Long-Chain Saturated Fatty Acids, Palmitic and Stearic Acids, Enhance the Repair of Photosystem II
Abstract
1. Introduction
2. Results
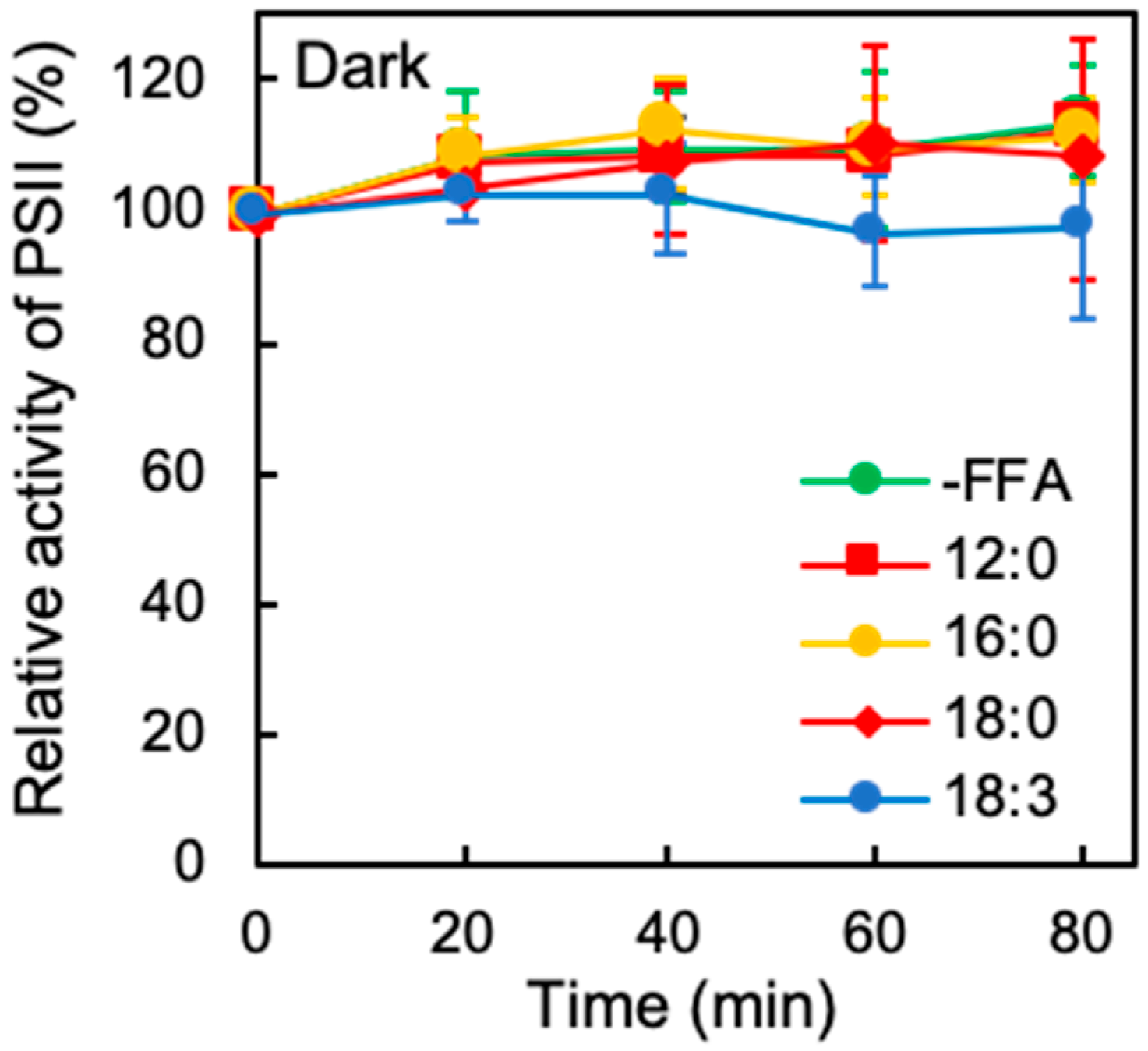
2.1. Effects of Exogenous FFA on the Photoinhibition of PSII Under Strong Light
2.2. Effects of Exogenous FFA on Photodamage to PSII under Strong Light
2.3. Effects of FFA on the De Novo Synthesis of D1 under Strong Light
3. Discussion
3.1. Acceleration of Photoinhibition of PSII by Medium-Chain Fatty Acids and Polyunsaturated Fatty Acids
3.2. Alleviation of Photoinhibition of PSII by Saturated Long Chain Fatty Acids
3.3. Future Perspectives
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Photoinhibition of PSII
4.3. Labeling of Proteins Synthsized De Novo
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FFA | Free fatty acids |
| PSII | Photosystem II |
| GMO | Genetically modified organisms |
| PG | Phosphatidylglycerol |
| DGDG | Digalactosyldiacylglycerol |
| PUFA | Polyunsaturated fatty acids |
| MCFA | Medium-chain fatty acids |
| AAS | Acyl-ACP synthetase |
| ROS | Reactive oxygen species |
References
- Liu, X.; Sheng, J.; Curtiss, R.; Curtiss, R. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef]
- Kato, A.; Takatani, N.; Use, K.; Uesaka, K.; Ikeda, K.; Chang, Y.; Kojima, K.; Aichi, M.; Ihara, K.; Nakahigashi, K.; et al. Identification of a cyanobacterial RND-type efflux system involved in export of free fatty acids. Plant Cell Physiol. 2015, 56, 2467–2477. [Google Scholar] [CrossRef]
- Sakamoto, T.; Delgaizo, V.B.; Bryant, D.A. Growth on urea can trigger death and peroxidation of the cyanobacterium Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol. 1998, 64, 2361–2366. [Google Scholar] [CrossRef]
- Ruffing, A.M.; Trahan, C.A. Biofuel toxicity and mechanisms of biofuel tolerance in three model cyanobacteria. Algal Res. 2014, 5, 121–132. [Google Scholar] [CrossRef]
- Tyystjarvi, E.; Aro, E.M. The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc. Natl. Acad. Sci. USA 1996, 93, 2213–2218. [Google Scholar] [CrossRef]
- Murata, N.; Nishiyama, Y. ATP is a driving force in the repair of photosystem II during photoinhibition. Plant Cell Environ. 2017, 41, 285–299. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta (BBA) Bioenerg. 2006, 1757, 742–749. [Google Scholar] [CrossRef]
- Aro, E.-M.; McCaffery, S.; Anderson, J.M. Photoinhibition and D1 protein degradation in Peas acclimated to different growth irradiances. Plant Physiol. 1993, 103, 835–843. [Google Scholar] [CrossRef]
- Jimbo, H.; Izuhara, T.; Hihara, Y.; Hisabori, T.; Nishiyama, Y. Light-inducible expression of translation factor EF-Tu during acclimation to strong light enhances the repair of photosystem II. Proc. Natl. Acad. Sci. USA 2019, 116, 21268–21273. [Google Scholar] [CrossRef]
- Morris, P.; Nash, G.V.; Hall, D.O. The stability of electron transport in in vitro chloroplast membranes. Photosynth. Res. 1982, 3, 227–240. [Google Scholar] [CrossRef]
- Takatani, N.; Use, K.; Kato, A.; Ikeda, K.; Kojima, K.; Aichi, M.; Maeda, S.-I.; Omata, T. Essential role of acyl-ACP synthetase in acclimation of the cyanobacterium Synechococcus elongatus strain PCC 7942 to High-Light Conditions. Plant Cell Physiol. 2015, 56, 1608–1615. [Google Scholar] [CrossRef]
- Von Berlepsch, S.; Kunz, H.-H.; Brodesser, S.; Fink, P.; Marin, K.; Flügge, U.-I.; Gierth, M. The Acyl-acyl carrier protein synthetase from Synechocystis sp. PCC 6803 mediates fatty acid import. Plant Physiol. 2012, 159, 606–617. [Google Scholar] [CrossRef]
- Kojima, K.; Keta, S.; Uesaka, K.; Kato, A.; Takatani, N.; Ihara, K.; Omata, T.; Aichi, M. A simple method for isolation and construction of markerless cyanobacterial mutants defective in acyl-acyl carrier protein synthetase. Appl. Microbiol. Biotechnol. 2016, 100, 10107–10113. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim. Biophys. Acta (BBA) Gen. Subj. 2007, 1767, 1032–1040. [Google Scholar] [CrossRef]
- Aro, E.-M.; Virgin, I.; Andersson, B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta (BBA) Bioenerg. 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Reduced photoinhibition under low irradiance enhanced Kacip fatimah (Labisia pumila Benth) secondary metabolites, phenyl alanine lyase and antioxidant activity. Int. J. Mol. Sci. 2012, 13, 5290–5306. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol. Plant. 2011, 142, 35–46. [Google Scholar] [CrossRef]
- Laczko-Dobos, H.; Fryčák, P.; Ughy, B.; Domonkos, I.; Wada, H.; Prokai, L.; Gombos, Z. Remodeling of phosphatidylglycerol in Synechocystis PCC6803. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2010, 1801, 163–170. [Google Scholar] [CrossRef]
- Sakurai, I.; Hagio, M.; Gombos, Z.; Tyystjärvi, T.; Paakkarinen, V.; Aro, E.-M.; Wada, H. Requirement of phosphatidylglycerol for maintenance of photosynthetic machinery. Plant Physiol. 2003, 133, 1376–1384. [Google Scholar] [CrossRef]
- Kruse, O.; Schmid, G.H. The Role of phosphatidylglycerol as a functional effector and membrane anchor of the D1-core peptide from photosystem II-particles of the cyanobacterium Oscillatoria chalybea. Z. Naturforsch. C 1995, 50, 380–390. [Google Scholar] [CrossRef]
- Takahashi, S.; Milward, S.E.; Fan, D.-Y.; Chow, W.S.; Badger, M.R. How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol. 2008, 149, 1560–1567. [Google Scholar] [CrossRef]
- Drath, M.; Kloft, N.; Batschauer, A.; Marin, K.; Novak, J.; Forchhammer, K. Ammonia triggers photodamage of photosystem II in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol. 2008, 147, 206–215. [Google Scholar] [CrossRef]
- Chan, T.; Shimizu, Y.; Pospisil, P.; Nijo, N.; Fujiwara, A.; Taninaka, Y.; Ishikawa, T.; Hori, H.; Nanba, D.; Imai, A.; et al. Quality control of photosystem II: Lipid peroxidation accelerates photoinhibition under excessive illumination. PLoS ONE 2012, 7, e52100. [Google Scholar] [CrossRef]
- Frankel, E. Lipid oxidation. Prog. Lipid Res. 1980, 19, 1–22. [Google Scholar] [CrossRef]
- Maeda, H.; Sakuragi, Y.; Bryant, D.A.; DellaPenna, D. Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol. 2005, 138, 1422–1435. [Google Scholar] [CrossRef]
- Inoue, S.; Ejima, K.; Iwai, E.; Hayashi, H.; Appel, J.; Tyystjärvi, E.; Murata, N.; Nishiyama, Y. Protection by α-tocopherol of the repair of photosystem II during photoinhibition in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1807, 236–241. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yang, S.; Tan, X. Photosynthetic conversion of carbon dioxide to oleochemicals by cyanobacteria: Recent advances and future perspectives. Front. Microbiol. 2020, 11, 634. [Google Scholar] [CrossRef]
- Komenda, J.; Kuviková, S.; Granvogl, B.; Eichacker, L.A.; Diner, B.A.; Nixon, P.J. Cleavage after residue Ala352 in the C-terminal extension is an early step in the maturation of the D1 subunit of Photosystem II in Synechocystis PCC 6803. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 829–837. [Google Scholar] [CrossRef]
- Yu, J.; Knoppová, J.; Michoux, F.; Bialek, W.; Cota, E.; Shukla, M.K.; Strašková, A.; Aznar, G.P.; Sobotka, R.; Komenda, J.; et al. Ycf48 involved in the biogenesis of the oxygen-evolving photosystem II complex is a seven-bladed beta-propeller protein. Proc. Natl. Acad. Sci. USA 2018, 115, E7824–E7833. [Google Scholar] [CrossRef]
- Mizusawa, N.; Sakurai, I.; Sato, N.; Wada, H. Lack of digalactosyldiacylglycerol increases the sensitivity of Synechocystis sp. PCC 6803 to high light stress. FEBS Lett. 2009, 583, 718–722. [Google Scholar] [CrossRef]
- Sakurai, I.; Mizusawa, N.; Wada, H.; Sato, N. Digalactosyldiacylglycerol Is required for stabilization of the oxygen-evolving complex in photosystem II. Plant Physiol. 2007, 145, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Wada, H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem. J. 1995, 308, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Wang, Y.; Zhang, Y.; Shu, S.; Sun, J.; Guo, S. Overexpression of transglutaminase from cucumber in tobacco increases salt tolerance through regulation of photosynthesis. Int. J. Mol. Sci. 2019, 20, 894. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kusama, Y.; Li, X.; Takaichi, S.; Nishiyama, Y. Overexpression of orange carotenoid protein protects the repair of PSII under strong light in Synechocystis sp. PCC 6803. Plant Cell Physiol. 2018, 60, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Kusama, Y.; Inoue, S.; Jimbo, H.; Takaichi, S.; Sonoike, K.; Hihara, Y.; Nishiyama, Y. Zeaxanthin and echinenone protect the repair of photosystem II from inhibition by singlet oxygen in Synechocystis sp. PCC 6803. Plant Cell Physiol. 2015, 56, 906–916. [Google Scholar] [CrossRef]
- Jimbo, H.; Yutthanasirikul, R.; Nagano, T.; Hisabori, T.; Hihara, Y.; Nishiyama, Y. Oxidation of translation factor EF-Tu inhibits the repair of photosystem II. Plant Physiol. 2018, 176, 2691–2699. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimbo, H.; Takagi, K.; Hirashima, T.; Nishiyama, Y.; Wada, H. Long-Chain Saturated Fatty Acids, Palmitic and Stearic Acids, Enhance the Repair of Photosystem II. Int. J. Mol. Sci. 2020, 21, 7509. https://doi.org/10.3390/ijms21207509
Jimbo H, Takagi K, Hirashima T, Nishiyama Y, Wada H. Long-Chain Saturated Fatty Acids, Palmitic and Stearic Acids, Enhance the Repair of Photosystem II. International Journal of Molecular Sciences. 2020; 21(20):7509. https://doi.org/10.3390/ijms21207509
Chicago/Turabian StyleJimbo, Haruhiko, Kensuke Takagi, Takashi Hirashima, Yoshitaka Nishiyama, and Hajime Wada. 2020. "Long-Chain Saturated Fatty Acids, Palmitic and Stearic Acids, Enhance the Repair of Photosystem II" International Journal of Molecular Sciences 21, no. 20: 7509. https://doi.org/10.3390/ijms21207509
APA StyleJimbo, H., Takagi, K., Hirashima, T., Nishiyama, Y., & Wada, H. (2020). Long-Chain Saturated Fatty Acids, Palmitic and Stearic Acids, Enhance the Repair of Photosystem II. International Journal of Molecular Sciences, 21(20), 7509. https://doi.org/10.3390/ijms21207509




