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.H.; Steffen, J.G.; Portereiko, M.F.; Lloyd, A.; Drews, G.N. The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. Plant Cell 2008, 20, 635–647. [Google Scholar] [CrossRef]
- Choe, J.E.; Kim, B.; Yoon, E.K.; Jang, S.; Kim, G.; Dhar, S.; Lee, S.A.; Lim, J. Characterization of the GRAS transcription factor SCARECROW-LIKE 28’s role in Arabidopsis root growth. J. Plant Biol. 2017, 60, 462–471. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Ogawa, M.; Fleet, C.M.; Zentella, R.; Hu, J.; Heo, J.O.; Lim, J.; Kamiya, Y.; Yamaguchi, S.; Sun, T.P. Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 2160–2165. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Friml, J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2010, 107, 12046–12051. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.L.; Nolan, R.O.R.; Jiang, H.; Tang, B.Y.; Zhang, M.C.; Li, Z.H.; Yin, Y.H. The AP2/ERF Transcription Factor TINY Modulates Brassinosteroid-Regulated Plant Growth and Drought Responses in Arabidopsis. Plant Cell 2019, 31, 1788–1806. [Google Scholar] [CrossRef]
- Magyar, Z.; De Veylder, L.; Atanassova, A.; Bako, L.; Inze, D.; Bogre, L. The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. Plant Cell 2005, 17, 2527–2541. [Google Scholar] [CrossRef]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A Toolkit for Quality Control of Next Generation Sequencing Data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.Q.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Kim, D.; Landmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Usadel, B.; Nagel, A.; Steinhauser, D.; Gibon, Y.; Blasing, O.E.; Redestig, H.; Sreenivasulu, N.; Krall, L.; Hannah, M.A.; Poree, F.; et al. PageMan: An interactive ontology tool to generate, display, and annotate overview graphs for profiling experiments. BMC Bioinform. 2006, 7, 8. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 13. [Google Scholar] [CrossRef]
- Steinegger, M.; Soding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Tian, F.; Yang, D.C.; Meng, Y.Q.; Jin, J.P.; Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2020, 48, D1104–D1113. [Google Scholar] [CrossRef]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. Clusterprofiler: An R Package for Comparing Biological Themes among Gene Clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Machanick, P.; Bailey, T.L. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics 2011, 27, 1696–1697. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Yi, C.; Ma, J.; Wang, S.; Peirats-Llobet, M.; Lewsey, M.G.; Whelan, J.; Shou, H. Analysis of Spatio-Temporal Transcriptome Profiles of Soybean (Glycine max) Tissues during Early Seed Development. Int. J. Mol. Sci. 2020, 21, 7603. https://doi.org/10.3390/ijms21207603
Sun S, Yi C, Ma J, Wang S, Peirats-Llobet M, Lewsey MG, Whelan J, Shou H. Analysis of Spatio-Temporal Transcriptome Profiles of Soybean (Glycine max) Tissues during Early Seed Development. International Journal of Molecular Sciences. 2020; 21(20):7603. https://doi.org/10.3390/ijms21207603
Chicago/Turabian StyleSun, Shuo, Changyu Yi, Jing Ma, Shoudong Wang, Marta Peirats-Llobet, Mathew G. Lewsey, James Whelan, and Huixia Shou. 2020. "Analysis of Spatio-Temporal Transcriptome Profiles of Soybean (Glycine max) Tissues during Early Seed Development" International Journal of Molecular Sciences 21, no. 20: 7603. https://doi.org/10.3390/ijms21207603
APA StyleSun, S., Yi, C., Ma, J., Wang, S., Peirats-Llobet, M., Lewsey, M. G., Whelan, J., & Shou, H. (2020). Analysis of Spatio-Temporal Transcriptome Profiles of Soybean (Glycine max) Tissues during Early Seed Development. International Journal of Molecular Sciences, 21(20), 7603. https://doi.org/10.3390/ijms21207603






