Platelet Induced Functional Alteration of CD4+ and CD8+ T Cells in HNSCC
Abstract
1. Introduction
2. Results
2.1. Aggregate Formation Depends on the PLT:PBMC Ratio and Occurs More Frequent in PBMC of Healthy Donors
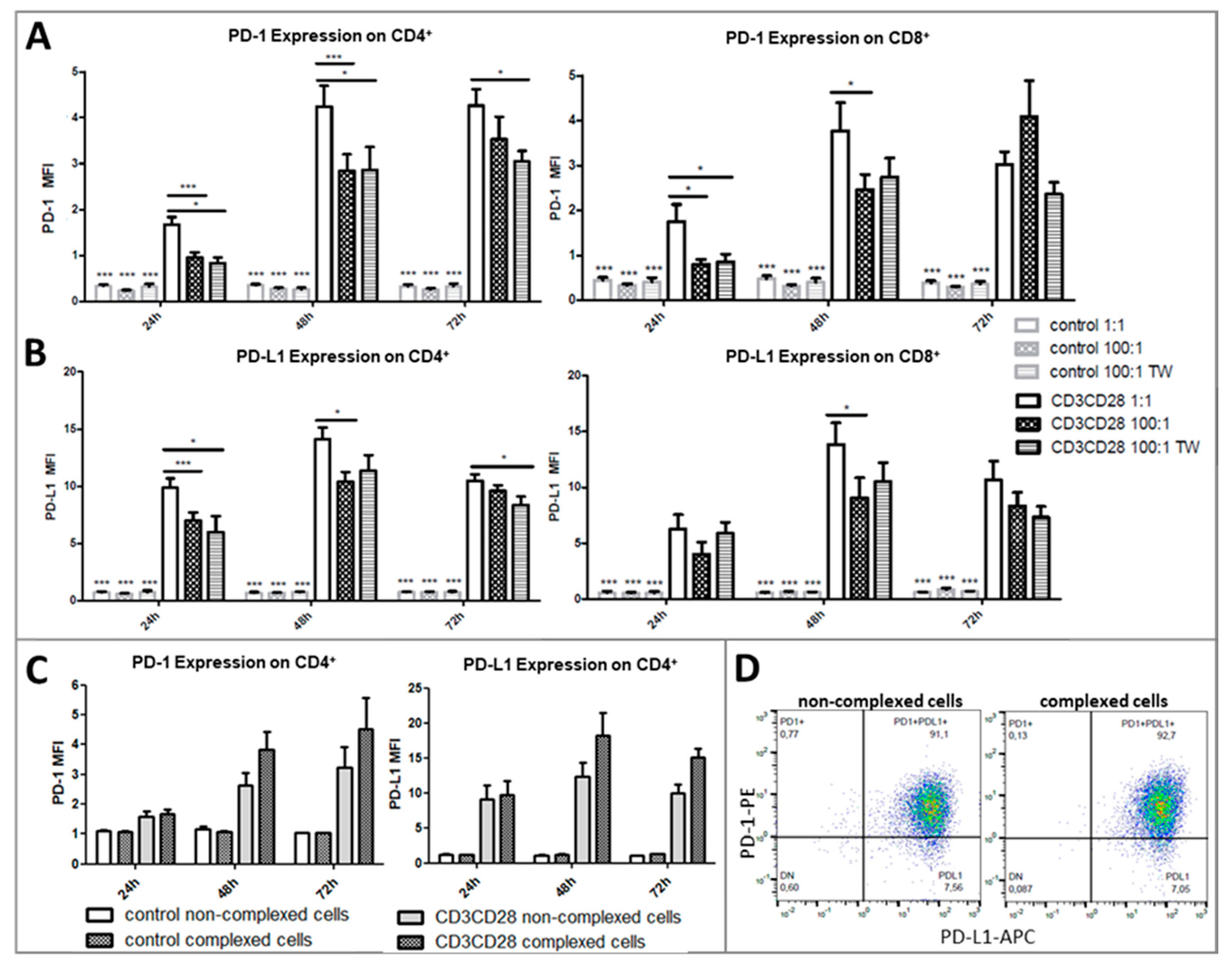
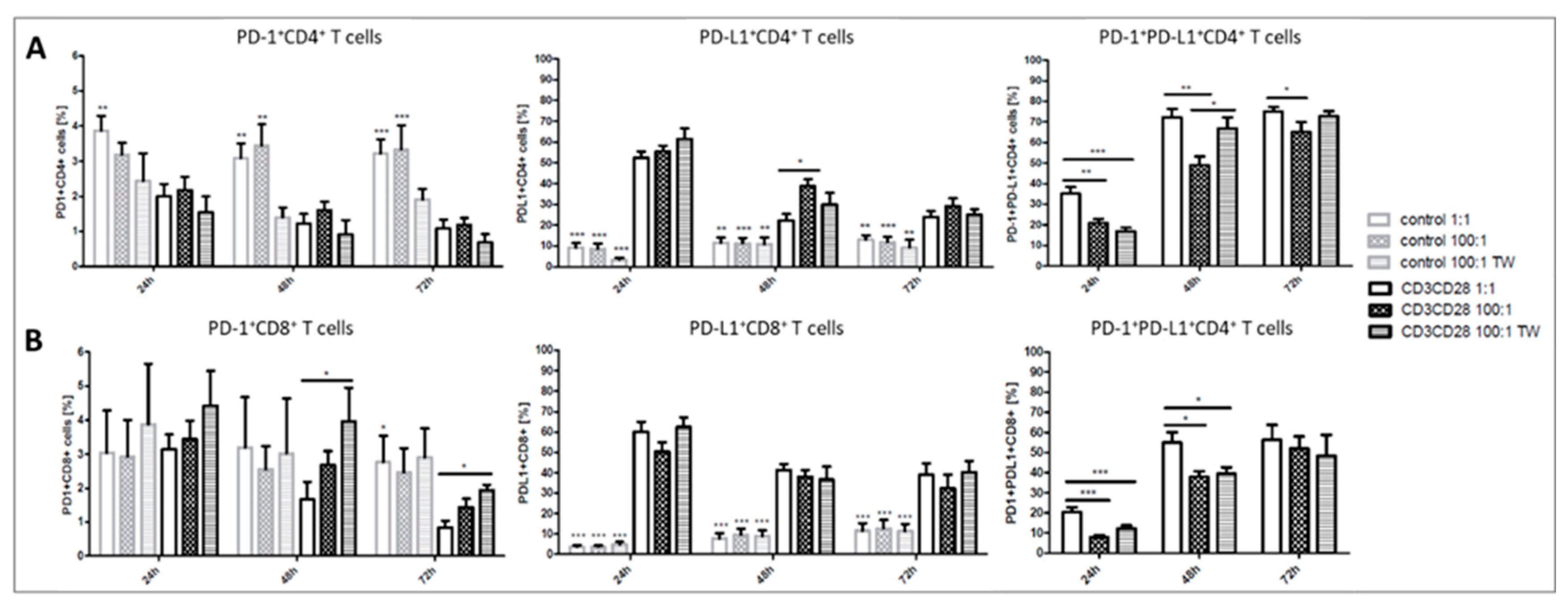
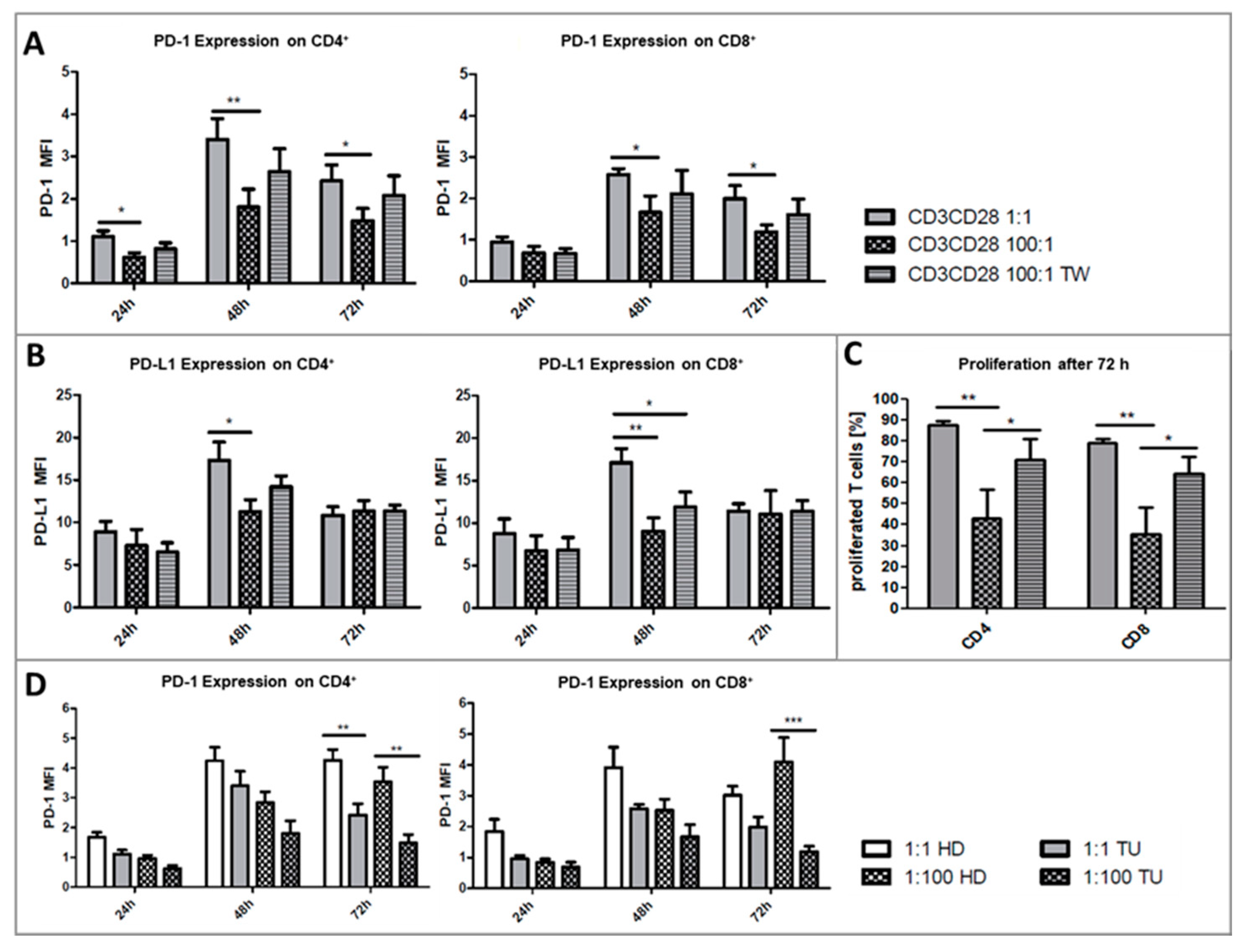
2.2. Platelets Inhibit TCR-Triggered PD-1/PD-L1 Expression on T Cells in PBMC from Healthy Donors
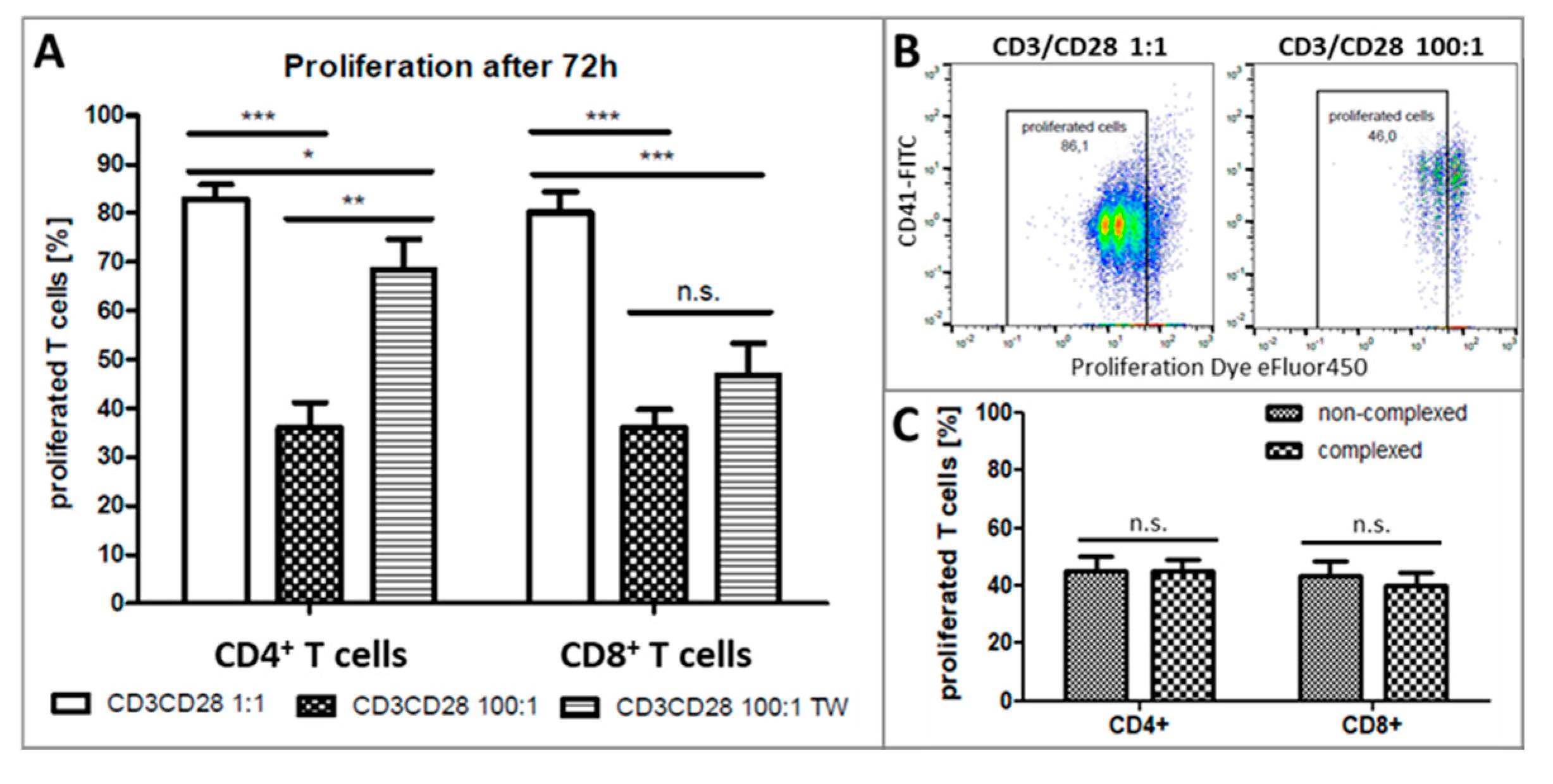
2.3. Platelets Inhibit T-Cell Proliferation in PBMC from Healthy Donors
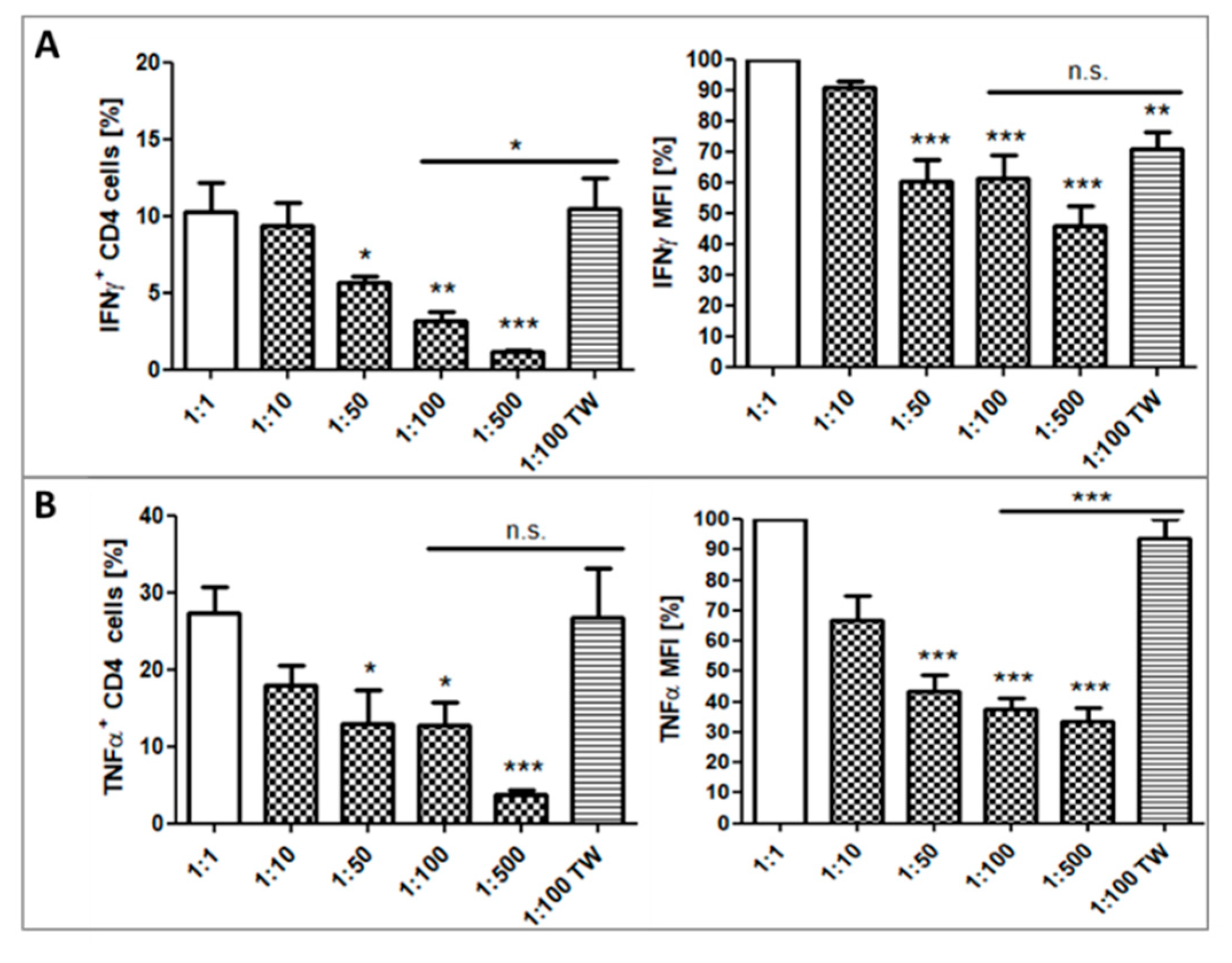
2.4. Impact of Platelets on Cytokine Production of CD4+ T Cells
2.5. Impact of Platelets on PD-1/PD-L1 Expression and Proliferation on T-Cells in PBMC from HNSCC Patients
3. Discussion
4. Materials and Methods
4.1. Blood Collection
4.2. Platelet and PBMC Isolation from Whole Blood
4.3. Cell Count and Purity Assessment of Isolated Cells by Flow Cytometry
4.4. Platelet and PBMC Activation and Co-Culture Experiments
4.5. Investigation of Cytokine Production of CD4+ T Cells
4.6. Flow Cytometry
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ledbetter, J.A.; Imboden, J.B.; Schieven, G.L.; Grosmaire, L.S.; Rabinovitch, P.S.; Lindsten, T.; Thompson, C.B.; June, C.H. CD28 ligation in T-cell activation: Evidence for two signal transduction pathways. Blood 1990, 75, 1531–1539. [Google Scholar] [CrossRef]
- Malissen, B.; Bongrand, P. Early T cell activation: Integrating biochemical, structural, and biophysical cues. Annu. Rev. Immunol. 2015, 33, 539–561. [Google Scholar] [CrossRef]
- Shipkova, M.; Wieland, E. Surface markers of lymphocyte activation and markers of cell proliferation. Clin. Chim. Acta 2012, 413, 1338–1349. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Thompson, C.B.; Lindsten, T.; Ledbetter, J.A.; Kunkel, S.L.; Young, H.A.; Emerson, S.G.; Leiden, J.M.; June, C.H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl. Acad. Sci. USA 1989, 86, 1333–1337. [Google Scholar] [CrossRef]
- Wells, A.; Gudmundsdottir, H.; Turka, L. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Investig. 1997, 100, 3173–3183. [Google Scholar] [CrossRef]
- Chapman, N.; Chi, H. Hallmarks of T-cell Exit from Quiescence. Cancer Immunol. Res. 2018, 6, 502–508. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Anderson, A.; Joller, N.; Kuchroo, V. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Crawford, A.; Wherry, E. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr. Opin. Immunol. 2009, 21, 179–186. [Google Scholar] [CrossRef]
- Kaufmann, D.; Walker, B. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 2009, 182, 5891–5897. [Google Scholar] [CrossRef]
- Keir, M.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Vibhakar, R.; Juan, G.; Traganos, F.; Darzynkiewicz, Z.; Finger, L.R. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp. Cell Res. 1997, 232, 25–28. [Google Scholar] [CrossRef]
- Hofmeyer, K.; Jeon, H.; Zang, X. The PD-1/PD-L1 (B7-H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef]
- Allie, S.; Zhang, W.; Fuse, S.; Usherwood, E.J. Programmed death 1 regulates development of central memory CD8 T cells after acute viral infection. J. Immunol. 2011, 186, 6280–6286. [Google Scholar] [CrossRef]
- Zarour, H.M. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef]
- McKinney, E.F.; Lee, J.C.; Jayne, D.R.W.; Lyons, P.A.; Smith, K.G.C. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015, 523, 612–616. [Google Scholar] [CrossRef]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Weber, C. Platelets as immune cells: Bridging inflammation and cardiovascular disease. Circ. Res. 2007, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Chapman, L.M.; Aggrey, A.A.; Field, D.J.; Srivastava, K.; Ture, S.; Yui, K.; Topham, D.J.; Baldwin, W.M.; Morrell, C.N. Platelets present antigen in the context of MHC class I. J. Immunol. 2012, 189, 916–923. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Li, Y.; Lang, S.; Yougbare, I.; Zhu, G.; Chen, P.; Ni, H. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Adv. Hematol. 2012, 2012, 384685. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.; Italiano, J.J.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Koenen, R. The prowess of platelets in immunity and inflammation. Thromb. Haemost. 2016, 116, 605–612. [Google Scholar] [CrossRef]
- Ponomarev, E. Fresh Evidence for Platelets as Neuronal and Innate Immune Cells: Their Role in the Activation, Differentiation, and Deactivation of Th1, Th17, and Tregs during Tissue Inflammation. Front. Immunol. 2018, 9, 406. [Google Scholar] [CrossRef]
- Meikle, C.K.S.; Kelly, C.A.; Garg, P.; Wuescher, L.M.; Ali, R.A.; Worth, R.G. Cancer and Thrombosis: The Platelet Perspective. Front. Cell Dev. Biol. 2017, 5, 147. [Google Scholar] [CrossRef]
- Joseph, J.E.; Harrison, P.; Mackie, I.J.; Isenberg, D.A.; Machin, S.J. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br. J. Haematol. 2001, 115, 451–459. [Google Scholar] [CrossRef]
- Bunescu, A.; Seideman, P.; Lenkei, R.; Levin, K.; Egberg, N. Enhanced Fcgamma receptor I, alphaMbeta2 integrin receptor expression by monocytes and neutrophils in rheumatoid arthritis: Interaction with platelets. J. Rheumatol. 2004, 31, 2347–2355. [Google Scholar]
- Gros, A.; Ollivier, V.; Ho-Tin-Noé, B. Platelets in inflammation: Regulation of leukocyte activities and vascular repair. Front. Immunol. 2015, 5, 678. [Google Scholar] [CrossRef] [PubMed]
- Totani, L.; Evangelista, V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2357–2361. [Google Scholar] [CrossRef]
- Zamora, C.; Cantó, E.; Nieto, J.C.; Ortiz, M.A.; Diaz-Torné, C.; Diaz-Lopez, C.; Llobet, J.M.; Juarez, C.; Vidal, S. Functional consequences of platelet binding to T lymphocytes in inflammation. J. Leukoc. Biol. 2013, 94, 521–529. [Google Scholar] [CrossRef]
- Polasky, C.; Wallesch, M.; Loyal, K.; Pries, R.; Wollenberg, B. Measurement of leukocyte-platelet aggregates (LPA) by FACS: A comparative analysis. Platelets 2020, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Smyth, S.S.; McEver, R.P.; Weyrich, A.S.; Morrell, C.N.; Hoffman, M.R.; Arepally, G.M.; French, P.A.; Dauerman, H.L.; Becker, R.C. Platelet functions beyond hemostasis. J. Thromb. Haemost. 2009, 7, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Zuchtriegel, G.; Uhl, B.; Puhr-Westerheide, D.; Pörnbacher, M.; Lauber, K.; Krombach, F.; Reichel, C.A. Platelets Guide Leukocytes to Their Sites of Extravasation. PLoS Biol. 2016, 14, e1002459. [Google Scholar] [CrossRef]
- Zamora, C.; Cantó, E.; Nieto, J.C.; Bardina, J.; Diaz-Torné, C.; Moya, P.; Magallares, B.; Ortiz, M.A.; Julià, G.; Juarez, C.; et al. Binding of Platelets to Lymphocytes: A Potential Anti-Inflammatory Therapy in Rheumatoid Arthritis. J. Immunol. 2017, 198, 3099–3108. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, Z.; Stålesen, R.; Hansson, G.K.; Li, N. Platelets provoke distinct dynamics of immune responses by differentially regulating CD4+ T-cell proliferation. J. Thromb. Haemost. 2014, 12, 1156–1165. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, J.W.; Yoo, H.M.; Park, C.H.; Song, K.Y. The Platelet-to-Lymphocyte Ratio versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Ann. Surg. Oncol. 2015, 22, 4363–4370. [Google Scholar] [CrossRef]
- Moschini, M.; Suardi, N.; Pellucchi, F.; Rocchini, L.; La Croce, G.; Capitanio, U.; Briganti, A. Impact of preoperative thrombocytosis on pathological outcomes and survival in patients treated with radical cystectomy for bladder carcinoma. Anticancer Res. 2014, 34, 3225–3230. [Google Scholar]
- Voutsadakis, I. Thrombocytosis as a prognostic marker in gastrointestinal cancers. World J. Gastrointest. Oncol. 2014, 6, 34–40. [Google Scholar] [CrossRef]
- Chadha, A.S.; Kocak-Uzel, E.; Das, P.; Minsky, B.D.; Delclos, M.E.; Mahmood, U.; Guha, S. Paraneoplastic thrombocytosis independently predicts poor prognosis in patients with locally advanced pancreatic cancer. Acta Oncol. 2015, 54, 971–978. [Google Scholar] [CrossRef]
- Ji, Y.; Sheng, L.; Du, X.; Qiu, G.; Su, D. Elevated platelet count is a strong predictor of poor prognosis in stage I non-small cell lung cancer patients. Platelets 2015, 26, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood 5et, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois 1et, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating With the Enemy. Front. Immunol. 2019, 10, 1805. [Google Scholar] [CrossRef]
- Olsson, A.; Cedervall, J. The pro-inflammatory role of platelets in cancer. Platelets 2018, 29, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yousef, G.; Ni, H. Cancer and platelet crosstalk: Opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018, 131, 1777–1789. [Google Scholar] [CrossRef]
- Vieira-de-Abreu, A.; Campbell, R.A.; Weyrich, A.S.; Zimmerman, G.A. Platelets: Versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin. Immunopathol. 2012, 34, 5–30. [Google Scholar] [CrossRef]
- Gudbrandsdottir, S.; Hasselbalch, H.; Nielsen, C. Activated platelets enhance IL-10 secretion and reduce TNF-α secretion by monocytes. J. Immunol. 2013, 191, 4059–4067. [Google Scholar] [CrossRef]
- Green, S.A.; Smith, M.; Hasley, R.B.; Stephany, D.; Harned, A.; Nagashima, K.; Abdullah, S.; Pittaluga, S.; Imamichi, T.; Qin, J.; et al. Activated platelet-T-cell conjugates in peripheral blood of patients with HIV infection: Coupling coagulation/inflammation and T cells. AIDS 2015, 29, 1297–1308. [Google Scholar] [CrossRef]
- Mudd, J.C.; Panigrahi, S.; Kyi, B.; Moon, S.H.; Manion, M.M.; Younes, S.A.; Sieg, S.F.; Funderburg, N.T.; Zidar, D.A.; Lederman, M.M.; et al. Inflammatory Function of CX3CR1+ CD8+ T Cells in Treated HIV Infection Is Modulated by Platelet Interactions. J. Infect. Dis. 2016, 214, 1808–1816. [Google Scholar] [CrossRef]
- Łukasik, Z.M.; Makowski, M.; Makowska, J.S. From blood coagulation to innate and adaptive immunity: The role of platelets in the physiology and pathology of autoimmune disorders. Rheumatol. Int. 2018, 38, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Meikle, C.K.; Meisler, A.J.; Bird, C.M.; Jeffries, J.A.; Azeem, N.; Garg, P.; Crawford, E.L.; Kelly, C.A.; Gao, T.Z.; Wuescher, L.M.; et al. Platelet-T cell aggregates in lung cancer patients: Implications for thrombosis. PLoS ONE 2020, 15, e0236966. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ji, Q.; Hjemdahl, P. Platelet-lymphocyte conjugation differs between lymphocyte subpopulations. J. Thromb. Haemost. 2006, 4, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Dinkla, S.; van Cranenbroek, B.; van der Heijden, W.A.; He, X.; Wallbrecher, R.; Dumitriu, I.E.; van der Ven, A.J.; Bosman, G.J.; Koenen, H.J.; Joosten, I. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood 2016, 127, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Stoiber, D.; Assinger, A. Platelet-Leukocyte Interplay in Cancer Development and Progression. Cells 2020, 9, 855. [Google Scholar] [CrossRef]
- Kral, J.B.; Schrottmaier, W.C.; Salzmann, M.; Assinger, A. Platelet Interaction with Innate Immune Cells. Transfus. Med. Hemother. 2016, 43, 78–88. [Google Scholar] [CrossRef]
- Latchman, Y.E.; Liang, S.C.; Wu, Y.; Chernova, T.; Sobel, R.A.; Klemm, M.; Kuchroo, V.K.; Freeman, G.J.; Sharpe, A.H. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10691–10696. [Google Scholar] [CrossRef]
- Chevolet, I.N.E.S.; Speeckaert, R.; Schreuer, M.; Neyns, B.; Krysko, O.; Bachert, C.; Hennart, B.; Allorge, D.; van Geel, N.; Van Gele, M.; et al. Characterization of the in vivo immune network of IDO, tryptophan metabolism, PD-L1, and CTLA-4 in circulating immune cells in melanoma. Oncoimmunology 2015, 4, e982382. [Google Scholar] [CrossRef]
- Kim, H.R.; Ha, S.J.; Hong, M.H.; Heo, S.J.; Koh, Y.W.; Choi, E.C.; Kim, E.K.; Pyo, K.H.; Jung, I.; Seo, D.; et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci. Rep. 2016, 6, 36956. [Google Scholar] [CrossRef]
- Fang, W.; Chen, Y.; Sheng, J.; Zhou, T.; Zhang, Y.; Zhan, J.; Liu, L.; Huang, J.; Peng, P.; Zhang, L. Association between PD-L1 Expression on Tumour-Infiltrating Lymphocytes and Overall Survival in Patients with Gastric Cancer. J. Cancer 2017, 8, 1579–1585. [Google Scholar] [CrossRef]
- Weber, M.; Wehrhan, F.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Preidl, R.; Neukam, F.W.; Ries, J. PD-L1 expression in tumor tissue and peripheral blood of patients with oral squamous cell carcinoma. Oncotarget 2017, 8, 112584–112597. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carlsson, R.; Comabella, M.; Wang, J.; Kosicki, M.; Carrion, B.; Hasan, M.; Wu, X.; Montalban, X.; Dziegiel, M.H.; et al. FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS. Nat. Med. 2014, 20, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Liao, R.Q.; Tu, H.Y.; Wang, W.J.; Dong, Z.Y.; Huang, S.M.; Guo, W.B.; Gou, L.Y.; Sun, H.W.; Zhang, Q.; et al. Stromal PD-L1-Positive Regulatory T cells and PD-1-Positive CD8-Positive T cells Define the Response of Different Subsets of Non-Small Cell Lung Cancer to PD-1/PD-L1 Blockade Immunotherapy. J. Thorac. Oncol. 2018, 13, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8+ T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef]
- Arrieta, O.; Montes-Servín, E.; Hernandez-Martinez, J.M.; Cardona, A.F.; Casas-Ruiz, E.; Crispín, J.C.; Motola, D.; Flores-Estrada, D.; Barrera, L. Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget 2017, 8, 101994–102005. [Google Scholar] [CrossRef]
- Carter, L.L.; Fouser, L.A.; Jussif, J.; Fitz, L.; Deng, B.; Wood, C.R.; Collins, M.; Honjo, T.; Freeman, G.J.; Carreno, B.M. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur. J. Immunol. 2002, 32, 634–643. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Weyrich, A.; Zimmerman, G. Platelets: Signaling cells in the immune continuum. Trends Immunol. 2004, 25, 489–495. [Google Scholar] [CrossRef]
- Ombrello, C.; Block, R.; Morrell, C. Our expanding view of platelet functions and its clinical implications. J. Cardiovasc. Transl. Res. 2010, 3, 538–546. [Google Scholar] [CrossRef]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M.B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar]
- Rolfes, V.; Idel, C.; Pries, R.; Plötze-Martin, K.; Habermann, J.; Gemoll, T.; Bohnet, S.; Latz, E.; Ribbat-Idel, J.; Franklin, B.S.; et al. PD-L1 is expressed on human platelets and is affected by immune checkpoint therapy. Oncotarget 2018, 9, 27460–27470. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polasky, C.; Wendt, F.; Pries, R.; Wollenberg, B. Platelet Induced Functional Alteration of CD4+ and CD8+ T Cells in HNSCC. Int. J. Mol. Sci. 2020, 21, 7507. https://doi.org/10.3390/ijms21207507
Polasky C, Wendt F, Pries R, Wollenberg B. Platelet Induced Functional Alteration of CD4+ and CD8+ T Cells in HNSCC. International Journal of Molecular Sciences. 2020; 21(20):7507. https://doi.org/10.3390/ijms21207507
Chicago/Turabian StylePolasky, Christina, Franziska Wendt, Ralph Pries, and Barbara Wollenberg. 2020. "Platelet Induced Functional Alteration of CD4+ and CD8+ T Cells in HNSCC" International Journal of Molecular Sciences 21, no. 20: 7507. https://doi.org/10.3390/ijms21207507
APA StylePolasky, C., Wendt, F., Pries, R., & Wollenberg, B. (2020). Platelet Induced Functional Alteration of CD4+ and CD8+ T Cells in HNSCC. International Journal of Molecular Sciences, 21(20), 7507. https://doi.org/10.3390/ijms21207507





