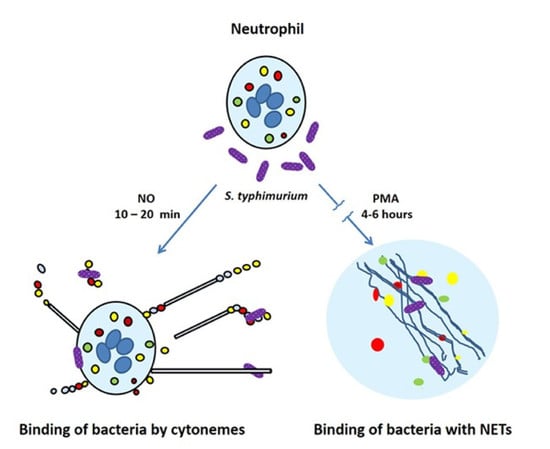
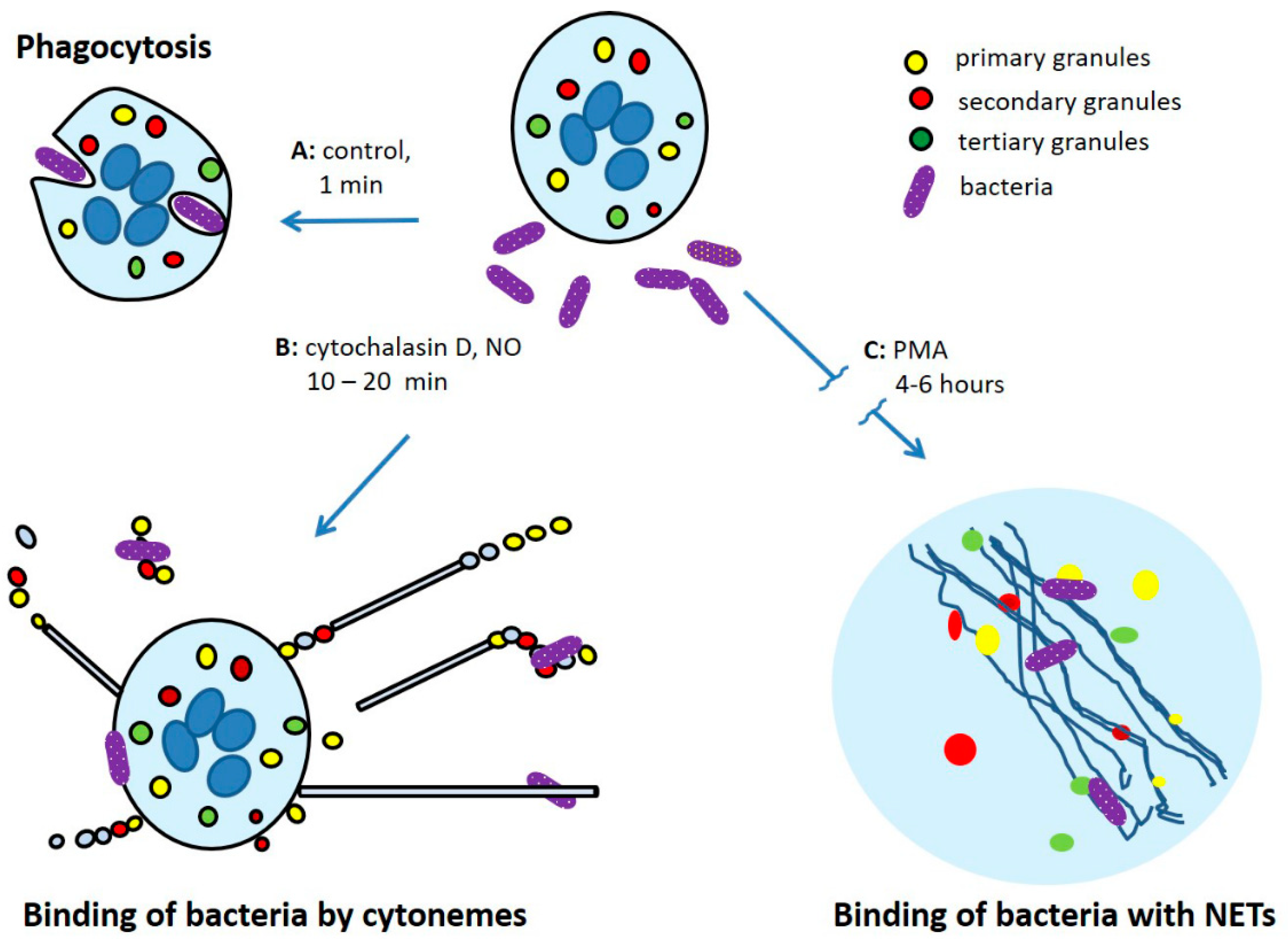
Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes
Abstract
1. Introduction
2. Structure, Composition, and Size of Cytonemes
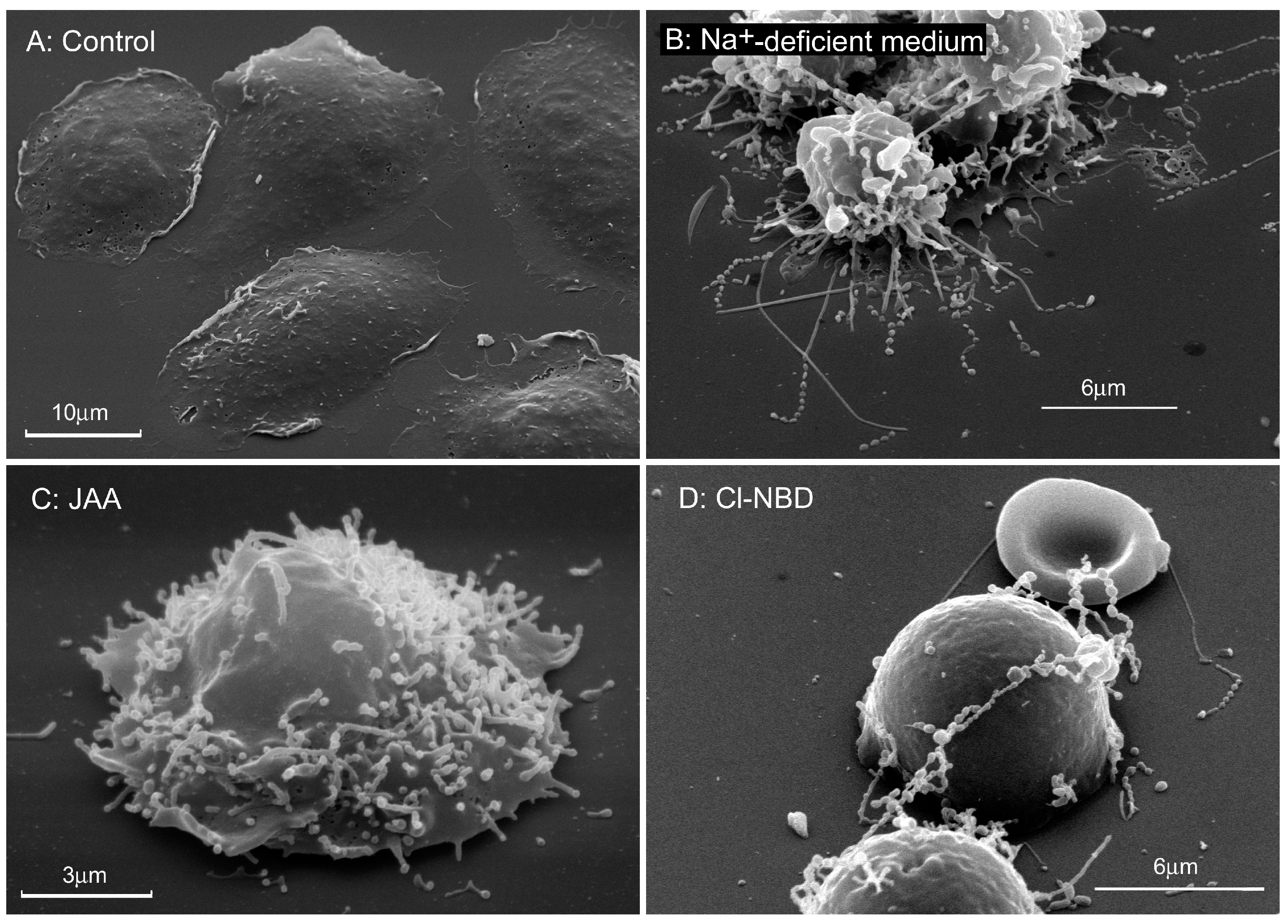
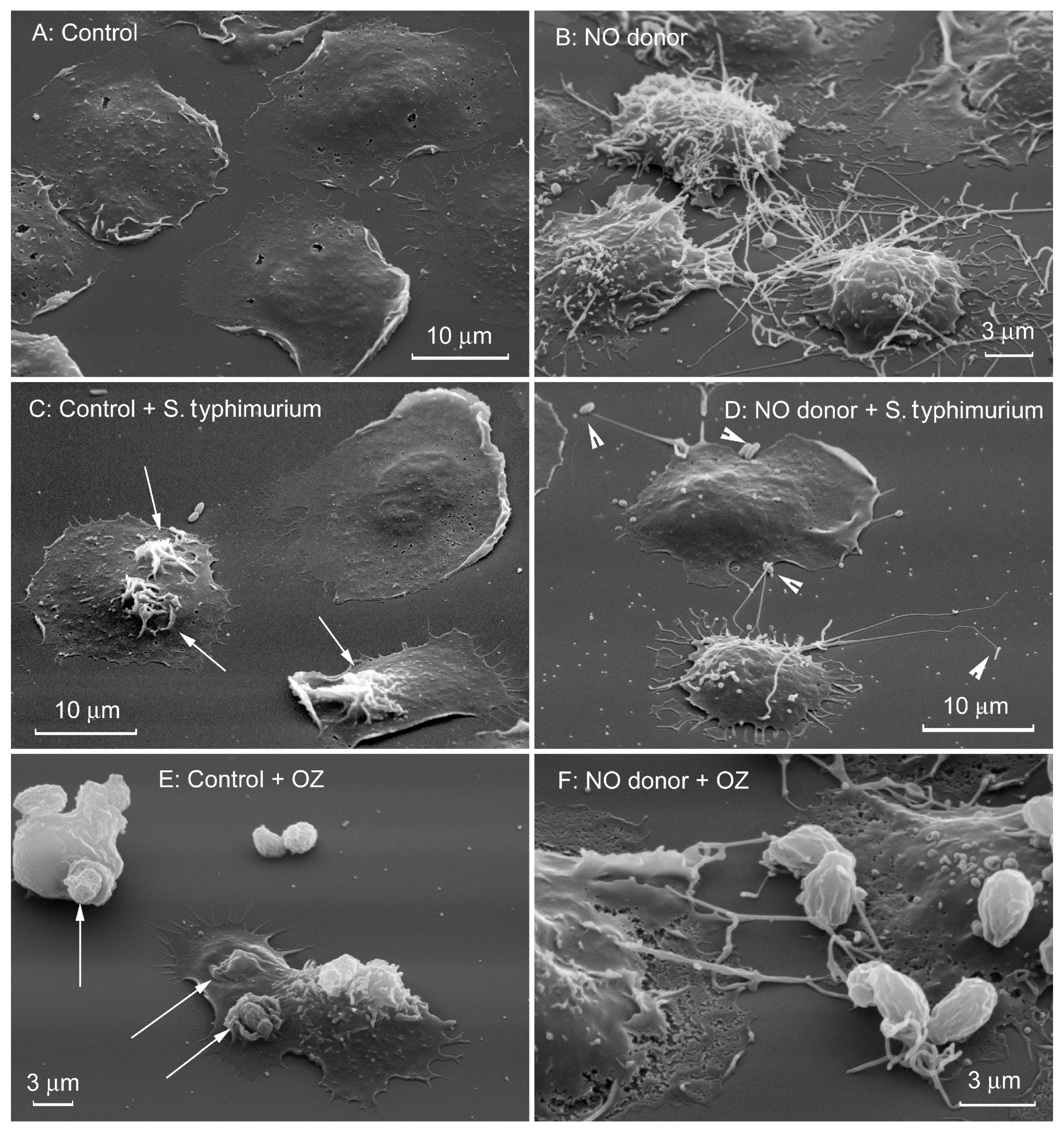
3. Factors that Affect Cytonemes Formation
4. How Cytonemes Scavenge Microbes: Advantages Over Phagocytosis
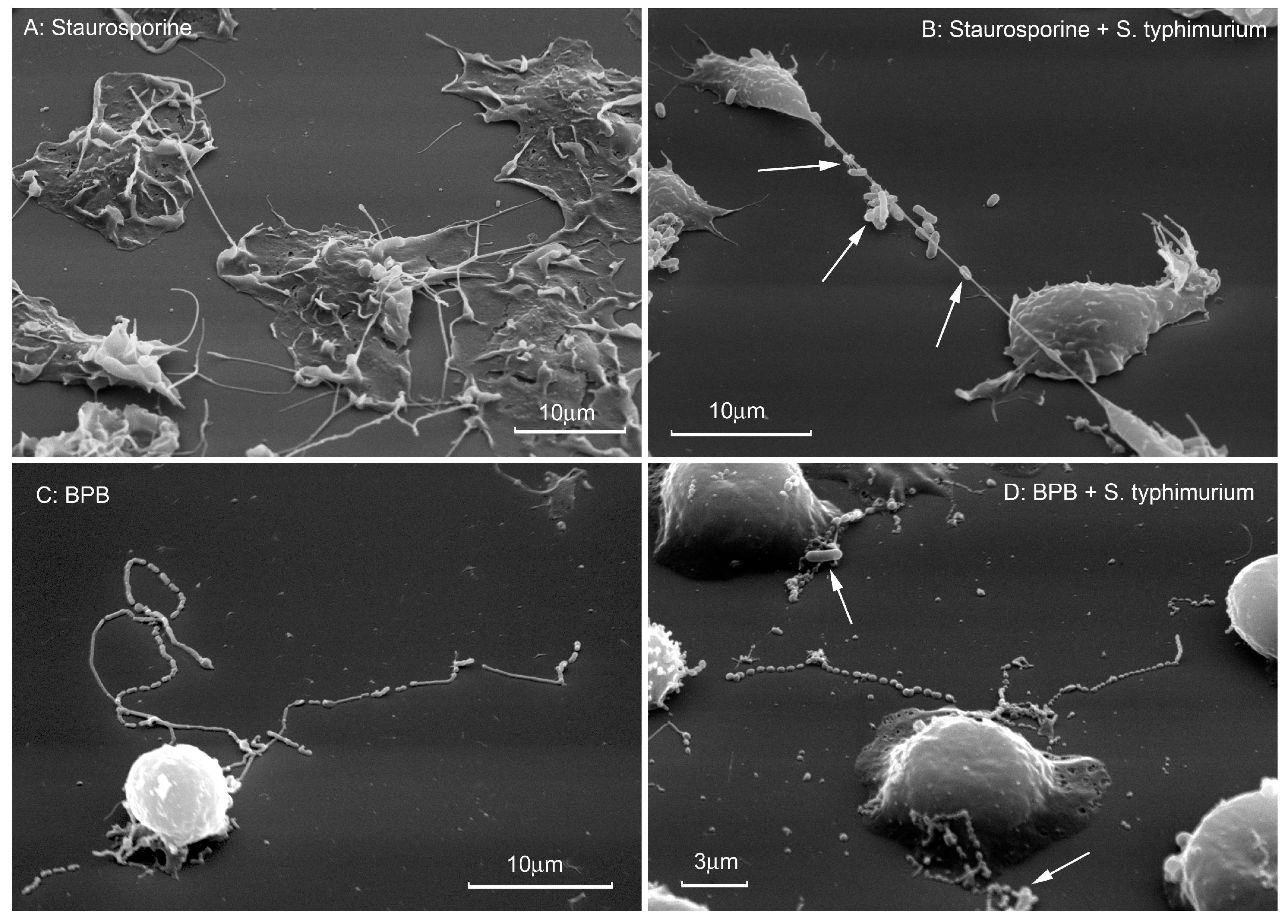
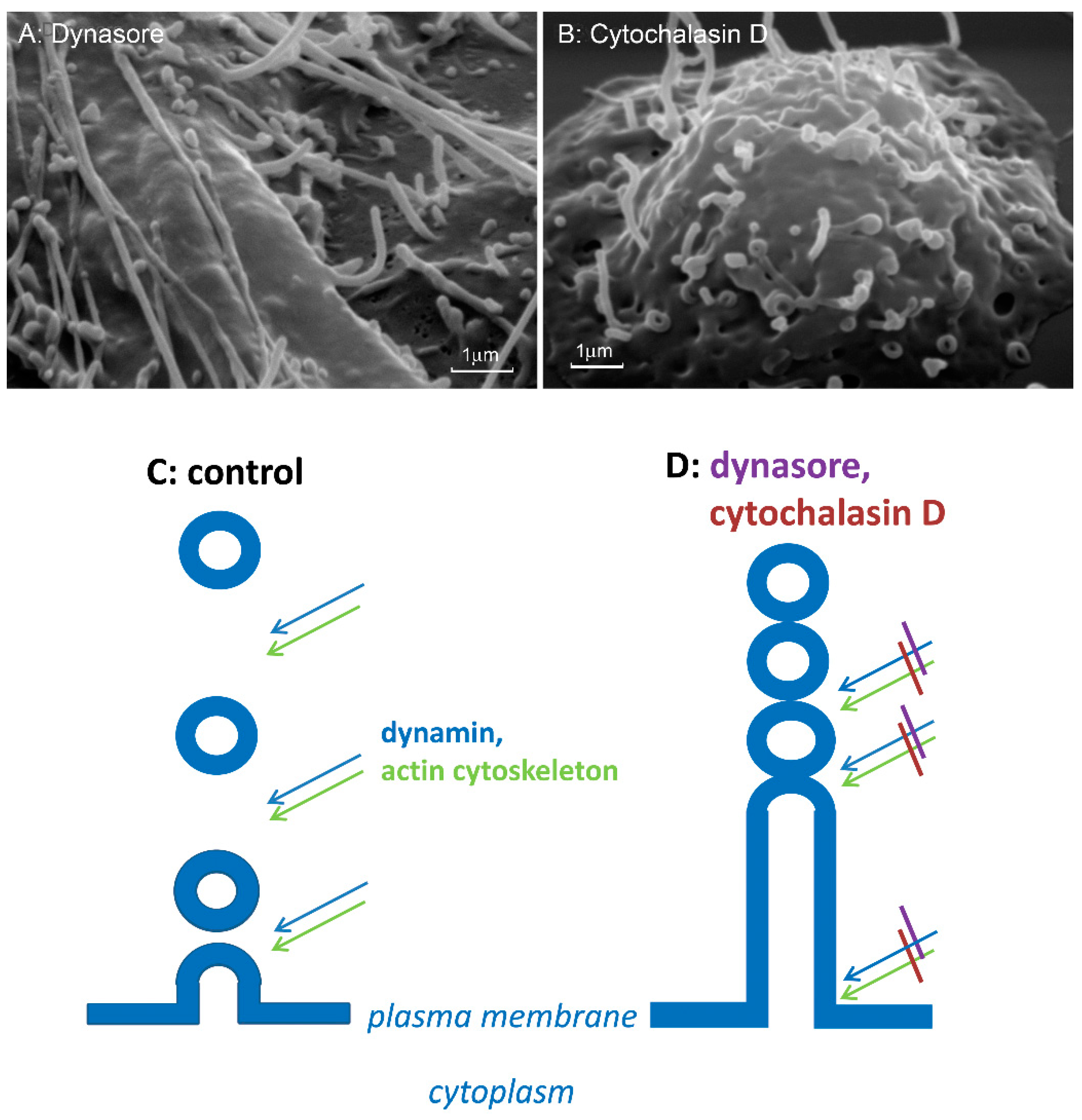
5. The Role of Microbial Alkaloids Depolymerizing Actin in the Formation of Cytonemes
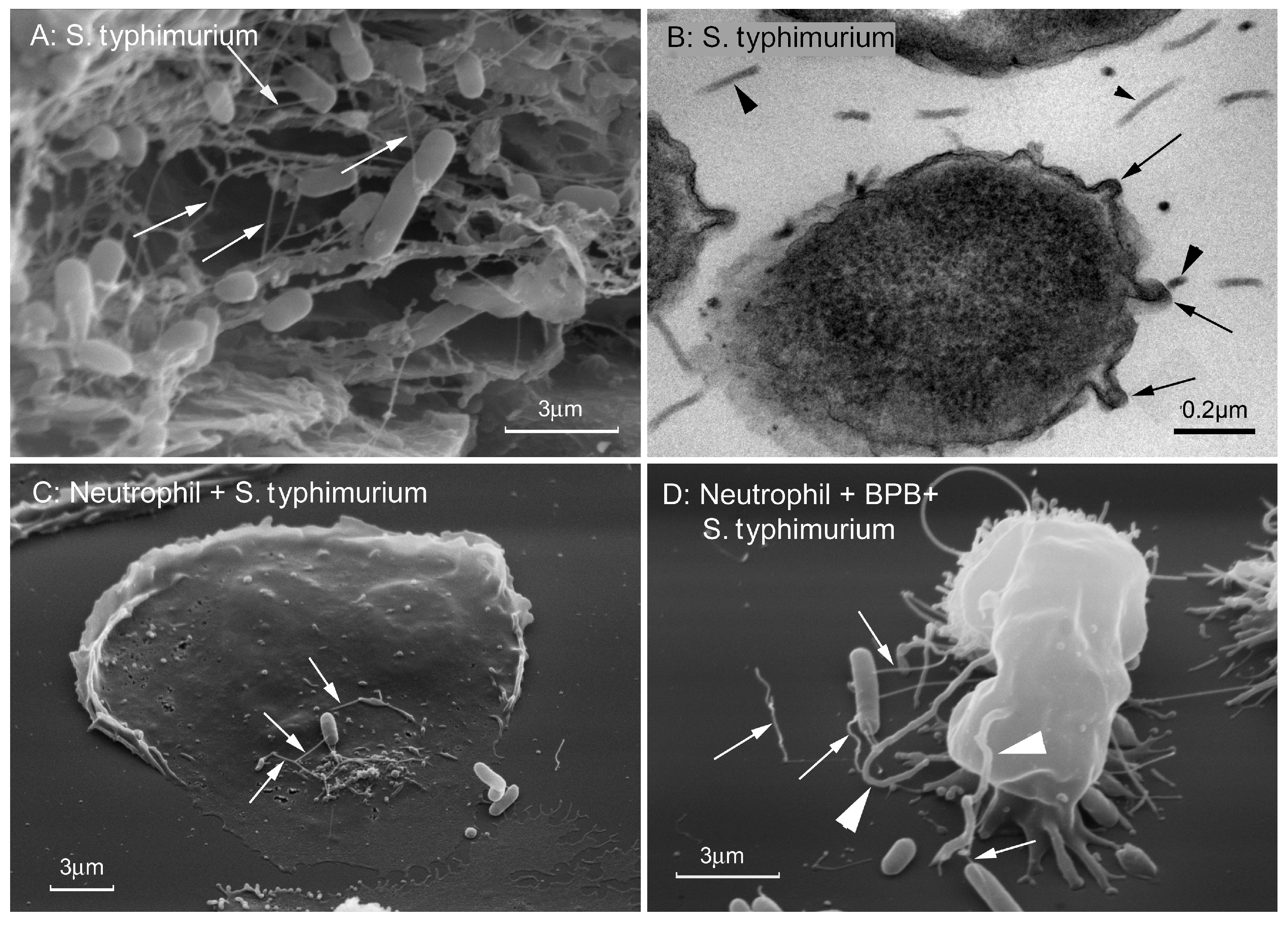
6. Cytonemes in Embryonic, Blood and Other Eukaryotic Cells
7. Cytonemes in Cell Adhesion and Communication of Parasitic Protozoa and Bacteria
8. Structure, Size, and Composition of NETs
9. NETs Formation In-Vitro and In-Vivo
10. How NETs Kill Microbes
11. NETs in Thrombosis, Autoimmunity, and Inflammation
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NETs | neutrophil extracellular traps |
| NO | nitric oxide |
| PMA | phorbol 12-myristate 13-acetate |
| LPS | lipopolysaccharide from Salmonella enterica serovar Typhimurium |
| fMLP | N-formylmethionyl-leucyl-phenylalanine |
| TIMPs | tissue inhibitors of metalloproteinase |
References
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes. Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Lominadze, G.; Powell, D.W.; Luerman, G.C.; Link, A.J.; Ward, R.A.; McLeish, K.R. Proteomic analysis of human neutrophil granules. Mol. Cell. Proteomics 2005, 4, 1503–1521. [Google Scholar] [CrossRef]
- Rorvig, S.; Ostergaard, O.; Heegaard, N.H.; Borregaard, N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: Correlation with transcriptome profiling of neutrophil precursors. J. Leukoc. Biol. 2013, 94, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Sud’ina, G.F.; Ullrich, V. Inhibition of neutrophil spreading during adhesion to fibronectin reveals formation of long tubulovesicular cell extensions (cytonemes). Exp. Cell. Res. 2001, 266, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Romanova, J.M.; Golyshev, S.A.; Stadnichuk, V.I.; Baratova, L.A.; Sud’ina, G.F.; Klein, T. Proteome analysis identified human neutrophil membrane tubulovesicular extensions (cytonemes, membrane tethers) as bactericide trafficking. Biochim. Biophys. Acta 2012, 1820, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Molotkovsky, J.G.; Ullrich, V.; Sud’ina, G.F. Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis. The effect of nitric oxide. Exp. Cell. Res. 2005, 304, 620–629. [Google Scholar] [CrossRef]
- Galkina, S.I.; Romanova, J.M.; Stadnichuk, V.I.; Molotkovsky, J.G.; Sud’ina, G.F.; Klein, T. Nitric oxide-induced membrane tubulovesicular extensions (cytonemes) of human neutrophils catch and hold Salmonella enterica serovar Typhimurium at a distance from the cell surface. FEMS Immunol. Med. Microbiol. 2009, 56, 162–171. [Google Scholar] [CrossRef]
- Galkina, S.I.; Stadnichuk, V.I.; Molotkovsky, J.G.; Romanova, J.M.; Sud’ina, G.F.; Klein, T. Microbial alkaloid staurosporine induces formation of nanometer-wide membrane tubular extensions (cytonemes, membrane tethers) in human neutrophils. Cell Adhes. Migr. 2010, 4, 32–38. [Google Scholar] [CrossRef][Green Version]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Arifulin, E.A.; Stadnichuk, V.I.; Gaponova, T.V.; Baratova, L.A.; Sud’ina, G.F. Inhibition of the, GTPase dynamin or actin depolymerisation initiates outward plasma membrane tubulation/vesiculation (cytoneme formation) in neutrophils. Biol. Cell 2015, 107, 144–158. [Google Scholar] [CrossRef]
- Corriden, R.; Self, T.; Akong-Moore, K.; Nizet, V.; Kellam, B.; Briddon, S.J.; Hill, S.J. Adenosine-A3 receptors in neutrophil microdomains promote the formation of bacteria-tethering cytonemes. EMBO Rep. 2013, 14, 726–732. [Google Scholar] [CrossRef]
- Schwan, C.; Stecher, B.; Tzivelekidis, T.; van Ham, M.; Rohde, M.; Hardt, W.D.; Wehland, J.; Aktories, K. Clostridium difficile toxin, CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009, 5, e1000626. [Google Scholar] [CrossRef] [PubMed]
- Schwan, C.; Kruppke, A.S.; Nolke, T.; Schumacher, L.; Koch-Nolte, F.; Kudryashev, M.; Stahlberg, H.; Aktories, K. Clostridium difficile toxin, CDT hijacks microtubule organization and reroutes vesicle traffic to increase pathogen adherence. Proc. Natl. Acad. Sci. USA 2014, 111, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Schwan, C.; Aktories, K. Formation of Nanotube-Like Protrusions, Regulation of Septin Organization and Re-guidance of Vesicle Traffic by Depolymerization of the Actin Cytoskeleton Induced by Binary Bacterial Protein Toxins. Curr. Top Microbiol. Immunol. 2017, 399, 35–51. [Google Scholar] [PubMed]
- Mezouar, S.; Vitte, J.; Gorvel, L.; Ben Amara, A.; Desnues, B.; Mege, J.L. Mast Cell Cytonemes as a Defense Mechanism against Coxiella burnetii. MBio 2019, 10, e02669-18. [Google Scholar] [CrossRef]
- Szempruch, A.J.; Sykes, S.E.; Kieft, R.; Dennison, L.; Becker, A.C.; Gartrell, A.; Martin, W.J.; Nakayasu, E.S.; Almeida, I.C.; Hajduk, S.L.; et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell 2016, 164, 246–257. [Google Scholar] [CrossRef]
- McCaig, W.D.; Koller, A.; Thanassi, D.G. Production of outer membrane vesicles and outer membrane tubes by Francisella novicida. J. Bacteriol. 2013, 195, 1120–1132. [Google Scholar] [CrossRef]
- Rupp, I.; Sologub, L.; Williamson, K.C.; Scheuermayer, M.; Reininger, L.; Doerig, C.; Eksi, S.; Kombila, D.U.; Frank, M.; Pradel, G. Malaria parasites form filamentous cell-to-cell connections during reproduction in the mosquito midgut. Cell Res. 2011, 21, 683–696. [Google Scholar] [CrossRef]
- Galkina, S.I.; Romanova, J.M.; Bragina, E.E.; Tiganova, I.G.; Stadnichuk, V.I.; Alekseeva, N.V.; Polyakov, V.Y.; Klein, T. Membrane tubules attach Salmonella Typhimurium to eukaryotic cells and bacteria. FEMS Immunol. Med. Microbiol. 2011, 61, 114–124. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Kruger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 2017, 6, e24437. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced, NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, O.E.; Borregaard, N. Neutrophil extracellular traps—The dark side of neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Tilley, D.O.; Zychlinsky, A. Neutrophil Extracellular Traps: The Biology of Chromatin Externalization. Dev. Cell 2018, 44, 542–553. [Google Scholar] [CrossRef]
- Pinegin, B.; Vorobjeva, N.; Pinegin, V. Neutrophil extracellular traps and their role in the development of chronic inflammation and autoimmunity. Autoimmun Rev. 2015, 14, 633–640. [Google Scholar] [CrossRef]
- Boeltz, S.; Amini, P.; Anders, H.J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to, NET: Current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef]
- Ramirez-Weber, F.A.; Kornberg, T.B. Cytonemes: Cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 1999, 97, 599–607. [Google Scholar] [CrossRef]
- Galkina, S.I.; Sud’ina, G.F.; Klein, T. Metabolic regulation of neutrophil spreading, membrane tubulovesicular extensions (cytonemes) formation and intracellular pH upon adhesion to fibronectin. Exp. Cell Res. 2006, 312, 2568–2579. [Google Scholar] [CrossRef]
- Galkina, S.I.; Fedorova, N.V.; Stadnichuk, V.I.; Sud’ina, G.F. Membrane tubulovesicular extensions (cytonemes): Secretory and adhesive cellular organelles. Cell Adhes. Migr. 2013, 7, 174–186. [Google Scholar] [CrossRef]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Arifulin, E.A.; Stadnichuk, V.I.; Baratova, L.A.; Sud’ina, G.F. Mold Alkaloid Cytochalasin D Modifies the Morphology and Secretion of fMLP-, LPS-, or, PMA-Stimulated Neutrophils upon Adhesion to Fibronectin. Mediat. Inflamm. 2017, 2017, 4308684. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995, 268, L699–L722. [Google Scholar] [CrossRef] [PubMed]
- Bang, I.S.; Liu, L.; Vazquez-Torres, A.; Crouch, M.L.; Stamler, J.S.; Fang, F.C. Maintenance of nitric oxide and redox homeostasis by the salmonella flavohemoglobin hmp. J. Biol. Chem. 2006, 281, 28039–28047. [Google Scholar] [CrossRef] [PubMed]
- Stevanin, T.M.; Poole, R.K.; Demoncheaux, E.A.; Read, R.C. Flavohemoglobin Hmp protects Salmonella enterica serovar typhimurium from nitric oxide-related killing by human macrophages. Infect. Immun. 2002, 70, 4399–4405. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.R., Jr. Nitric oxide: A brief overview of chemical and physical properties relevant to therapeutic applications. Future Sci. OA 2015, 1, FSO59. [Google Scholar] [CrossRef]
- Wallerath, T.; Gath, I.; Aulitzky, W.E.; Pollock, J.S.; Kleinert, H.; Forstermann, U. Identification of the NO synthase isoforms expressed in human neutrophil granulocytes, megakaryocytes and platelets. Thromb. Haemost. 1997, 77, 163–167. [Google Scholar] [CrossRef]
- Greenberg, S.S.; Ouyang, J.; Zhao, X.; Giles, T.D. Human and rat neutrophils constitutively express neural nitric oxide synthase mRNA. Nitric Oxide. 1998, 2, 203–212. [Google Scholar] [CrossRef]
- Sessa, W.C. The nitric oxide synthase family of proteins. J. Vasc. Res. 1994, 31, 131–143. [Google Scholar] [CrossRef]
- Alam, M.S.; Akaike, T.; Okamoto, S.; Kubota, T.; Yoshitake, J.; Sawa, T.; Miyamoto, Y.; Tamura, F.; Maeda, H. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect Immun. 2002, 70, 3130–3142. [Google Scholar] [CrossRef]
- Klink, M.; Cedzynski, M.; St Swierzko, A.; Tchorzewski, H.; Sulowska, Z. Involvement of nitric oxide donor compounds in the bactericidal activity of human neutrophils in vitro. J. Med. Microbiol. 2003, 52, 303–308. [Google Scholar] [CrossRef]
- Vazquez-Torres, A.; Jones-Carson, J.; Mastroeni, P.; Ischiropoulos, H.; Fang, F.C. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 2000, 192, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Frawley, E.R.; Karlinsey, J.E.; Singhal, A.; Libby, S.J.; Doulias, P.T.; Ischiropoulos, H.; Fang, F.C. Nitric Oxide Disrupts Zinc Homeostasis in Salmonella enterica Serovar Typhimurium. MBio 2018, 9, e01040-18. [Google Scholar] [CrossRef] [PubMed]
- Iovine, N.M.; Pursnani, S.; Voldman, A.; Wasserman, G.; Blaser, M.J.; Weinrauch, Y. Reactive nitrogen species contribute to innate host defense against Campylobacter jejuni. Infect. Immun. 2008, 76, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Urbano, R.; Karlinsey, J.E.; Libby, S.J.; Doulias, P.T.; Ischiropoulos, H.; Warheit-Niemi, H.I.; Liggitt, D.H.; Horswill, A.R.; Fang, F.C. Host Nitric Oxide Disrupts Microbial Cell-to-Cell Communication to Inhibit Staphylococcal Virulence. Cell Host Microbe 2018, 23, 594–606.e7. [Google Scholar] [CrossRef]
- Mannick, J.B. Immunoregulatory and antimicrobial effects of nitrogen oxides. Proc. Am. Thorac. Soc. 2006, 3, 161–165. [Google Scholar] [CrossRef]
- Zaki, M.H.; Akuta, T.; Akaike, T. Nitric oxide-induced nitrative stress involved in microbial pathogenesis. J. Pharmacol. Sci. 2005, 98, 117–129. [Google Scholar] [CrossRef]
- Zagryazhskaya, A.N.; Lindner, S.C.; Grishina, Z.V.; Galkina, S.I.; Steinhilber, D.; Sud’ina, G.F. Nitric oxide mediates distinct effects of various LPS chemotypes on phagocytosis and leukotriene synthesis in human neutrophils. Int. J. Biochem. Cell Biol. 2010, 42, 921–931. [Google Scholar] [CrossRef]
- Machado, J.D.; Segura, F.; Brioso, M.A.; Borges, R. Nitric oxide modulates a late step of exocytosis. J. Biol. Chem. 2000, 275, 20274–20279. [Google Scholar] [CrossRef]
- Matsushita, K.; Morrell, C.N.; Cambien, B.; Yang, S.X.; Yamakuchi, M.; Bao, C.; Hara, M.R.; Quick, R.A.; Cao, W.; O’Rourke, B.; et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 2003, 115, 139–150. [Google Scholar] [CrossRef]
- Morrell, C.N.; Matsushita, K.; Chiles, K.; Scharpf, R.B.; Yamakuchi, M.; Mason, R.J.; Bergmeier, W.; Mankowski, J.L.; Baldwin, W.M.; Faraday, N.; et al. Regulation of platelet granule exocytosis by S-nitrosylation. Proc. Natl. Acad. Sci. USA 2005, 102, 3782–3787. [Google Scholar] [CrossRef]
- Ferlito, M.; Irani, K.; Faraday, N.; Lowenstein, C.J. Nitric oxide inhibits exocytosis of cytolytic granules from lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11689–11694. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, C.J. Nitric oxide regulation of protein trafficking in the cardiovascular system. Cardiovasc. Res. 2007, 75, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Parra, K.J.; Kane, P.M. Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol. Cell Biol. 1998, 18, 7064–7074. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhou, A.; Al-Lamki, R.S.; Karet, F.E. The a-subunit of the V-type H+-ATPase interacts with phosphofructokinase-1 in humans. J. Biol. Chem. 2003, 278, 20013–20018. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Sautin, Y.Y.; Holliday, L.S.; Gluck, S.L. The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H+-ATPase. J. Biol. Chem. 2004, 279, 8732–8739. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Hallak, H.; de Boitte, A.; Lapetina, E.G.; Brune, B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 1999, 274, 9427–9430. [Google Scholar] [CrossRef]
- Wu, K.; Aoki, C.; Elste, A.; Rogalski-Wilk, A.A.; Siekevitz, P. The synthesis of ATP by glycolytic enzymes in the postsynaptic density and the effect of endogenously generated nitric oxide. Proc. Natl. Acad. Sci. USA 1997, 94, 13273–13278. [Google Scholar] [CrossRef]
- Forgac, M. The vacuolar H+-ATPase of clathrin-coated vesicles is reversibly inhibited by S-nitrosoglutathione. J. Biol. Chem. 1999, 274, 1301–1305. [Google Scholar] [CrossRef]
- Decker, B.L.; Wickner, W.T. Enolase activates homotypic vacuole fusion and protein transport to the vacuole in yeast. J. Biol. Chem. 2006, 281, 14523–14528. [Google Scholar] [CrossRef]
- Glaser, P.E.; Gross, R.W. Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3-phosphate dehydrogenase: Discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry 1995, 34, 12193–12203. [Google Scholar] [CrossRef]
- Glaser, P.E.; Han, X.; Gross, R.W. Tubulin is the endogenous inhibitor of the glyceraldehyde 3-phosphate dehydrogenase isoform that catalyzes membrane fusion: Implications for the coordinated regulation of glycolysis and membrane fusion. Proc. Natl. Acad. Sci. USA 2002, 99, 14104–14109. [Google Scholar] [CrossRef] [PubMed]
- Hessler, R.J.; Blackwood, R.A.; Brock, T.G.; Francis, J.W.; Harsh, D.M.; Smolen, J.E. Identification of glyceraldehyde-3-phosphate dehydrogenase as a Ca2+-dependent fusogen in human neutrophil cytosol. J. Leukoc. Biol. 1998, 63, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hirano, Y.; Inomata, A.; Yokota, S.; Miyachi, K.; Kaneda, M.; Umeda, M.; Furukawa, K.; Omata, S.; Horigome, T. Participation of a fusogenic protein, glyceraldehyde-3-phosphate dehydrogenase, in nuclear membrane assembly. J. Biol. Chem. 2003, 278, 20395–20404. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Bayer, M.J.; Buhler, S.; Andersen, J.S.; Mann, M.; Mayer, A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 2001, 409, 581–588. [Google Scholar] [CrossRef]
- Bayer, M.J.; Reese, C.; Buhler, S.; Peters, C.; Mayer, A. Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J. Cell Biol. 2003, 162, 211–222. [Google Scholar] [CrossRef]
- Strasser, B.; Iwaszkiewicz, J.; Michielin, O.; Mayer, A. The V-ATPase proteolipid cylinder promotes the lipid-mixing stage of SNARE-dependent fusion of yeast vacuoles. EMBO J. 2011, 30, 4126–4141. [Google Scholar] [CrossRef]
- Desfougeres, Y.; Vavassori, S.; Rompf, M.; Gerasimaite, R.; Mayer, A. Organelle acidification negatively regulates vacuole membrane fusion in vivo. Sci. Rep. 2016, 6, 29045. [Google Scholar] [CrossRef]
- Couoh-Cardel, S.; Hsueh, Y.C.; Wilkens, S.; Movileanu, L. Yeast V-ATPase Proteolipid Ring Acts as a Large-conductance Transmembrane Protein Pore. Sci. Rep. 2016, 6, 24774. [Google Scholar] [CrossRef]
- Baars, T.L.; Petri, S.; Peters, C.; Mayer, A. Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Mol. Biol. Cell 2007, 18, 3873–3882. [Google Scholar] [CrossRef]
- Clancy, R.; Leszczynska, J.; Amin, A.; Levartovsky, D.; Abramson, S.B. Nitric oxide stimulates ADP ribosylation of actin in association with the inhibition of actin polymerization in human neutrophils. J. Leukoc. Biol. 1995, 58, 196–202. [Google Scholar] [CrossRef]
- Jog, N.R.; Rane, M.J.; Lominadze, G.; Luerman, G.C.; Ward, R.A.; McLeish, K.R. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am. J. Physiol. Cell Physiol. 2007, 292, C1690–C1700. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Lo, A.; Logan, M.R.; Lacy, P.; Eitzen, G. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am. J. Physiol. Cell Physiol. 2008, 295, C1354–C1365. [Google Scholar] [CrossRef] [PubMed]
- Muallem, S.; Kwiatkowska, K.; Xu, X.; Yin, H.L. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol. 1995, 128, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987, 105, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.P.; Marks, P.G.; Wang, J.Y.; Falls, D.L.; Badwey, J.A. A protein kinase from neutrophils that specifically recognizes Ser-3 in cofilin. J. Biol. Chem. 2000, 275, 2869–2876. [Google Scholar] [CrossRef]
- Rosales, C.; Jones, S.L.; McCourt, D.; Brown, E.J. Bromophenacyl bromide binding to the actin-bundling protein l-plastin inhibits inositol trisphosphate-independent increase in Ca2+ in human neutrophils. Proc. Natl. Acad. Sci. USA 1994, 91, 3534–3538. [Google Scholar] [CrossRef]
- Kornberg, T.B.; Roy, S. Cytonemes as specialized signaling filopodia. Development 2014, 141, 729–736. [Google Scholar] [CrossRef]
- Casas-Tinto, S.; Portela, M. Cytonemes, Their Formation, Regulation, and Roles in Signaling and Communication in Tumorigenesis. Int. J. Mol. Sci. 2019, 20, 5641. [Google Scholar] [CrossRef]
- Gonzalez-Mendez, L.; Gradilla, A.C.; Guerrero, I. The cytoneme connection: Direct long-distance signal transfer during development. Development 2019, 146, dev174607. [Google Scholar] [CrossRef]
- Ji, Y.; Ferracci, G.; Warley, A.; Ward, M.; Leung, K.Y.; Samsuddin, S.; Leveque, C.; Queen, L.; Reebye, V.; Pal, P.; et al. Beta-Actin regulates platelet nitric oxide synthase 3 activity through interaction with heat shock protein. Proc. Natl. Acad. Sci. USA 2007, 104, 8839–8844. [Google Scholar] [CrossRef]
- Su, Y.; Edwards-Bennett, S.; Bubb, M.R.; Block, E.R. Regulation of endothelial nitric oxide synthase by the actin cytoskeleton. Am. J. Physiol. Cell Physiol. 2003, 284, C1542–C1549. [Google Scholar] [CrossRef] [PubMed]
- Kondrikov, D.; Fonseca, F.V.; Elms, S.; Fulton, D.; Black, S.M.; Su, Y. {beta}-actin association with endothelial, N.O.; synthase modulates, N.O.; and superoxide generation from the enzyme. J. Biol. Chem. 2009, 285, 4319–4327. [Google Scholar] [CrossRef]
- Mi, Q.; Chen, N.; Shaifta, Y.; Xie, L.; Lu, H.; Liu, Z.; Chen, Q.; Hamid, C.; Becker, S.; Ji, Y.; et al. Activation of endothelial nitric oxide synthase is dependent on its interaction with globular actin in human umbilical vein endothelial cells. J. Mol. Cell Cardiol. 2011, 51, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Fels, J.; Jeggle, P.; Kusche-Vihrog, K.; Oberleithner, H. Cortical actin nanodynamics determines nitric oxide release in vascular endothelium. PLoS ONE 2012, 7, e41520. [Google Scholar] [CrossRef] [PubMed]
- Clarke, F.M.; Masters, C.J. On the association of glycolytic enzymes with structural proteins of skeletal muscle. Biochim. Biophys. Acta 1975, 381, 37–46. [Google Scholar] [CrossRef]
- Xu, P.; Crawford, M.; Way, M.; Godovac-Zimmermann, J.; Segal, A.W.; Radulovic, M. Subproteome analysis of the neutrophil cytoskeleton. Proteomics 2009, 9, 2037–2049. [Google Scholar] [CrossRef]
- Holliday, L.S.; Lu, M.; Lee, B.S.; Nelson, R.D.; Solivan, S.; Zhang, L.; Gluck, S.L. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J. Biol. Chem. 2000, 275, 32331–32337. [Google Scholar] [CrossRef]
- Chen, S.H.; Bubb, M.R.; Yarmola, E.G.; Zuo, J.; Jiang, J.; Lee, B.S.; Lu, M.; Gluck, S.L.; Hurst, I.R.; Holliday, L.S. Vacuolar H+-ATPase binding to microfilaments: Regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J. Biol. Chem. 2004, 279, 7988–7998. [Google Scholar] [CrossRef]
- Vitavska, O.; Merzendorfer, H.; Wieczorek, H. The V-ATPase subunit C binds to polymeric F-actin as well as to monomeric G-actin and induces cross-linking of actin filaments. J. Biol. Chem. 2005, 280, 1070–1076. [Google Scholar] [CrossRef]
- Dalli, J.; Montero-Melendez, T.; Norling, L.V.; Yin, X.; Hinds, C.; Haskard, D.; Mayr, M.; Perretti, M. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol. Cell. Proteom. 2013, 12, 2205–2219. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Schifferli, J.A. Ectosomes as modulators of inflammation and immunity. Clin. Exp. Immunol. 2011, 163, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Gasser, O.; Hess, C.; Miot, S.; Deon, C.; Sanchez, J.C.; Schifferli, J.A. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003, 285, 243–257. [Google Scholar] [CrossRef]
- Hess, C.; Sadallah, S.; Hefti, A.; Landmann, R.; Schifferli, J.A. Ectosomes released by human neutrophils are specialized functional units. J. Immunol. 1999, 163, 4564–4573. [Google Scholar] [CrossRef]
- Stein, J.M.; Luzio, J.P. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem. J. 1991, 274, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Timar, C.I.; Lorincz, A.M.; Csepanyi-Komi, R.; Valyi-Nagy, A.; Nagy, G.; Buzas, E.I.; Ivanyi, Z.; Kittel, A.; Powell, D.W.; McLeish, K.R.; et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood 2013, 121, 510–518. [Google Scholar] [CrossRef]
- Sweitzer, S.M.; Hinshaw, J.E. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 1998, 93, 1021–1029. [Google Scholar] [CrossRef]
- Antonny, B.; Burd, C.; De Camilli, P.; Chen, E.; Daumke, O.; Faelber, K.; Ford, M.; Frolov, V.A.; Frost, A.; Hinshaw, J.E.; et al. Membrane fission by dynamin: What we know and what we need to know. EMBO J. 2016, 35, 2270–2284. [Google Scholar] [CrossRef]
- Campelo, F.; Malhotra, V. Membrane fission: The biogenesis of transport carriers. Annu. Rev. Biochem. 2012, 81, 407–427. [Google Scholar] [CrossRef]
- Itoh, T.; Erdmann, K.S.; Roux, A.; Habermann, B.; Werner, H.; De Camilli, P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell 2005, 9, 791–804. [Google Scholar] [CrossRef]
- Merrifield, C.J.; Feldman, M.E.; Wan, L.; Almers, W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 2002, 4, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Fraser, S.E.; McClay, D. Dynamics of thin filopodia during sea urchin gastrulation. Development 1995, 121, 2501–2511. [Google Scholar]
- Aktories, K.; Lang, A.E.; Schwan, C.; Mannherz, H.G. Actin as target for modification by bacterial protein toxins. FEBS J. 2011, 278, 4526–4543. [Google Scholar] [CrossRef]
- Aktories, K.; Papatheodorou, P.; Schwan, C. Binary Clostridium difficile toxin (CDT)—A virulence factor disturbing the cytoskeleton. Anaerobe 2018, 53, 21–29. [Google Scholar] [CrossRef]
- Whipple, R.A.; Cheung, A.M.; Martin, S.S. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp. Cell Res. 2007, 313, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, K.R.; Whipple, R.A.; Boggs, A.E.; Hessler, L.K.; Bhandary, L.; Vitolo, M.I.; Thompson, K.; Martin, S.S. Pharmacologic regulation of AMPK in breast cancer affects cytoskeletal properties involved with microtentacle formation and re-attachment. Oncotarget 2015, 6, 36292–36307. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; DeFranco, A.L. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Mol. Biol. Cell 2003, 14, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Atkin-Smith, G.K.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.J.; Ravichandran, K.S.; Hulett, M.D.; Poon, I.K. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015, 6, 7439. [Google Scholar] [CrossRef]
- Raghunathan, A.; Sivakamasundari, R.; Wolenski, J.; Poddar, R.; Weissman, S.M. Functional analysis of B144/LST1: A gene in the tumor necrosis factor cluster that induces formation of long filopodia in eukaryotic cells. Exp. Cell Res. 2001, 268, 230–244. [Google Scholar] [CrossRef]
- Kuhni-Boghenbor, K.; Ma, M.; Lemgruber, L.; Cyrklaff, M.; Frischknecht, F.; Gaschen, V.; Stoffel, M.; Baumgartner, M. Actin-mediated plasma membrane plasticity of the intracellular parasite Theileria annulata. Cell Microbiol. 2012, 14, 1867–1879. [Google Scholar] [CrossRef]
- Sattler, J.M.; Ganter, M.; Hliscs, M.; Matuschewski, K.; Schuler, H. Actin regulation in the malaria parasite. Eur. J. Cell Biol. 2011, 90, 966–971. [Google Scholar] [CrossRef]
- Follett, E.A.; Gordon, J. An Electron Microscope Study of Vibrio Flagella. J. Gen. Microbiol. 1963, 32, 235–239. [Google Scholar] [CrossRef]
- Seidler, R.J.; Starr, M.P. Structure of the flagellum of Bdellovibrio bacteriovorus. J. Bacteriol. 1968, 95, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Geis, G.; Suerbaum, S.; Forsthoff, B.; Leying, H.; Opferkuch, W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J. Med. Microbiol. 1993, 38, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.D. Baumann P Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J. Bacteriol. 1971, 107, 295–302. [Google Scholar] [CrossRef]
- Ginocchio, C.C.; Olmsted, S.B.; Wells, C.L.; Galan, J.E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell 1994, 76, 717–724. [Google Scholar] [CrossRef]
- Reed, K.A.; Clark, M.A.; Booth, T.A.; Hueck, C.J.; Miller, S.I.; Hirst, B.H.; Jepson, M.A. Cell-contact-stimulated formation of filamentous appendages by Salmonella typhimurium does not depend on the type III secretion system encoded by Salmonella pathogenicity island1. Infect. Immun. 1998, 66, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Vassallo, C.N.; Pathak, D.T.; Wall, D. Myxobacteria produce outer membrane-enclosed tubes in unstructured environments. J. Bacteriol. 2014, 196, 1807–1814. [Google Scholar] [CrossRef]
- Remis, J.P.; Wei, D.; Gorur, A.; Zemla, M.; Haraga, J.; Allen, S.; Witkowska, H.E.; Costerton, J.W.; Berleman, J.E.; Auer, M. Bacterial social networks: Structure and composition of Myxococcus xanthus outer membrane vesicle chains. Environ. Microbiol. 2014, 16, 598–610. [Google Scholar] [CrossRef]
- Marguet, E.; Gaudin, M.; Gauliard, E.; Fourquaux, I.; le Blond du Plouy, S.; Matsui, I.; Forterre, P. Membrane vesicles, nanopods and/or nanotubes produced by hyperthermophilic archaea of the genus Thermococcus. Biochem. Soc. Trans. 2013, 41, 436–442. [Google Scholar] [CrossRef]
- Pirbadian, S.; Barchinger, S.E.; Leung, K.M.; Byun, H.S.; Jangir, Y.; Bouhenni, R.A.; Reed, S.B.; Romine, M.F.; Saffarini, D.A.; Shi, L.; et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl. Acad. Sci. USA 2014, 111, 12883–12888. [Google Scholar] [CrossRef]
- Subramanian, P.; Pirbadian, S.; El-Naggar, M.Y.; Jensen, G.J. Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proc. Natl. Acad. Sci. USA 2018, 115, E3246–E3255. [Google Scholar] [CrossRef] [PubMed]
- Onouchi, T.; Shiogama, K.; Matsui, T.; Mizutani, Y.; Sakurai, K.; Inada, K.; Tsutsumi, Y. Visualization of Neutrophil Extracellular Traps and Fibrin Meshwork in Human Fibrinopurulent Inflammatory Lesions: II. Ultrastructural Study. Acta Histochem. Cytochem. 2016, 49, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F., 3rd; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11 b subunit of the integrin Mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Braughton, K.R.; Palazzolo-Ballance, A.M.; Kennedy, A.D.; Sampaio, E.; Kristosturyan, E.; Whitney, A.R.; Sturdevant, D.E.; Dorward, D.W.; Holland, S.M.; et al. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J. Innate Immun. 2010, 2, 560–575. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Byrd, A.S.; O’Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 2013, 190, 4136–4148. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Jenne, C.N.; Surewaard, B.G.; Thanabalasuriar, A.; Lee, W.Y.; Sanz, M.J.; Mowen, K.; Opdenakker, G.; Kubes, P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 2015, 6, 6673. [Google Scholar] [CrossRef]
- Martinod, K.; Witsch, T.; Farley, K.; Gallant, M.; Remold-O’Donnell, E.; Wagner, D.D. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J. Thromb. Haemost. 2016, 14, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Khan, S.N.; Radic, M. Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 2008, 180, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Halverson, T.W.; Wilton, M.; Poon, K.K.; Petri, B.; Lewenza, S. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015, 11, e1004593. [Google Scholar] [CrossRef]
- Hirsch, J.G. Bactericidal action of histone. J. Exp. Med. 1958, 108, 925–944. [Google Scholar] [CrossRef]
- Menegazzi, R.; Decleva, E.; Dri, P. Killing by neutrophil extracellular traps: Fact or folklore? Blood 2012, 119, 1214–1216. [Google Scholar] [CrossRef]
- Gettins, P.G. Serpin structure, mechanism, and function. Chem. Rev. 2002, 102, 4751–4804. [Google Scholar] [CrossRef]
- Benarafa, C.; Simon, H.U. Role of granule proteases in the life and death of neutrophils. Biochem. Biophys. Res. Commun. 2017, 482, 473–481. [Google Scholar] [CrossRef]
- Nauseef, W.M. Editorial: Nyet to NETs? A pause for healthy skepticism. J. Leukoc. Biol. 2012, 91, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M.; Kubes, P. Pondering neutrophil extracellular traps with healthy skepticism. Cell Microbiol. 2016, 18, 1349–1357. [Google Scholar] [CrossRef]
- Martinod, K.; Fuchs, T.A.; Zitomersky, N.L.; Wong, S.L.; Demers, M.; Gallant, M.; Wang, Y.; Wagner, D.D. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015, 125, 1948–1956. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Wagner, D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1777–1783. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Demers, M.; Fuchs, T.A.; Wong, S.L.; Brill, A.; Gallant, M.; Hu, J.; Wang, Y.; Wagner, D.D. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 8674–8679. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, L.M.; Posch, F.; Martinod, K.; Grilz, E.; Daullary, T.; Hell, L.; Brostjan, C.; Zielinski, C.; Ay, C.; Wagner, D.D.; et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Jimenez-Alcazar, M.; Rangaswamy, C.; Panda, R.; Bitterling, J.; Simsek, Y.J.; Long, A.T.; Bilyy, R.; Krenn, V.; Renne, C.; Renne, T.; et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017, 358, 1202–1206. [Google Scholar] [CrossRef]
- Pratesi, F.; Dioni, I.; Tommasi, C.; Alcaro, M.C.; Paolini, I.; Barbetti, F.; Boscaro, F.; Panza, F.; Puxeddu, I.; Rovero, P.; et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann. Rheum. Dis. 2014, 73, 1414–1422. [Google Scholar] [CrossRef]
- Dwivedi, N.; Neeli, I.; Schall, N.; Wan, H.; Desiderio, D.M.; Csernok, E.; Thompson, P.R.; Dali, H.; Briand, J.P.; Muller, S.; et al. Deimination of linker histones links neutrophil extracellular trap release with autoantibodies in systemic autoimmunity. FASEB J. 2014, 28, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Causey, C.P.; Knuckley, B.; Slack-Noyes, J.L.; Thompson, P.R. Protein arginine deiminase 4 (PAD4): Current understanding and future therapeutic potential. Curr. Opin. Drug Discov. Dev. 2009, 12, 616–627. [Google Scholar]
- Panda, R.; Krieger, T.; Hopf, L.; Renne, T.; Haag, F.; Rober, N.; Conrad, K.; Csernok, E.; Fuchs, T.A. Neutrophil Extracellular Traps Contain Selected Antigens of Anti-Neutrophil Cytoplasmic Antibodies. Front. Immunol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Nakazawa, D.; Shida, H.; Kusunoki, Y.; Miyoshi, A.; Nishio, S.; Tomaru, U.; Atsumi, T.; Ishizu, A. The responses of macrophages in interaction with neutrophils that undergo, NETosis. J. Autoimmun. 2016, 67, 19–28. [Google Scholar] [CrossRef]
- Farrera, C.; Fadeel, B. Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T. Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: A cooperative mechanism in the control of infection and infectious inflammation. J. Leukoc. Biol. 2011, 89, 675–683. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galkina, S.I.; Fedorova, N.V.; Golenkina, E.A.; Stadnichuk, V.I.; Sud’ina, G.F. Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes. Int. J. Mol. Sci. 2020, 21, 586. https://doi.org/10.3390/ijms21020586
Galkina SI, Fedorova NV, Golenkina EA, Stadnichuk VI, Sud’ina GF. Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes. International Journal of Molecular Sciences. 2020; 21(2):586. https://doi.org/10.3390/ijms21020586
Chicago/Turabian StyleGalkina, Svetlana I., Natalia V. Fedorova, Ekaterina A. Golenkina, Vladimir I. Stadnichuk, and Galina F. Sud’ina. 2020. "Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes" International Journal of Molecular Sciences 21, no. 2: 586. https://doi.org/10.3390/ijms21020586
APA StyleGalkina, S. I., Fedorova, N. V., Golenkina, E. A., Stadnichuk, V. I., & Sud’ina, G. F. (2020). Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes. International Journal of Molecular Sciences, 21(2), 586. https://doi.org/10.3390/ijms21020586