Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer
Abstract
1. Introduction
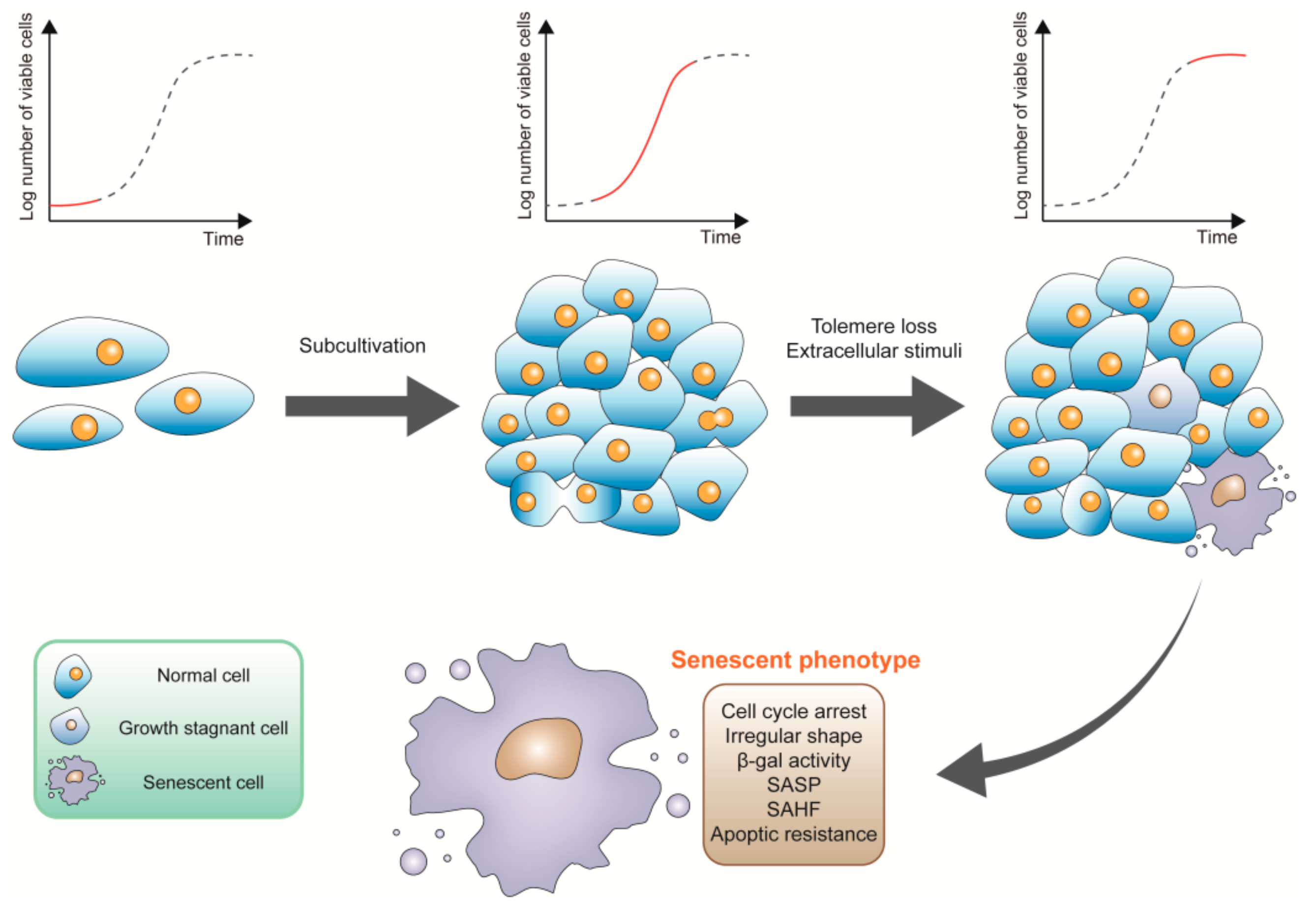
2. The Characteristics of Senescent Cells
3. Natural Polyphenols for Cancer Therapy
3.1. Sources of Polyphenols
3.2. Synergistic Effects of Combined Polyphenols Administration
3.3. The Molecular Mechanisms of Polyphenols for Cancer Therapy
4. Mechanisms of Polyphenols Targeting Senescence for Cancer Therapy and Prevention
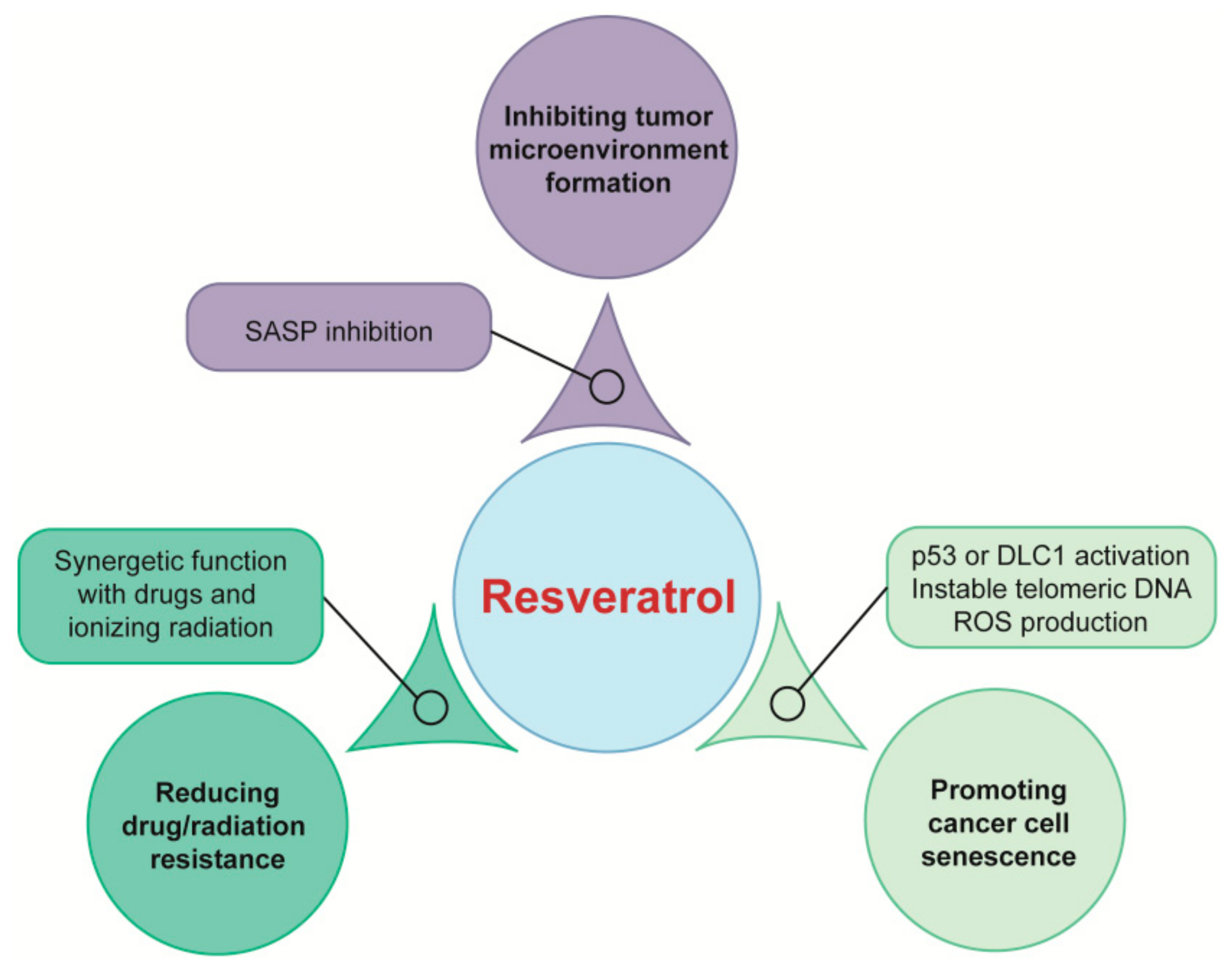
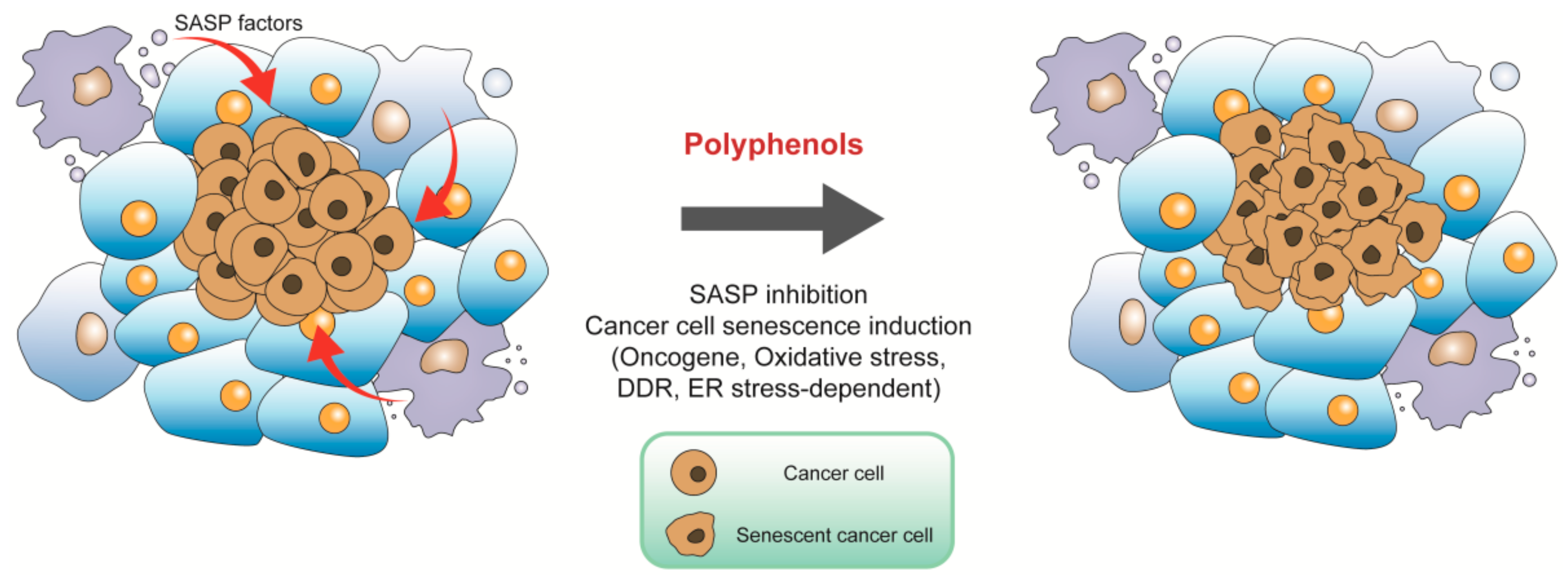
4.1. Targeting Tumor Microenvironment for Cancer Prevention
4.2. Inducing Cancer Cell Senescence for Cancer Therapy
4.2.1. Oncogene-Induced Senescence (OIS)
4.2.2. Oxidative Stress-Induced Senescence
4.2.3. DDR-Induced Senescence
4.2.4. Endoplasmic Reticulum (ER) Stress-Induced Senescence
4.2.5. Other Senescence Mechanisms Induced by Polyphenols
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016. A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [CrossRef]
- Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [CrossRef]
- Bray, F.; Ferlay, J. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Costa, A.; Bonner, M.Y. Use of Polyphenolic Compounds in Dermatologic Oncology. Am. J. Clin. Dermatol. 2016, 17, 369–385. [Google Scholar] [CrossRef]
- Das, J.; Ramani, R. Polyphenol compounds and PKC signaling. Biochim. Biophys. Acta. 2016, 1860, 2107–2121. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, S.; Majeed, M. Curcumin as an Adjunct Therapy and microRNA Modulator in Breast Cancer. Curr. Pharm. Des. 2018, 24, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Meeran, S.M. Epigenetic regulation by selected dietary phytochemicals in cancer chemoprevention. Cancer Lett. 2014, 355, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Penta, D. Epigenetics of Breast Cancer: Clinical Status of Epi-drugs and Phytochemicals. Adv. Exp. Med. Biol. 2019, 1152, 293–310. [Google Scholar] [PubMed]
- Arora, I.; Sharma, M. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef] [PubMed]
- Juli, G.; Oliverio, M. Anti-tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma. Cancers 2019, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, L.; De Luca, A. Impact of natural dietary agents on multiple myeloma prevention and treatment: molecular insights and potential for clinical translation. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Patel, S.N. A novel prodrug of epigallocatechin-3-gallate: differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. 2011, 4, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Vuoso, D.C. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cell growth and survival inhibition. Sci. Rep. 2019, 9, 13045. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, T.; Choi, Y.W. Pinus radiata bark extract induces caspase-independent apoptosis-like cell death in MCF-7 human breast cancer cells. Cell Biol. Toxicol. 2016, 32, 451–464. [Google Scholar] [CrossRef]
- Sharif, T.; Auger, C. Red wine polyphenols cause growth inhibition and apoptosis in acute lymphoblastic leukaemia cells by inducing a redox-sensitive up-regulation of p73 and down-regulation of UHRF1. Eur. J. Cancer. 2010, 46, 983–994. [Google Scholar] [CrossRef]
- Vuong, T.; Mallet, J.F. Role of a polyphenol-enriched preparation on chemoprevention of mammary carcinoma through cancer stem cells and inflammatory pathways modulation. J. Transl. Med. 2016, 14, 13. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Xue, K.S.; Tang, L. Mitigation of Fumonisin Biomarkers by Green Tea Polyphenols in a High-Risk Population of Hepatocellular Carcinoma. Sci. Rep. 2015, 5, 17545. [Google Scholar] [CrossRef]
- Serrano, M.; Lin, A.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Braig, M.; Lee, S. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 2005, 436, 660–665. [Google Scholar] [CrossRef]
- Chen, Z.; Trotman, L.C. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Benatar, T.; Yang, W. IGFBP7 reduces breast tumor growth by induction of senescence and apoptosis pathways. Breast Cancer Res. Treat. 2012, 133, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Takeshita, F. miR-22 represses cancer progression by inducing cellular senescence. J. Cell Biol. 2011, 193, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; He, Z. Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int. J. Oncol. 2013, 43, 1503–1510. [Google Scholar] [CrossRef]
- Sun, R.; Zhai, R. Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Med. 2019. [Google Scholar] [CrossRef]
- Rebbaa, A.; Zheng, X. The role of histone acetylation versus DNA damage in drug-induced senescence and apoptosis. Cell Death Differ. 2006, 13, 1960–1967. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef]
- Nagano, T.; Nakano, M. Identification of cellular senescence-specific genes by comparative transcriptomics. Sci. Rep. 2016, 6, 31758. [Google Scholar] [CrossRef]
- Hayflick LMoorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Hayflick, L.J.E.C.R. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.; Mas, A. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Espin, D.; Canamero, M. Programmed cell senescence during mammalian embryonic development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, R.; Romero, R. Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor. J. Immunol. Res. 2019, 2019, 3128010. [Google Scholar] [CrossRef] [PubMed]
- Del Rey, M.J.; Valin, A. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immun. Ageing 2019, 16, 29. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ingram, J.A. Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J. 2009, 9, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Reyfman, P.A.; Walter, J.M. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Lee SSchmitt, C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar]
- Seluanov, A.; Gorbunova, V. Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53. Mol. Cell Biol. 2001, 21, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995, 55, 2284–2292. [Google Scholar] [PubMed]
- Zhang, J.; Patel, J.M. Enhanced apoptosis in prolonged cultures of senescent porcine pulmonary artery endothelial cells. Mech. Ageing Dev. 2002, 123, 613–625. [Google Scholar] [CrossRef]
- Watanabe, S.; Kawamoto, S. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Ross, E. Representative literature on the phytonutrients category: Phenolic acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef]
- Ji, S.; Zheng, Z. Resveratrol promotes oxidative stress to drive DLC1 mediated cellular senescence in cancer cells. Exp. Cell Res. 2018, 370, 292–302. [Google Scholar] [CrossRef]
- Teponno, R.B.; Kusari, S. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Zamin, L.L.; Filippi-Chiela, E.C. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009, 100, 1655–1662. [Google Scholar] [CrossRef]
- Filippi-Chiela, E.C.; Thome, M.P. Resveratrol abrogates the temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the temozolomide-induced senescence in glioma cells. BMC Cancer 2013, 13, 147. [Google Scholar] [CrossRef]
- Sprouse AAHerbert, B.S. Resveratrol augments paclitaxel treatment in MDA-MB-231 and paclitaxel-resistant MDA-MB-231 breast cancer cells. Anticancer Res. 2014, 34, 5363–5374. [Google Scholar]
- Zhu, Y.; He, W. Resveratrol overcomes gefitinib resistance by increasing the intracellular gefitinib concentration and triggering apoptosis, autophagy and senescence in PC9/G NSCLC cells. Sci. Rep. 2015, 5, 17730. [Google Scholar] [CrossRef]
- Luo, H.; Wang, L. Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells. Int. J. Oncol. 2013, 43, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, D. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Schettino, C. Synergistic Interplay between Curcumin and Polyphenol-Rich Foods in the Mediterranean Diet: Therapeutic Prospects for Neurofibromatosis 1 Patients. Nutrients 2017, 9, 783. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sethi, G. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007, 109, 2293–2302. [Google Scholar] [CrossRef]
- Song, N.R.; Chung, M.Y. Quercetin suppresses invasion and migration of H-Ras-transformed MCF10A human epithelial cells by inhibiting phosphatidylinositol 3-kinase. Food Chem. 2014, 142, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhu, J. Differential effects of polyphenols-enriched extracts from hawthorn fruit peels and fleshes on cell cycle and apoptosis in human MCF-7 breast carcinoma cells. Food Chem. 2013, 141, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Verdura, S.; Cuyas, E. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hou, D. Resveratrol sequentially induces replication and oxidative stresses to drive p53-CXCR2 mediated cellular senescence in cancer cells. Sci. Rep. 2017, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Rusin, M.; Zajkowicz, A. Resveratrol induces senescence-like growth inhibition of U-2 OS cells associated with the instability of telomeric DNA and upregulation of BRCA1. Mech. Ageing Dev. 2009, 130, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Heiss, E.H.; Schilder, Y.D. Chronic treatment with resveratrol induces redox stress-and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells. J. Biol. Chem. 2007, 282, 26759–26766. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, M.S. Resveratrol induces cellular senescence with attenuated mono-ubiquitination of histone H2B in glioma cells. Biochem. Biophys. Res. Commun. 2011, 407, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, J. Resveratrol Represses Pokemon Expression in Human Glioma Cells. Mol. Neurobiol. 2016, 53, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, B. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS ONE 2013, 8, e70627. [Google Scholar] [CrossRef]
- Back, J.H.; Zhu, Y. Resveratrol-mediated downregulation of Rictor attenuates autophagic process and suppresses UV-induced skin carcinogenesis. Photochem. Photobiol. 2012, 88, 1165–1172. [Google Scholar] [CrossRef]
- Chen, R.J.; Wu, P.H. P53-dependent downregulation of hTERT protein expression and telomerase activity induces senescence in lung cancer cells as a result of pterostilbene treatment. Cell Death Dis. 2017, 8, e2985. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y. ATM/CHK/p53 Pathway Dependent Chemopreventive and Therapeutic Activity on Lung Cancer by Pterostilbene. PLoS ONE 2016, 11, e0162335. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, X. Antitumor effects of naturally occurring oligomeric resveratrol derivatives. FASEB J. 2013, 27, 4561–4571. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Sosinska, P. High Potency of a Novel Resveratrol Derivative, 3,3′,4,4′-Tetrahydroxy-trans-stilbene, against Ovarian Cancer Is Associated with an Oxidative Stress-Mediated Imbalance between DNA Damage Accumulation and Repair. Oxid. Med. Cell. Longev. 2015, 2015, 135691. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Jiang, Q. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int. 2015, 80, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, C.X. Beta-naphthoflavone (DB06732) mediates estrogen receptor-positive breast cancer cell cycle arrest through AhR-dependent regulation of PI3K/AKT and MAPK/ERK signaling. Carcinogenesis 2014, 35, 703–713. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L. Baicalin induces cellular senescence in human colon cancer cells via upregulation of DEPP and the activation of Ras/Raf/MEK/ERK signaling. Cell. Death Dis. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Provinciali, M.; Papalini, F. Effect of the silybin-phosphatidylcholine complex (IdB 1016) on the development of mammary tumors in HER-2/neu transgenic mice. Cancer Res. 2007, 67, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Banerjee KMandal, M. Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells. Redox Biol. 2015, 5, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Yuk, H.J. Coumestrol induces senescence through protein kinase CKII inhibition-mediated reactive oxygen species production in human breast cancer and colon cancer cells. Food Chem. 2013, 141, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, X. Isradipine prevents rotenone-induced intracellular calcium rise that accelerates senescence in human neuroblastoma SH-SY5Y cells. Neuroscience 2013, 246, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, S.N.; Kala, R. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp. Cell Res. 2014, 324, 40–53. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, L. Oroxin A inhibits breast cancer cell growth by inducing robust endoplasmic reticulum stress and senescence. Anticancer Drugs. 2016, 27, 204–215. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rasool, R.U. Cristacarpin promotes ER stress-mediated ROS generation leading to premature senescence by activation of p21(waf-1). Age (Dordr) 2016, 38, 62. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Q. Inhibition of glioma growth by flavokawain B is mediated through endoplasmic reticulum stress induced autophagy. Autophagy 2018, 14, 2007–2022. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, S. Involvement of autophagy induction in penta-1,2,3,4,6-O-galloyl-beta-D-glucose-induced senescence-like growth arrest in human cancer cells. Autophagy 2014, 10, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Kim, J.H. Regulation of SIRT1/AMPK axis is critically involved in gallotannin-induced senescence and impaired autophagy leading to cell death in hepatocellular carcinoma cells. Arch. Toxicol. 2018, 92, 241–257. [Google Scholar] [CrossRef]
- Krtolica, A.; Parrinello, S. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ayers, J.L. Senescence-associated tissue microenvironment promotes colon cancer formation through the secretory factor GDF15. Aging Cell. 2019, 18, e13013. [Google Scholar] [CrossRef] [PubMed]
- Menicacci, B.; Margheri, F. Chronic Resveratrol Treatment Reduces the Pro-angiogenic Effect of Human Fibroblast “Senescent-Associated Secretory Phenotype” on Endothelial Colony-Forming Cells: The Role of IL8. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Menicacci, B.; Laurenzana, A. Chronic Resveratrol Treatment Inhibits MRC5 Fibroblast SASP-Related Protumoral Effects on Melanoma Cells. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1187–1195. [Google Scholar] [CrossRef]
- Lim, H.; Park, H. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef]
- Perrott, K.M.; Wiley, C.D. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience 2017, 39, 161–173. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Z. Resveratrol reduces senescence-associated secretory phenotype by SIRT1/NF-kappaB pathway in gut of the annual fish Nothobranchius guentheri. Fish Shellfish Immunol. 2018, 80, 473–479. [Google Scholar] [CrossRef]
- Liu, T.; Ma, L. Resveratrol inhibits age-dependent spontaneous tumorigenesis by SIRT1-mediated post-translational modulations in the annual fish Nothobranchius guentheri. Oncotarget 2017, 8, 55422–55434. [Google Scholar] [CrossRef][Green Version]
- Hickson, L.J.; Langhi Prata, L.G.P. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yin, Y. Downregulation of B-myb promotes senescence via the ROS-mediated p53/p21 pathway, in vascular endothelial cells. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wen, H. Loss of KMT2D induces prostate cancer ROS-mediated DNA damage by suppressing the enhancer activity and DNA binding of antioxidant transcription factor FOXO3. Epigenetics 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yang, A. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS ONE 2013, 8, e60065. [Google Scholar] [CrossRef]
- Ling, X.; Yang, W. TERT regulates telomere-related senescence and apoptosis through DNA damage response in male germ cells exposed to BPDE in vitro and to B[a]P in vivo. Environ. Pollut. 2018, 235, 836–849. [Google Scholar] [CrossRef]
- Chen, J.; Crutchley, J. Identification of a DNA Damage-Induced Alternative Splicing Pathway That Regulates p53 and Cellular Senescence Markers. Cancer Discov. 2017, 7, 766–781. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y. The p38-activated ER stress-ATF6alpha axis mediates cellular senescence. FASEB J. 2019, 33, 2422–2434. [Google Scholar] [CrossRef]
- Qi, X.F.; Chen, Z.Y. FoxO3a suppresses the senescence of cardiac microvascular endothelial cells by regulating the ROS-mediated cell cycle. J. Mol. Cell Cardiol. 2015, 81, 114–126. [Google Scholar] [CrossRef]
- Mowla, S.N.; Lam, E.W. Cellular senescence and aging: The role of B-MYB. Aging Cell. 2014, 13, 773–779. [Google Scholar] [CrossRef]
- Masselink, H.; Vastenhouw, N. B-myb rescues ras-induced premature senescence, which requires its transactivation domain. Cancer Lett. 2001, 171, 87–101. [Google Scholar] [CrossRef]
- Rouaud, F.; Hamouda-Tekaya, N. E2F1 inhibition mediates cell death of metastatic melanoma. Cell Death Dis. 2018, 9, 527. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Q. A gain-of-function senescence bypass screen identifies the homeobox transcription factor DLX2 as a regulator of ATM-p53 signaling. Genes Dev. 2016, 30, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yao, X. Punicalagin induces senescent growth arrest in human papillary thyroid carcinoma BCPAP cells via NF-kappaB signaling pathway. Biomed. Pharmacother. 2018, 103, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Liu, R. Identification and functional characterization of the transcription factor NF-kappaB subunit p65 in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2019, 95, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.E.; Filippi-Chiela, E.C. Inhibition of HDAC increases the senescence induced by natural polyphenols in glioma cells. Biochem. Cell. Biol. 2014, 92, 297–304. [Google Scholar] [CrossRef] [PubMed]

| Classification | Compounds | Concentration | Pathways | Cancer Type | Reference |
|---|---|---|---|---|---|
| Resveratrol and its derivatives | Resveratrol | 25/50 (μM) | p53/CXCR2 | Osteosarcoma Fibrosarcoma Lung cancer | [60] |
| 50 (μM) | BRCA1/DDR | Osteosarcoma | [61] | ||
| 30 (μM) | ROS/DDR | Colon cancer | [62] | ||
| 100 (μM) | ROS/DLC1/SASP | Breast cancer Lung cancer | [46] | ||
| 6/20 (μM) 100 (μM) | Histone H2B Pokemon | Glioma | [63,64] | ||
| 25/50 (μM) | SIRT1 | Gastric cancer | [65] | ||
| 50 (μM) | Rictor/RhoA-GTPase | Nonmelanoma skin cancer | [66] | ||
| Pterostilbene | 2.5/5/50 (μM) | hTERT/DDR | Lung cancer | [67,68] | |
| Pauciflorol B | 10 (μM) | p16/Rb | Lung cancer | [69] | |
| 3,3’,4,4’-tetrahydroxy-trans-stilbene | 10/50/100 (μM) | ROS DDR | Ovarian cancer | [70] | |
| Flavonoids | Quercetin | 50/100/200 (μM) | RAS/MAPK/ERK PI3K/AKT | Glioma | [71] |
| Beta-naphthoflavone | 10 (μM) | PI3K/AKT/cyclinD1/D3 MAPK/ERK | Breast cancer | [72] | |
| Baicalin | 10/20/40 (μM) | DEPP/RAS/Raf/MEK/ERK DEPP/p16/Rb | Colon cancer | [73] | |
| IdB 1016 | 63.2/126.5 (μg/mL) | HER-2/neu p53 | Breast cancer | [74] | |
| Diosmin | 5/10 (μM) | ROS DDR | Breast cancer | [27] | |
| Apigenin | Above 25 (μM) | ROS/RNS p16/cyclin D1/p-Rb p21/cyclin E/p-Rb | Colorectal cancer | [75] | |
| Coumestrol | 50 (μM) | CKII/ROS/p53/p21 | Breast cancer Colon cancer | [76] | |
| Rotenone | 0.4 (μM) | Ca2+/ROS | Neuroblastoma | [77] | |
| Epigallocatechin gallate | 10 (μM) | DDR | Colon cancer | [78] | |
| Oroxin A | 5/10/15/20 (μM) | p38/ER stress | Breast cancer | [79] | |
| Cristacarpin | 1 (μM) | p38/ER stress/ROS/p21 | Pancreatic Breast cancer | [80] | |
| Flavokawain B | 3 (μg/mL) | ATF4/DDIT3/TRIB3/AkT/mTOR | Glioblastoma multiforme | [81] | |
| Phenolic acids | Penta-1,2,3,4,6-Ogalloyl-β-d-glucose | 25 (μM) | UPR | Liver cancer Breast cancer Lung cancer | [82] |
| Gallotannin | 40 (μM) | AMPK/SIRT1 | Hepatocellular carcinoma | [83] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Y.; Wei, J.; Zhao, C.; Li, G. Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer. Int. J. Mol. Sci. 2020, 21, 684. https://doi.org/10.3390/ijms21020684
Bian Y, Wei J, Zhao C, Li G. Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer. International Journal of Molecular Sciences. 2020; 21(2):684. https://doi.org/10.3390/ijms21020684
Chicago/Turabian StyleBian, Yan, Juntong Wei, Changsheng Zhao, and Guorong Li. 2020. "Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer" International Journal of Molecular Sciences 21, no. 2: 684. https://doi.org/10.3390/ijms21020684
APA StyleBian, Y., Wei, J., Zhao, C., & Li, G. (2020). Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer. International Journal of Molecular Sciences, 21(2), 684. https://doi.org/10.3390/ijms21020684




