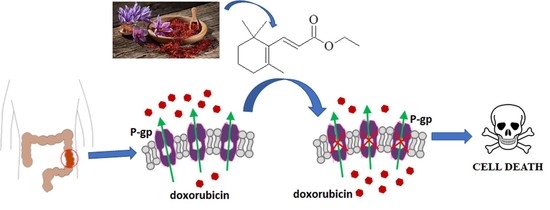
TMPE Derived from Saffron Natural Monoterpene as Cytotoxic and Multidrug Resistance Reversing Agent in Colon Cancer Cells
Abstract
1. Introduction
2. Results
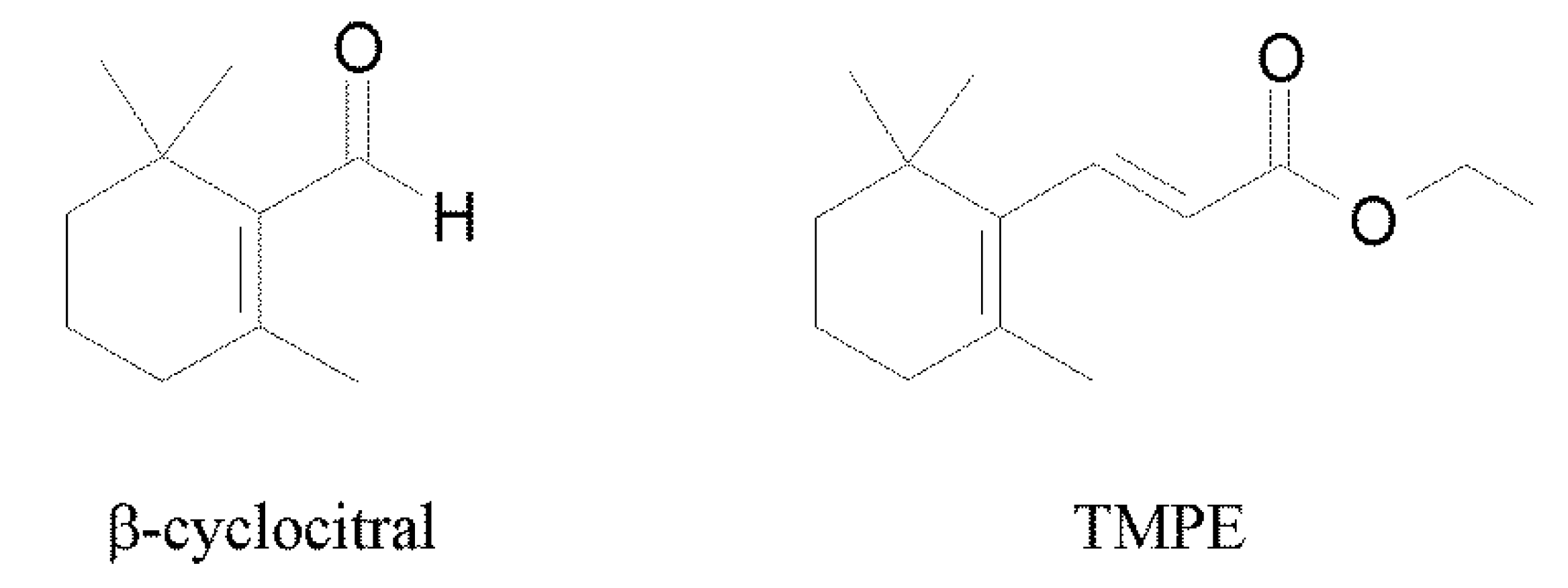
2.1. Synthesis of the Compound
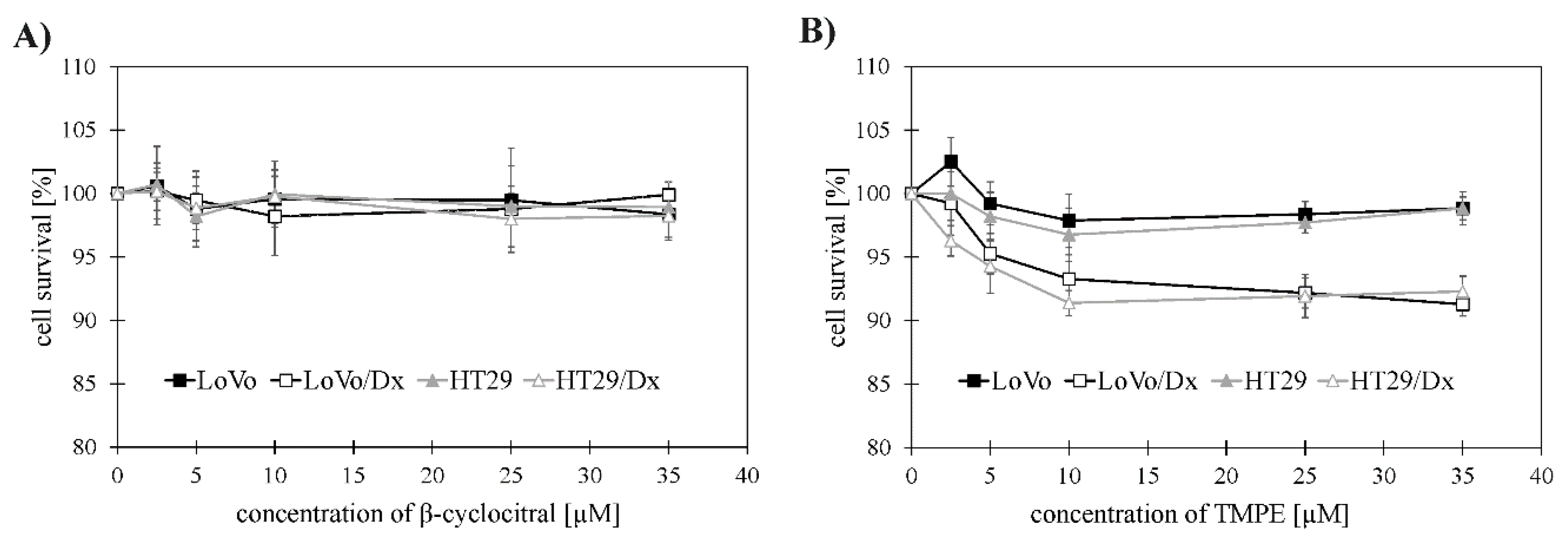
2.2. Cytotoxic Activity of β-Cyclocitral and its Derivative TMPE against Colon Cancer Cells
2.3. Chemosensitizing Effects
2.3.1. Change in Doxorubicin Cytotoxicity in the Resistant Colon Cancer Cells
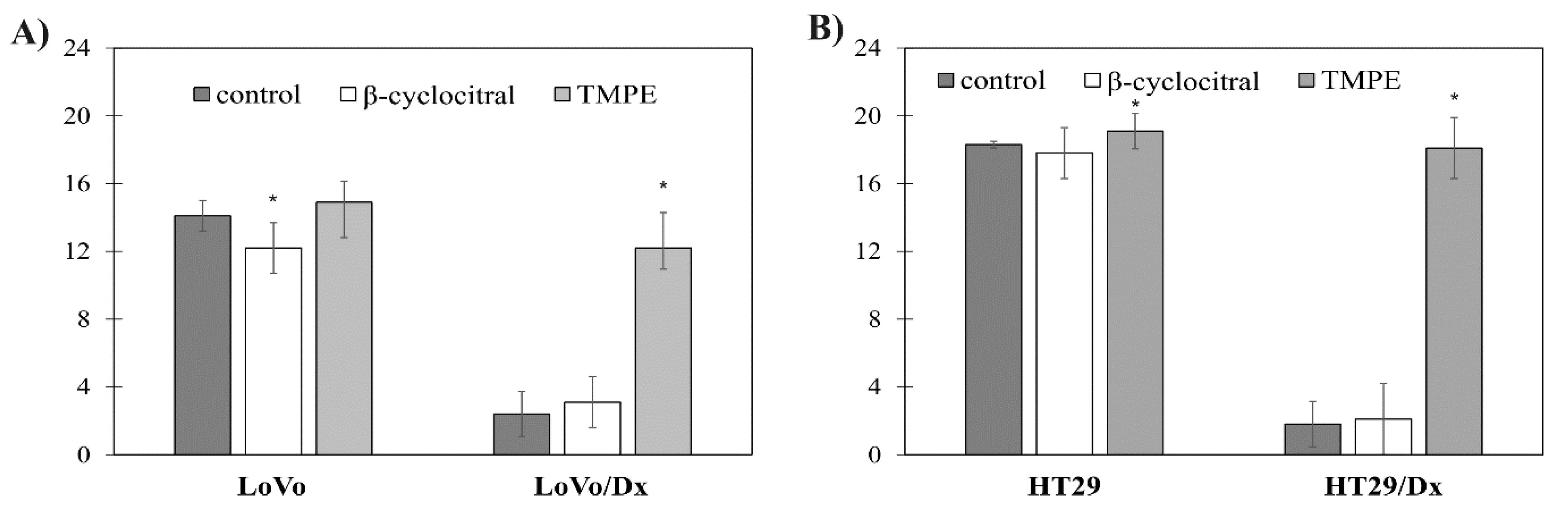
2.3.2. Increase in Doxorubicin Accumulation in Resistant Colon Cancer Cells
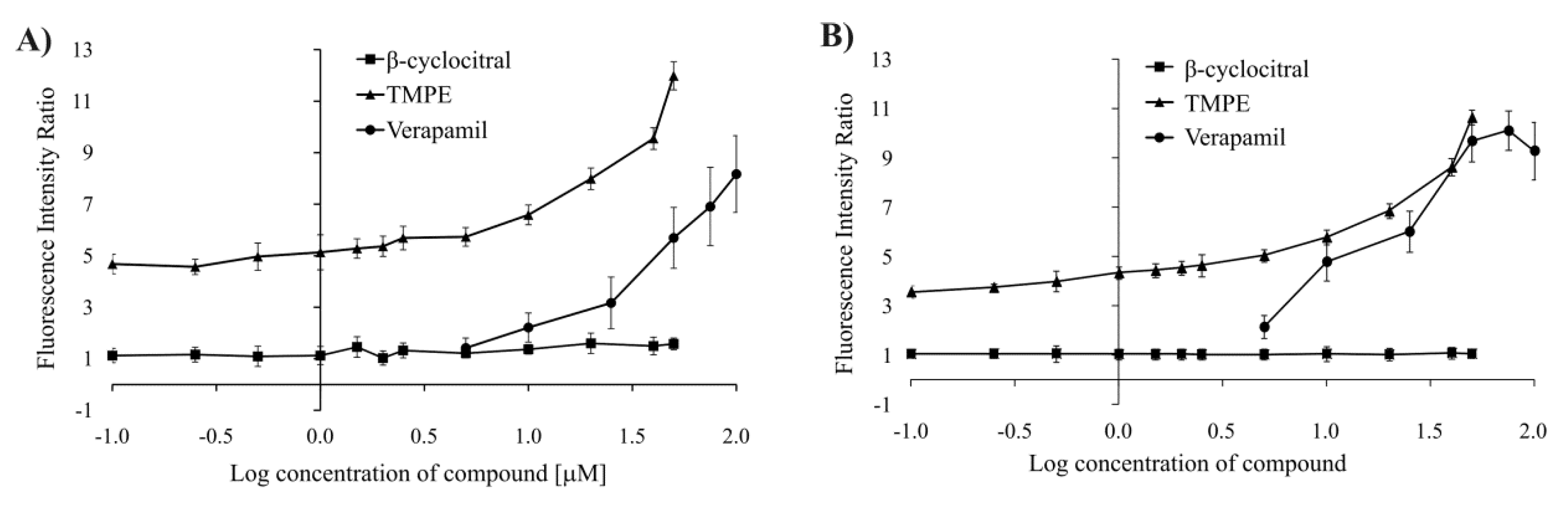
2.3.3. Increase in Rhodamine 123 Accumulation in Colon Cancer Cells
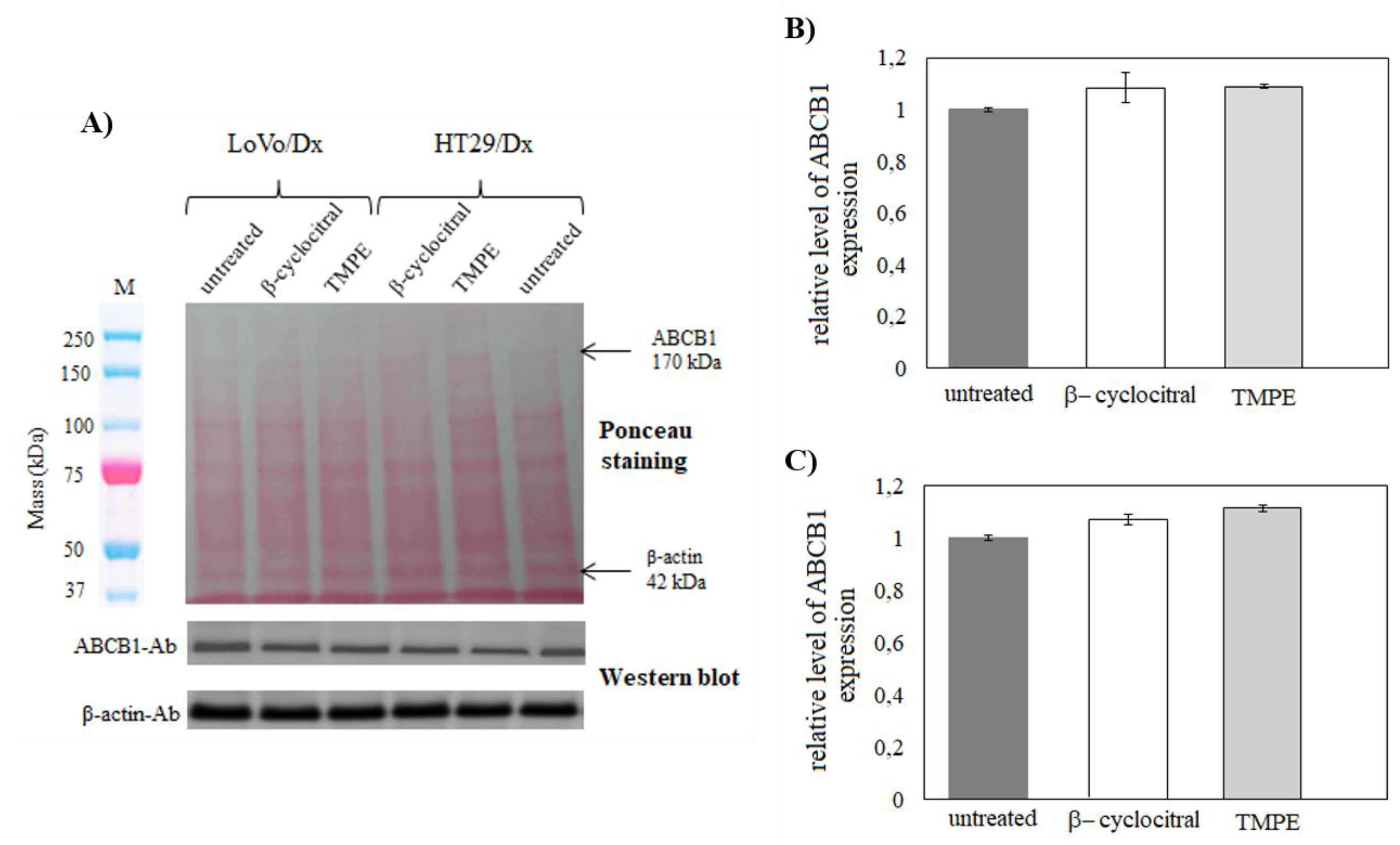
2.3.4. ABCB1 Expression in LoVo/Dx and HT29/Dx Cells
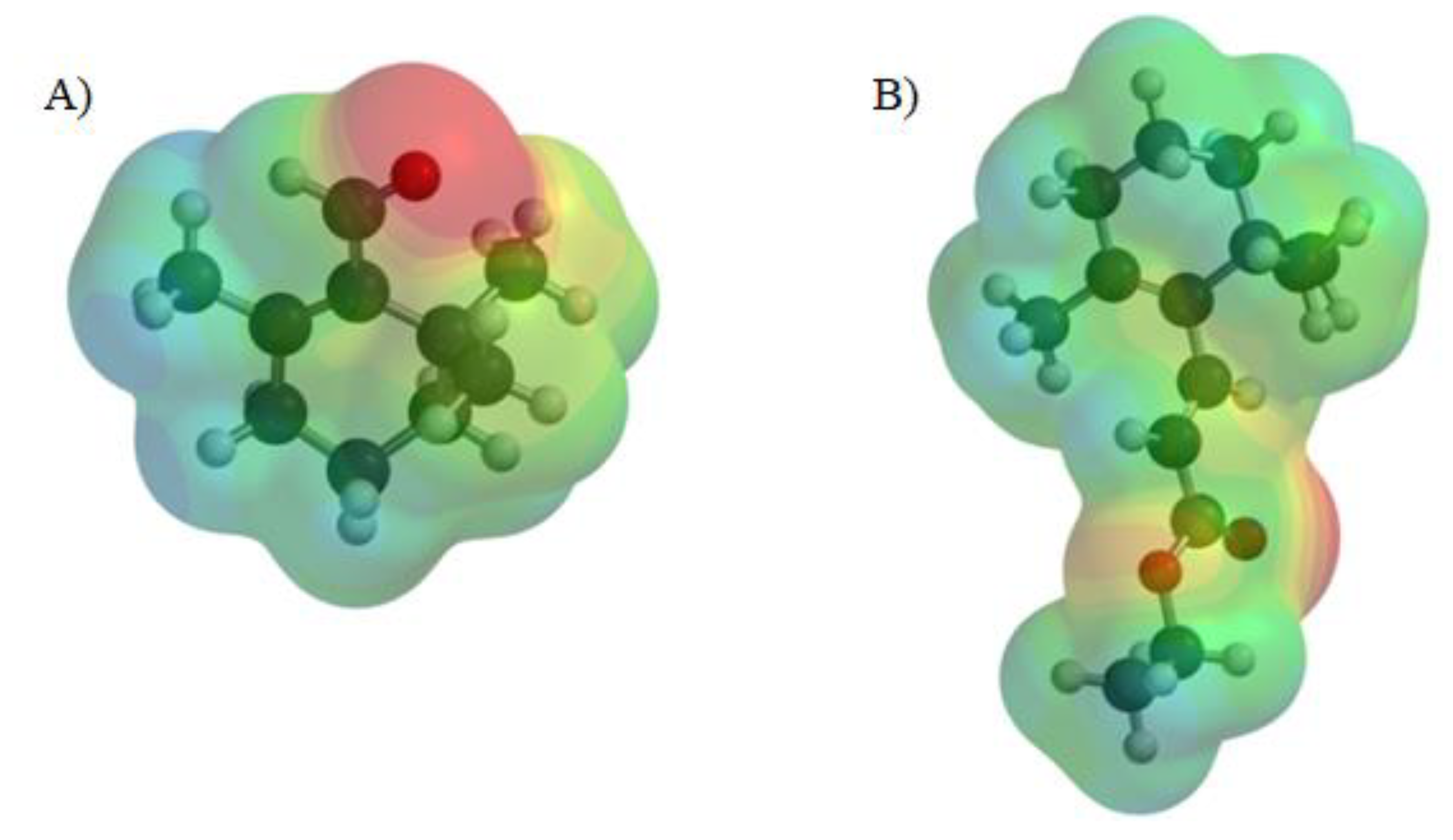
2.4. Molecular Calculations
2.5. Prediction of Toxicological and Physicochemical Properties
3. Discussion
4. Materials and Methods
4.1. Chemicals
Synthesis
4.2. Cell Cultures
4.3. Cell Viability Assay
4.4. Polymerase Chain Reaction
4.5. Western Blot Analysis
4.6. Intracellular Accumulation of Doxorubicin
4.7. Accumulation of Rhodamine 123 in Cancer Cells
4.8. Isobolographic Analysis
4.9. Molecular Calculations
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Wink, M. Plant secondary metabolism: Diversity, function and its evolution. Nat. Prod. Commun. 2008, 3, 1205–1216. [Google Scholar] [CrossRef]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Fernandes, J. Antitumor monoterpenes. In Bioactive Essential Oils and Cancer; de Sousa, D., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 175–200. [Google Scholar]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 3, 130. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn. Res. 2014, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, F.I.; Espinosa-Aguirre, J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004, 28, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Moradzadeh, M.; Kalani, M.R.; Avan, A. The antileukemic effects of saffron (Crocus sativus L.) and its related molecular targets: A mini review. J. Cell. Biochem. 2019, 120, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Moraga, A.R.; Rambla, J.L.; Ahrazem, O.; Granell, A.; Gomez-Gomez, L. Metabolite and target transcript analyses during Crocus sativus stigma development. Phytochemistry 2009, 70, 1009–1016. [Google Scholar] [CrossRef]
- Szakacs, G.; Hall, M.D.; Gottesman, M.M.; Boumendjel, A.; Kachadourian, R.; Day, B.J.; Baubichon-Cortay, H.; Di Pietro, A. Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chem. Rev. 2014, 114, 5753–5774. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sroda-Pomianek, K.; Michalak, K.; Palko-Labuz, A.; Pola, A.; Dziegiel, P.; Pula, B.; Swiatek, P.; Wesolowska, O. Cytotoxic and multidrug resistance reversal activity of phenothiazine derivative is strongly enhanced by theobromine, a phytochemical from cocoa. Eur. J. Pharmacol. 2019, 849, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef]
- Fogh, J.; Trempe, G. New human tumor cell lines. In Human Tumor Cell In Vitro; Fogh, J., Ed.; Springer: New York, NY, USA, 1975; pp. 115–159. [Google Scholar]
- Drewinko, B.; Romsdahl, M.M.; Yang, L.Y.; Ahearn, M.J.; Trujillo, J.M. Establishment of a human carcinoembryonic antigen-producing colon adenocarcinoma cell line. Cancer Res. 1976, 36, 467–475. [Google Scholar]
- Cao, H.; Xu, E.; Liu, H.; Wan, L.; Lai, M. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol. Res. Pract. 2015, 211, 557–569. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Grandi, M.; Geroni, C.; Giuliani, F.C. Isolation and characterization of a human colon adenocarcinoma cell line resistant to doxorubicin. Br. J. Cancer 1986, 54, 515–518. [Google Scholar] [CrossRef]
- Riganti, C.; Miraglia, E.; Viarisio, D.; Costamagna, C.; Pescarmona, G.; Ghigo, D.; Bosia, A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [Google Scholar]
- Wesolowska, O.; Wisniewski, J.; Sroda, K.; Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Dus, D.; Michalak, K. 8-Prenylnaringenin is an inhibitor of multidrug resistance-associated transporters, P-glycoprotein and MRP1. Eur. J. Pharmacol. 2010, 644, 32–40. [Google Scholar] [CrossRef]
- Wesolowska, O.; Wisniewski, J.; Sroda-Pomianek, K.; Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Dus, D.; Duarte, N.; Ferreira, M.J.U.; Michalak, K. Multidrug resistance reversal and apoptosis induction in human colon cancer cells by some flavonoids present in Citrus plants. J. Nat. Prod. 2012, 75, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.; Wesolowska, O.; Sroda-Pomianek, K.; Paprocka, M.; Bielawska-Pohl, A.; Krawczenko, A.; Duarte, N.; Ferreira, M.J.U.; Duś Dus, D.; Michalak, K. Euphorbia species-derived diterpenes and coumarins as multidrug resistance modulators in human colon carcinoma cells. Anticancer Res. 2016, 36, 2259–2264. [Google Scholar] [PubMed]
- Sanches, L.J.; Marinello, P.C.; Panis, C.; Fagundes, T.R.; Morgado-Diaz, J.A.; de-Freitas-Junior, J.C.M.; Cecchini, R.; Cecchini, A.L.; Luiz, R.C. Cytotoxicity of citral against melanoma cells: The involvement of oxidative stress generation and cell growth protein reduction. Tumour Biol. 2017, 39, 1010428317695914. [Google Scholar] [CrossRef]
- Nigjeh, S.E.; Yeap, S.K.; Nordin, N.; Kamalideghan, B.; Ky, H.; Rosli, R. Citral induced apoptosis in MDA-MB-231 spheroid cells. BMC Complement. Altern. Med. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, T.; Kitanaka, A.; Kubota, Y.; Yamaoka, G.; Kameda, T.; Imataki, O.; Dobashi, H.; Bandoh, S.; Kadowaki, N.; Tanaka, T. Lemongrass essential oil and citral inhibit Src/Stat3 activity and suppress the proliferation/survival of small-cell lung cancer cells, alone or in combination with chemotherapeutic agents. Int. J. Oncol. 2018, 52, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Dang, Y.Y.; Huang, M.; Xu, W.S.; Chen, X.P.; Wang, Y.T. Anti-cancer properties of terpenoids isolated from Rhizoma curcumae—A review. J. Ethnopharmacol. 2012, 143, 406–411. [Google Scholar] [CrossRef]
- Escribano, J.; Alonso, G.L.; Coca-Prados, M.; Fernandez, J.A. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996, 100, 23–30. [Google Scholar] [CrossRef]
- Slamenova, D.; Horvathova, E.; Sramkova, M.; Marsslkova, L. DNA-protective effects of two components of essential plant oils carvacrol and thymol on mammalian cells cultured in vitro. Neoplasma 2007, 54, 108–112. [Google Scholar]
- Jaafari, A.; Tilaoui, M.; Ait Mouse, H.; Ait M’bark, L.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Braz. J. Pharmacogn. 2012, 22, 534–540. [Google Scholar] [CrossRef]
- De Ines, C.; Argandona, V.H.; Rovirosa, J.; San-Martin, A.; Diaz-Marrero, A.R.; Cueto, M.; Gonzalez-Coloma, A. Cytotoxic activity of halogenated monoterpenes from Plocamium cartilagineum. Z. Naturforsch. C J. Biosci. 2004, 59, 339–344. [Google Scholar] [CrossRef]
- Toffoli, G.; Viel, A.; Tumiotto, L.; Biscontin, G.; Rossi, C.; Boiocchi, M. Pleiotropic resistant phenotype is a multifactorial phenomenon in human colon carcinoma cell lines. Br. J. Cancer 1991, 63, 51–56. [Google Scholar] [CrossRef]
- Riganti, C.; Rolando, B.; Kopecka, J.; Campia, I.; Chegaev, K.; Lazzarato, L.; Federico, A.; Fruttero, R.; Ghigo, D. Mitochondrial-targeting nitrooxy-doxorubicin: A new approach to overcome drug resistance. Mol. Pharm. 2013, 10, 161–174. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine 2012, 19, 1288–1297. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Eldin, E.; Eldin, M.N.; Fatani, S.H.; Wink, M. Influence of combinations of digitonin with selected phenolics, terpenoids, and alkaloids on the expression and activity of P-glycoprotein in leukaemia and colon cancer cells. Phytomedicine 2013, 21, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Takagi, A.; Kitazawa, H.; Kawakami, J.; Adachi, I. Inhibition of P-glycoprotein-mediated transport by extracts of and monoterpenoids contained in Zanthoxyli fructus. Toxicol. Appl. Pharmacol. 2005, 209, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Takada, T.; Yamamura, Y.; Adachi, I.; Suzuki, H.; Kawakami, J. Inhibitory effects of terpenoids on multidrug resistance-associated protein 2- and breast cancer resistance protein-mediated transport. Drug Metab. Dispos. 2008, 36, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Dogra, A.; Kotwal, P.; Gour, G.; Bhatt, S.; Singh, G.; Mukherjee, D.; Nandi, U. Description of duglike properties of safranal and its chemistry behind low oral exposure. ACS Omega 2020, 5, 9885–9891. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.; Ahmed, O.B.; Spengler, G.; Molnar, J.; Lage, H.; Ferreira, M.J.U. Jatrophane diterpenes and cancer multidrug resistance—ABCB1 efflux modulation and selective cell death induction. Phytomedicine 2016, 23, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.; Gyemant, N.; Abreu, P.M.; Molnar, J.; Ferreira, M.J. New macrocyclic lathyrane diterpenes, from Euphorbia lagascae, as inhibitors of multidrug resistance of tumour cells. Planta Med. 2006, 72, 162–168. [Google Scholar] [CrossRef]
- Miyashita, M.; Sadzuka, Y. Effect of linalool as a component of Humulus lupulus on doxorubicin-induced antitumor activity. Food Chem. Toxicol. 2013, 53, 174–179. [Google Scholar] [CrossRef]
- Singh, S.; Fatima, Z.; Ahmad, K.; Hameed, S. Fungicidal action of geraniol against Candida albicans is potentiated by abrogated CaCdr1p drug efflux and fluconazole synergism. PLoS ONE 2018, 13, e0203079. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Niu, H.; Zhang, W.; Mu, H.; Sun, C.; Duan, J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett. Appl. Microbiol. 2015, 60, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Didziapetris, R.; Japertas, P.; Avdeef, A.; Petrauskas, A. Classification analysis of P-glycoprotein substrate specificity. J. Drug Target. 2003, 11, 391–406. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Uryga, A.; Kostrzewa-Suslow, E.; Michalak, K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed. Pharmacother. 2017, 88, 232–241. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wesolowska, O.; Michalak, K.; Blaszczyk, M.; Molnar, J.; Sroda-Pomianek, K. Organosilicon compounds, SILA-409 and SILA-421, as doxorubicin resistance-reversing agents in human colon cancer cells. Molecules 2020, 25, 1654. [Google Scholar] [CrossRef]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
- OSIRIS Property Explorer; Actelion Pharmaceuticals: Allschwil, Switzerland, 2010; Available online: http://www.organic-chemistry.org/prog/peo/ (accessed on 28 August 2020).



| (A) | ||||
| Concentration (µM) | Ratio | Combination Index | ||
| Dox | β-Cyclocitral | TMPE | ||
| 10 | 5 | 2:1 | 1.11 | |
| 10 | 5 | 2:1 | 0.63 | |
| (B) | ||||
| Concentration (µM) | Ratio | Combination Index | ||
| Dox | β-Cyclocitral | TMPE | ||
| 10 | 5 | 2:1 | 1.201 | |
| 10 | 5 | 2:1 | 0.852 | |
| Parameter | β-Cyclocitral Aqua | TMPE Aqua |
|---|---|---|
| ELUMO (eV) | 0.28 | 0.42 |
| EHOMO (eV) | −8.92 | −8.35 |
| Energy gap (eV) | 9.20 | 8.48 |
| Chemical hardness (eV) | 4.60 | 4.24 |
| LogP | 2.14 | 3.52 |
| Dipole moment (D) | 6.06 | 3.15 |
| Global electrophilicity index (eV) | 1.793 | 1.915 |
| Property | Compound | ||
|---|---|---|---|
| β-Cyclocitral | TMPE | ||
| Physico-chemical properties | cLogP | 2.118 | 3.472 |
| Solubility (LogS) | −2.214 | −2.721 | |
| Molecular weight | 152 | 222 | |
| TPSA | 17.07 | 26.30 | |
| Druglikeness | −7.062 | −11.216 | |
| Drug Score | 0.281 | 0.426 | |
| Toxicity risks | Mutagenic | N | N |
| Tumorigenic | N | N | |
| Irritant | H | N | |
| Reproductive effect | N | N | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Środa-Pomianek, K.; Palko-Łabuz, A.; Poła, A.; Ferens-Sieczkowska, M.; Wesołowska, O.; Kozioł, A. TMPE Derived from Saffron Natural Monoterpene as Cytotoxic and Multidrug Resistance Reversing Agent in Colon Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7529. https://doi.org/10.3390/ijms21207529
Środa-Pomianek K, Palko-Łabuz A, Poła A, Ferens-Sieczkowska M, Wesołowska O, Kozioł A. TMPE Derived from Saffron Natural Monoterpene as Cytotoxic and Multidrug Resistance Reversing Agent in Colon Cancer Cells. International Journal of Molecular Sciences. 2020; 21(20):7529. https://doi.org/10.3390/ijms21207529
Chicago/Turabian StyleŚroda-Pomianek, Kamila, Anna Palko-Łabuz, Andrzej Poła, Mirosława Ferens-Sieczkowska, Olga Wesołowska, and Agata Kozioł. 2020. "TMPE Derived from Saffron Natural Monoterpene as Cytotoxic and Multidrug Resistance Reversing Agent in Colon Cancer Cells" International Journal of Molecular Sciences 21, no. 20: 7529. https://doi.org/10.3390/ijms21207529
APA StyleŚroda-Pomianek, K., Palko-Łabuz, A., Poła, A., Ferens-Sieczkowska, M., Wesołowska, O., & Kozioł, A. (2020). TMPE Derived from Saffron Natural Monoterpene as Cytotoxic and Multidrug Resistance Reversing Agent in Colon Cancer Cells. International Journal of Molecular Sciences, 21(20), 7529. https://doi.org/10.3390/ijms21207529