DGKα in Neutrophil Biology and Its Implications for Respiratory Diseases
Abstract
1. Introduction
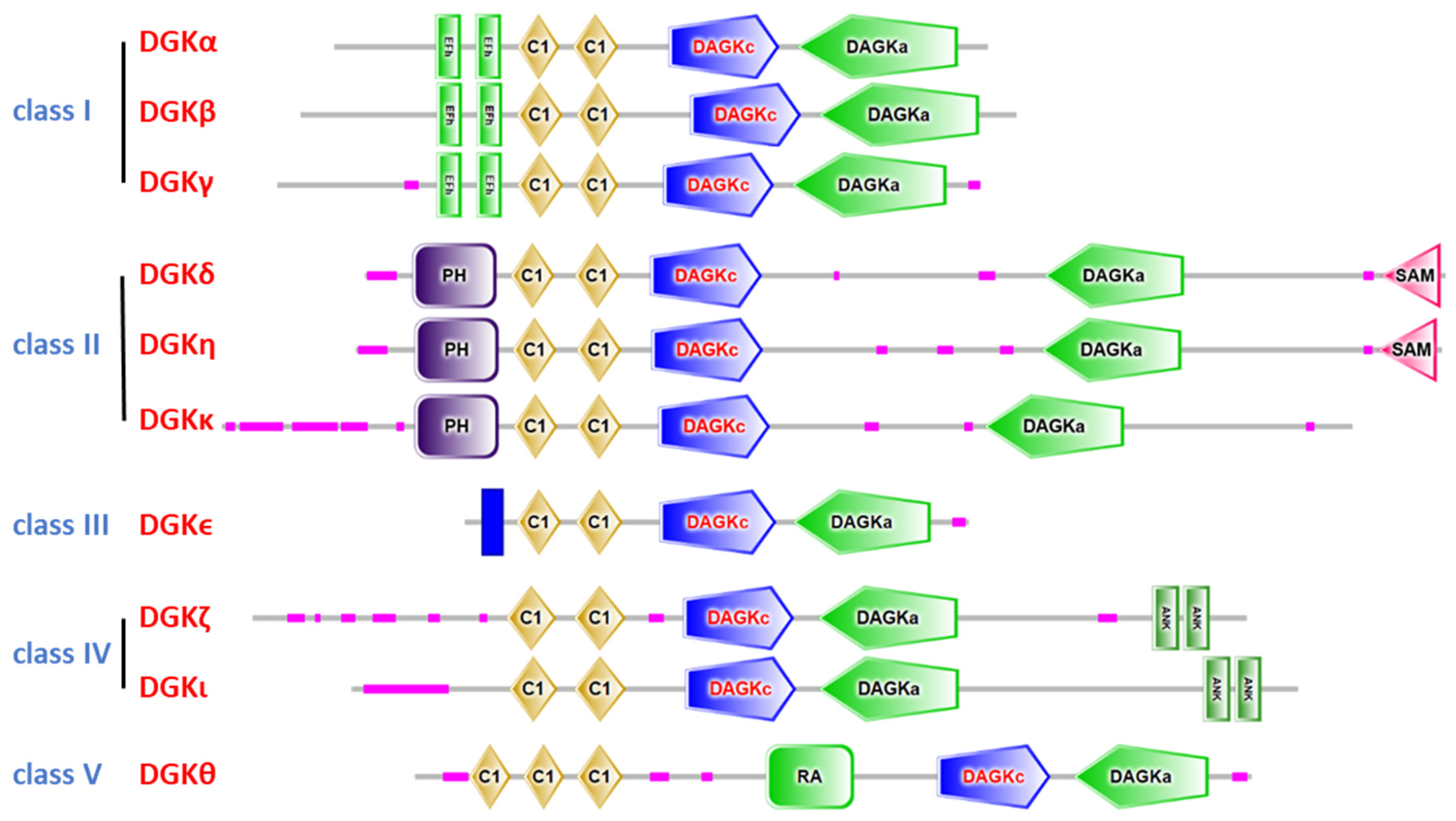
2. The Diacylglycerol Kinase Family
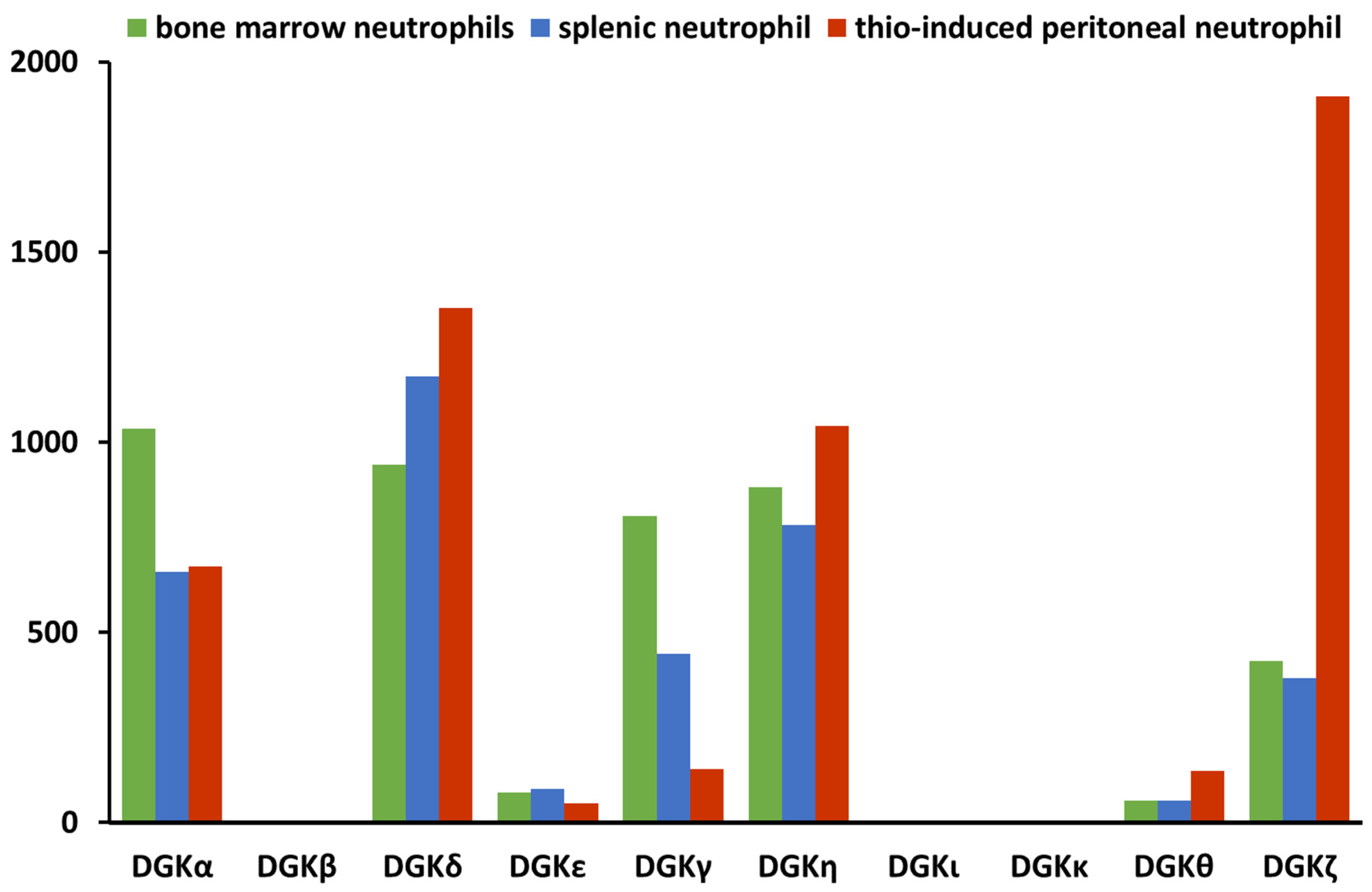
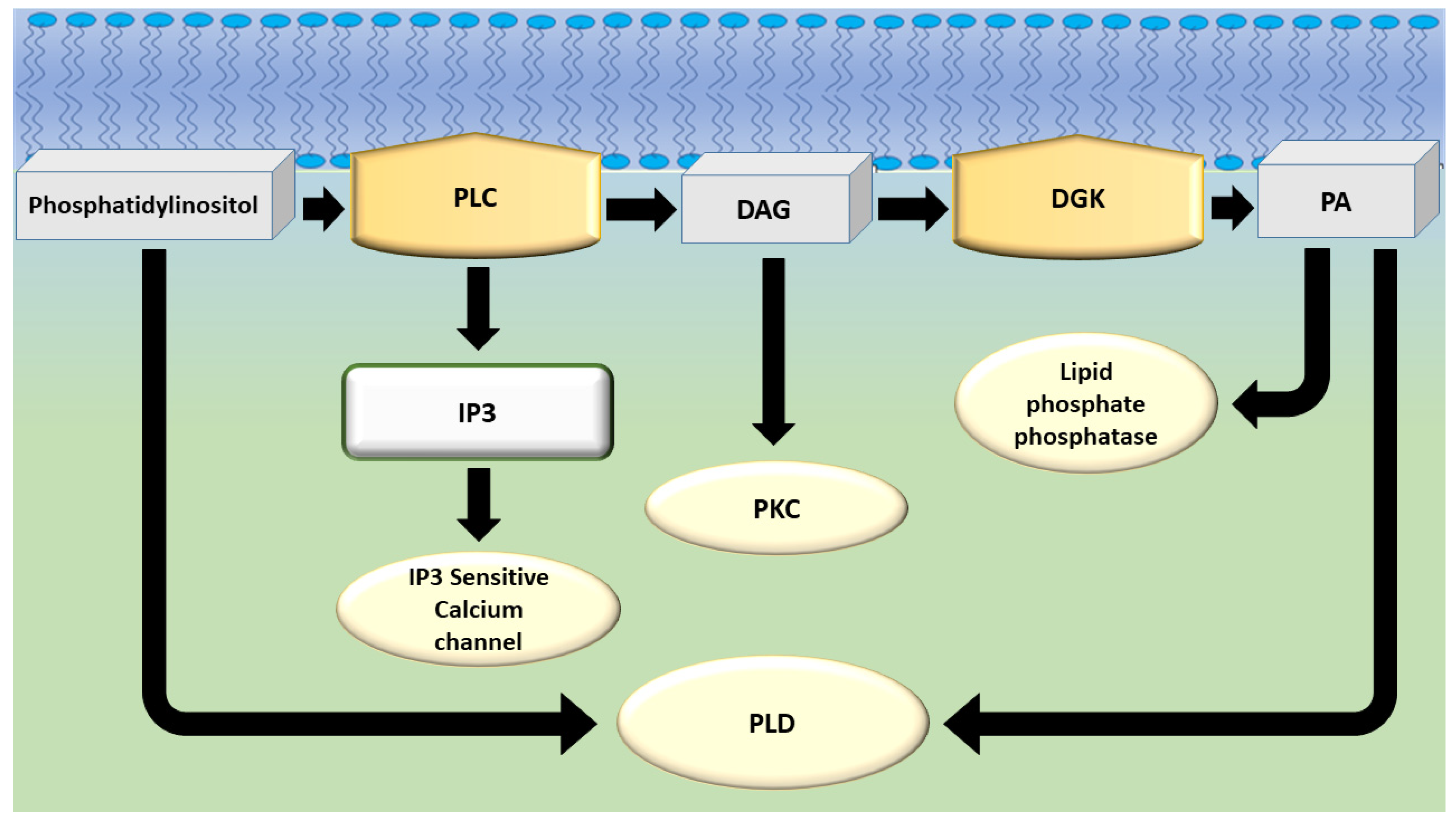
3. DGKα in Neutrophil Biology
4. DGKs in Respiratory Diseases
5. Discussion
Funding
Conflicts of Interest
Abbreviations
| Arp3 | Actin related protein 3 |
| CXCL8 | C-X-C motif chemokine ligand-8 |
| CXCR1 | C-X-C motif chemokine receptor-1 |
| CXCR2 | C-X-C motif chemokine receptor-2. |
| COPD | Chronic obstructive pulmonary disease |
| DAG | Diacylglycerol |
| DGK | Diacylglycerol kinase |
| IP3 | Inositol trisphosphate |
| NET | Neutrophil extracellular traps |
| PA | Phosphatidic acid |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| PLD | Phospholipase D |
| RCP | Rab-coupling protein |
| RICD | Restimulation-induced cell death |
| TCR | T cell receptor |
| XLP-1 | X-linked lymphoproliferative disease 1 |
References
- Mérida, I.; Avila-Flores, A.; Merino, E. Diacylglycerol kinases: At the hub of cell signalling. Biochem. J. 2008, 409, 1–18. [Google Scholar] [CrossRef]
- Topham, M.K. Signaling roles of diacylglycerol kinases. J. Cell. Biochem. 2006, 97, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Los, A.P.; van Baal, J.; de Widt, J.; Divecha, N.; van Blitterswijk, W.J. Structure-activity relationship of diacylglycerol kinase theta. Biochim. Biophys. Acta 2004, 1636, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Schaap, D.; van der Wal, J.; van Blitterswijk, W.J. Consensus sequences for ATP-binding sites in protein kinases do not apply to diacylglycerol kinases. Biochem. J. 1994, 304 Pt 2, 661–662. [Google Scholar] [CrossRef]
- Kazanietz, M.G. Targeting protein kinase C and “non-kinase” phorbol ester receptors: Emerging concepts and therapeutic implications. Biochim. Biophys. Acta 2005, 1754, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Sakane, F.; Kai, M.; Wada, I.; Imai, S.; Kanoh, H. The C-terminal part of diacylglycerol kinase alpha lacking zinc fingers serves as a catalytic domain. Biochem. J. 1996, 318 Pt 2, 583–590. [Google Scholar] [CrossRef]
- Shindo, M.; Irie, K.; Masuda, A.; Ohigashi, H.; Shirai, Y.; Miyasaka, K.; Saito, N. Synthesis and phorbol ester binding of the cysteine-rich domains of diacylglycerol kinase (DGK) isozymes. DGKgamma and DGKbeta are new targets of tumor-promoting phorbol esters. J. Biol. Chem. 2003, 278, 18448–18454. [Google Scholar] [CrossRef]
- Shindo, M.; Irie, K.; Ohigashi, H.; Kuriyama, M.; Saito, N. Diacylglycerol kinase gamma is one of the specific receptors of tumor-promoting phorbol esters. Biochem. Biophys. Res. Commun. 2001, 289, 451–456. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Sakane, F.; Yamada, K.; Imai, S.; Kanoh, H. Porcine 80-kDa diacylglycerol kinase is a calcium-binding and calcium/phospholipid-dependent enzyme and undergoes calcium-dependent translocation. J. Biol. Chem. 1991, 266, 7096–7100. [Google Scholar]
- Takahashi, M.; Yamamoto, T.; Sakai, H.; Sakane, F. Calcium negatively regulates an intramolecular interaction between the N-terminal recoverin homology and EF-hand motif domains and the C-terminal C1 and catalytic domains of diacylglycerol kinase α. Biochem. Biophys. Res. Commun. 2012, 423, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Suzuki, K.; Sakamoto, T.; Iwamoto, T.; Murata, T.; Sakane, F. Crystal structure and calcium-induced conformational changes of diacylglycerol kinase α EF-hand domains. Protein Sci. 2019, 28, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Kume, A.; Kawase, K.; Komenoi, S.; Usuki, T.; Takeshita, E.; Sakai, H.; Sakane, F. The Pleckstrin Homology Domain of Diacylglycerol Kinase η Strongly and Selectively Binds to Phosphatidylinositol 4,5-Bisphosphate. J. Biol. Chem. 2016, 291, 8150–8161. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sakiyama, S.; Usuki, T.; Sakai, H.; Sakane, F. Diacylglycerol kinase δ1 transiently translocates to the plasma membrane in response to high glucose. Biochim. Biophys. Acta 2012, 1823, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Sakane, F. Recent progress on type II diacylglycerol kinases: The physiological functions of diacylglycerol kinase δ, η and κ and their involvement in disease. J. Biochem. 2012, 152, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Hozumi, Y.; Tanaka, T.; Okada, M.; Nakano, T.; Suzuki, Y.; Iseki, K.; Kakehata, S.; Topham, M.K.; Goto, K. Role of the N-terminal hydrophobic residues of DGKε in targeting the endoplasmic reticulum. Biochim. Biophys. Acta 2014, 1842, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.; Epand, R.M. Catalytic activity and acyl-chain selectivity of diacylglycerol kinase ɛ are modulated by residues in and near the lipoxygenase-like motif. J. Mol. Biol. 2012, 416, 619–628. [Google Scholar] [CrossRef]
- Zhu, J.; Chaki, M.; Lu, D.; Ren, C.; Wang, S.S.; Rauhauser, A.; Li, B.; Zimmerman, S.; Jun, B.; Du, Y.; et al. Loss of diacylglycerol kinase epsilon in mice causes endothelial distress and impairs glomerular Cox-2 and PGE2 production. Am. J. Physiol. Renal Physiol. 2016, 310, F895–F908. [Google Scholar] [CrossRef]
- Luo, B.; Prescott, S.M.; Topham, M.K. Protein kinase C alpha phosphorylates and negatively regulates diacylglycerol kinase zeta. J. Biol. Chem. 2003, 278, 39542–39547. [Google Scholar] [CrossRef]
- Los, A.P.; de Widt, J.; Topham, M.K.; van Blitterswijk, W.J.; Divecha, N. Protein kinase C inhibits binding of diacylglycerol kinase-zeta to the retinoblastoma protein. Biochim. Biophys. Acta 2007, 1773, 352–357. [Google Scholar] [CrossRef][Green Version]
- Luo, B.; Prescott, S.M.; Topham, M.K. Association of diacylglycerol kinase zeta with protein kinase C alpha: Spatial regulation of diacylglycerol signaling. J. Cell Biol. 2003, 160, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Houssa, B.; Schaap, D.; van der Wal, J.; Goto, K.; Kondo, H.; Yamakawa, A.; Shibata, M.; Takenawa, T.; van Blitterswijk, W.J. Cloning of a novel human diacylglycerol kinase (DGKtheta) containing three cysteine-rich domains, a proline-rich region, and a pleckstrin homology domain with an overlapping Ras-associating domain. J. Biol. Chem. 1997, 272, 10422–10428. [Google Scholar] [CrossRef] [PubMed]
- Topham, M.K.; Epand, R.M. Mammalian diacylglycerol kinases: Molecular interactions and biological functions of selected isoforms. Biochim. Biophys. Acta 2009, 1790, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Olenchock, B.A.; Guo, R.; Carpenter, J.H.; Jordan, M.; Topham, M.K.; Koretzky, G.A.; Zhong, X.P. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 2006, 7, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Kouzuki, T.; Kakefuda, K.; Moriguchi, S.; Oyagi, A.; Horie, K.; Morita, S.Y.; Shimazawa, M.; Fukunaga, K.; Takeda, J.; et al. Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKbeta in neurite spine formation, contributing to cognitive function. PLoS ONE 2010, 5, e11602. [Google Scholar] [CrossRef] [PubMed]
- Crotty, T.; Cai, J.; Sakane, F.; Taketomi, A.; Prescott, S.M.; Topham, M.K. Diacylglycerol kinase delta regulates protein kinase C and epidermal growth factor receptor signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 15485–15490. [Google Scholar] [CrossRef] [PubMed]
- Mannerås-Holm, L.; Schönke, M.; Brozinick, J.T.; Vetterli, L.; Bui, H.H.; Sanders, P.; Nascimento, E.B.M.; Björnholm, M.; Chibalin, A.V.; Zierath, J.R. Diacylglycerol kinase ε deficiency preserves glucose tolerance and modulates lipid metabolism in obese mice. J. Lipid Res. 2017, 58, 907–915. [Google Scholar] [CrossRef]
- Isozaki, T.; Komenoi, S.; Lu, Q.; Usuki, T.; Tomokata, S.; Matsutomo, D.; Sakai, H.; Bando, K.; Kiyonari, H.; Sakane, F. Deficiency of diacylglycerol kinase η induces lithium-sensitive mania-like behavior. J. Neurochem. 2016, 138, 448–456. [Google Scholar] [CrossRef]
- Regier, D.S.; Higbee, J.; Lund, K.M.; Sakane, F.; Prescott, S.M.; Topham, M.K. Diacylglycerol kinase iota regulates Ras guanyl-releasing protein 3 and inhibits Rap1 signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 7595–7600. [Google Scholar] [CrossRef]
- Zhong, X.P.; Hainey, E.A.; Olenchock, B.A.; Jordan, M.S.; Maltzman, J.S.; Nichols, K.E.; Shen, H.; Koretzky, G.A. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat. Immunol. 2003, 4, 882–890. [Google Scholar] [CrossRef]
- De Chaffoy de Courcelles, D.; Roevens, P.; Van Belle, H.; Kennis, L.; Somers, Y.; De Clerck, F. The role of endogenously formed diacylglycerol in the propagation and termination of platelet activation. A biochemical and functional analysis using the novel diacylglycerol kinase inhibitor, R 59 949. J. Biol. Chem. 1989, 264, 3274–3285. [Google Scholar] [PubMed]
- De Chaffoy de Courcelles, D.C.; Roevens, P.; Van Belle, H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J. Biol. Chem. 1985, 260, 15762–15770. [Google Scholar] [PubMed]
- Sato, M.; Liu, K.; Sasaki, S.; Kunii, N.; Sakai, H.; Mizuno, H.; Saga, H.; Sakane, F. Evaluations of the selectivities of the diacylglycerol kinase inhibitors r59022 and r59949 among diacylglycerol kinase isozymes using a new non-radioactive assay method. Pharmacology 2013, 92, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sakane, F.; Kanoh, H.; Walsh, J.P. Selectivity of the diacylglycerol kinase inhibitor 3-[2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl]-2, 3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochem. Pharmacol. 2000, 59, 763–772. [Google Scholar] [CrossRef]
- Ruffo, E.; Malacarne, V.; Larsen, S.E.; Das, R.; Patrussi, L.; Wülfing, C.; Biskup, C.; Kapnick, S.M.; Verbist, K.; Tedrick, P.; et al. Inhibition of diacylglycerol kinase α restores restimulation-induced cell death and reduces immunopathology in XLP-1. Sci. Transl. Med. 2016, 8, 321ra7. [Google Scholar] [CrossRef]
- Dominguez, C.L.; Floyd, D.H.; Xiao, A.; Mullins, G.R.; Kefas, B.A.; Xin, W.; Yacur, M.N.; Abounader, R.; Lee, J.K.; Wilson, G.M.; et al. Diacylglycerol kinase α is a critical signaling node and novel therapeutic target in glioblastoma and other cancers. Cancer Discov. 2013, 3, 782–797. [Google Scholar] [CrossRef]
- Boroda, S.; Niccum, M.; Raje, V.; Purow, B.W.; Harris, T.E. Dual activities of ritanserin and R59022 as DGKα inhibitors and serotonin receptor antagonists. Biochem. Pharmacol. 2017, 123, 29–39. [Google Scholar] [CrossRef]
- McCloud, R.L.; Franks, C.E.; Campbell, S.T.; Purow, B.W.; Harris, T.E.; Hsu, K.L. Deconstructing Lipid Kinase Inhibitors by Chemical Proteomics. Biochemistry 2018, 57, 231–236. [Google Scholar] [CrossRef]
- Franks, C.E.; Campbell, S.T.; Purow, B.W.; Harris, T.E.; Hsu, K.L. The Ligand Binding Landscape of Diacylglycerol Kinases. Cell Chem. Biol. 2017, 24, 870–880.e5. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Malek-Hosseini, M.; Ghoreishi, A.; Raznahan, M.; Rezazadeh, S.A. Effect of ritanserin, a 5HT2A/2C antagonist, on negative symptoms of schizophrenia: A double-blind randomized placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1879–1883. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Mohajari, H.; Reza Mohammadi, M.; Amini, H. Ritanserin as an adjunct to lithium and haloperidol for the treatment of medication-naive patients with acute mania: A double blind and placebo controlled trial. BMC Psychiatry 2003, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.W.; Maany, I.; Fudala, P.J.; Ehrman, R.N.; Robbins, S.J.; O’Brien, C.P. A randomized, double-blind, placebo-controlled study of ritanserin pharmacotherapy for cocaine dependence. Drug Alcohol Depend. 2001, 61, 183–189. [Google Scholar] [CrossRef]
- Wiesbeck, G.A.; Weijers, H.G.; Chick, J.; Naranjo, C.A.; Boening, J. Ritanserin in relapse prevention in abstinent alcoholics: Results from a placebo-controlled double-blind international multicenter trial. Ritanserin in Alcoholism Work Group. Alcohol Clin. Exp. Res. 1999, 23, 230–235. [Google Scholar] [PubMed]
- Olin, R.; Klein, R.; Berg, P.A. A randomised double-blind 16-week study of ritanserin in fibromyalgia syndrome: Clinical outcome and analysis of autoantibodies to serotonin, gangliosides and phospholipids. Clin. Rheumatol. 1998, 17, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Duinkerke, S.J.; Botter, P.A.; Jansen, A.A.; van Dongen, P.A.; van Haaften, A.J.; Boom, A.J.; van Laarhoven, J.H.; Busard, H.L. Ritanserin, a selective 5-HT2/1C antagonist, and negative symptoms in schizophrenia. A placebo-controlled double-blind trial. Br. J. Psychiatry 1993, 163, 451–455. [Google Scholar] [CrossRef]
- Bersani, G.; Grispini, A.; Marini, S.; Pasini, A.; Valducci, M.; Ciani, N. 5-HT2 antagonist ritanserin in neuroleptic-induced parkinsonism: A double-blind comparison with orphenadrine and placebo. Clin. Neuropharmacol. 1990, 13, 500–506. [Google Scholar] [CrossRef]
- Den Boer, J.A.; Westenberg, H.G. Serotonin function in panic disorder: A double blind placebo controlled study with fluvoxamine and ritanserin. Psychopharmacology 1990, 102, 85–94. [Google Scholar] [CrossRef]
- Hedner, T.; Persson, B. Experience with ketanserin and ritanserin in hypertensive patients. J. Cardiovasc. Pharmacol. 1988, 11 (Suppl. 1), S44–S48. [Google Scholar]
- Paiva, T.; Arriaga, F.; Wauquier, A.; Lara, E.; Largo, R.; Leitao, J.N. Effects of ritanserin on sleep disturbances of dysthymic patients. Psychopharmacology 1988, 96, 395–399. [Google Scholar] [CrossRef]
- Bressa, G.M.; Marini, S.; Gregori, S. Serotonin S2 receptors blockage and generalized anxiety disorders. A double-blind study on ritanserin and lorazepam. Int. J. Clin. Pharmacol. Res. 1987, 7, 111–119. [Google Scholar]
- Maertens de Noordhout, A.; Delwaide, P.J. Open pilot trial of ritanserin in parkinsonism. Clin. Neuropharmacol. 1986, 9, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, D.L.; Hoppenbrouwers, M.L.; Gelders, Y.G.; Reyntjens, A.J. The influence of ritanserin, a serotonin antagonist, in anxiety disorders: A double-blind placebo-controlled study versus lorazepam. Pharmacopsychiatry 1985, 18, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Olmez, I.; Love, S.; Xiao, A.; Manigat, L.; Randolph, P.; McKenna, B.D.; Neal, B.P.; Boroda, S.; Li, M.; Brenneman, B.; et al. Targeting the mesenchymal subtype in glioblastoma and other cancers via inhibition of diacylglycerol kinase alpha. Neuro Oncol. 2018, 20, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Velnati, S.; Ruffo, E.; Massarotti, A.; Talmon, M.; Varma, K.S.S.; Gesu, A.; Fresu, L.G.; Snow, A.L.; Bertoni, A.; Capello, D.; et al. Identification of a novel DGKα inhibitor for XLP-1 therapy by virtual screening. Eur. J. Med. Chem. 2019, 164, 378–390. [Google Scholar] [CrossRef]
- Liu, K.; Kunii, N.; Sakuma, M.; Yamaki, A.; Mizuno, S.; Sato, M.; Sakai, H.; Kado, S.; Kumagai, K.; Kojima, H.; et al. A novel diacylglycerol kinase α-selective inhibitor, CU-3, induces cancer cell apoptosis and enhances immune response. J. Lipid Res. 2016, 57, 368–379. [Google Scholar] [CrossRef]
- Gray, R.D.; Lucas, C.D.; MacKellar, A.; Li, F.; Hiersemenzel, K.; Haslett, C.; Davidson, D.J.; Rossi, A.G. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J. Inflamm. 2013, 10, 12. [Google Scholar] [CrossRef]
- Suire, S.; Lécureuil, C.; Anderson, K.E.; Damoulakis, G.; Niewczas, I.; Davidson, K.; Guillou, H.; Pan, D.; Clark, J.; Hawkins, P.T.; et al. GPCR activation of Ras and PI3Kc in neutrophils depends on PLCb2/b3 and the RasGEF RasGRP4. EMBO J. 2012, 31, 3118–3129. [Google Scholar] [CrossRef]
- Speranza, F.; Mahankali, M.; Henkels, K.M.; Gomez-Cambronero, J. The molecular basis of leukocyte adhesion involving phosphatidic acid and phospholipase D. J. Biol. Chem. 2014, 289, 28885–28897. [Google Scholar] [CrossRef]
- Tou, J.S.; Gill, J.S. Lysophosphatidic acid increases phosphatidic acid formation, phospholipase D activity and degranulation by human neutrophils. Cell Signal. 2005, 17, 77–82. [Google Scholar] [CrossRef]
- Erickson, R.W.; Langel-Peveri, P.; Traynor-Kaplan, A.E.; Heyworth, P.G.; Curnutte, J.T. Activation of human neutrophil NADPH oxidase by phosphatidic acid or diacylglycerol in a cell-free system. Activity of diacylglycerol is dependent on its conversion to phosphatidic acid. J. Biol. Chem. 1999, 274, 22243–22250. [Google Scholar] [CrossRef]
- Karathanassis, D.; Stahelin, R.V.; Bravo, J.; Perisic, O.; Pacold, C.M.; Cho, W.; Williams, R.L. Binding of the PX domain of p47(phox) to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002, 21, 5057–5068. [Google Scholar] [CrossRef]
- Batista, E.L.; Warbington, M.; Badwey, J.A.; Van Dyke, T.E. Differentiation of HL-60 cells to granulocytes involves regulation of select diacylglycerol kinases (DGKs). J. Cell. Biochem. 2005, 94, 774–793. [Google Scholar] [CrossRef]
- Yamada, K.; Sakane, F.; Imai, S.; Tsushima, S.; Murakami, T.; Kanoh, H. Regulatory role of diacylglycerol kinase gamma in macrophage differentiation of leukemia cells. Biochem. Biophys. Res. Commun. 2003, 305, 101–107. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tanaka, T.; Hozumi, Y.; Saino-Saito, S.; Nakano, T.; Tajima, K.; Kato, T.; Goto, K. Expression of mRNAs for the diacylglycerol kinase family in immune cellsduring an inflammatory reaction. Biomed. Res. 2014, 35, 61–68. [Google Scholar] [CrossRef]
- Oyaizu, K.; Kantarci, A.; Maeda, H.; Batista, E.L.; Hasturk, H.; Murayama, Y.; Badwey, J.A.; Van Dyke, T.E. Identification of mRNAs for the various diacylglycerol kinase isoforms in neutrophils from patients with localized aggressive periodontitis. J. Periodontal Res. 2003, 38, 488–495. [Google Scholar] [CrossRef]
- Heng, T.S.; Painter, M.W.; Consortium, I.G.P. The Immunological Genome Project: Networks of gene expression in immune cells. Nat. Immunol. 2008, 9, 1091–1094. [Google Scholar] [CrossRef]
- Perkins, R.S.; Lindsay, M.A.; Barnes, P.J.; Giembycz, M.A. Early signalling events implicated in leukotriene B4-induced activation of the NADPH oxidase in eosinophils: Role of Ca2+, protein kinase C and phospholipases C and D. Biochem. J. 1995, 310 Pt 3, 795–806. [Google Scholar] [CrossRef]
- Reali, E.; Spisani, S.; Gavioli, R.; Lanza, F.; Moretti, S.; Traniello, S. IL-8 enhances antibody-dependent cellular cytotoxicity in human neutrophils. Immunol. Cell Biol. 1995, 73, 234–238. [Google Scholar] [CrossRef]
- Muid, R.E.; Penfield, A.; Dale, M.M. The diacylglycerol kinase inhibitor, R59022, enhances the superoxide generation from human neutrophils induced by stimulation of fMet-Leu-Phe, IgG and C3b receptors. Biochem. Biophys. Res. Commun. 1987, 143, 630–637. [Google Scholar] [CrossRef]
- Gomez-Cambronero, J.; Molski, T.F.; Becker, E.L.; Sha’afi, R.I. The diacylglycerol kinase inhibitor R59022 potentiates superoxide production but not secretion induced by fMet-Leu-Phe: Effects of leupeptin and the protein kinase C inhibitor H-7. Biochem. Biophys. Res. Commun. 1987, 148, 38–46. [Google Scholar] [CrossRef]
- Hurttia, H.; Leino, L. Subcellular localization of diacylglycerol kinase activity in stimulated and unstimulated human peripheral blood lymphocytes and neutrophils. Biochem. Mol. Biol. Int. 1996, 40, 579–585. [Google Scholar] [CrossRef]
- Tao, W.; Molski, T.F.; Sha’afi, R.I. Arachidonic acid release in rabbit neutrophils. Biochem. J. 1989, 257, 633–637. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Hiura, M.; Yoshida, K.; Okamura, N.; Ishibashi, S. A diacylglycerol kinase inhibitor, R 59 022, potentiates superoxide anion production and 46-kDa protein phosphorylation in guinea pig polymorphonuclear leukocytes. J. Biol. Chem. 1990, 265, 15418–15423. [Google Scholar]
- Boonen, G.J.; de Koster, B.M.; VanSteveninck, J.; Elferink, J.G. Neutrophil chemotaxis induced by the diacylglycerol kinase inhibitor R59022. Biochim. Biophys. Acta 1993, 1178, 97–102. [Google Scholar] [CrossRef]
- Silva, L.M.; Brenchley, L.; Moutsopoulos, N.M. Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol. Rev. 2019, 287, 226–235. [Google Scholar] [CrossRef]
- Tyagi, S.R.; Uhlinger, D.J.; Lambeth, J.D.; Champagne, C.; Van Dyke, T.E. Altered diacylglycerol level and metabolism in neutrophils from patients with localized juvenile periodontitis. Infect. Immun. 1992, 60, 2481–2487. [Google Scholar]
- Leino, L.; Hurttia, H.; Peltonen, E. Diacylglycerol in peripheral blood neutrophils from patients with localized juvenile periodontitis. J. Periodontal Res. 1994, 29, 334–338. [Google Scholar] [CrossRef]
- Hurttia, H.M.; Pelto, L.M.; Leino, L. Evidence of an association between functional abnormalities and defective diacylglycerol kinase activity in peripheral blood neutrophils from patients with localized juvenile periodontitis. J. Periodontal Res. 1997, 32, 401–407. [Google Scholar] [CrossRef]
- Hurttia, H.; Saarinen, K.; Leino, L. Increased adhesion of peripheral blood neutrophils from patients with localized juvenile periodontitis. J. Periodontal Res. 1998, 33, 292–297. [Google Scholar] [CrossRef]
- Gronert, K.; Kantarci, A.; Levy, B.D.; Clish, C.B.; Odparlik, S.; Hasturk, H.; Badwey, J.A.; Colgan, S.P.; Van Dyke, T.E.; Serhan, C.N. A molecular defect in intracellular lipid signaling in human neutrophils in localized aggressive periodontal tissue damage. J. Immunol. 2004, 172, 1856–1861. [Google Scholar] [CrossRef]
- Batista, E.L.; Kantarci, A.I.; Hasturk, H.; Van Dyke, T.E. Alternative Splicing Generates a Diacylglycerol Kinase α (DGKα) Transcript That Acts as a Dominant Negative Modulator of Superoxide Production in Localized Aggressive Periodontitis. J. Periodontol. 2014, 85, 934–943. [Google Scholar] [CrossRef]
- Kettritz, R. How anti-neutrophil cytoplasmic autoantibodies activate neutrophils. Clin. Exp. Immunol. 2012, 169, 220–228. [Google Scholar] [CrossRef]
- Jarrot, P.A.; Kaplanski, G. Pathogenesis of ANCA-associated vasculitis: An update. Autoimmun. Rev. 2016, 15, 704–713. [Google Scholar] [CrossRef]
- Williams, J.M.; Pettitt, T.R.; Powell, W.; Grove, J.; Savage, C.O.; Wakelam, M.J. Antineutrophil cytoplasm antibody-stimulated neutrophil adhesion depends on diacylglycerol kinase-catalyzed phosphatidic acid formation. J. Am. Soc. Nephrol. 2007, 18, 1112–1120. [Google Scholar] [CrossRef]
- Alonso, R.; Mazzeo, C.; Rodriguez, M.C.; Marsh, M.; Fraile-Ramos, A.; Calvo, V.; Avila-Flores, A.; Merida, I.; Izquierdo, M. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011, 18, 1161–1173. [Google Scholar] [CrossRef]
- Rainero, E.; Caswell, P.T.; Muller, P.A.; Grindlay, J.; McCaffrey, M.W.; Zhang, Q.; Wakelam, M.J.; Vousden, K.H.; Graziani, A.; Norman, J.C. Diacylglycerol kinase α controls RCP-dependent integrin trafficking to promote invasive migration. J. Cell Biol. 2012, 196, 277–295. [Google Scholar] [CrossRef]
- Xie, S.; Naslavsky, N.; Caplan, S. Diacylglycerol kinase α regulates tubular recycling endosome biogenesis and major histocompatibility complex class I recycling. J. Biol. Chem. 2014, 289, 31914–31926. [Google Scholar] [CrossRef]
- Rincón, E.; Sáez de Guinoa, J.; Gharbi, S.I.; Sorzano, C.O.; Carrasco, Y.R.; Mérida, I. Translocation dynamics of sorting nexin 27 in activated T cells. J. Cell Sci. 2011, 124, 776–788. [Google Scholar] [CrossRef]
- Nagaya, H.; Wada, I.; Jia, Y.J.; Kanoh, H. Diacylglycerol kinase delta suppresses ER-to-Golgi traffic via its SAM and PH domains. Mol. Biol Cell 2002, 13, 302–316. [Google Scholar] [CrossRef]
- Goldschmidt, H.L.; Tu-Sekine, B.; Volk, L.; Anggono, V.; Huganir, R.L.; Raben, D.M. DGKθ Catalytic Activity Is Required for Efficient Recycling of Presynaptic Vesicles at Excitatory Synapses. Cell Rep. 2016, 14, 200–207. [Google Scholar] [CrossRef]
- Holden, N.J.; Savage, C.O.; Young, S.P.; Wakelam, M.J.; Harper, L.; Williams, J.M. A dual role for diacylglycerol kinase generated phosphatidic acid in autoantibody-induced neutrophil exocytosis. Mol. Med. 2011, 17, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Kulkarni, N.S.; Hollins, F.; Sutcliffe, A.; Saunders, R.; Shah, S.; Siddiqui, S.; Gupta, S.; Haldar, P.; Green, R.; Pavord, I.; et al. Eosinophil protein in airway macrophages: A novel biomarker of eosinophilic inflammation in patients with asthma. J. Allergy Clin. Immunol. 2010, 126, 61–69.e63. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Bagnasco, D.; Borriello, F.; Heffler, E.; Canonica, G.W. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: Evidence and unmet needs. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 186–200. [Google Scholar] [CrossRef]
- Del Giacco, S.R.; Bakirtas, A.; Bel, E.; Custovic, A.; Diamant, Z.; Hamelmann, E.; Heffler, E.; Kalayci, Ö.; Saglani, S.; Sergejeva, S.; et al. Allergy in severe asthma. Allergy 2017, 72, 207–220. [Google Scholar] [CrossRef]
- Green, R.H.; Brightling, C.E.; Woltmann, G.; Parker, D.; Wardlaw, A.J.; Pavord, I.D. Analysis of induced sputum in adults with asthma: Identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002, 57, 875–879. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Ma, Q.; Zhang, Y.; Li, Z.; Wang, C. DGKα DNA vaccine relieves airway allergic inflammation in asthma model possibly via induction of T cell anergy. Int. J. Clin. Exp. Pathol. 2013, 6, 2404–2411. [Google Scholar]
- Martínez-Moreno, M.; García-Liévana, J.; Soutar, D.; Torres-Ayuso, P.; Andrada, E.; Zhong, X.P.; Koretzky, G.A.; Mérida, I.; Ávila-Flores, A. FoxO-dependent regulation of diacylglycerol kinase α gene expression. Mol. Cell. Biol. 2012, 32, 4168–4180. [Google Scholar] [CrossRef]
- Mérida, I.; Andrada, E.; Gharbi, S.I.; Ávila-Flores, A. Redundant and specialized roles for diacylglycerol kinases α and ζ in the control of T cell functions. Sci. Signal. 2015, 8, re6. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, P.; Krishna, S.; Wang, J.; Lin, X.; Huang, H.; Xie, D.; Gorentla, B.; Huang, R.; Gao, J.; et al. Unexpected positive control of NFκB and miR-155 by DGKα and ζ ensures effector and memory CD8+ T cell differentiation. Oncotarget 2016, 7, 33744–33764. [Google Scholar] [CrossRef]
- Snow, A.L.; Pandiyan, P.; Zheng, L.; Krummey, S.M.; Lenardo, M.J. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol. Rev. 2010, 236, 68–82. [Google Scholar] [CrossRef]
- Shin, J.; O’Brien, T.F.; Grayson, J.M.; Zhong, X.P. Differential regulation of primary and memory CD8 T cell immune responses by diacylglycerol kinases. J. Immunol. 2012, 188, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Mérida, I.; Avila-Flores, A.; García, J.; Merino, E.; Almena, M.; Torres-Ayuso, P. Diacylglycerol kinase alpha, from negative modulation of T cell activation to control of cancer progression. Adv. Enzym. Regul. 2009, 49, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, M.A.; Jones, D.R.; Izquierdo, M.; Mérida, I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J. Cell Biol. 2001, 153, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, M.A.; Pradet-Balade, B.; Jones, D.R.; Martínez-A, C.; Stone, J.C.; Garcia-Sanz, J.A.; Mérida, I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: A novel mechanism for Ras attenuation. J. Immunol. 2003, 170, 2877–2883. [Google Scholar] [CrossRef]
- Chauveau, A.; Le Floc’h, A.; Bantilan, N.S.; Koretzky, G.A.; Huse, M. Diacylglycerol kinase α establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci. Signal. 2014, 7, ra82. [Google Scholar] [CrossRef]
- Carrasco, S.; Mérida, I. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 2007, 32, 27–36. [Google Scholar] [CrossRef]
- Stace, C.L.; Ktistakis, N.T. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim. Biophys. Acta 2006, 1761, 913–926. [Google Scholar] [CrossRef]
- Kooijman, E.E.; Chupin, V.; de Kruijff, B.; Burger, K.N. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic 2003, 4, 162–174. [Google Scholar] [CrossRef]
- Damaj, B.B.; McColl, S.R.; Neote, K.; Hébert, C.A.; Naccache, P.H. Diverging signal transduction pathways activated by interleukin 8 (IL-8) and related chemokines in human neutrophils. IL-8 and Gro-alpha differentially stimulate calcium influx through IL-8 receptors A and B. J. Biol. Chem. 1996, 271, 20540–20544. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Wolf, M.; Qin, S.; Mackay, C.R.; Baggiolini, M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc. Natl. Acad. Sci. USA 1996, 93, 6682–6686. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, W.A.; Kharitonov, S.A.; Barnes, P.J. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax 2003, 58, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Sapey, E.; Stockley, J.A.; Greenwood, H.; Ahmad, A.; Bayley, D.; Lord, J.M.; Insall, R.H.; Stockley, R.A. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 183, 1176–1186. [Google Scholar] [CrossRef]
- Iyer, S.S.; Kusner, D.J. Assay of phospholipase D activity in cell-free systems. Methods Mol. Biol. 2006, 332, 281–298. [Google Scholar] [CrossRef]
- Lehman, N.; Di Fulvio, M.; McCray, N.; Campos, I.; Tabatabaian, F.; Gomez-Cambronero, J. Phagocyte cell migration is mediated by phospholipases PLD1 and PLD2. Blood 2006, 108, 3564–3572. [Google Scholar] [CrossRef]
- Antonescu, C.N.; Danuser, G.; Schmid, S.L. Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis. Mol. Biol. Cell 2010, 21, 2944–2952. [Google Scholar] [CrossRef]
- Cai, K.; Sewer, M.B. cAMP-stimulated transcription of DGKθ requires steroidogenic factor 1 and sterol regulatory element binding protein 1. J. Lipid Res. 2013, 54, 2121–2132. [Google Scholar] [CrossRef]
- Cai, K.; Lucki, N.C.; Sewer, M.B. Silencing diacylglycerol kinase-theta expression reduces steroid hormone biosynthesis and cholesterol metabolism in human adrenocortical cells. Biochim. Biophys. Acta 2014, 1841, 552–562. [Google Scholar] [CrossRef]
- Xu, X.; Jin, T. The Novel Functions of the PLC/PKC/PKD Signaling Axis in G Protein-Coupled Receptor-Mediated Chemotaxis of Neutrophils. J. Immunol. Res. 2015, 2015, 817604. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef] [PubMed]
- Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, With Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am. J. Respir. Crit. Care Med. 2000, 161, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, J.C.; Kropski, J.A.; Blackwell, T.S. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018, 71–72, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Suryadevara, V.; Huang, L.; Kim, S.J.; Cheresh, S.; Shaaya, M.; Bandela, M.; Fu, P.; Feghali-Bostwick, C.A.; Di Paolo, G.; Kamp, D.W.; et al. Role of phospholipase D in bleomycin-induced mitochondrial reactive oxygen species generation, mitochondrial DNA damage and pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L175–L187. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Kumar, R.K.; Iyer, A.; Boswell, S.; Gerarduzzi, C.; Dadhania, V.P.; Herbert, Z.; Joshi, N.; Luyendyk, J.P.; Humphreys, B.D.; et al. Targeting Phospholipase D4 Attenuates Kidney Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Lu, W.; Schmidt Paustian, A.M.; Ge, M.Q.; Koziol-White, C.J.; Flayer, C.H.; Killingbeck, S.S.; Wang, N.; Dong, X.; Riese, M.J.; et al. Diacylglycerol kinase ζ promotes allergic airway inflammation and airway hyperresponsiveness through distinct mechanisms. Sci. Signal. 2019, 12, eaax3332. [Google Scholar] [CrossRef]
- Malerba, M.; Ricciardolo, F.; Radaeli, A.; Torregiani, C.; Ceriani, L.; Mori, E.; Bontempelli, M.; Tantucci, C.; Grassi, V. Neutrophilic inflammation and IL-8 levels in induced sputum of alpha-1-antitrypsin PiMZ subjects. Thorax 2006, 61, 129–133. [Google Scholar] [CrossRef]
- Hazari, Y.M.; Bashir, A.; Habib, M.; Bashir, S.; Habib, H.; Qasim, M.A.; Shah, N.N.; Haq, E.; Teckman, J.; Fazili, K.M. Alpha-1-antitrypsin deficiency: Genetic variations, clinical manifestations and therapeutic interventions. Mutat. Res. 2017, 773, 14–25. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldanzi, G.; Malerba, M. DGKα in Neutrophil Biology and Its Implications for Respiratory Diseases. Int. J. Mol. Sci. 2019, 20, 5673. https://doi.org/10.3390/ijms20225673
Baldanzi G, Malerba M. DGKα in Neutrophil Biology and Its Implications for Respiratory Diseases. International Journal of Molecular Sciences. 2019; 20(22):5673. https://doi.org/10.3390/ijms20225673
Chicago/Turabian StyleBaldanzi, Gianluca, and Mario Malerba. 2019. "DGKα in Neutrophil Biology and Its Implications for Respiratory Diseases" International Journal of Molecular Sciences 20, no. 22: 5673. https://doi.org/10.3390/ijms20225673
APA StyleBaldanzi, G., & Malerba, M. (2019). DGKα in Neutrophil Biology and Its Implications for Respiratory Diseases. International Journal of Molecular Sciences, 20(22), 5673. https://doi.org/10.3390/ijms20225673





